Abstract
Aims
Myocardial injury (MI) in coronavirus disease‐19 (COVID‐19) is quite prevalent at admission and affects prognosis. Little is known about troponin trajectories and their prognostic role. We aimed to describe the early in‐hospital evolution of MI and its prognostic impact.
Methods and results
We performed an analysis from an Italian multicentre study enrolling COVID‐19 patients, hospitalized from 1 March to 9 April 2020. MI was defined as increased troponin level. The first troponin was tested within 24 h from admission, the second one between 24 and 48 h. Elevated troponin was defined as values above the 99th percentile of normal values. Patients were divided in four groups: normal, normal then elevated, elevated then normal, and elevated. The outcome was in‐hospital death. The study population included 197 patients; 41% had normal troponin at both evaluations, 44% had elevated troponin at both assessments, 8% had normal then elevated troponin, and 7% had elevated then normal troponin. During hospitalization, 49 (25%) patients died. Patients with incident MI, with persistent MI, and with MI only at admission had a higher risk of death compared with those with normal troponin at both evaluations (P < 0.001). At multivariable analysis, patients with normal troponin at admission and MI injury on Day 2 had the highest mortality risk (hazard ratio 3.78, 95% confidence interval 1.10–13.09, P = 0.035).
Conclusions
In patients admitted for COVID‐19, re‐test MI on Day 2 provides a prognostic value. A non‐negligible proportion of patients with incident MI on Day 2 is identified at high risk of death only by the second measurement.
Keywords: Myocardial injury, COVID‐19, Troponin trajectories, COVID‐19 outcome
Introduction
During severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic healthcare providers are being required to face with many unconventional features in their professional daily life. 1 Moreover, even though the epidemiology curve was flattened by prevention measures, 2 the incidence of new cases is progressively increasing after the reduction of social restrictions. 3 In this epidemiological scenario, the multidisciplinary assessment of coronavirus disease‐19 (COVID‐19) patients is crucial to accomplish a careful prognostic stratification, in order to identify those who will require a higher intensity of care. 4 The cardiac involvement in COVID‐19, detected by increased serum troponin at admission, has been extensively investigated, and its prognostic impact has been found as significant in different cohorts. 5 , 6 , 7 However, acute respiratory distress syndrome (ARDS), including that due to SARS‐CoV‐2, frequently requires prolonged hospitalizations, 8 with possible evolutions of the clinical and laboratory status. In this perspective, the development of myocardial injury during hospitalization might be a relevant prognostic marker, as already demonstrated in ARDS with other aetiologies than SARS‐CoV‐2. 9 In a previous report, the importance of troponin trends was explored in an Asian population including 187 patients, finding out that a progressive increasing in troponin serum concentration was a strong negative prognostic marker, 7 while an Italian experience on 50 patients demonstrated that patient with myocardial injury in at least one assessment within 24 h from admission had a more severe disease. 10 However, systematic and focused reports exploring the prognostic role of troponin increase during severe COVID‐19 and the exact timing for troponin reassessment in large Caucasian population are still lacking.
Aim of our study was to explore the prevalence and prognostic impact of early serum troponin concentration increase in a large Caucasian population admitted for severe COVID‐19, in order to identify patients that might require a higher intensity of care.
Methods
Population and outcome
We performed a multicentre observational study enrolling Caucasian patients with laboratory‐confirmed SARS‐CoV‐2 infection (i.e. positive swab or bronchoalveolar lavage), referred to 13 Italian Cardiology Units from 1 March to 9 April 2020. 6 , 11 Diagnosis of COVID‐19 was made by real‐time reverse transcriptase–polymerase chain reaction (RT‐PCR) assay of nasal and pharyngeal swabs. 12 RT‐PCR of lower respiratory tract aspirates was also performed when clinically indicated. For this specific analysis, we included all consecutive patients with at least two available assessments of circulating levels of high‐sensitivity plasma troponin (either troponin I or troponin T): the first at admission (i.e. within 24 h from admission) and the second assessment between 24 and 48 h from admission. In case of multiple troponin evaluations performed within the same day, the first one was considered. Patients with an acute cardiovascular diagnosis (i.e. acute heart failure, acute coronary syndrome, and new onset arrhythmias) upon admission were excluded. The study complied with the ethics of the Declaration of Helsinki. The outcome measure of the study was in‐hospital all cause death.
