Abstract
Coronavirus disease 2019 (COVID‐19) has been shown to be associated with a lot of neurological complications, of whom Guillain‐Barre syndrome (GBS) is an important post‐infectious consequentiality. More than 220 patients with GBS have been reported thus far. We intend to share our experience with five patients of GBS where one of them had severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in the cerebrospinal fluid (CSF). This is the first‐ever report demonstrating the presence of SARS‐CoV‐2 in the CSF of an adult patient; a similar occurrence has recently been described in a pediatric patient. We wish to emphasize the fact that commonly GBS occurs as a result of a post‐infectious process but in a few cases where the symptoms of COVID‐19 and GBS occur concurrently, corresponding to the viremic phase, separate pathogenesis needs to be thought of. This para‐infectious nature is exemplified by the presence of virus in the cerebrospinal fluid of one of our patients. We review the neuroinvasive potential of SARS‐Cov‐2 in this regard and draw parallels with Cytomegalovirus, Zika virus, and Human Immunodeficiency virus‐associated occurrences of GBS.
Keywords: cerebrospinal fluid, COVID‐19, Guillain‐Barre syndrome, reverse‐transcriptase polymerase chain reaction
1. INTRODUCTION
Severe acute respiratory syndrome (SARS‐CoV‐2) infection, resulting in coronavirus disease 2019 (COVID‐19) pandemic, has been shown to be associated with a variety of neurological manifestations ranging from involvement of the brain and spinal cord to neuromuscular complications. Guillain‐Barre syndrome (GBS) is one such complication that has been reported from different parts of the world.
We are a tertiary‐care University Hospital, serving additionally as an apex (Level 3 unit) COVID‐19 referral facility,in the Northern part of India. Of 3928 reverse‐transcriptase polymerase chain reaction (RT‐PCR) positive patients for SARS‐CoV‐2 seen between March 11, 2020 and March 19, 2021, eight patients were diagnosed having GBS;1 of these five patients matched Level 1 of diagnostic certainty as per Brighton Criteria.2, 3
We share our experience of these five patients and discuss in detail the pertinence of cerebrospinal fluid (CSF) positivity for SARS‐Cov‐2, observed in one of our cases. A summary of all cases is provided in Table 1.
Table 1.
Summary of COVID‐19‐associated Guillain‐Barre syndrome cases
| Case no. | Age, sex | Days between onset of COVID‐19 symptoms and GBS | EPS | CSFa (RT‐PCR) | mEGOS | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1‐DE | 27, M | 5 days | AMAN | Negative | 6 | IVIg | Discharged on 14th day |
| 2‐SH | 35, F | 9 days | AIDP | Negative | 0b | Supportive | Discharged on 5th day |
| 3‐DU | 40, F | 20 days | AIDP | Negative | 9 | IVIg | Died on 8th day |
| 4‐MA | 48, F | 1 day | AIDP | Negative | 4 | IVIg | Discharged on 10th day |
| 5‐KA | 50, M | 2 days | AMSAN | Positive | 10 | IVIg | Discharged on 27th day |
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor sensory axonal neuropathy; CSF, cerebrospinal fluid; EPS, electrophysiological study; GBS, Guillain‐Barre syndrome; IVIg, intravenous immunoglobulin given as 0.4 gm/kg bodyweight for 5 days; mEGOS, modified Erasmus GBS Outcome Score; RT‐PCR, reverse transcriptase polymerase chain reaction.
Albuminocytologic dissociation was noted in all patients except in 3‐DU where the cell count was 12.
Score at discharge.
2. CASE 1
A 27‐year‐old gentleman (1‐DE) had complaints of fever, sore throat, and myalgia for 5 days. In the presence of myalgia, an objective presence of weakness got masked until the difficulty in going upstairs and wearing footwear was noticed; the weakness progressed to involve the upper limbs over the next 3 days. By the 6th day, he was unable to walk unaided and required support for routine chores. There was no suggestion of bulbar or pulmonary involvement, bladder or bowel complaints, or cranial nerve deficits. On examination, the patient was alert and responsive. There was generalized hypotonia; the MRC sum score was 38/60, with loss of deep tendon response (DTR) at both ankles and hyporeflexia in the remainder. Both plantars were flexor. He was detected positive by RT‐PCR (naso‐ and oro‐pharyngeal sample) for SARS‐CoV‐2; the clinical severity was labeled as moderate category. CSF examination revealed albuminocytologic dissociation (protein 185.7 mg/dL). Nerve conduction study (NCS) of all four limbs was suggestive of a motor axonal pattern. The patient was initiated on intravenous immunoglobulin (IVIg) at a dose of 0.4 gm/kg/day for 5 days; ivermectin and remdesivir were given as per protocol to manage COVID‐19. The patient responded to treatment (walking with minimal support) and was discharged on the 14th day.
