Abstract
Aims
We examined the value of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) in patients admitted for coronavirus disease 2019 (COVID‐19) without prior history of heart failure (HF) or cardiomyopathy.
Methods and results
Retrospective cohort of consecutive adults (N = 679; median age 59 years; 38.7% women; 87.5% White; 7.1% Black; 5.4% Asian; 34.3% Hispanic) admitted with documented COVID‐19 in an academic centre in Long Island, NY. Admission NT‐proBNP was categorized using the European Society of Cardiology Heart Failure Association age‐specific criteria for acute presentations. We examined (i) mortality and the composite of death or mechanical ventilation and (ii) out‐of‐hospital, intensive care unit (ICU)‐free, and ventilator‐free days at 28 days. Estimates were adjusted for confounders using a lasso selection process. Using age‐specific criteria, 417 patients (61.4%) had low, 141 (20.8%) borderline, and 121 (17.8%) high NT‐proBNP. Mortality was 5.8%, 20.6%, and 36.4% for patients with low, borderline, and high NT‐proBNP, respectively. In lasso‐adjusted models, high NT‐proBNP was associated with higher mortality [hazard ratio (HR) 2.15; 95% confidence interval (CI) 1.06–4.39; P = 0.034] and composite endpoint rates (HR 1.66; 95%CI 1.04–2.66; P = 0.035). Patients with high NT‐proBNP had 32%, 33%, and 33% fewer out‐of‐hospital, ICU‐free, and ventilator‐free days compared with low NT‐proBNP counterparts. Results were consistent across age, sex, and race, and regardless of coronary artery disease or hypertension, except for stronger mortality signal with high NT‐proBNP in women.
Conclusions
In patients with COVID‐19 and no HF history, high admission NT‐proBNP is associated with higher mortality and healthcare resources utilization. Preventive strategies may be required for these patients.
Keywords: COVID‐19, NT‐proBNP, Outcomes, Healthcare resources utilization, Mortality, Mechanical ventilation
Introduction
Coronavirus disease 2019 (COVID‐19) results from infection with the SARS‐COV‐2 virus and has been associated with significant morbidity, including respiratory failure, acute kidney injury, thromboembolic complications, and cardiovascular involvement. 1 Although overt cardiac involvement during the acute phase is unusual, cardiac magnetic resonance imaging studies have shown subclinical ongoing inflammation in patients with recovered COVID‐19, 2 and autopsy studies have shown infection of the myocardium in severe COVID‐19. 3 Furthermore, risk factors for cardiovascular disease, including hypertension, diabetes, and obesity, as well as prior cardiovascular disease, including heart failure and coronary artery disease, predict worse outcomes in patients with COVID‐19. 4
Elevations of troponin and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) predict worse outcomes in patients hospitalized with COVID‐19, including death and mechanical ventilation in both single centre studies 5 , 6 and meta‐analyses. 7 , 8 NT‐proBNP has also been incorporated as part of a model to risk stratify patients in an attempt to potentially improve healthcare resource allocation. 9 Outside of COVID‐19, elevations in NT‐proBNP is associated with worse outcomes in hospitalized patients with and without history of heart failure (HF). 10 However, data on the clinical value NT‐proBNP levels in patients with COVID‐19 without prior history of HF are limited. Progressive inflammation, hypoxemia, sepsis, and volume overload in COVID‐19 may increase myocardial stress, 11 and in conjunction with the primary lung impairment and renal injury may predispose patients with COVID‐19 to develop an acute HF phenotype, 12 for which NT‐proBNP may serve as a surrogate.
Prior studies have examined NT‐proBNP levels in all‐comers with COVID‐19. In this work, we have individually adjudicated the absence of HF history or cardiomyopathy in a cohort of patients admitted with COVID‐19 in an academic referral centre during the first wave of the pandemic in Long Island, NY. Using the age‐specific diagnostic classification of NT‐proBNP for acute presentations endorsed by the Heart Failure Association of the European Society of Cardiology (ESC), 13 we examined the association between admission NT‐proBNP and 28 day clinical outcomes and healthcare resources utilization (HCRU). We hypothesized that elevated admission NT‐proBNP is independently associated with worse outcomes and more HCRU in patients with COVID‐19.
Methods
Study population
This is a retrospective cohort study of consecutive patients who were admitted with confirmed COVID‐19 pneumonia at a single academic centre (Stony Brook Medical Center, Stony Brook, NY) between 3/1/2020 and 4/16/2020, that is, during the first wave of the COVID‐19 pandemic in New York. We reviewed the medical records of adults who tested positive for SARS‐CoV‐2 by polymerase chain reaction and were determined to have COVID‐19 pneumonia with at least moderate disease based on World Health Organization criteria, defined as adults with COVID‐19 and clinical signs of pneumonia including fever, cough, dyspnoea, or tachypnea. 14 From the initial inception cohort of 1020 patients, we excluded patients who (i) were previously intubated prior to transfer to the academic centre, (ii) had documented history of HF or cardiomyopathy or were undergoing diagnostic evaluation for these entities, and (iii) did not have NT‐proBNP available on admission.