Data collection
Patients' data including demographics, medical history (with particular attention to cardiovascular medical history), in‐hospital clinical course, and outcomes were extracted from the in‐hospitals medical records. The in‐hospital outcome was ascertained until 23 April 2020. Renal function was measured as estimated glomerular filtration rate and was calculated by the chronic kidney disease epidemiology collaboration equation 13 ; chronic kidney disease was defined when estimated glomerular filtration rate was <60 mL/kg/1.73m2. Both venous and arterial blood samples for biochemistry and gas analysis were collected at the time of hospitalization and during the hospital stay as appropriate. Cardiac troponin (either troponin I or troponin T) was considered elevated if serum level was above the 99% percentile of normal values as per manufacturer indications. Thereafter, patients were categorized according to their troponin level on the first and on the second assay in four groups: normal (i.e. normal troponin level at both assessments), normal‐elevated (i.e. normal troponin level at admission and elevated troponin between 24 and 48 h), elevated‐normal (i.e. elevated troponin at admission and normal troponin value at Day 2), elevated (i.e. elevated troponin value at both evaluations).
Statistical analysis
Continuous variables are shown as means and standard deviations, skewed variables as medians and interquartile ranges, dichotomous variables as counts and percentages. Comparisons between groups were made, respectively, using ANOVA test for means, Kruskal–Wallis test for medians, and χ 2 test (or Fisher's exact test whenever appropriate) for proportions. A bar chart was drawn to show intra‐hospital mortality according to the trend of troponin level.
Cumulative incidence function of death was computed taking into account hospital discharge as a competing event. Overall and pairwise comparisons of cumulative incidence functions amongst subgroups were performed by means of Gray test. 14
Variables clinically relevant and significantly associated with the risk of death at the univariable analysis were tested in a multiple Cox regression model to identify independent risk factors. Sex and age were included in the final model as primary adjusting factors without considering their statistical significance, and other clinical covariates were selected using a backward procedure, using a P value <0.10 for model retention. The hazard ratios (HRs), 95% confidence intervals (CIs), and P values from a Wald test were reported.
To evaluate possible selection bias due to missing troponin data during the first 2 days of hospitalization, clinical characteristics between patients included in the present analysis and those recruited in the multicentre study were compared.
A two‐tailed P value <0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Population characteristics and troponin trends
Overall, 614 patients were recruited in the multicentre study. For the present analysis, the final study population consisted of 197 patients. The remaining patients were excluded due to the lack of a second troponin assay within 48 h after admission. In Table S1 a comparison between the group included in the current analysis and the group excluded is shown. No relevant differences emerged amongst the two groups for most of clinical and laboratory parameters. Concerning troponin trends between 24 and 48 h after admission, 15 (8%) patients showed an increasing troponin (i.e. normal‐elevated group), 87 (44%) a constantly elevated troponin, 14 (7%) a decreasing troponin (i.e. elevated‐normal group), and 81 (41%) a normal troponin concentration at both evaluations. Compared with other groups, patients with persistently normal troponin value were younger (63 ± 13 years) and had the lowest comorbidity profile compared with the other three groups. Noteworthy, patients who experienced myocardial injury only on Day 2 had a cardiovascular background similar to those with myocardial injury already present at admission: in detail, 14% had history of heart failure, 79% hypertension, 21% atrial fibrillation, and 29% diabetes (Table 1 ).
Table 1.
