Abstract
Background
The COVID‐19 pandemic may induce post‐traumatic stress disorder (PTSD) symptoms among patients with cancer, who also face adaptations to their treatment. The authors assessed the occurrence of PTSD symptoms, investigated pandemic‐induced adjustments in medical oncology practice in patients with cancer, and explored risk factors for PTSD and the association between PTSD symptoms, insomnia, and quality of life (QoL).
Methods
This prospective French study was conducted in patients with solid/hematologic tumors who were receiving medical treatment in the day care departments of 2 cancer centers during the lockdown. Adjustments to medical oncology practice were collected from medical records. PTSD (measured using the Impact of Event Scale‐Revised), insomnia (measured using the Insomnia Severity Index), QoL (measured using the Functional Assessment of Cancer Therapy‐General instrument), and cognitive complaints (measured using the Functional Assessment of Cancer Therapy–Cognitive Function instrument) were collected through validated questionnaires.
Results
Clinical data and questionnaires were available for 734 and 576 patients, respectively. The median patient age was 64 years, and 69% of patients were women. Twenty‐one percent of patients had PTSD. Twenty‐seven percent (95% CI, 23%‐30%) had an adjustment in their medical oncology program, including adjournments (29%), treatment interruptions (16%), modified treatment plans (27%), or adapted monitoring (27%). Women and patients experiencing an adjustment in oncology practice had a higher odds of PTSD (odds ratio= 2.10 [95% CI, 1.07‐4.14] and 1.65 [95% CI, 1.03‐2.63]; P < .05). PTSD symptoms were correlated with worse scores for QoL, cognition, and insomnia.
Conclusions
Twenty‐one percent of patients with cancer experienced PTSD symptoms associated with poor QoL during the first COVID‐19–induced lockdown. Medical oncology practice was adjusted in approximately one‐quarter of patients and was associated with the occurrence of PTSD symptoms. Psychosocial support should be offered in cancer centers to promote emotional resilience and avoid PTSD symptoms in patients.
Keywords: COVID‐19, lockdown, patients with cancer, post‐traumatic stress disorder (PTSD), treatment adjustments
Short abstract
Post‐traumatic stress disorder symptomatology occurred in 21% of patients with cancer during the first lockdown due to COVID‐19, was more frequent among women, and was associated with adjustment in medical oncology treatments. Caregivers should pay special attention to the psychological needs of patients with cancer to prevent or manage post‐traumatic stress disorder symptoms.
Introduction
COVID‐19 1 was first diagnosed in France on January 24, 2020. 2 The pandemic rapidly spread, leading to the implementation of a nationwide lockdown from March 17, 2020, to May 11, 2020. A major disease outbreak like the COVID‐19 pandemic may induce symptoms of post‐traumatic stress disorder (PTSD), 3 especially in people at high risk of infection. Several studies have reported a higher risk of COVID‐19 infection and induced complications in patients with cancer. 4 In response to both this higher vulnerability of patients with cancer to COVID‐19 and the lockdown measures, guidelines were issued to adjust oncologic care during the COVID‐19 pandemic. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 This led to frequent delays and disruptions in cancer health care. 15 Altogether, the fear of becoming infected, 16 , 17 the fear of cancer care disruption, and social isolation because of the lockdown measures 18 may have added stress in an already vulnerable cancer population. Indeed, cancer diagnosis and treatment may already be experienced as traumatic in a substantial proportion of patients with cancer, especially those who have a history of trauma, psychiatric conditions, low socioeconomic status, young age, advanced disease, invasive treatment, reduced quality of life (QoL), and poor social support. 19 Various surveys have shown that the psychological impact of the COVID‐19 pandemic has led to increased levels of stress and anxiety in patients with cancer. 20 , 21 , 22 , 23 To our knowledge, only 1 small study in hematologic patients used a dedicated scale to explore the frequency of PTSD symptoms in patients with cancer during the first COVID‐19 lockdown. 20 That study identified high levels of psychological distress, especially among younger patients and women. To date, no study has focused on the association between PTSD symptoms and COVID‐19–induced adjustments in terms of changes in medical oncology practice. However, adjustments may include delayed or modified treatments and cancellation or postponement of monitoring visits, all of which are likely to induce stress in patients with cancer. In addition, a relation between PTSD and QoL has been observed in patients who have cancer, 24 and both post‐traumatic experiences and poor QoL were associated with sleep disturbances in those with advanced disease. 25 Moreover, previous studies have suggested that perceived cognitive dysfunction in patients with breast cancer may be mediated by post‐traumatic stress. 26 The relations between PTSD and QoL, insomnia, and cognitive complaints have not been yet explored in patients with cancer during the COVID‐19 pandemic.
We conducted a large, prospective study in patients with solid/hematologic malignancy who were receiving medical treatment during the first COVID‐19 lockdown in the outpatient clinics of 2 regional French cancer centers. Our objectives were: 1) to estimate the proportion of patients with cancer who had PTSD symptomatology during the first lockdown period, 2) to assess pandemic‐induced adjustments in medical oncology practice and their association with PTSD symptoms, 3) to assess demographic and clinical factors associated with PTSD symptoms, and 4) to investigate the association between PTSD symptoms and insomnia, QoL, and cognitive complaints in patients with cancer.
Materials and Methods
Study Design and Participants
COVIPACT is a prospective study conducted in 2 French cancer centers. The study population includes adult outpatients who were receiving an oncologic treatment at the day care hospital that was initiated before or during the pandemic lockdown, from the beginning of the first nationwide lockdown on March 17, 2020, until May 29, 2020. Patients who were at least 18 years old and were being treated for solid or hematologic cancers during the first lockdown were included.