Data collection and processing
Data were extracted from our electronic medical records system including demographics, comorbid conditions, medication use, self‐reported smoking history, vital signs, and laboratory values including NT‐proBNP levels. Vital signs and laboratory values were collected on the day of admission. Standard census definitions were used regarding race and ethnicity. We excluded patients with known or suspected HF or cardiomyopathy. History of HF or cardiomyopathy was adjudicated individually for each patient, based on past medical history, medications, previous imaging data, and cardiology documentation in the electronic medical records. Briefly, presence of HF was verified if, in addition to a physician diagnosis, there was (i) documentation of symptoms (e.g. shortness of breath) or signs (e.g. oedema) of HF; (ii) supporting echocardiographic findings; and (iii) HF therapy, including diuretics, beta‐blockers, or renin‐angiotensin‐aldosterone system inhibitors. We used the guidance provided by the ESC guidelines for corroborating echocardiographic criteria to diagnose HF in cases without reduced left ventricular ejection fraction. 15 Follow up data were collected until death, hospital discharge, or readmission for COVID‐19‐related causes. We used the 28 day framework for outcomes and HCRU, which has been standard in recent COVID‐19 trials. 16 , 17 Patients not readmitted by 28 days were considered alive and out of the hospital for the purposes of HCRU analysis. Patients still hospitalized by the database lock (July 22, 2020) were censored as alive for length of stay, mechanical ventilation, and mortality analysis. This study was approved by the Institutional Review Board of Stony Brook University (IRB2020‐00211).
Inpatient echocardiography was performed sparingly during the first pandemic wave, after screening of echocardiogram requests by experienced cardiac imaging faculty, out of concerns about patient and personnel safety and the need to carefully allocate constrained human resources and personal protective equipment. As a result, few patients had echocardiograms performed during their COVID‐19 hospitalization or shortly thereafter, and several studies were partial, that is, an abbreviated protocol was employed to reduce physician and patient exposure.
N‐terminal pro‐B‐type natriuretic peptide
We used the ESC‐endorsed, age‐specific criteria for diagnostic classification of NT‐proBNP during acute presentations 13 to categorize admission NT‐proBNP levels into the following: (i) low (<300 pg/mL, suggesting HF is unlikely); (ii) borderline (300–450 ng/mL for ages < 50; 300–900 ng/mL for ages 50–75; and 300–1800 ng/mL for ages > 75, characterized as ‘grey zone’ for HF diagnosis); and (iii) high (>450 ng/mL for ages < 50; >900 ng/mL for ages 50–75; and >1800 ng/mL for ages > 75, suggesting that HF is likely).
Endpoints
The primary endpoint was 28 day mortality. The secondary endpoint was the composite of death or mechanical ventilation at 28 days. We assessed HCRU using the 28 day framework that has been previously used in COVID‐19 studies. 18 Metrics of HCRU included (i) days alive out of hospital (‘hospital‐free’ days); (ii) days alive outside the intensive care unit (ICU) (‘ICU‐free’ days); and (iii) days alive not on mechanical ventilation (‘ventilator‐free’ days), during the first 28 days.
Statistical analysis
Demographic and presenting characteristics were compared across NT‐proBNP categories with non‐parametric tests for trend. The association between admission NT‐proBNP and the primary and secondary endpoints (time‐to‐event analysis) was evaluated with adjusted Cox proportional hazards models. Given the large number of potential confounders and relatively small number of events, we used a machine learning approach (lasso inferential models) to select the most relevant adjustment model without overfitting. 19 Specifically, we used a double‐selection lasso logistic regression (process dslogit in STATA and a plugin selection method) with each endpoint (i.e. death at 28 days and the composite of death or mechanical ventilation at 28 days, in separate regressions) as dependent variables of interest, NT‐proBNP (categorized as described above) as the exposure of interest, and all the variables in Table 1 as potential confounders (‘control’ variables). 20 This process estimates separate lassos both for the outcomes and for the exposures of interest to select the optimal degree of penalization (‘lambda’). We confirmed the covariate selection with an alternative lasso‐based inferential method, the partialling‐out lasso, 21 which reached the same covariates. Before running the lasso processes, we used multiple imputations (N = 5) with chained equations for missing covariate values and ran each lasso separately for each imputed data set; the lasso‐selected covariates were stable across the imputed data sets. Similarly, we ran adjusted Cox regression models in the imputed data sets and combined the estimates as previously described. 22 The following covariates were selected by the double‐selection lasso process and were included in adjusted Cox models: age, sex, race, history of hypertension, coronary artery disease, and chronic kidney disease, days from symptom onset, systolic blood pressure, temperature, creatinine, d‐dimers, troponin T, alanine aminotransferase, procalcitonin, and QTc interval on presentation. For HCRU analysis, we used logistic regression models to examine need for ICU and mechanical ventilation and negative binomial regression models to examine hospital‐free, ICU‐free, and ventilator‐free days. All models were adjusted for the covariates described above, and estimates were derived from imputed data sets for missing values. Cox models met the proportional hazards assumption as evaluated with the Schoenfeld residuals. In pre‐specified analyses, we examined the association of NT‐proBNP with outcomes in the following subgroups: age ≥ 65 vs. <65; male vs. female; White vs. non‐White patients; history of coronary artery disease; and presence of hypertension. For the subset of patients with available echocardiographic data, we also calculated the HFA‐PEFF score, which is recommended by the Heart Failure Association of ESC as a means to support the diagnosis of HF with preserved ejection fraction, 23 and we examined the correlation of echocardiographic characteristics with NT‐proBNP categories. Statistical analyses were performed using STATA 16.1 (StataCorp, College Station, TX).
Table 1.