Demographic and clinical characteristics of the study population at admission stratified by the trend of troponin level during the first 2 days of hospitalization (N = 197)
| Troponin trend | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Normal then elevated | Elevated then normal | Elevated | ||||||
| N | N | N | N | P value | |||||
| Age (years) | 81 | 63 ± 13 a | 15 | 64 ± 15 | 14 | 70 ± 12 | 87 | 70 ± 13 | 0.006 |
| Sex (male) | 81 | 58 (72) | 15 | 10 (67) | 14 | 10 (71) | 87 | 57 (66) | 0.850 |
| Oxygen saturation (ambient air, %) | 79 | 91 (85–95) | 15 | 96 (90–97) | 14 | 90 (85–97) | 86 | 92 (86–96) | 0.198 |
| White blood cell count (μL) | 79 | 6600 (5150–8610) b , a | 14 | 6527 (5085–7465) b | 14 | 9860 (8575–10 538) | 87 | 8670 (5770–11 380) | 0.001 |
| Lymphocytes (μL) | 73 | 810 (600–1331) | 14 | 1045 (728–1778) | 13 | 1020 (605–1400) | 78 | 945 (693–1288) | 0.678 |
| CRP (mg/dL) | 79 | 44 (13–139) | 14 | 11 (3–27) | 14 | 33 (12–157) | 85 | 23 (6–144) | 0.083 |
| Lactate dehydrogenase (U/L) | 73 | 416 (273–596) | 14 | 301 (195–418) | 13 | 250 (180–428) | 73 | 332 (257–498) | 0.075 |
| ABG test lactate (mmol/L) | 61 | 1.2 (0.9–1.5) | 13 | 1.0 (0.7–1.9) | 7 | 1.6 (1.5–1.9) | 68 | 1.4 (0.9–1.7) | 0.152 |
| PaO2/FiO2 (mmHg/%) | 75 | 180 (97–290) | 11 | 310 (243–381) | 11 | 267 (131–302) | 71 | 257 (126–333) | 0.105 |
| Heart failure | 80 | 2 (3) b , a | 14 | 2 (14) | 14 | 4 (29) | 86 | 22 (26) | <0.001 |
| Coronary artery disease | 80 | 9 (11) a | 14 | 4 (29) | 14 | 6 (43) | 86 | 26 (30) | 0.004 |
| Atrial fibrillation | 80 | 5 (6) a | 14 | 3 (21) | 14 | 4 (29) | 86 | 24 (28) | 0.001 |
| Chronic obstructive pulmonary disease | 80 | 4 (5) | 14 | 3 (21) | 14 | 2 (14) | 86 | 10 (12) | 0.111 |
| Diabetes | 80 | 16 (20) | 14 | 4 (29) | 14 | 3 (21) | 86 | 26 (30) | 0.498 |
| Hypertension | 80 | 32 (40) a | 14 | 11 (79) | 14 | 11 (79) | 86 | 55 (64) | 0.001 |
| Chronic kidney disease (eGFR < 60 mL/min/m2) | 80 | 6 (8) a | 14 | 1 (7) | 14 | 5 (36) | 86 | 23 (27) | 0.001 |
| Prior ACEi/ARB therapy | 69 | 21 (30) c | 11 | 9 (82) | 13 | 9 (69) | 74 | 33 (45) | 0.002 |
Legend: ABG, arterial blood gas; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; FiO2, fraction of inspired oxygen; PaO2, oxygen partial pressure at arterial gas analysis.
Data are shown as mean ± standard deviation, median (interquartile range), or count (%). Subgroup comparisons were made with the same test used for the overall analysis, adjusting for multiple comparisons with either Bonferroni method for χ 2, Fisher and ANOVA tests, or with the Dwass, Steel, Critchlow–Flign (DSCF) method for the Kruskal–Wallis tests.
Subgroups analyses: indicates statistically significant comparison vs. ‘elevated’.
Subgroups analyses: indicates statistically significant comparison vs. ‘elevated then normal’.
Subgroups analyses: indicates statistically significant comparison vs. ‘normal then elevated’.
Outcome prediction
Over a median in‐hospital stay of 16 (interquartile range 9–26) days, 49 (25%) patients died. The main cause of death was respiratory failure (37 events), a minority of patients (five events) died for cardiovascular causes. The risk of death was 11% in the group with constantly normal troponin, 33% in the normal‐elevated group, 43% in elevated‐normal group and 33% in the persistently elevated group (P = 0.001, Figure 1 ). Moreover, 69% of non‐survivors were included in the elevated group or in the normal then elevated group, compared with only 46% of survivors. (Table S2 ).
Figure 1.