Data Collection
Medical evaluation
Demographics and clinical information, such as patient characteristics, initial cancer treatment, and pandemic‐induced adjustments in medical oncology practice, were extracted from medical records. History of psychological disorders was based on past or present consumption of psychotropic drugs or known condition, as reported in the medical records.
Enrolled patients were asked to complete validated self‐report questionnaires on PTSD symptoms, insomnia, QoL, and cognition. Questionnaires were administered from April 16, 2020, to May 29, 2020, ie, during the first lockdown in France.
Post‐traumatic stress evaluation
Participants completed the Impact of Event Scale‐Revised (IES‐R) questionnaire, 27 a 22‐item questionnaire that assesses subjective distress caused by traumatic events. Because the authors of the IES‐R allow instructions for the assessed event to be adapted, patients participating in the COVIPACT study were asked the following: “For each IES‐R item, indicate how much you were troubled over the last 7 days regarding the COVID‐19 pandemic.” Items are rated on a 5‐point scale, ranging from 0 (not at all) to 4 (extremely). The IES‐R yields a total score ranging from 0 to 88, and scores can also be calculated for the intrusion, avoidance, and hyperarousal subscales. A total IES‐R score ≥33 indicates PTSD symptomatology. 28
Quality‐of‐life evaluation
Patients answered validated self‐administered questionnaires to evaluate their QoL. The Functional Assessment of Cancer Therapy‐General (FACT‐G) is a 27‐item questionnaire designed to measure 4 domains of health‐related QoL in patients with cancer: physical, social, emotional, and functional well‐being (6 or 7 items each).
Cognitive complaints evaluation
The Functional Assessment of Cancer Therapy‐Cognitive Function (FACT‐Cog) assesses cognitive complaints on 4 subscales 29 : perceived cognitive impairments (20 items; score range, 0‐72), impact on QoL (4 items; score range, 0‐16), comments from others (4 items; score range, 0‐16), and perceived cognitive abilities(9 items; score range, 0‐28).
Insomnia evaluation
The 7‐item Insomnia Severity Index (ISI) was used to assess the severity of both nighttime and daytime components of insomnia. 30 A 5‐point Likert scale is used to rate each item from 0 (no problem) to 4 (very severe problem). The total score ranges from 0 to 28 and is interpreted as follows: absence of insomnia (0‐7), subthreshold insomnia (8‐14), moderate insomnia (15‐21), and severe insomnia (22‐28).
All questionnaires were administered to 576 patients. Only questionnaires with an overall item response rate >80% were considered valid for analyses (ie, between 560 and 567, depending on the questionnaires). The remaining missing items were imputed using the average of completed items.
Study Outcomes
The main outcomes were the proportion of patients with cancer who had PTSD symptomatology during the first lockdown period and the proportion of patients with pandemic‐induced adjustments in medical oncology practice.
Adjustment of medical oncology practice referred to any change from standard practice and treatment that was made to prevent the spread of the COVID‐19 during the first lockdown. The following were considered adjustments in medical oncology practice: any adaptation in type of treatment (chemotherapy, immunotherapy), in treatment plan (rhythm of administration, cycles of treatment), in method of administration (patient's home instead of day care), and in monitoring (phone or video consultation) and any treatment adjournment or interruption. Each adjustment was discussed and validated during multidisciplinary meetings. Secondary outcomes included QoL, insomnia, and cognition.
Statistical Analysis
The minimal number of participants needed to assess the proportion of patients with pandemic‐induced adjustments in medical oncology practice was estimated to be 385 patients with a 95% CI and a maximal 5% margin of error.
Characteristics of patients were described using numbers and proportions for categorical variables and means and standard deviations (or medians and interquartile ranges) for continuous variables. Clinical characteristics were compared according to adjustments in medical oncology practice using χ2 tests and Student tests.
We used logistic regression to assess the association between adjustments in medical oncology practice and clinical factors (factors were selected based on P values < .10 in univariate analysis) with odds of PTSD symptoms. We also used linear regression to analyze IES‐R total scores and subscale scores as continuous measures. Analyses were controlled for the study center.
Scales and subscales of QoL, cognitive complaints, and insomnia were described and compared according to PTSD symptoms using the Mann‐Whitney‐Wilcoxon test. Associations between PTSD symptoms and QoL, cognitive complaints, and insomnia were then assessed in linear models adjusted for the previously selected clinical factors. Differences >10% on QoL scales were considered clinically relevant. 31 , 32
All statistical analyses were carried out using R statistical software (4.0.3). A 2‐side P value < .05 was considered statistically significant.
Ethics Approval
Approval for the study was obtained from the local ethics committee (ref. 220 C07; South Mediterranean II Committee for the Protection of Persons). The study was conducted in compliance with the French research standard (MR‐003 “Research in the Field of Health Without Collection of Consent”; compliance commitment to MR‐003 for the Francois Baclesse Center [no. 2146328 v.0, dated from January 26, 2018]). All patients received information and none expressed any opposition to the use of their data. The trial is registered as Regional Center for Biology identifier 2020‐A00879‐30 (ClinicalTrials.gov identifier trial NCT04366154).
Results
In total, there were 734 patients in the study, including 576 who completed at least 1 self‐administered questionnaire on stress, insomnia, QoL, and cognition (Fig. 1). Characteristics of the whole sample are presented in Table 1.
Figure 1.