Baseline patient characteristics according to admission NT‐proBNP categories a
| Characteristic | Low NT‐proBNP (N = 417) | Borderline NT‐proBNP (N = 141) | High NT‐proBNP (N = 121) | P value b |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 55 (47–64) | 73 (64–83) | 71 (61–84) | <0.001 |
| Race, N (%) | 0.085 | |||
| White | 358 (85.9) | 122 (86.5) | 112 (92.6) | |
| Black | 33 (7.9) | 11 (7.8) | 3 (2.5) | |
| Asian | 26 (6.2) | 8 (5.7) | 6 (5.0) | |
| Hispanic, N (%) | 159 (38.1) | 38 (27.0) | 32 (26.4) | 0.009 |
| Female sex | 144 (34.5) | 68 (48.2) | 51 (42.1) | 0.011 |
| Body mass index (kg/m2) | 30.0 (26.4–35.4) | 27.7 (25.4–31.0) | 27.6 (24.4–32.8) | <0.001 |
| Comorbidities, N (%) | ||||
| Hypertension | 180 (43.2) | 106 (75.2) | 96 (80.0) | <0.001 |
| Diabetes | 109 (26.1) | 49 (34.8) | 40 (33.1) | 0.084 |
| Coronary artery disease | 33 (7.9) | 30 (21.3) | 41 (34.2) | <0.001 |
| Atrial fibrillation | 11 (3.0) | 17 (14.2) | 16 (15.7) | <0.001 |
| Chronic lung disease | 25 (6.0) | 26 (18.4) | 18 (15.0) | <0.001 |
| Chronic kidney disease | 11 (2.6) | 13 (9.2) | 30 (25.0) | <0.001 |
| Asthma | 40 (9.6) | 6 (4.3) | 9 (7.5) | 0.122 |
| Immunocompromised | 26 (6.5) | 19 (14.0) | 16 (14.0) | 0.006 |
| Medication use, N (%) | ||||
| ACE inhibitor | 58 (13.9) | 28 (19.9) | 21 (17.5) | 0.193 |
| Angiotensin receptor blocker | 61 (14.6) | 28 (19.9) | 27 (22.5) | 0.076 |
| Statins | 122 (29.3) | 66 (46.8) | 63 (52.5) | <0.001 |
| Initial vital signs, N (%) | ||||
| Systolic blood pressure (mmHg) | 123 (112–139) | 129 (113–146) | 130 (108–148) | 0.103 |
| Diastolic blood pressure (mmHg) | 75 (69–82) | 71 (63–80) | 68 (62–78) | <0.001 |
| Heart rate (bpm) | 100 (89–112) | 92 (78–104) | 91 (80.5–104) | <0.001 |
| Temperature (°C) | 37.8 (37.2–38.7) | 37.7 (37.2–38.7) | 37.4 (36.9–38.25) | <0.001 |
| Respiratory rate (bpm) | 20 (18–26) | 20 (18–26) | 20 (18–28) | 0.685 |
| Oxygen saturation (%) | 93 (91–95) | 93 (91–96) | 94 (90–96) | 0.280 |
| FiO2 requirement | 32 (28–50) | 32 (28–44) | 36 (28–100) | 0.002 |
| Clinical findings c , N (%) | ||||
| Symptom duration on presentation (days) | 7 (5–10) | 5 (3–7) | 4 (2–7) | <0.001 |
| QTc (ms) | 431 (415–449) | 442 (420.5–461) | 450 (427–472) | <0.001 |
| Creatinine (mg/dL) | 0.88 (0.7–1.02) | 0.98 (0.77–1.39) | 1.31 (0.97–2.69) | <0.001 |
| Alanine aminotransferase (IU/L) | 38 (24–61) | 26 (16–45) | 21 (15–37) | <0.001 |
| Aspartate aminotransferase (IU/L) | 45 (31–68) | 41 (29.5–61.5) | 36 (28–60) | 0.007 |
| Lymphocyte count (K/uL) | 0.91 (0.67–1.22) | 0.78 (0.51–1.13) | 0.76 (0.49–1.24) | <0.001 |
| International normalized ratio | 1.2 (1.1–1.2) | 1.1 (1.1–1.3) | 1.1 (1.1–1.3) | 0.875 |
| Troponin (ng/mL) | 0.01 (0.01–0.01) | 0.01 (0.01–0.02) | 0.04 (0.01–0.1) | <0.001 |
| ESR (mm/h) | 53 (31–73) | 57 (30–81) | 61 (37–81) | 0.025 |
| Ferritin (ng/mL) | 828 (418–1,550) | 654 (286–1206) | 912 (475–1898) | 0.754 |
| C‐reactive protein (mg/dL) | 9.2 (4.4–16.1) | 9.6 (4.8–16.4) | 11.0 (5.4–21.9) | 0.041 |
| d‐dimer (ng/mL) | 288 (195–441) | 459 (277–962) | 590 (347–1,401) | <0.001 |
| Procalcitonin (ng/mL) | 0.15 (0.10–0.28) | 0.19 (0.12–0.4) | 0.45 (0.2–1.2) | <0.001 |
| Lactate dehydrogenase (IU/L) d | 385 (301–495) | 354 (269–482) | 379 (290–521) | 0.996 |
| Creatine phosphokinase (IU/L) e | 146 (66–321) | 138 (59–285) | 118 (61–365) | 0.674 |
| Interleukin‐6 (pg/mL) | 49.2 (24.6–87.6) | 69.9 (34.3–111.4) | 54.4 (26.0–117.6) | 0.084 |
Values are N (%) or median (25th–75th percentile).
