Abstract
Objective
To evaluate seroreactivity and disease flares after COVID‐19 vaccination in a multiethnic/multiracial cohort of patients with systemic lupus erythematosus (SLE).
Methods
Ninety SLE patients and 20 healthy controls receiving a complete COVID‐19 vaccine regimen were included. IgG seroreactivity to the SARS–CoV‐2 spike receptor‐binding domain (RBD) and SARS–CoV‐2 microneutralization were used to evaluate B cell responses; interferon‐γ (IFNγ) production was measured by enzyme‐linked immunospot (ELISpot) assay in order to assess T cell responses. Disease activity was measured by the hybrid SLE Disease Activity Index (SLEDAI), and flares were identified according to the Safety of Estrogens in Lupus Erythematosus National Assessment–SLEDAI flare index.
Results
Overall, fully vaccinated SLE patients produced significantly lower IgG antibodies against SARS–CoV‐2 spike RBD compared to fully vaccinated controls. Twenty‐six SLE patients (28.8%) generated an IgG response below that of the lowest control (<100 units/ml). In logistic regression analyses, the use of any immunosuppressant or prednisone and a normal anti–double‐stranded DNA antibody level prior to vaccination were associated with decreased vaccine responses. IgG seroreactivity to the SARS–CoV‐2 spike RBD strongly correlated with the SARS–CoV‐2 microneutralization titers and correlated with antigen‐specific IFNγ production determined by ELISpot. In a subset of patients with poor antibody responses, IFNγ production was similarly diminished. Pre‐ and postvaccination SLEDAI scores were similar in both groups. Postvaccination flares occurred in 11.4% of patients; 1.3% of these were severe.
Conclusion
In a multiethnic/multiracial study of SLE patients, 29% had a low response to the COVID‐19 vaccine which was associated with receiving immunosuppressive therapy. Reassuringly, severe disease flares were rare. While minimal protective levels remain unknown, these data suggest that protocol development is needed to assess the efficacy of booster vaccination.
INTRODUCTION
As scientific advances have been applied with unprecedented speed during the COVID‐19 pandemic, physicians and their patients have pivoted from treatment of infection and passive immunization to full‐scale preventative measures, particularly in high‐risk individuals (1, 2). Patients with systemic lupus erythematosus (SLE) comprise a unique population with regard to risk for infection and outcomes associated with SARS–CoV‐2, given underlying demographics, associated organ damage, and comorbidities. In addition, medications commonly used to treat SLE have been associated with an increased risk of death from COVID‐19 (3). Early data provided evidence that patients with SLE have a high risk of hospitalization from COVID‐19, with factors including race/ethnicity, comorbidities such as cardiovascular disease and renal insufficiency, and higher body mass index identified as independent predictors of hospitalization (1, 4). Further raising concern, infection was reported to be associated with flares of disease (5). In subsequent studies, patients with SLE and confirmed COVID‐19 were demonstrated to generate and maintain serologic responses despite the use of a variety of immunosuppressants (6). These data provided reassurance regarding the efficacy and durability of humoral immunity and protection against reinfection with SARS–CoV‐2, as well as potential insights into the efficacy of active immunization in SLE patients.
Since the phase III clinical studies of all 3 vaccines excluded patients treated with immunosuppressants or immune‐modifying drugs within 6 months of enrollment, data on SLE are virtually absent (7, 8, 9). Furthermore, given the potential for disease flares following immunization, it is not surprising that a recent study reported hesitancy for vaccination in patients with rheumatic diseases, including SLE (10). Accordingly, the current study was initiated to address these critical gaps and examine the efficacy of these promising COVID‐19 vaccines in patients with SLE. This was accomplished by evaluating a multiethnic/multiracial cohort of SLE patients using assessments of serologic responses which were compared to healthy controls. The assays included antibodies to the spike protein receptor‐binding domain (RBD), virus‐neutralizing antibodies, and antigen‐specific T cell production of interferon‐γ (IFNγ), both prior to and after vaccination. Factors associated with the level of responsiveness were sought. In addition, SLE disease activity pre‐ and postvaccination was measured, as well as the rate of flare postvaccination.
PATIENTS AND METHODS
Study population and inclusion/exclusion criteria
Patients were recruited from the established New York University (NYU) Lupus Cohort, a prospective convenience registry open to enrolling any patient with SLE seen at NYU Langone Health and Bellevue Hospital Center since 2014. All SLE patients in the NYU Lupus Cohort are age 18 or older and fulfill ≥1 of the following criteria: 1) the American College of Rheumatology (ACR) revised classification criteria (11); 2) the Systemic Lupus International Collaborating Clinics classification criteria (12); and/or 3) the European Alliance of Associations for Rheumatology/ACR classification criteria (13). All NYU Lupus Cohort patients and controls provided written informed consent, which was available in English, Spanish, and Mandarin. All adult patients with SLE planning to receive any of the available COVID‐19 vaccines were eligible for inclusion. Exclusion criteria included unwillingness to provide blood after the second dose of the vaccine, incomplete vaccination schedule, and speaking a language other than English, Spanish, or Mandarin. Healthy controls were ≥18 years of age, had no known rheumatic diseases and were receiving no immunosuppressive medications. The study protocol and the NYU Lupus Cohort and recruitment of controls were approved by the NYU and Bellevue Hospital Institutional Review Boards.