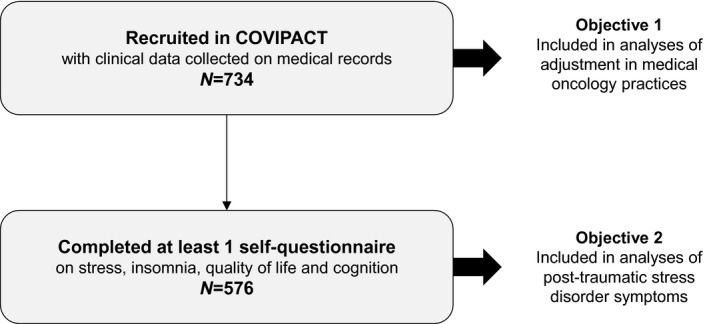
Flow chart of participants in the COVIPACT study (N = 734; ClinicalTrial.gov identifier NCT 04366154).
TABLE 1.
Univariate Association Between Demographic and Clinical Characteristics and Cancer Treatment Modifications, N = 734
| Patient Characteristic | No. of Patients (%) a | P b | ||
|---|---|---|---|---|
| Total Sample, N = 734 | Adjustment in Medical Oncology Practice, N = 195 (27%) | No Adjustment in Medical Oncology Practice, N = 539 (73%) | ||
| Age, y | ||||
| Mean ± SD | 62.3 ± 11.7 | 63.1 ± 10.6 | 62.0 ± 12.1 | .29 |
| ≥70 | 215 (29) | 56 (29) | 159 (29) | .91 |
| <70 | 519 (71) | 139 (71) | 380 (71) | |
| Sex | ||||
| Female | 509 (69) | 133 (68) | 376 (70) | .76 |
| Male | 225 (31) | 62 (32) | 163 (30) | |
| BMI: Mean ± SD, kg/m2 | 25.4 ± 5.1 | 25.0 ± 5.0 | 25.6 ± 5.2 | .16 |
| ECOG performance status | ||||
| 0 or 1 | 664 (91) | 168 (88) | 496 (93) | .54 |
| ≥2 | 63 (9) | 23 (12) | 40 (7) | |
| Type of cancer | <.01 c | |||
| Breast cancer | 304 (41) | 82 (42) | 222 (41) | .90 |
| Lung, head and neck cancer | 163 (22) | 65 (33) | 98 (18) | <.01 c |
| Digestive system cancer | 123 (17) | 12 (6) | 111 (21) | <.01 c |
| Gynecologic cancer | 78 (11) | 16 (8) | 62 (12) | .25 |
| Urologic cancer | 29 (4) | 6 (3) | 23 (4) | .61 |
| Other solid and hematologic cancer | 37 (5) | 14 (7) | 23 (4) | .16 |
| Stage of solid cancer d | ||||
| Metastatic | 435 (60) | 124 (65) | 311 (58) | .13 |
| Localized | 289 (40) | 67 (35) | 222 (42) | |
| De novo treatment | ||||
| Yes | 360 (49) | 76 (39) | 284 (53) | .01 c |
| No | 374 (51) | 119 (61) | 255 (47) | |
| Therapy | <.01 c | |||
| Chemotherapy alone | 361 (49) | 59 (30) | 302 (56) | <.01 c |
| Targeted therapy alone | 136 (19) | 62 (32) | 74 (14) | <.01 c |
| Chemotherapy and targeted therapy | 128 (17) | 31 (16) | 97 (18) | .58 |
| Immunotherapy alone | 61 (8) | 26 (13) | 35 (6) | <.01 c |
| Other treatment e | 48 (7) | 17 (9) | 31 (6) | .21 |
| Initiation of treatment | ||||
| Before lockdown f | 462 (63) | 157 (81) | 305 (57) | <.01 c |
| During lockdown | 272 (37) | 38 (19) | 234 (43) | |
| History of chronic conditions | ||||
| Hypertension | 248 (34) | 70 (36) | 178 (33) | .48 |
| Cardiovascular disease | 166 (23) | 27 (14) | 64 (12) | .57 |
| Pulmonary disease | 116 (16) | 38 (19) | 78 (14) | .10 |
| Other cancer | 105 (14) | 30 (15) | 75 (14) | .62 |
| Diabetes | 91 (12) | 27 (14) | 64 (12) | .48 |
| Psychological disorders | 58 (8) | 22 (11) | 36 (7) | .04 c |
| Kidney disease | 28 (4) | 11 (6) | 17 (3) | .12 |
| Immune disease | 16 (2) | 4 (2) | 12 (2) | .88 |
| Other chronic condition | 174 (24) | 49 (25) | 144 (27) | .66 |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
Values are or N (%) of nonmissing data, unless specified otherwise. Data were missing for <1% of patients (3 were missing BMI, 7 were missing ECOG performance status, and 1 was missing history of chronic conditions).
P values were derived from Student tests or χ2 tests.
This P value indicates a significant difference.
The analysis excluded 10 hematologic cancers.
Other treatment includes any combinations of immunotherapy or hormonotherapy with other therapy (all administered to <15 patients [<2%]).
Lockdown in France started on March 17, 2020.
Demographic and Clinical Characteristics
Among the 734 patients included in the study, the median age was 64 years, and 29% of patients were older than 70 years; 69% were women, and 91% had an Eastern Cooperative Oncology Group score of 0 or 1 (Table 1). The median time since cancer diagnosis was 14 months. Patients were mostly treated for breast cancer (41%); lung, head, and neck cancer (22%); digestive system cancer (17%); or gynecologic cancer (11%). Chemotherapy was the most frequent therapy (49%), followed by targeted therapy (19%), a combination of chemotherapy and targeted therapy (17%), and immunotherapy alone (8%). More than one‐half of patients had initiated their current cancer treatment before lockdown (63%), with a median time since treatment initiation of 2.2 months.