3. CASE 2
A 35‐year‐old lady (2‐SH), with no comorbidities, presented with complaints of fever and anosmia for 4 days; the fever subsided but after 9 days of onset of symptoms, she developed tingling in both lower limbs followed by mild weakness. There was no suggestion of bulbar or pulmonary involvement, bladder or bowel complaints, or cranial nerve deficits. On examination, lower limbs were hypotonic; the MRC sum score was 52/60, with loss of DTR at ankles and knees with hyporeflexia in the upper limbs. Plantars were flexor. She was detected positive for COVID‐19. CSF examination revealed albuminocytologic dissociation (protein 91.6 mg/dL); NCS showed a demyelinating polyneuropathy. The patient was kept under observation and managed as a case of COVID‐19 with mild severity. She was discharged on the 5th day with advice to report urgently in the event of any deterioration. At 6 weeks post‐discharge, the patient had improved completely.
4. CASE 3
A 40‐year‐old lady (3‐DU) was referred to our facility with areflexic quadriparesis (MRC sum score: 18/60) with suggestion of bifacial and bulbar involvement. After having developed fever and breathlessness, she had been diagnosed with COVID‐19 and was on treatment for the same. After around 20 days of onset of symptoms of COVID‐19, she experienced paresthesias in her lower limbs associated with weakness rapidly progressing from lower to upper limbs, followed by weakness of the respiratory muscles. Besides hepatic impairment, she had markedly elevated levels of C‐reactive protein (82.3 mg/L), d‐dimer (3.65), fibrinogen (655 mg/dL), and lactate dehydrogenase (5596 U/L). Her computed tomography severity score was 18/25. CSF examination showed 12 cells/mm3 (lymphocytes 75%), protein 157.2 mg/dL, and glucose 81.9 mg/dL (blood glucose 118 mg/dL). NCS was suggestive of demyelinating polyneuropathy. She had worsening pulmonary functions and was put on ventilatory support. Besides IVIg, antibiotics were continued along with methylprednisolone. She continued a downhill course and succumbed to her illness on the 8th of admission.
5. CASE 4
A 48‐year‐old lady (4‐MA), a known case of constrictive pericarditis, presented with complaints of both lower limb paresthesias and weakness for 3 days, preceded by a history of fever and sore throat for a day. She was detected positive by RT‐PCR for COVID‐19; the severity of her disease was classified as mild. There was no suggestion of bulbar or pulmonary involvement, bladder or bowel complaints, or cranial nerve deficits. On examination, there was hypotonia in the lower limbs; the MRC sum score was 44/60, with loss of DTR at both ankles and hyporeflexia in the remainder. Both plantars were flexor. CSF examination revealed albuminocytologic dissociation (protein 185.7 mg/dL). NCS was suggestive of demyelinating polyneuropathy. IVIg was given to the patient (0.4 gm/kg/bodyweight/day for 5 days) to which she showed good improvement. At discharge (10th day), her buckling of knees had improved but foot dorsiflexors were still weak (MRC 3/5). She had minimal weakness at ankles at 8 weeks post‐discharge.
6. CASE 5
A 50‐year‐old gentleman (5‐KA) presented with complaints of weakness of all limbs and breathlessness for 9 days. To start, the patient had a mild headache and sore throat for 2 days, which was followed by tingling sensation involving both feet. The tingling progressed to involve the upper limbs over another 2 days and culminated in weakness of the involved limbs. On examination, the patient was conscious and oriented. All four limbs had hypotonia; the MRC‐sum score was 22/60, with areflexia in the lower limbs and hyporeflexia in the upper limbs. Ocular and facial musculature was not involved. The plantars were non‐elicitable. Autonomic dysfunction per se could not be ascertained owing to ongoing tachypnea, hypoxemia, and associated tachycardia. Cerebrospinal fluid (CSF) showed 10 cells/mm3 (lymphocytes 90%), protein 90.2 mg/dL, and glucose‐96.6 mg/dL (blood glucose 126 mg/dL). The CSF was detected to be positive for SARS‐Cov‐2 virus by RT‐PCR (ct value: 31). Intrigued by CSF positivity, a repeat lumbar puncture was done after 48 h to look for SARS‐CoV‐2 virus; the CSF again tested positive for SARS‐CoV‐2 virus by RT‐PCR. The march of events has been depicted as a timeline in Figure 1.
Figure 1.