Categories of NT‐proBNP are defined per Heart Failure Association of the European Society of Cardiology practical guidance statement on natriuretic peptides, Eur J Heart Fail 2019:21; 715–731. Low NT‐proBNP (‘heart failure unlikely’) is defined as <300 ng/mL. Borderline NT‐proBNP (‘grey zone’) is defined as 300–450 ng/mL for ages <50; 300–900 ng/mL for ages 50–75; and 300–1800 ng/mL for ages >75. High NT‐proBNP (‘heart failure likely’) is defined as >450 ng/mL for ages <50; >900 ng/mL for ages 50–75; and >1800 ng/mL for ages >75.
Non‐parametric test for trend across ordered categories.
Findings within 48 h of admission.
Available in 367 patients.
Available in 362 patients.
ACE, angiotensin converting enzyme; ESR, erythrocyte sedimentation rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; QTc, corrected QT interval on electrocardiogram.
Results
Baseline characteristics
Of the 945 patients who fulfilled the clinical inclusion and exclusion criteria, 679 (71.9%) had NT‐proBNP on admission and were included in the analytic cohort (Supporting Information, Figure S1 ). Patients with available NT‐proBNP were older; more likely to be male; had higher prevalence of hypertension, diabetes, coronary artery disease, chronic lung disease, home use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and statin regimens; and had higher heart rate, temperature, respiratory rate, lower oxygen saturation, higher FiO2 requirement, longer QTc, and higher creatinine at presentation. These patients also had higher erythrocyte sedimentation rate, ferritin, C‐reactive protein, and procalcitonin and had lactate dehydrogenase values (Table S1 ).
The median NT‐proBNP on admission was 164 ng/mL (25th–75th percentile, 49–723). Using the ESC‐endorsed, age‐specific criteria for diagnostic NT‐proBNP categorization, 417 patients (61.4%) had low, 141 (20.8%) borderline, and 121 (17.8%) high NT‐proBNP levels. Patients with borderline or high NT‐proBNP were older; more likely to be non‐Hispanic and female; and had lower body mass index, more hypertension, coronary artery disease, atrial fibrillation, chronic lung disease, chronic kidney disease, immunocompromised state, and home statin use when compared with patients with low NT‐proBNP values. These patients also had shorter symptom duration, lower diastolic blood pressure, heart rate, and temperature, and required higher FiO2 at presentation. Patients with borderline or high NT‐proBNP had higher creatinine, troponin, erythrocyte sedimentation rate, C‐reactive protein, d‐dimer, and procalcitonin and had lower lymphocyte count on presentation (Table 1 ). During hospitalization, corticosteroids (which may cause or exacerbate fluid retention) were administered in 36.4%, 46.1%, and 38.8% of patients with low, borderline, and high admission NT‐proBNP, respectively (P = 0.129 for Fisher's exact test).
N‐terminal pro‐B‐type natriuretic peptide and outcomes
At 28 days, 97 patients had died (14.3%), and 207 patients (30.5%) met the 28 day secondary endpoint of death or mechanical ventilation. Mortality at 28 days was 5.8%, 20.6%, and 36.4% for patients with age‐specific low, borderline, and high admission NT‐proBNP, respectively (Figure 1 A ). The secondary endpoint was met by 23.7%, 35.5%, and 47.9% of patients with low, borderline, and high NT‐proBNP, respectively (Figure 1 B ).
Figure 1.
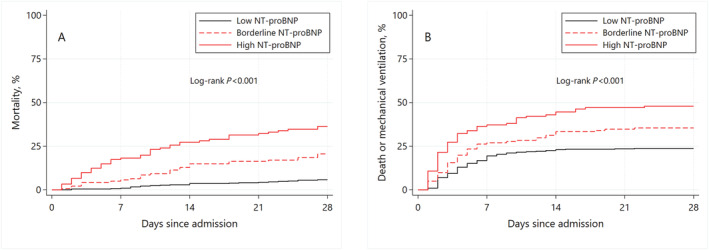
Kaplan–Meier estimates of 28‐day mortality (A) and composite of death or mechanical ventilation (B) according to admission N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). Categories of NT‐proBNP defined per the recommendations of the Heart Failure Association of the European Society of Cardiology. Low NT‐proBNP is defined as <300 ng/mL. Borderline NT‐proBNP is defined as 300–450 ng/mL for ages <50; 300–900 ng/mL for ages 50–75; and 300–1800 ng/mL for ages > 75. High NT‐proBNP is defined as >450 ng/mL for ages <50; >900 ng/mL for ages 50–75; and >1800 ng/mL for ages >75.
In Cox regression models adjusting for significant confounders, patients with high NT‐proBNP levels were at significantly higher risk for death [hazard ratio (HR) 2.15; 95% confidence interval (CI) 1.06–4.39; P = 0.034] and the composite of death or mechanical ventilation (HR 1.66; 95%CI 1.04–2.66; P = 0.035) at 28 days. Confounders were selected with lasso models for inference (age, sex, race, history of hypertension, coronary artery disease, chronic kidney disease, days from symptom onset, systolic blood pressure, temperature, creatinine, d‐dimers, troponin T, alanine aminotransferase, and procalcitonin on presentation). Patients with low and borderline NT‐proBNP were not at significantly higher risk for the primary or secondary endpoint (Table 2 ).
Table 2.