Changes in Medical Oncology Practice
Medical oncology practice was modified in 195 patients (27%; 95% CI, 23%‐30%). Changes were more frequent in patients who were treated with immunotherapy alone (which concerned mainly patients with lung, head, and neck cancer) or targeted therapy alone (mainly patients with breast cancer) (Table 1). Patients who received de novo treatment and who initiated their treatment after the beginning of the first lockdown were less likely to experience an adjustment in their medical oncologic treatment.
Among these 195 patients, adjustments in medical oncology practice included adjournments (29%), treatment interruptions (16%), adapted treatment plan (mostly adaptation in the rhythm of administration; 27%), adapted monitoring (mostly phone/video consultation; 27%), and other modifications. such as a change of treatment type or administration method (10%). Details of modifications by clinical subgroups are provided in Figure 2.
Figure 2.
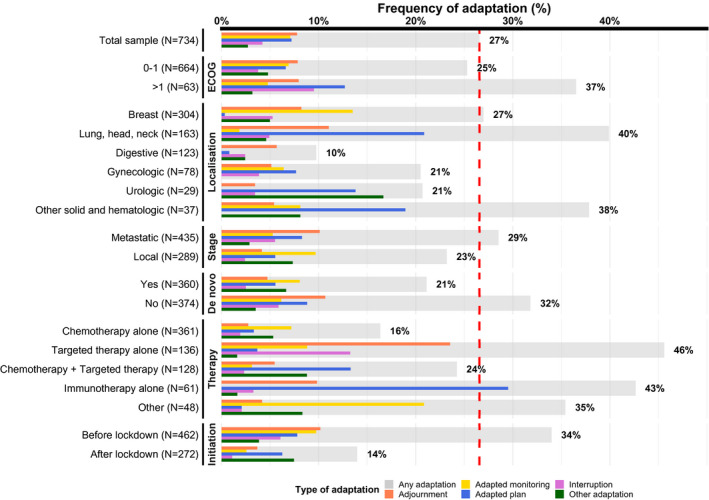
Adjustment in medical oncology practice by clinical characteristics. Adjustment in medical oncology practice (any adaptation or specific types of adaptations) are presented as percentages of the total sample by clinical characteristics. The red dashed line indicates the percentage of any adaptation in the total patient sample (27%). ECOG indicates Eastern Cooperative Oncology Group.
PTSD Symptoms and Associated Factors
The subgroup of 576 patients who completed at least 1 self‐administered questionnaire had the same characteristics as the overall sample (Table 2; see Supporting Table 1). Approximately 21% of them had PTSD symptomatology. All IES‐R subscale scores were higher in patients who had PTSD symptoms: median scores in patients with versus without PTSD were 15 versus 6 for avoidance, 18 versus 5 for intrusion, and 11 versus 2 for hyperarousal (see Supporting Table 2).
TABLE 2.
Multivariate Associations Between Clinical Factors and Post‐Traumatic Stress Disorder Symptomatology, N = 563
| Variable | No. of Patients (%) | OR [95% CI] a | P | |
|---|---|---|---|---|
| No PTSD Symptoms: IES‐R Score <33 | PTSD Symptoms: IES‐R Score ≥33 | |||
| Total sample | 443 (79) | 120 (21) | ||
| Age, y | .26 | |||
| <70 | 322 (77) | 95 (23) | 1.34 [0.81‐2.26] | |
| ≥70 | 121 (83) | 25 (17) | 1.00 | |
| Sex | .030 b | |||
| Male | 133 (86) | 21 (14) | 1.00 | |
| Female | 310 (76) | 99 (24) | 2.10 [1.07‐4.14] | |
| Type of cancer | .88 | |||
| Digestive system cancer | 80 (86) | 13 (14) | 1.00 | |
| Breast cancer | 191 (76) | 60 (24) | 1.17 [0.58‐2.51] | |
| Lung, head and neck cancer | 87 (80) | 22 (20) | 1.43 [0.66‐3.23] | |
| Gynecologic cancer | 46 (74) | 16 (26) | 1.46 [0.61‐3.57] | |
| Urologic cancer | 20 (87) | 3 (13) | 1.04 [0.22‐3.68] | |
| Other solid and hematologic cancer | 19 (76) | 6 (24) | 1.75 [0.53‐5.42] | |
| History of psychological disorders | .13 | |||
| No | 412 (80) | 105 (20) | 1.00 | |
| Yes | 31 (67) | 15 (33) | 1.68 [0.85‐3.23] | |
| Adjustment in medical oncology practice | .037 b | |||
| No | 336 (81) | 78 (19) | 1.00 | |
| Yes | 107 (72) | 42 (28) | 1.65 [1.03‐2.63] | |
Abbreviations: IES‐R, Impact of Event Scale‐Revised; OR, odds ratio; PTSD, post‐traumatic stress disorder.
ORs (95% CIs) for PTSD symptomatology were estimated using logistic regression, and the model was adjusted for study center.
This P value indicates a significant difference.
In univariate analysis, PTSD symptomatology was more frequent in patients younger than 70 years (23%), women (24%), patients with breast cancer (24%) or gynecologic cancer (26%), those who had a history of psychological disorder (33%), and those whose medical oncology practice was adjusted (28%) (Table 2). We observed no difference in time since cancer diagnosis according to PTSD symptomatology. In multivariate models adjusted for age, study center, type of cancer, and history of psychological disorders, women and patients whose medical oncology practice was adjusted had statistically higher odds of PTSD symptoms (OR, 2.10 [95% CI, 1.07‐4.14] and 1.65 [95% CI, 1.03‐2.63]; P < .05). When considering the IES‐R score as a continuous measure, only sex remained significantly associated with more symptoms on the global scale and subscales.