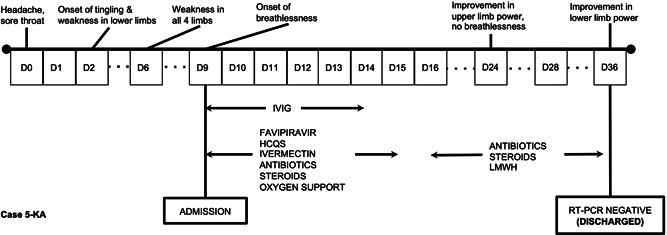
Depicts the time‐line of events during the hospital stay of Case 5‐KA
7. DISCUSSION
GBS is the commonest neuromuscular complication reported with COVID‐19. We report five patients of COVID‐19‐associated GBS where CSF of one of them was tested positive for SARS‐Cov‐2 by RT‐PCR. Recent publications have attempted to define the associated mechanisms as either dysregulated inflammatory responses or autoantibody mediated.4 Dysimmune response has been considered as the most important mechanism of GBS in patients with COVID‐19. However, this might not be the only mechanism leading to GBS, and CSF‐positivity in one of our cases makes us inquisitive to seek a deeper insight into the pathophysiological processes. Direct injury has also been hypothesized in these cases; our Case (5‐KA) attests to the possibility of such an occurrence.4
More than 220 occurrences of GBS have been reported to date, with possibly more in the process of getting published.5, 6, 7 A review of literature comprising of patients with GBS associated with COVID‐19 shows that the age range at presentation is wide (8 to 94 years) with a male preponderance (male:female = 2.18). The duration between the onset of GBS and COVID‐19 may be variable ranging from −10 days (preceding COVID‐19) to +90 days (succeeding COVID‐19 symptoms) with most patients (~95%) occurring after the onset of COVID‐19 symptoms. Amongst the GBS‐variants (calculated as‐reported), acute inflammatory demyelinating polyneuropathy (~75%) is the most common type followed by acute motor axonal neuropathy (~11%) and acute motor‐sensory axonal neuropathy (~7%) variety; other variants such as Miller–Fisher syndrome, polyneuritis cranialis, and the pharyngeal, cervical, and brachial variant, are uncommon to rare. It has also been suggested that COVID‐19‐associated GBS has a relatively poorer outcome than GBS occurring otherwise.7
The immune‐mediated phenomenon in COVID‐associated GBS seems plausible for most patients but another pathophysiological process needs to be thought of in a group of outliers. This group of outliers is constituted by patients in whom the symptoms of GBS either precede or are almost concurrent with the symptoms of COVID‐19. The point in question is of deciding whether the association fits into a postinfectious process or with a para‐infectious process. For a postinfectious process, it is reasonably assumed that the time elapsed between the onset of symptoms of COVID‐19 was sufficient enough to lead to the generation of antibodies demonstrating molecular mimicry and triggering an autoimmune process. In contrast, in a para‐infectious process, such an interval is not present, and an alternative mechanism needs to be looked into. Regarding the latter, it may be assumed that either the symptoms were non‐manifest in nature and directly led to the dysimmune complication, or that the SARS‐Cov‐2 virus was capable of inflicting direct damage to radicles and nerves.
In at least four published cases, and the one that we discussed, COVID‐associated GBS can be presumed to have occurred as a result of a para‐infectious process. In four of these cases, the manifestation of GBS followed the onset of symptoms of COVID‐19 by 1, 2, and 8 (two cases) days while the fifth case did not have any COVID‐related symptom. Two of our Cases (4‐MA and 5‐KA) also fall into this category. Our view is attested by a recent report demonstrating the SARS‐Cov‐2 virus in the CSF of a 17‐year‐old girl with GBS, during the phase of viremia.8 We feel that these patients are outliers and do not conform to the typical immune‐mediated process. An insight into this type of involvement can be had from the outbreak of Zika virus infection when similar neurological manifestations were described.9, 10 In Zika virus infection, 48% of patients have neurological symptoms occurring either concurrently or shortly after the initial symptoms of Zika virus disease, that is para‐infectious in onset (corresponding to viremia). Besides demonstrating corresponding antibody responses in the CSF, the Zika virus genome was also detected in three patients proving its neurotropic potential and the phenomenon of neuro‐invasiveness. Our Case (5‐KA), similarly, raises the possibility of direct SARS‐CoV‐2 virus‐mediated radicular injury by demonstrating the genome in the CSF in two samples taken 48 h apart. It may be appreciated that the CSF has not been tested for SARS‐CoV‐2 in all cases of COVID‐19‐associated GBS reported in the literature; thus, we may not be having the actual statistics on its presence in the CSF.5, 6, 7