Patient outcomes according to admission NT‐proBNP
| Characteristic | Low a NT‐proBNP (N = 417) | P value | Borderline NT‐proBNP (N = 141) | P value | High NT‐proBNP (N = 121) | P value |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| Events at 28 days, N (%) | 24 (5.8) | 29 (20.6) | 44 (36.4) | |||
| Unadjusted HR (95%CI) | 1.00 | Ref. | 3.87 (2.26–6.62) | <0.001 | 7.86 (4.78–12.9) | <0.001 |
| Adjusted HR (95%CI) b | 1.00 | Ref. | 1.44 (0.70–2.97) | 0.322 | 2.15 (1.06–4.39) | 0.034 |
| Composite | ||||||
| Events at 28 days, N (%) | 99 (23.7) | 50 (35.5) | 58 (47.9) | |||
| Unadjusted HR (95%CI) | 1.00 | Ref. | 1.61 (1.15–2.25) | 0.006 | 2.47 (1.78–3.43) | <0.001 |
| Adjusted HR (95%CI) b | 1.00 | Ref. | 1.34 (0.87–2.07) | 0.186 | 1.66 (1.04–2.66) | 0.035 |
Categories of NT‐proBNP defined as in Table 1 .
Adjusted for age, sex, race, history of hypertension, coronary artery disease, and chronic kidney disease, days from symptom onset, systolic blood pressure, temperature, creatinine, d‐dimer, troponin T, alanine aminotransferase, procalcitonin, and QTc on presentation.
CI, confidence interval; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
In analyses with log10‐NT‐proBNP examined as a continuous variable, each log10 higher NT‐proBNP at baseline was associated with a HR of 1.58 (95%CI 1.10–2.27; P = 0.013) for mortality and 1.33 (95%CI 1.05–1.69; P = 0.017) for the composite of death or mechanical ventilation in adjusted models including the covariates described above.
N‐terminal pro‐B‐type natriuretic peptide and healthcare resources utilization
Median length of stay in the hospital was 9 days (5–16). In total, 188 patients (27.7%) required ICU admission, with median ICU length of stay of 13 days (6–26), and 163 (24.0%) required mechanical ventilation with median duration of 12 days (7–22). In adjusted models, elevated admission NT‐proBNP (borderline or high) was not significantly associated with need for ICU admission or mechanical ventilation (Table 3 ).
Table 3.
Healthcare resources utilization at 28 days according to admission NT‐proBNP
| Variable | Low NT‐proBNP a | Borderline NT‐proBNP | High NT‐proBNP | |||
|---|---|---|---|---|---|---|
| (N = 417) | P value | (N = 141) | P value | (N = 121) | P value | |
| ICU admission | ||||||
| Events, N (%) | 111 (26.6) | 39 (27.7) | 38 (31.4) | |||
| OR (95%CI) b | 1.00 | Ref. | 1.28 (0.71–2.30) | 0.41 | 1.37 (0.67–2.81) | 0.39 |
| Mechanical ventilation | ||||||
| Events, N (%) | 92 (22.1) | 38 (27.0) | 33 (27.3) | |||
| OR (95%CI) | 1.00 | Ref. | 1.64 (0.90–2.99) | 0.10 | 1.58 (0.76–3.30) | 0.22 |
| Hospital‐free days | ||||||
| Days, mean (SD) | 15.9 (9.0) | 12.6 (9.8) | 10.7 (10.7) | |||
| RR (95%CI) | 1.00 | Ref. | 0.83 (0.65–1.07) | 0.15 | 0.65 (0.45–0.94) | 0.02 |
| ICU‐free days | ||||||
| Days, mean (SD) | 23.3 (9.0) | 19.4 (12.2) | 15.6 (13.5) | |||
| RR (95%CI) | 1.00 | Ref. | 0.87 (0.71–1.06) | 0.16 | 0.58 (0.41–0.83) | 0.003 |
| Ventilator‐free days | ||||||
| Days, mean (SD) | 23.9 (8.7) | 19.7 (12.0) | 15.9 (13.5) | |||
| RR (95%CI) | 1.00 | Ref. | 0.88 (0.72–1.08) | 0.23 | 0.61 (0.43–0.87) | 0.006 |
Categories of NT‐proBNP defined as in Table 1 .
All estimates have been adjusted for age, sex, race, history of hypertension, coronary artery disease, and chronic kidney disease, days from symptom onset, systolic blood pressure, temperature, creatinine, d‐dimer, troponin T, alanine aminotransferase, procalcitonin, and QTc on presentation.
CI, confidence interval; OR, odds ratio; RR, rate ratio; SD, standard deviation.
At 28 days, patients had a median of 18 hospital‐free days (0–23), 28 ICU‐free days (15–28), and 28 ventilator‐free days (18–28). In adjusted analyses, patients with high admission NT‐proBNP spent fewer hospital‐free, ICU‐free, and ventilator‐free days compared with those with low admission NT‐proBNP, by 32%, 33%, and 33%, respectively (Table 3 ). Borderline or low admission NT‐proBNP was not associated with fewer HCRU‐free days.
Subgroup analyses
In pre‐specified analyses, the association of admission NT‐proBNP (examined as continuous log10‐NT‐proBNP) was not significantly different between patients 65 years or older vs. younger; in White vs. non‐White patients; and in patients with or without history of coronary artery disease or hypertension (Figure S2A and S2B —unadjusted estimates). However, there was a signal for stronger association of NT‐proBNP with mortality (but not with the composite of death or mechanical ventilation) in women vs. men. The adjusted HR estimate per log10‐NT‐proBNP was 5.29 (95%CI 2.16–12.97; P < 0.001) in women vs.1.20 (95%CI 0.78–1.84; P = 0.40) in men, P < 0.001 for the interaction. However, the number of deaths in women was small, and therefore, this result needs to be interpreted with caution, as the association of NT‐proBNP with the composite endpoint did not differ between men and women.