PTSD Symptoms: Quality of Life, Cognition, and Insomnia
Patients with PTSD symptomatology had the worst scores on all dimensions of QoL (FACT‐G; except on the FACT‐G social well‐being subscale), cognition (FACT‐Cog), and insomnia (ISI) (Fig. 3; see Supporting Table 2). Average differences in scores among patients with or without PTSD symptoms were −17.6 [95% CI, −20.5, −14.8] for the total FACT‐G score, −13.8 [95% CI, −16.1; −11.5] for the FACT‐Cog perceived subscale score, and 6.8 [95% CI, 5.6‐8.0] for the ISI (all P < .001) (Table 3). Associations remained similar and statistically significant after adjustment for age, sex, study center, type of cancer, history of psychological disorder, and adjustments in medical oncology practice during the first lockdown (Table 3).
Figure 3.
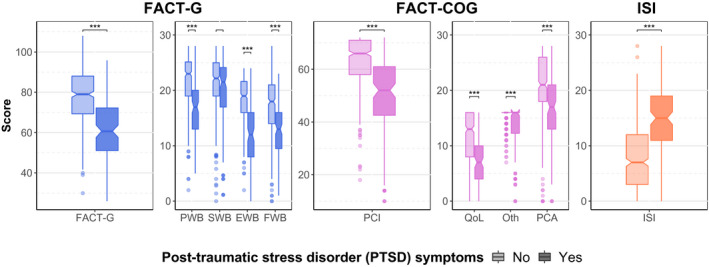
Scales and subscales of quality of life (QoL), cognition, and insomnia by post‐traumatic stress disorder (PTSD) symptoms. For the Functional Assessment of Cancer Therapy‐General (FACT‐G) and the FACT‐Cognitive Function (FACT‐Cog), higher scores indicate better QoL and cognition, respectively. For the Insomnia Severity Index (ISI), higher scores indicate greater severity of insomnia. Pairwise comparisons are from the Mann‐Whitney‐Wilcoxon test. Three asterisks indicate P values < .001. EWB indicates emotional well‐being; FWB, functional well‐being; Oth, other; PCA, perceived cognitive abilities; PCI, perceived cognitive impairments; PWB, physical well‐being; SWB, social well‐being.
TABLE 3.
Multivariate Associations Between Post‐Traumatic Stress Disorder Symptoms and Indices of Quality of Life, Cognition, and Insomnia
| Measure | No PTSD Symptoms: IES‐R Score <33 | PTSD Symptoms: IES‐R Score ≥33 | P |
|---|---|---|---|
| Total sample | 79% | 21% | |
| FACT‐G, n = 551 | |||
| Mean score ± SD | 78.7 ± 13.8 | 61.3 ± 14.0 | |
| Unadjusted β [95% CI] a | Reference | −17.6 [−20.5, −14.8] | <.01 b |
| Adjusted β [95% CI] | Reference | −17.5 [−20.3, −14.6] | <.01 b |
| FACT‐Cog PCI, n = 553 | |||
| Mean score ± SD | 63.1 ± 9.8 | 49.3 ± 15.3 | |
| Unadjusted β [95% CI] | Reference | −13.8 [−16.1, −11.5] | <.01 b |
| Adjusted β [95% CI] | Reference | −13.6 [−15.9, −11.3] | <.01 b |
| ISI, n = 552 | |||
| Mean score ± SD | 8.2 ± 6.0 | 14.9 ± 5.7 | |
| Unadjusted β [95% CI] | Reference | 6.8 [5.6‐8.0] | <.01 b |
| Adjusted β [95% CI] | Reference | 6.4 [5.1‐7.6] | <.01 b |
Abbreviations: FACT‐Cog, Functional Assessment of Cancer Therapy‐Cognitive Function; FACT‐G, Functional Assessment of Cancer Therapy‐General; IES‐R, Impact of Event Scale‐Revised; ISI, Insomnia Severity Index; PCI, perceived cognitive impairments subscale;PTSD, post‐traumatic stress disorder.
β‐coefficients (95% CIs) were calculated from linear models. Adjustment includes age of patients, sex, study center, type of cancer, history of psychological disorders, and adjustment in medical oncology practice during lockdown.
This P value indicates a significant difference.
Discussion
To our knowledge, COVIPACT is the first large study to focus on both the occurrence of PTSD symptoms and adjustments in medical oncology practice in patients with cancer during the first COVID‐19 lockdown using validated self‐report questionnaires and data from medical files. A few smaller studies assessed either PTSD symptoms or treatment adjustments in patients with cancer during the COVID‐19 pandemic and found results in line with ours.
The occurrence of PTSD symptoms in patients with cancer during the first COVID‐19 lockdown was first investigated by Romito et al 20 in a study of 77 outpatients with lymphoma in Italy. Those authors found that 36% of patients had PTSD symptoms according to the IES‐R. This higher proportion of PTSD compared with that found in our patients might be caused by differences between study populations, particularly differences in the incidence of COVID‐19. Romito et al also found more PTSD symptoms in younger patients and women, which is in agreement with our results. However, they did not collect adjustments in medical treatment and thus could not investigate an association with PTSD symptoms. In a Chinese cross‐sectional study that included 660 patients with breast cancer, Juanjuan et al 22 reported that 20.8% of patients showed severe distress symptoms during the COVID‐19 pandemic according to the IES‐R, which is similar to our findings. In addition, 46.2% patients self‐reported that they had to discontinue or modify their treatment during the outbreak, which was associated with a higher risk of distress, as found in our study using medical records. Swainston et al 23 analyzed the psychological effect of self‐reported treatment disruption in patients with breast cancer. In that study, 31.6% of participants reported a change in their medical oncology treatment, and women who experienced a service disruption reported poorer perceived cognitive function. Altogether, these results are in line with our findings in patients with heterogeneous types of cancers.