Besides the possibility of direct virus‐mediated injury in COVID‐19‐associated GBS, a different type of immune‐modulation, that is, immunodeficiency merits discussion. Either as a consequence of cytokine storm leading to a state of immune‐exhaustion or secondary to the reduced functional diversity of T‐cells, there seems to a state of relative immunodeficiency in patients with COVID‐19.11, 12, 13 A corollary may be drawn from at least 12 published reports of cytomegalovirus (CMV) infection leading to the development of GBS in patients with renal transplantation. This almost established association, where CMV has been demonstrated in the CSF of patients with GBS, provides us a surrogate how immunodeficiency may enhance neuroinvasiveness of an otherwise commonly detected virus (CMV) in renal transplant patients.14 Similarly, GBS has been seen in patients infected with human immunodeficiency virus (HIV), either in the state of pure immunodeficiency or as a part of immune‐reconstitution syndrome.15
Looking at the literature on COVID‐19, it will be appropriate to assume that most patients with GBS occur as a result of an autoimmune process. The remainder, however, may fit into a para‐infectious course suggestive of the neuroinvasive potential of SARS‐CoV‐2 virus, occurring during viremia. We, therefore, feel that such a possibility should be considered in these patients and CSF must be tested in all patients suspected of COVID‐19‐associated GBS for SARS‐CoV‐2 to have a better understanding of an alternative pathogenesis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Concept and design of the study: Farman Khan, Saurabh Pandey, Deepak Sharma, Suruchi Shukla, Himanshu Dandu, Ravindra Kumar Garg, and Hardeep Singh Malhotra. Acquisition of data: Farman Khan, Praveen Sharma, Saurabh Pandey, Deepak Sharma, Vijayavarman V, Neeraj Kumar, Himanshu Dandu, Amita Jain, and Hardeep Singh Malhotra. Analysis and interpretation of data: Praveen Sharma, Vijayavarman V, Neeraj Kumar, Suruchi Shukla, Amita Jain, Ravindra Kumar Garg, and Hardeep Singh Malhotra. Drafting of manuscript: Farman Khan, Saurabh Pandey, Deepak Sharma, Neeraj Kumar, Suruchi Shukla, Himanshu Dandu, Amita Jain, Ravindra Kumar Garg, and Hardeep Singh Malhotra. Final approval: All the authors.
ACKNOWLEDGMENT
Funding was not involved in the conduct of this study.
Khan F, Sharma P, Pandey S, et al. COVID‐19‐associated Guillain‐Barre syndrome: Postinfectious alone or neuroinvasive too? J Med Virol. 2021;93:6045‐6049. 10.1002/jmv.27159
REFERENCES
- 1.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barre syndrome. Ann Neurol. 1990;27(Suppl):S21‐S24. [DOI] [PubMed] [Google Scholar]
- 2.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain‐Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33‐43. [DOI] [PubMed] [Google Scholar]
- 3.Goodfellow JA, Willison HJ. Guillain‐Barré syndrome: a century of progress. Nat Rev Neurol. 2016;12:723‐731. [DOI] [PubMed] [Google Scholar]
- 4.Freire M, Andrade A, Sopeña B, et al. Guillain Barré syndrome associated with COVID‐19‐lessons learned about its pathogenesis during the first year of the pandemic, a systematic review. Autoimmun Rev. 2021:102875. 10.1016/j.autrev.2021.102875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu‐Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain‐Barré syndrome spectrum associated with COVID‐19: an up‐to‐date systematic review of 73 cases. J Neurol. 2021;268:1133‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanda S, Handa R, Prasad A, et al. Covid‐19 associated Guillain‐Barre Syndrome: contrasting tale of four patients from a tertiary care centre in India. Am J Emerg Med. 2021;39:125‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finsterer J, Scorza FA. Guillain‐Barre syndrome in 220 patients with COVID‐19. Egypt J Neurol Psychiatr Neurosurg. 2021;57:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araújo NM, Ferreira LC, Dantas DP, et al. First report of SARS‐CoV‐2 detection in cerebrospinal fluid in a child with Guillain‐Barré syndrome. Pediatr Infect Dis J. 2021;40(7):e274‐e276. 10.1097/INF.0000000000003146 [DOI] [PubMed] [Google Scholar]
- 9.Parra B, Lizarazo J, Jiménez‐Arango JA, et al. Guillain–Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375:1513‐1523. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz LS, Parra B, Pardo CA. Neuroviruses emerging in the Americas Study: neurological implications of Zika virus infection in adults. J Infect Dis. 2017;216(suppl_10):S897‐S905. 10.1093/infdis/jix511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17:541‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS‐CoV‐2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792. 10.1016/j.autrev.2021.102792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merzkani M, Israel E, Sachdeva M. Primary cytomegalovirus infection causing Guillain‐Barré syndrome in a living renal allograft recipient. Case Rep Transplant. 2017;2017:7264793. 10.1155/2017/7264793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brannagan TH III, Zhou Y. HIV‐associated Guillain–Barré syndrome. J Neurol Sci. 2003;208:39‐42. [DOI] [PubMed] [Google Scholar]