Echocardiography subset
Among the 679 patients in the study, 57 (8.4%) had an echocardiogram during their COVID‐19 hospitalization or shortly thereafter (maximum time from admission to echocardiography was 4 months). The echocardiographic findings and non‐parametric correlation with NT‐proBNP are summarized in Table S2 . Patients with a larger left atrium tended to have higher NT‐proBNP levels. Using the HFA‐PEFF score, 5 of 57 patients would meet criteria for HF with preserved ejection fraction (5 or 6 points), assuming continuing presence of symptoms.
Discussion
In this single‐centre retrospective study, elevated admission NT‐proBNP was associated with higher mortality and higher rates of death or mechanical ventilation in hospitalized COVID‐19 patients without history of HF or cardiomyopathy. When NT‐proBNP was categorized into low, borderline, or high (using age‐specific criteria endorsed by ESC), high NT‐proBNP (vs. low) was associated with two‐fold higher 28 day mortality and 1.5‐fold elevated risk for death or mechanical ventilation after accounting for covariates selected with a machine learning algorithm. Results were similar when admission NT‐proBNP was evaluated as a continuous variable. Although high NT‐proBNP was not associated with higher rates of ICU admission or need for mechanical ventilation in our study, overall, 28 day HCRU was higher among patients with high NT‐proBNP, as evident from fewer out‐of‐hospital, ICU‐free, and ventilator‐free days in this patient group. Importantly, the association of elevated NT‐proBNP with outcomes was consistent in patients with vs. without history of coronary artery disease or hypertension, and across age, race, and gender groups. There was a signal for higher mortality risk with elevated NT‐proBNP in women, but the number of events was small, and this finding needs confirmation in larger studies.
Previous studies have shown that elevations in cardiac biomarkers including troponin and NT‐proBNP predict worse outcomes in patients with COVID‐19. 5 Subsequent meta‐analysis have shown that higher NT‐proBNP is associated with severe clinical condition, higher rates of mechanical ventilation, and mortality. 7 , 24 However, these studies have assessed NT‐proBNP in populations based on requirement for mechanical ventilation, 25 presence of cardiac injury, 7 and development of acute HF. 6 We specifically examined NT‐proBNP in non‐selected hospitalized patients with COVID‐19 without history of HF or cardiomyopathy, demonstrating that high NT‐proBNP is associated with poor outcomes in these COVID‐19 patients. Our findings have several clinical and pathophysiological implications.
Healthcare resources allocation has been a challenge as COVID‐19 has placed substantial strain on healthcare systems. Cardiac biomarkers can risk stratify patients and therefore have been proposed as tools to improve resource utilization, although how to use those findings is not entirely clear. 26 Our study did confirm that patients with high admission NT‐proBNP need longer hospitalization and more ICU and ventilator days, which was not consistently demonstrated in previous studies, potentially as a result of smaller sample size. 27 From a management perspective, patients with COVID‐19 and age‐specific high NT‐proBNP on admission may need cardiac imaging even in the absence of history of Stage C HF. Dormant Stage B disease may place patients at higher risk, 28 and structural abnormalities combined with myocardial injury are associated with increased mortality. 15 Elevated NT‐proBNP in patients admitted with COVID‐19 with no known cardiac conditions may point to COVID‐19‐mediated cardiac complications, and therefore, these patients should be considered for cardiac imaging. For example, point of care ultrasound may be used to screen for important cardiac disease and congestion, for example, through inferior vena cava collapsibility and the presence of B‐lines or pleural effusion. As higher NT‐proBNP is associated with a worse prognosis independent of troponin, elevated NT‐proBNP should perhaps prompt stratified management, for example, closer fluid balance monitoring. 15 Serial NT‐proBNP determinations could also supplement clinical assessment of changes in volume status. In any case, these strategies would need prospective evaluation.
An advantage of our study is the use of consensus‐based, age‐specific cut‐off points for NT‐proBNP. In studies with data‐driven cut‐offs to define elevated NT‐proBNP, the associated risk in patients with COVID‐19 was potentially overestimated due to overfitting. For example, a previous large cohort study reported an adjusted HR for mortality in high vs low NT‐proBNP patients of 5.11 (95%CI, 3.50–7.47). 29 However, this study utilized an operational cut‐off based on institutional distribution of biomarkers. Besides providing more moderate and potentially less biased risk estimates, the standardized NT‐proBNP definitions suggested by the ESC may also provide a more generalizable context in interpreting NT‐proBNP in COVID‐19.