In addition, our results confirm the strong relation between PTSD symptoms and QoL, cognition, and insomnia observed in patients with and without cancer. Indeed, insomnia is commonly associated with PTSD both in veterans 33 and in the general population. 34 Associations between PTSD and poorer cognition 26 , 35 or lower QoL 36 have also been documented in patients with cancer.
PTSD symptoms during the COVID‐19 pandemic should be interpreted with caution in patients with cancer because cancer diagnosis and treatment are traumatic experiences in themselves. 19 In a recent meta‐analysis, prevalence estimates of cancer‐related PTSD ranged from 7.3% to 13.8% using screening questionnaires other than the IES‐R. 37 Regarding the IES‐R, several cutoff values for PTSD symptomatology have been suggested, ranging from 24 to 33, depending on the population. 38 Although these values have not been validated in patients with cancer, several studies reported proportions of approximately 6% to 29% of cancer‐related PTSD using the conservative cutoff score of 33, 39 , 40 , 41 which is in the same range as the proportion of 21% that we found in the COVID‐19 context. However, those prior studies used cancer as the main traumatic stressor, whereas the COVID‐19 pandemic was the main stressor in the current study. In addition, we did not observe any association between PTSD symptoms and clinical oncology factors such as disease stage or time since cancer diagnosis, which are risk factors for cancer‐related PTSD. 37 Overall, it is unlikely that we captured PTSD symptoms related to cancer only or the pandemic only; rather, we identified an exacerbation of cancer‐related stress symptoms linked to lockdown‐induced constraints that led to adjustments in practice and lack of social support.
Our findings on COVID‐19 pandemic‐induced adjustments in medical oncology practice in a large sample of patients with cancer in France are original. Other studies investigated changes in care for patients with cancer but focused on specific localizations, such as head and neck 10 or gynecologic cancers. 42 Moreover, treatment modifications were often self‐reported by patients or physicians and were not collected from medical records. In France, a national study (PRATICOVID; Commission for Data Protection and Liberties reference number 2217722v0) 43 conducted in 9 hospitals to describe the adaptation of care for patients with cancer induced by the pandemic reported that 44% of medical cancer treatments were adapted in 268 patients receiving medical treatment, which is higher than in our study. However, one‐half of those patients received an oral chemotherapy protocol, and most (70%) were followed by telemedicine. Moreover, patients were recruited in military and general hospitals versus cancer centers in the current study.
This study has some limitations. First, we did not consider all dimensions of management for patients with cancer because we focused on medical oncology practice. Second, patients were not diagnosed with PTSD based on structured clinical diagnostic interviews: we used the IES‐R, which is a validated and widely used instrument to screen for PTSD symptoms. Moreover, analyzing the IES‐R score as both a continuous and a dichotomous variable led to some discrepancies in findings. Although the use of regression methods on continuous data is generally favored, the choice to dichotomize the IES‐R score was based on its right‐skewed distribution. 44 In addition, dichotomized measures represent distinct groups of individuals better according to the presence of PTSD symptoms; thus our analyses aimed to assess group differences rather than individual differences, which are clinically more meaningful. However, we cannot rule out the possibility that misclassification of patients may explain discrepancy. Third, the cross‐sectional design of our analysis precludes any conclusions regarding causality. Finally, these results represent the early onset of PTSD symptoms, which could then worsen after a few months or, conversely, decline with adjustment over time, and deserve further investigation in longitudinal studies.
Despite these limitations, our study is the first to assess PTSD symptomatology and to report its association with adjustments in medical oncology practice during the first COVID‐19–induced lockdown among many patients with cancer using validated questionnaires and data collected from medical files. This is important because the COVID‐19 pandemic is ongoing and will continue to lead to major changes in oncology practice worldwide. In a population already at high risk for psychological distress, changes in patient care may act as an additional stressor that must be considered by physicians when deciding to implement treatment adjustments. More attention should be paid to the psychological needs of patients with cancer to prevent or detect and manage PTSD symptoms in this vulnerable population. We recommend rapidly implementing psychosocial support for treated patients with cancer to promote emotional resilience and to avoid the onset of PTSD symptoms. In parallel, psychological support should be proposed to patients who have already developed PTSD during this long pandemic with its successive lockdowns.
Funding Support
This work was supported by a research grant from Fondation ARC (COVID202001320) and financial support from the GEFLUC Normandie (Les Entreprises Contre le Cancer/Campaigns Against Cancer, Rouen‐Normandie).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Florence Joly: Conceptualization, writing–original draft, and supervision. Olivier Rigal: Resources and project administration. Lydia Guittet: Writing–review and editing. Sophie Lefèvre‐Arbogast: Formal analysis and writing–original draft. Jean‐Michel Grellard: Project administration. Giulia Binarelli: Investigation and writing–review and editing. Marie Lange: Investigation and writing–review and editing. Chantal Rieux: Investigation. Marie Fernette: Investigation. Laure Tron: Investigation. François Gernier: Investigation. Romain Travers: Data curation. Adeline Morel: Resources. Doriane Richard: Investigation and project administration. Bénédicte Griffon: Supervision. Alexandra Leconte: Project administration. Etienne Bastien: Investigation. Florian Quilan: Investigation. Louis‐Ferdinand Pépin: Supervision. Fabrice Jardin: Resources. Marianne Leheurteur: Resources. Bénédicte Clarisse: Conceptualization, methodology, funding acquisition, writing–review and editing, and supervision. Justine Lequesne: Methodology, formal analysis, and writing–original draft. Audrey Faveyrial: Resources, visualization, and writing–review and editing.