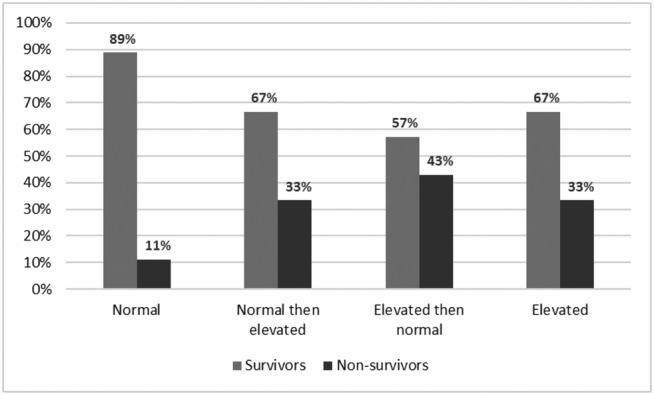
Intra‐hospital mortality stratifying patients according to the trend of troponin level during the first two days of hospitalization [normal troponin (N = 81) vs. normal troponin then elevated (N = 15) vs. elevated troponin then normal (N = 14) vs. elevated troponin (N = 87), overall P value: 0.001].
Cumulative incidence function analysis (Figure 2 ) showed that patients with normal troponin concentration at both evaluations had the lowest risk of in‐hospital death (overall P < 0.001). Compared with this group, patients with persistently elevated troponin (P < 0.001) and normal‐elevated group (P = 0.015) had a higher risk of in‐hospital death. This trend was consistent throughout the hospitalization period.
Figure 2.
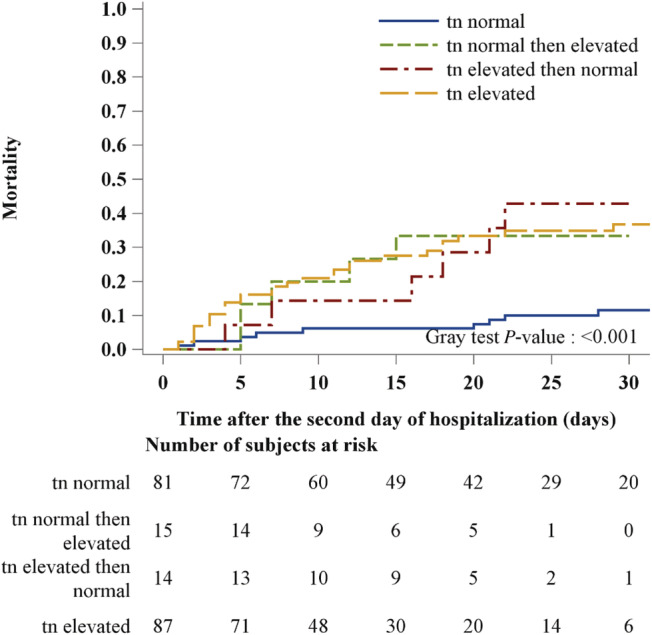
Cumulative incidence function for intra‐hospital mortality stratifying patients according to the trend of troponin level during the first 2 days of hospitalization: normal troponin vs. normal troponin then elevated vs. elevated troponin then normal vs. elevated troponin.
Univariable analysis for outcome showed that, compared with patients with normal troponin at admission and on Day 2, those with persistently elevated troponin levels, or those who developed elevated troponin levels between 24 and 48 h after admission had an increased risk of death (HR 4.26, 95% CI 2.00–9.07; P < 0.001 and HR 3.96, 95% CI 1.32–11.92; P = 0.014, respectively, Table 2 ). Multivariable Cox regression analysis, adjusted for age, sex, oxygen saturation, C‐reactive protein, and chronic kidney disease, confirmed that persistently increased troponin concentration (HR 2.61, 95% CI 1.11–6.16, P = 0.029) and early increase in troponin within 48 h from admission (HR 3.78, 95% CI 1.10–13.07, P = 0.035) were both predictors of in‐hospital death. Interestingly, the latter emerged as the strongest factor. The other independent predictors of in‐hospital death were older age, chronic kidney disease, and low arterial oxygen saturation at admission (Table 2 ).
Table 2.