Several mechanisms have been proposed to explain the association of NT‐proBNP with outcomes in patients with COVID‐19. These include progressive inflammation, hypoxemia, sepsis, and volume overload states, which may increase myocardial stress. 11 , 30 Vascular complications of COVID‐19 including pulmonary embolism and acute kidney injury may also aggravate myocardial stress. These mechanisms may predispose patients with COVID‐19 to manifest an acute HF phenotype. 12 Unfortunately, imaging, including cardiac imaging, was only sparingly performed during the first wave of the pandemic, as a result of constrained resources. Therefore, the mechanistic insights that could be gained from detailed imaging are lacking. In the limited subset of patients with echocardiographic data available, however, most parameters of systolic and diastolic function were within normal limits, suggesting additional mechanisms that may underlie cardiac stretch and elevated NT‐proBNP, for example, fluid retention. However, these data should be interpreted with extreme caution, as the numbers were small, echocardiography studies were limited in scope, and echocardiography was performed per clinical indication; thus, this subset of does not represent a random subset. Further mechanistic studies, including cardiac imaging and systematic volume status assessment, would help further understand the implications of elevated NT‐proBNP in COVID‐19. The signal for a stronger association of high NT‐proBNP with mortality in women is alarming and should be evaluated in prospective studies. Notably, a similar differential association, albeit with long‐term mortality, has been reported by a clinical trial with BNP used as a diagnostic tool in acute presentations. 31
Limitations
There are several limitations to our study. First, NT‐proBNP was not systematically assessed during admission for COVID‐19 in our institution, which may have led to inclusion of patients with a higher burden of comorbidities, inherently higher risk, and more severe presenting clinical condition. Second, as healthcare systems in our area were under significant stress, cardiac imaging was used sparingly, and thus, we could not provide information on underlying Stage B HF in our patients. In addition, several patients admitted for COVID‐19 had no previous records in our institution and thus previous workup for HF symptoms may have been performed elsewhere but not transmitted timely, leading to incomplete adjudication elements. Both these limitations might be especially relevant for the adjudication of HF with preserved ejection fraction, and thus, misclassification of this entity in our study is possible. Third, NT‐proBNP was not serially assessed, and thus, we could not evaluate the incremental value of serial data. Fourth, despite studying a non‐selected COVID‐19 population, patients admitted during the first wave of the pandemic in our region had generally at least moderate to severe presentations, as evident by overall high mortality and need for mechanical ventilation. Subsequent populations may exhibit better prognosis despite similar NT‐proBNP levels, as healthcare systems are better prepared and more effective treatments become available. Finally, although this study identified significant trends in HCRU, larger cohorts with more power may identify significant differences in ICU admission or mechanical ventilation.
Conclusion
In patients with COVID‐19 without HF history, age‐specific high NT‐proBNP on admission was independently associated with worse clinical outcomes and higher HCRU, with a homogenous effect across major subgroups. Whether we can use this information to better manage patients admitted with COVID‐19—potentially through point‐of‐care imaging, close fluid management, volume status monitoring, or other measures—with the goal to improve outcomes and reduce HCRU, will need prospective investigation.
Conflict of interest
The authors have no disclosures.
Funding
The authors have no internal or external sources of funding.
Author contributions
Jeanwoo Yoo and Andreas P. Kalogeropoulos performed the study conception and design. Jeanwoo Yoo, Prabhjot Grewal, Jessica Hotelling, Aikaterini Papamanoli, Kerry Cao, Simrat Dhaliwal, Robin Jacob, Azad Mojahedi, Andreas Kalogeropoulos carried out the acquisition, analysis, or interpretation of data. All authors performed the drafting and revision of the work as well as final approval of the submitted manuscript.
Supporting information
Table S1. Patient Characteristics According to Availability of NT‐proBNP on Admission.
Table S2. Inpatient Echocardiography Data.
Figure S1. Flowchart of study population.
Figure S2A. Unadjusted Associations of Admission Log10‐NT‐proBNP with Mortality at 28 Days in Subgroups of Interest.
Figure S2B. Unadjusted Associations of Admission Log10‐NT‐proBNP with the Composite of Death or Mechanical ventilation at 28 days in Subgroups of Interest.
Yoo, J. , Grewal, P. , Hotelling, J. , Papamanoli, A. , Cao, K. , Dhaliwal, S. , Jacob, R. , Mojahedi, A. , Bloom, M. E. , Marcos, L. A. , Skopicki, H. A. , and Kalogeropoulos, A. P. (2021) Admission NT‐proBNP and outcomes in patients without history of heart failure hospitalized with COVID‐19. ESC Heart Failure, 8: 4278–4287. 10.1002/ehf2.13548.
References
- 1. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N Engl J Med 2020; 383: 2451–2460. [DOI] [PubMed] [Google Scholar]
- 2. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H‐P, Blankenberg S, Püschel K, Westermann D. Association of cardiac infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol 2020; 5: 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rey JR, Caro‐Codón J, Rosillo SO, Iniesta ÁM, Castrejón‐Castrejón S, Marco‐Clement I, Martín‐Polo L, Merino‐Argos C, Rodríguez‐Sotelo L, García‐Veas JM, Martínez‐Marín LA, Martínez‐Cossiani M, Buño A, Gonzalez‐Valle L, Herrero A, López‐Sendón JL, Merino JL. Heart failure in COVID‐19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020; 22: 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bansal A, Kumar A, Patel D, Puri R, Kalra A, Kapadia SR, Reed GW. Meta‐analysis comparing outcomes in patients with and without cardiac injury and coronavirus disease 2019 (COVID 19). Am J Cardiol 2021; 141: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li JW, Han TW, Woodward M, Anderson CS, Zhou H, Chen YD, Neal B. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta‐analysis. Prog Cardiovasc Dis 2020; 63: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McRae MP, Simmons GW, Christodoulides NJ, Lu Z, Kang SK, Fenyo D, Alcorn T, Dapkins IP, Sharif I, Vurmaz D, Modak SS, Srinivasan K, Warhadpande S, Shrivastav R, McDevitt JT. Clinical decision support tool and rapid point‐of‐care platform for determining disease severity in patients with COVID‐19. Lab Chip 2020; 20: 2075–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. York MK, Gupta DK, Reynolds CF, Farber‐Eger E, Wells QS, Bachmann KN, Xu M, Harrell FE, Wang TJ. B‐Type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol 2018; 71: 2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kazory A, Ronco C, McCullough PA. SARS‐CoV‐2 (COVID‐19) and intravascular volume management strategies in the critically ill. Proc (Bayl Univ Med Cent) 2020; 0: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azevedo RB, Botelho BG, Hollanda JVG, Ferreira LVL, Junqueira de Andrade LZ, Oei SSML, Mello TS, Muxfeldt ES. Covid‐19 and the cardiovascular system: a comprehensive review. J Hum Hypertens 2021; 35: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M, Januzzi JL Jr, Cardiology obotHFAotESo . Heart failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21: 715–731. [DOI] [PubMed] [Google Scholar]
- 14. Organization WH . COVID‐19 Clinical management: living guidance. 2021;2021.