Supporting information
Table S1‐S2
Joly F, Rigal O, Guittet L, Lefèvre‐Arbogast S, Grellard J‐M, Binarelli G, Lange M, Rieux C, Fernette M, Tron L, Gernier F, Travers R, Morel A, Richard D, Griffon B, Leconte A, Bastien E, Quilan F, Pépin L‐F, Jardin F, Leheurteur M, Clarisse B, Lequesne J, Faveyrial A. Post‐traumatic stress symptomatology and adjustment of medical oncology practice during the COVID‐19 pandemic among adult patients with cancer in a day care hospital. Cancer. 2021. 10.1002/cncr.33856
We acknowledge the Northwest Data Center (CTD‐CNO) for managing the data. It is supported by grants from the French National League Against Cancer and the French National Cancer Institute. We thank the Clinical Research Associates Bérénice Legrand, Clotilde Pupin, Delphine Bridelance, and Loréna Masseline for data collection as well as Bérengère de Gourmont for support in data entry. We acknowledge Kayigan Wilson d'Almeida for her assistance in medical writing. We also thank all patients who agreed to participate.
References
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernard Stoecklin S, Rolland P, Silue Y, et al. First cases of coronavirus disease 2019 (COVID‐19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25:2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mak IW, Chu CM, Pan PC, Yiu MG, Chan VL. Long‐term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31:318‐326. doi: 10.1016/j.genhosppsych.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma A, Malviya R, Kumar V, Gupta R, Awasthi R. Severity and risk of COVID‐19 in cancer patients: an evidence‐based learning. Dermatol Ther. 2020;33:e13778. doi: 10.1111/dth.13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raymond E, Thieblemont C, Alran S, Faivre S. Impact of the COVID‐19 outbreak on the management of patients with cancer. Target Oncol. 2020;15:249‐259. doi: 10.1007/s11523-020-00721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. You B, Ravaud A, Canivet A, et al. The official French guidelines to protect patients with cancer against SARS‐CoV‐2 infection. Lancet. 2020;21:619‐621. doi: 10.1016/S1470-2045(20)30204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fakhry N, Schultz P, Morinière S, et al. French consensus on management of head and neck cancer surgery during COVID‐19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137:159‐160. doi: 10.1016/j.anorl.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinkove R, McQuilten ZK, Adler J, et al. Managing haematology and oncology patients during the COVID‐19 pandemic: interim consensus guidance. Med J Aust. 2020;212:481‐489. doi: 10.5694/mja2.50607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jafari A, Rezaei‐Tavirani M, Karami S, Yazdani M, Zali H, Jafari Z. Cancer care management during the COVID‐19 pandemic. Risk Manag Healthc Policy. 2020;13:1711‐1721. doi: 10.2147/RMHP.S261357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiong KL, Guo T, Yao CMKL, et al. Changing practice patterns in head and neck oncologic surgery in the early COVID‐19 era. Head Neck. 2020;42:1179‐1186. doi: 10.1002/hed.26202 [DOI] [PubMed] [Google Scholar]
- 11. Panzuto F, Maccauro M, Campana D, et al. Impact of the SARS‐CoV2 pandemic dissemination on the management of neuroendocrine neoplasia in Italy: a report from the Italian Association for Neuroendocrine Tumors (Itanet). J Endocrinol Invest. 2021;44:989‐994. doi: 10.1007/s40618-020-01393-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakkar S, Al‐Omar K, Aljarrah Q, et al. Impact of COVID‐19 on thyroid cancer surgery and adjunct therapy. Updates Surg. 2020;72:867‐869. doi: 10.1007/s13304-020-00833-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasparri ML, Gentilini OD, Lueftner D, Kuehn T, Kaidar‐Person O, Poortmans P. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST). Breast. 2020;52:110‐115. doi: 10.1016/j.breast.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ralli M, Minni A, Candelori F, Cialente F, Greco A, de Vincentiis M. Effects of COVID‐19 pandemic on otolaryngology surgery in Italy: the experience of our university hospital. Otolaryngol Head Neck Surg. 2020;163:86‐88. doi: 10.1177/0194599820928970 [DOI] [PubMed] [Google Scholar]
- 15. Riera R, Bagattini AM, Pacheco RL, Pachito DV, Roitberg F, Ilbawi A. Delays and disruptions in cancer health care due to COVID‐19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311‐323. doi: 10.1200/GO.20.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lou E, Teoh D, Brown K, et al. Perspectives of cancer patients and their health during the COVID‐19 pandemic. PLoS One. 2020;15:e0241741. doi: 10.1371/journal.pone.0241741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moraliyage H, De Silva D, Ranasinghe W, et al. Cancer in lockdown: impact of the COVID‐19 pandemic on patients with cancer. Oncologist. 2021;26:e342‐e344. doi: 10.1002/onco.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miaskowski C, Paul SM, Snowberg K, et al. Loneliness and symptom burden in oncology patients during the COVID‐19 pandemic. Cancer. Published online April 27, 2021. doi: 10.1002/cncr.33603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cordova MJ, Riba MB, Spiegel D. Post‐traumatic stress disorder and cancer. Lancet Psychiatry. 2017;4:330‐338. doi: 10.1016/S2215-0366(17)30014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romito F, Dellino M, Loseto G, et al. Psychological distress in outpatients with lymphoma during the COVID‐19 pandemic. Front Oncol. 