Univariable and multivariable Cox regression model for intra‐hospital mortality
| Univariable | Multivariable (N = 188) | ||||
|---|---|---|---|---|---|
| Level/units | HR (95% CI) | P value | HR (95% CI) | P value | |
| Troponin trend (vs. normal) | Elevated | 4.26 (2.00–9.07) | <0.001 | 2.61 (1.11–6.16) | 0.029 |
| Normal then elevated | 3.96 (1.32–11.92) | 0.014 | 3.78 (1.10–13.07) | 0.035 | |
| Elevated then normal | 4.32 (1.53–12.19) | 0.006 | 2.15 (0.70–6.56) | 0.180 | |
| Age | +5 years | 1.33 (1.17–1.52) | <0.001 | 1.25 (1.06–1.47) | 0.009 |
| Sex | M vs. F | 1.04 (0.55–1.97) | 0.907 | 1.54 (0.76–3.12) | 0.234 |
| Oxygen saturation | +5% | 0.80 (0.69–0.93) | 0.003 | 0.73 (0.61–0.88) | <0.001 |
| White body cell count | +1000 U/μL | 1.08 (1.02–1.13) | 0.005 | ||
| Lymphocytes count | +100 U/μL | 0.94 (0.89–1.00) | 0.055 | ||
| CRP | +10 mg/L | 1.04 (1.01–1.06) | 0.005 | 1.03 (1.00–1.05) | 0.075 |
| Lactate dehydrogenase | +1000 mg/dL | 1.10 (0.99–1.21) | 0.072 | ||
| ABG test lactate | +1 mmol/L | 1.18 (1.07–1.30) | 0.001 | ||
| PaO2/FiO2 | +50 mmHg/% | 0.85 (0.73–0.98) | 0.029 | ||
| Heart failure | Yes vs. no | 2.74 (1.47–5.11) | 0.002 | ||
| Coronary artery disease | Yes vs. no | 1.69 (0.92–3.10) | 0.093 | ||
| Atrial fibrillation | Yes vs. no | 1.94 (1.01–3.73) | 0.047 | ||
| Chronic obstructive pulmonary disease | Yes vs. no | 1.59 (0.71–3.54) | 0.259 | ||
| Diabetes | Yes vs. no | 1.86 (1.02–3.38) | 0.044 | ||
| Hypertension | Yes vs. no | 1.86 (1.01–3.42) | 0.045 | ||
| Chronic kidney disease | Yes vs. no | 3.56 (2.00–6.34) | <0.001 | 3.03 (1.51–6.08) | 0.002 |
| Prior ACEi/ARB therapy | Yes vs. no | 1.88 (1.04–3.39) | 0.035 | ||
Legend: ABG, arterial blood gas; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CRP, C‐reactive protein; FiO2, fraction of inspired oxygen; HR, hazard ratio; PaO2, oxygen partial pressure at arterial gas analysis; RBC, red blood cell.
Discussion
In severe COVID‐19 infection, requiring oxygen supplementation, it is mandatory to perform an adequate and prompt prognostic stratification of patients hospitalized, in order to identify those who may most benefit from intensive and specific care. 4 The current study expanded the current knowledge about the relevance of a systematic evaluation of myocardial injury not only at admission but also within the first 48 h of in‐hospital stay in a large Caucasian population. Indeed, elevated troponin level on the second assessment emerged as a predictor of mortality, regardless of troponin value at admission. These data suggest that COVID‐19 patients deserve a serial and comprehensive assessment, beyond baseline values, because it may provide an additive prognostic role. Indeed, in our analysis, a non‐negligible proportion of patients (8%) with normal troponin level at admission and a pathological serum concentration on second assessment were re‐classified as high risk of in‐hospital death patients, similarly to those with myocardial injury at admission. In this perspective, serial troponin dosage is a low‐cost, univocal, and valuable measurement that provided an incremental prognostic value compared with the isolated assessment of myocardial injury at admission and, therefore, could be helpful in the clinical management of COVD‐19 patients, in addition to inflammation and respiratory parameters.