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M and Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 16. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz‐Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH. Baricitinib plus remdesivir for hospitalized adults with Covid‐19. N Engl J Med 2021; 384: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo‐Hernandez H, Rochwerg B, Lamontagne F, Han MA, Liu Q, Agarwal A, Agoritsas T, Chu DK, Couban R, Darzi A, Devji T, Fang B, Fang C, Flottorp SA, Foroutan F, Ghadimi M, Heels‐Ansdell D, Honarmand K, Hou L, Hou X, Ibrahim Q, Khamis A, Lam B, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Qasim A, Rada G, Riaz IB, Sadeghirad B, Sekercioglu N, Sheng L, Sreekanta A, Switzer C, Tendal B, Thabane L, Tomlinson G, Turner T, Vandvik PO, Vernooij RW, Viteri‐Garcia A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello‐Petersen R, Qasim A, Martinez JPD, Cusano E. Drug treatments for covid‐19: living systematic review and network meta‐analysis. BMJ 2020; 370: m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch B, Vock DM, Wolfson J. Covariate selection with group lasso and doubly robust estimation of causal effects. Biometrics 2018; 74: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belloni A, Chernozhukov V, Wei Y. Post‐selection inference for generalized linear models with many controls. J Bus Econ Stat 2016; 34: 606–619. [Google Scholar]
- 21. Belloni A, Chernozhukov V, Hansen C. High‐dimensional methods and inference on structural and treatment effects. J Econ Perspect 2014; 28: 29–50. [Google Scholar]
- 22. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 24. Sorrentino S, Cacia M, Leo I, Polimeni A, Sabatino J, Spaccarotella CAM, Mongiardo A, De Rosa S, Indolfi C. B‐Type natriuretic peptide as biomarker of COVID‐19 disease severity—a meta‐analysis. J Clin Med 2020; 9: 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gavin W, Campbell E, Zaidi S‐A, Gavin N, Dbeibo L, Beeler C, Kuebler K, Abdel‐Rahman A, Luetkemeyer M, Kara A. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID‐19. Am J Infect Control 2020. S0196–6553(20)30689–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stefanini GG, Chiarito M, Ferrante G, Cannata F, Azzolini E, Viggiani G, de Marco A, Briani M, Bocciolone M, Bragato R, Corrada E, Gasparini GL, Marconi M, Monti L, Pagnotta PA, Panico C, Pini D, Regazzoli D, My I, Kallikourdis M, Ciccarelli M, Badalamenti S, Aghemo A, Reimers B, Condorelli G. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID‐19. Heart 2020; 106: 1512–1518. [DOI] [PubMed] [Google Scholar]
- 27. Abdeen Y, Kaako A, Alnabulsi M, Okeh A, Meng W, Miller R. The prognostic effect of brain natriuretic peptide levels on outcomes of hospitalized patients with COVID‐19. Avicenna J Med 2021; 11: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghany R, Palacio A, Chen G, Dawkins E, McCarter D, Forbes E, Chung B, Tamariz L. Prior cardiovascular risk and screening echocardiograms predict hospitalization and severity of coronavirus infection among elderly medicare patients. Am J Prev Cardiol 2020; 3: 100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin JJ, Cheng X, Zhou F, Lei F, Akolkar G, Cai J, Zhang XJ, Blet A, Xie J, Zhang P, Liu YM, Huang Z, Zhao LP, Lin L, Xia M, Chen MM, Song X, Bai L, Chen Z, Zhang X, Xiang D, Chen J, Xu Q, Ma X, Touyz RM, Gao C, Wang H, Liu L, Mao W, Luo P, Yan Y, Ye P, Chen M, Chen G, Zhu L, She ZG, Huang X, Yuan Y, Zhang BH, Wang Y, Liu PP, Li H. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID‐19. Hypertension 2020; 76: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babapoor‐Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID‐19: possible mechanisms. Life Sci 2020; 253: 117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christ M, Laule‐Kilian K, Hochholzer W, Klima T, Breidthardt T, Perruchoud AP, Mueller C. Gender‐specific risk stratification with B‐type natriuretic peptide levels in patients with acute dyspnea: insights from the B‐type natriuretic peptide for acute shortness of breath evaluation study. J Am Coll Cardiol 2006; 48: 1808–1812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics According to Availability of NT‐proBNP on Admission.
Table S2. Inpatient Echocardiography Data.
Figure S1. Flowchart of study population.
Figure S2A. Unadjusted Associations of Admission Log10‐NT‐proBNP with Mortality at 28 Days in Subgroups of Interest.
Figure S2B. Unadjusted Associations of Admission Log10‐NT‐proBNP with the Composite of Death or Mechanical ventilation at 28 days in Subgroups of Interest.