2020;10:1270. doi: 10.3389/fonc.2020.01270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miaskowski C, Paul SM, Snowberg K, et al. Stress and symptom burden in oncology patients during the COVID‐19 pandemic. J Pain Symptom Manage. 2020;60:e25‐e34. doi: 10.1016/j.jpainsymman.2020.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juanjuan L, Santa‐Maria CA, Hongfang F, et al. Patient‐reported outcomes of patients with breast cancer during the COVID‐19 outbreak in the epicenter of China: a cross‐sectional survey study. Clin Breast Cancer. 2020;20:e651‐e662. doi: 10.1016/j.clbc.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swainston J, Chapman B, Grunfeld EA, Derakshan N. COVID‐19 lockdown and its adverse impact on psychological health in breast cancer. Front Psychol. 2020;11:2033. doi: 10.3389/fpsyg.2020.02033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gold JI, Douglas MK, Thomas ML, Elliott JE, Rao SM, Miaskowski C. The relationship between posttraumatic stress disorder, mood states, functional status, and quality of life in oncology outpatients. J Pain Symptom Manage. 2012;44:520‐531. doi: 10.1016/j.jpainsymman.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 25. Mystakidou K, Parpa E, Tsilika E, Gennatas C, Galanos A, Vlahos L. How is sleep quality affected by the psychological and symptom distress of advanced cancer patients? Palliat Med. 2009;23:46‐53. doi: 10.1177/0269216308098088 [DOI] [PubMed] [Google Scholar]
- 26. Hermelink K, Bühner M, Sckopke P, et al. Chemotherapy and post‐traumatic stress in the causation of cognitive dysfunction in breast cancer patients. J Natl Cancer Inst. 2017;109:djx057. doi: 10.1093/jnci/djx057 [DOI] [PubMed] [Google Scholar]
- 27. Weiss DS, Marmar CR. The Impact of Event Scale‐Revised. In: Wilson K, Keane TM, eds. Assessing Psychological Trauma and PTSD. Guilford; 1996:399‐411. [Google Scholar]
- 28. Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale‐Revised. Behav Res Ther. 2003;41:1489‐1496. doi: 10.1016/j.brat.2003.07.010 [DOI] [PubMed] [Google Scholar]
- 29. Joly F, Lange M, Rigal O, et al. French version of the Functional Assessment of Cancer Therapy‐Cognitive Function (FACT‐Cog) version 3. Support Care Cancer. 2012;20:3297‐3305. doi: 10.1007/s00520-012-1439-2 [DOI] [PubMed] [Google Scholar]
- 30. Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601‐608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ringash J, O'Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient‐reported outcomes. Cancer. 2007;110:196‐202. doi: 10.1002/cncr.22799 [DOI] [PubMed] [Google Scholar]
- 32. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol. 1998;16:139‐144. doi: 10.1200/JCO.1998.16.1.139 [DOI] [PubMed] [Google Scholar]
- 33. Taylor DJ, Pruiksma KE, Hale WJ, et al. Prevalence, correlates, and predictors of insomnia in the US Army prior to deployment. Sleep. 2016;39:1795‐1806. doi: 10.5665/sleep.6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24:1‐15. doi: 10.1016/j.janxdis.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Y, Hendrix CC. Cancer‐related cognitive impairment in breast cancer patients: influences of psychological variables. Asia Pac J Oncol Nurs. 2018;5:296‐306. doi: 10.4103/apjon.apjon_16_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morrill EF, Brewer NT, O'Neill SC, et al. The interaction of post‐traumatic growth and post‐traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology. 2008;17:948‐953. doi: 10.1002/pon.1313 [DOI] [PubMed] [Google Scholar]
- 37. Abbey G, Thompson SB, Hickish T, Heathcote D. A meta‐analysis of prevalence rates and moderating factors for cancer‐related post‐traumatic stress disorder. Psychooncology. 2015;24:371‐381. doi: 10.1002/pon.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rash CJ, Coffey SF, Baschnagel JS, Drobes DJ, Saladin ME. Psychometric properties of the IES‐R in traumatized substance dependent individuals with and without PTSD. Addict Behav. 2008;33:1039‐1047. doi: 10.1016/j.addbeh.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Civilotti C, Castelli L, Binaschi L, et al. Dissociative symptomatology in cancer patients. Front Psychol. 2015;6:118. doi: 10.3389/fpsyg.2015.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miles A, McClements PL, Steele RJ, Redeker C, Sevdalis N, Wardle J. Perceived diagnostic delay and cancer‐related distress: a cross‐sectional study of patients with colorectal cancer. Psychooncology. 2017;26:29‐36. doi: 10.1002/pon.4093 [DOI] [PubMed] [Google Scholar]
- 41. Kazlauskiene J, Bulotiene G. Prevalence of post‐traumatic stress disorder among Lithuanian breast cancer patients and its risk factors. J Psychosom Res. 2020;131:109939. doi: 10.1016/j.jpsychores.2020.109939 [DOI] [PubMed] [Google Scholar]
- 42. Frey MK, Fowlkes RK, Badiner NM, et al. Gynecologic oncology care during the COVID‐19 pandemic at three affiliated New York City hospitals. Gynecol Oncol. 2020;159:470‐475. doi: 10.1016/j.ygyno.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Helissey C, Cessot A, Boudin L, et al. Evaluation of medical practices in oncology in the context of the COVID‐19 pandemic in France: physicians' point of view: the PRATICOVID study. Cancer Med. 2020;9:8875‐8883. doi: 10.1002/cam4.3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7:19‐40. doi: 10.1037/1082-989X.7.1.19 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