Myocardial injury assessment: Timing and significance
Myocardial injury at admission is a known strong prognostic marker in patients hospitalized for SARS‐CoV‐2 infection. 6 , 7 , 15 Different pathophysiological mechanisms underlying this phenomenon have been advocated, such as systemic inflammation, respiratory failure, in‐hospital complications, and direct myocardial damage carried by the virus. 5 , 16 All the mechanisms that may determine the troponin release, can be perpetuated during the hospitalization, especially within the first days, when specific treatments are not effective yet. In this view, analogously to the acute heart failure setting, 17 the re‐evaluation of serum troponin concentration within 48 h from admission was found to have a prognostic role, potentially useful on top of baseline assessment of patients with severe COVID‐19, in the absence of any acute cardiovascular disease. In a Chinese report focused on myocardial injury, the trend of troponin was described in a small subset of patients, showing that troponin changes are possible during the hospitalization, but strong prognostic information derived from these changings were not provided. 18 On the other hand, the prognostic role of the second troponin assessment was partially explored by Guo et al. who described that patients going through a serum troponin increase have a higher rate of adverse events. 7 However, these and other additional data, all exclusively including Asian patients, 19 , 20 are limited by the fact that the timing of the second evaluation was not defined and the increase could be of any amount within either normal or abnormal range, thus providing only a partial clinical support. In our study, switching from normal to increased troponin or maintaining an abnormal level between 24 and 48 h after admission was independently associated to a decreased in‐hospital survival rate. In this view, the repeated troponin assay allowed to detect an additional 8% of patients at high risk of adverse outcome despite normal troponin at admission, thus showing that an early troponin increase is a negative prognostic index.
Clinical implications
In our opinion, our results may be helpful in the clinical management of patients hospitalized with severe COVID‐19. The prognostic role of reassessing myocardial injury within the first phases of hospitalization, as depicted in our analysis, might represent a new tool to improve the challenging clinical management of these patients. Indeed, the baseline assessment alone is not always sufficient to identify patients who will not respond to standard care and might rapidly evolve in a more severe disease. 21 , 22 In this perspective, the persistence or the development of myocardial injury at 48 h from hospitalization may accurately identify patients at higher risk of death, and, therefore, that would possibly most benefit from a higher intensity of care. Considering the similarity between the cardiovascular background of patients with myocardial injury at admission and those who will increase troponin level on Day 2, the presence of a heavy cardiovascular comorbidity profile should be considered a risk factor for myocardial injury occurrence. Nevertheless, the main cause of death for patients with increased troponin levels was non‐cardiovascular. This indicates that troponin release might be an index of general inflammation and respiratory failure severity, leading to both myocardial injury and in‐hospital non‐cardiovascular death. 5 , 16 In this perspective, the early increased troponin levels has proved to be a stronger prognosis predictor compared with the heavy cardiovascular profile. Indeed, despite history of heart failure, history of atrial fibrillation, and hypertension were significant at univariable analysis, they lost significance in the backward selection procedure we used to define our model. This finding supports the hypothesis that reassessing troponin on Day 2 is a useful aid not only in patients with myocardial injury at admission but also in all COVID‐19 patients requiring hospitalization. Finally, as previously claimed, 16 considering that we accurately excluded patients with an acute cardiac disease at admission, we support the hypothesis that myocardial injury assessment is a useful prognostic tool also in COVID‐19 patients without an acute cardiovascular disease. Therefore, a precise protocol to define the timing to assess myocardial injury and the clinical significance of elevated troponin would be helpful.
Limitations
Some limitations should be acknowledged. The main one is that troponin levels were determined by different assays in different hospitals. Therefore, we were not able to analyse troponin as a continuous variable. However, this limitation was partially overcome by the dichotomization of troponin in normal vs. elevated according to the single assay. This is a retrospective analysis based on a multicentre study; therefore, some patients were excluded due to the absence of a second troponin assessment or because they experienced a fatal outcome within 48 h. Despite the absence of relevant differences in most of clinical and laboratory parameters between the study population and the excluded patients partially overcame this limitation, a selection bias still exists, represented by the choice of the clinician to perform a second troponin assay in selected patients. Moreover, despite recent reports that suggest a possible persistent cardiac involvement after COVID‐19, 23 , 24 we could not follow the trend of troponin during the whole hospitalization period neither after discharge, focusing our attention just on the first 2 days. We had not the highest serum concentration of troponin systematically available amongst the measurements performed between 24 and 48 h, due to the retrospective nature and the design of the study. Our study would benefit from a validation in a different population, even though, to the best of our knowledge, our population is the best characterized amongst those with multiple troponin samples. A limitation of our study is that the prognostic effect of troponin changes from the first to the second day of hospitalization was explored with a multivariable model based on 49 events, and an independent validation of this effect in an external set of patients would be therefore needed. Finally, an accurate analysis to find which factors could predict the incidence of myocardial injury within 48 h from admission was not feasible due to the limited sample size. For the same reason, we could not investigate the significance of troponin changes in specific subsets of patients, such as amongst different age groups. It remains an open question, which would require future, focused studies.
Conclusions
The assessment of early development or persistent myocardial injury at precisely defined timepoints emerged as a relevant prognostic tool in a large Caucasian population admitted for severe COVID‐19. Re‐testing troponin on Day 2 after hospital admission is an accurate and useful tool to detect a non‐negligible share of patients at high risk of in‐hospital death, regardless of the troponin value at admission.
Conflict of interest
Dr Carubelli received consulting honoraria from CVie Therapeutics Limited, Servier, and Windtree Therapeutics outside the submitted work. Dr Ameri reported having received speaker and advisor honoraria from Novartis, AstraZeneca, Vifor, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, GlaxoSmithKline, and Merck, Sharp & Dohme and nonfinancial support from Actelion outside the submitted work. Dr Leonardi reported grants and personal fees from AstraZeneca and personal fees from BMS/Pfizer, Novo Nordisk, and Chiesi outside the submitted work. Dr Agostoni reported nonfinancial support from Menarini, Novartis, and Boehringer; grants from Daiichiò Sankyo and Bayer; and grants and nonfinancial support from Actelion outside the submitted work. Dr Mortara reports personal consulting honoraria from Novartis, Servier, Astra Zeneca for participation to advisory board meetings and receives grants from Novartis and Niccomo for research trials. Dr Piepoli reported having received research grants and speaking fees from Novartis, Servier, and TRX and nonfinancial support from Vifor outside the submitted work. Dr Metra reported personal consulting honoraria from Abbott Vascular, Amgen, Bayer, Edwards Therapeutics, Servier, Vifor Pharma, and Windtree Therapeutics for participation to advisory board meetings and executive committees of clinical trials. Dr Senni reported personal fees from Novartis, Abbott, Merck, Bayer, Boehringer, Vifor, and AstraZeneca outside the submitted work.
Supporting information
Table S1. Comparison of relevant clinical characteristics between patients excluded and included in the analysis.
Table S2. Demographic and clinical characteristics of the study population at admission stratified by vital status (N = 197).
Nuzzi, V. , Merlo, M. , Specchia, C. , Lombardi, C. M. , Carubelli, V. , Iorio, A. , Inciardi, R. M. , Bellasi, A. , Canale, C. , Camporotondo, R. , Catagnano, F. , Dalla Vecchia, L. A. , Giovinazzo, S. , Maccagni, G. , Mapelli, M. , Margonato, D. , Monzo, L. , Oriecuia, C. , Peveri, G. , Pozzi, A. , Provenzale, G. , Sarullo, F. , Tomasoni, D. , Ameri, P. , Gnecchi, M. , Leonardi, S. , Agostoni, P. , Carugo, S. , Danzi, G. B. , Guazzi, M. , La Rovere, M. T. , Mortara, A. , Piepoli, M. , Porto, I. , Volterrani, M. , Senni, M. , Metra, M. , and Sinagra, G. (2021) The prognostic value of serial troponin measurements in patients admitted for COVID‐19. ESC Heart Failure, 8: 3504–3511. 10.1002/ehf2.13462.
Vincenzo Nuzzi and Marco Merlo equally contributed as first authors.
References
- 1. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi‐Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol 2020; 75: 2352–2371.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lau H, Khosrawipour V, Kocbach P, Mikolajczyk A, Schubert J, Bania J, Khosrawipour T. The positive impact of lockdown in Wuhan on containing the COVID‐19 outbreak in China. J Travel Med 2020; 27: taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization https://covid19.who.int/ (19 January 2021).
- 4. De Simone G, Mancusi C. COVID‐19: timing is important. Eur J Intern Med 2020; 77: 134–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, Metra M. COVID‐19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 2020; 22: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Merlo M, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Specchia C, Metra M, Senni M. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol 2020; 5: 1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, Zhu Q, Hu M, Li XY, Li Y, Liang LR, Tong ZH, Sun B, Peng P, Shi HZ. Comparison of hospitalized patients with ARDS caused by COVID‐19 and H1N1. Chest 2020; 158: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metkus TS, Guallar E, Sokoll L, Morrow DA, Tomaselli G, Brower R, Kim BS, Schulman S, Korley FK. Progressive myocardial injury is associated with mortality in the acute respiratory distress syndrome. J Crit Care 2018; 48: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaninotto M, Mion MM, Padoan A, Babuin L, Plebani M. Cardiac troponin I in SARS‐CoV‐2‐patients: the additional prognostic value of serial monitoring. Clin Chim Acta 2020; 511: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, Barbieri L, Bellasi A, Camporotondo R, Canale C, Carubelli V, Carugo S, Catagnano F, Dalla Vecchia LA, Danzi GB, Di Pasquale M, Gaudenzi M, Giovinazzo S, Gnecchi M, Iorio A, La Rovere MT, Leonardi S, Maccagni G, Mapelli M, Margonato D, Merlo M, Monzo L, Mortara A, Nuzzi V, Piepoli M, Porto I, Pozzi A, Sarullo F, Sinagra G, Volterrani M, Zaccone G, Guazzi M, Senni M, Metra M. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID‐19. Results of the cardio‐COVID‐Italy multicentre study. Eur J Heart Fail 2020; 22: 2238–2247. [DOI] [PubMed] [Google Scholar]
- 12. Organization WHO . Pneumonia of unknown cause—China. https://wwwwhoint/csr/don/05‐january‐4222020‐pneumonia‐of‐unkown‐cause‐china/en/.2020 (19 January 2021).
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408]. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray R. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- 15. Ferrante G, Fazzari F, Cozzi O, Maurina M, Bragato R, D'Orazio F, Torrisi C, Lanza E, Indolfi E, Donghi V, Mantovani R, Liccardo G, Voza A, Azzolini E, Balzarini L, Reimers B, Stefanini GG, Condorelli G, Monti L. Risk factors for myocardial injury and death in patients with COVID‐19: insights from a cohort study with chest computed tomography. Cardiovasc Res 2020; 116: 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID‐19: JACC review topic of the week. J Am Coll Cardiol 2020; 76: 1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connor CM, Fiuzat M, Lombardi C, Fujita K, Jia G, Davison BA, Cleland J, Bloomfield D, Dittrich HC, Delucca P, Givertz MM, Mansoor G, Ponikowski P, Teerlink JR, Voors AA, Massie BM, Cotter G, Metra M. Impact of serial troponin release on outcomes in patients with acute heart failure: analysis from the PROTECT pilot study. Circ Heart Fail 2011; 4: 724–732. [DOI] [PubMed] [Google Scholar]
- 18. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020; 41: 2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan F, Yang L, Li Y, Liang B, Li L, Ye T, Li L, Liu D, Gui S, Hu Y, Zheng C. Factors associated with death outcome in patients with severe coronavirus disease‐19 (COVID‐19): a case‐control study. Int J Med Sci 2020; 17: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruch Y, Kaeuffer C, Guffroy A, Lefebvre N, Hansmann Y, Danion F. Rapid radiological worsening and cytokine storm syndrome in COVID‐19 pneumonia. Eur J Case Rep Intern Med 2020; 7: 001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC, Chan KS, Haja Mohideen S. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID‐19 infection. Ann Acad Med Singapore 2020; 49: 108–118. [PubMed] [Google Scholar]
- 23. Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, Rowe D, Bogoch II, Smith PT, Baggish AL, Putukian M, Engel DJ. Prevalence of inflammatory heart disease among professional athletes with prior COVID‐19 infection who received systematic return‐to‐play cardiac screening. JAMA Cardiol 2021; 4: e210565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nuzzi V, Castrichini M, Collini V, Roman‐Pognuz E, Di Bella S, Luzzati R, Berlot G, Confalonieri M, Merlo M, Stolfo D, Sinagra G. Impaired right ventricular longitudinal strain without pulmonary hypertension in patients who have recovered from COVID‐19. Circ Cardiovasc Imaging 2021; 14: e012166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of relevant clinical characteristics between patients excluded and included in the analysis.
Table S2. Demographic and clinical characteristics of the study population at admission stratified by vital status (N = 197).