Study design and data collection
Patients were recruited using convenience sampling, with inclusion and exclusion criteria as stated above. For most patients, blood samples were available pre‐ and postvaccination. Disease activity measures and laboratory data prior to vaccination were available as part of the NYU Lupus Cohort but were limited to patients seen within 4 months of their first vaccine dose. Postvaccination follow‐ups were scheduled ~2 weeks after the second dose of the messenger RNA (mRNA) vaccines (i.e., BNT162b2 [Pfizer/BioNTech] or mRNA‐1273 [Moderna]) or after 1 dose of Ad26.COV2.S (Johnson & Johnson) to collect postvaccination blood samples and assess for any change in SLE activity. Disease activity was measured by the hybrid Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)–SLE Disease Activity Index (SLEDAI) (urine protein:creatinine ratios >0.5 were always counted), and flares were assessed by the SELENA–SLEDAI flare index (14, 15, 16). In addition, the use and doses of immunosuppressive medications were recorded at each visit, including, among others, glucocorticoids, hydroxychloroquine (HCQ), azathioprine, mycophenolate mofetil (MMF), methotrexate (MTX), belimumab, and tacrolimus. Any cyclophosphamide, obinutuzumab, or rituximab administered within 6 months of the patient's visit was also recorded. Considering that our patients were largely enrolled before the ACR updated their guidelines to temporarily hold MMF used to treat more severe manifestations such as nephritis, that medication was not held. We did advise patients to hold MTX and adjusted other medications as recommended per this guidance (17).
Enzyme‐linked immunosorbent assay (ELISA) for recombinant SARS–CoV‐2 spike protein
Ninety‐six–well plates were coated with 1 μg/ml recombinant SARS–CoV‐2 spike RBD (no. BT10500; R&D Systems), diluted in phosphate buffered saline (PBS) and incubated overnight at 4°C. Plates were blocked with 0.1% gelatin in PBS. Plasma (spun 10,000 rpm for 1 minute) were diluted 1:200–1:100,000 and added to the plate for 1 hour at room temperature. Samples were run in triplicate. With each run, 2 positive controls were included in the 96‐well plate: plasma from control (non‐SLE) participants postvaccination, with high and low IgG titers, each diluted 1:500 to ensure that measurements were captured across the assay range. Detection relies on an enzyme‐labeled secondary antibody, alkaline phosphatase–conjugated rabbit anti‐human IgG (γ‐chain–specific) (Sigma) diluted 1:2,000. After developing with the addition of phosphatase substrate, the optical density (OD) was measured at 405 nm, and the reaction was evaluated when the low positive control reached an OD of 1. The OD measured for a tested sample was multiplied by the dilution factor, which gave an OD in the range of 0.3–0.8.
SARS–CoV‐2 microneutralization assay
Viral neutralization activity of plasma was measured in an immunofluorescence‐based microneutralization assay by detecting the neutralization of infectious virus in cultured Vero E6 cells (no. CRL‐1586, African green monkey kidney cells; ATCC). Cells were maintained according to standard ATCC protocols. Briefly, Vero E6 cells were grown in minimum essential medium (MEM) supplemented with 10% heat‐inactivated fetal bovine serum (FBS), 2 mM l‐glutamine, and 1% of MEM Nonessential Amino Acid Solution (no. MT25025CI; Fisher). Cell cultures were grown in 75 or 150 cm2 flasks at 37°C with 5% CO2 and passaged 2–3 times per week using trypsin–EDTA. Cell cultures used for virus testing were prepared as subconfluent monolayers. All incubations containing cells were performed at 37°C with 5% CO2. All SARS–CoV‐2 infection assays were performed in the Centers for Disease Control and Prevention (CDC)/US Department of Agriculture–approved biosafety level 3 facility in compliance with NYU Grossman School of Medicine guidelines for biosafety level 3. SARS–CoV‐2 isolate USA‐WA1/2020, deposited by the CDC, was obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NR‐52281, GenBank accession no. MT233526). Serial dilutions of heat‐inactivated plasma (56°C for 1 hour) were incubated with USA‐WA1/2020 stock (at fixed 1 × 106 plaque‐forming units/ml) for 1 hour at 37°C. One hundred microliters of the plasma–virus mix was then added to the cells and incubated at 37°C with 5% CO2. Twenty‐four hours postinfection, cells were fixed with 10% formalin solution (4% active formaldehyde) for 1 hour, stained with an anti‐SARS–CoV‐2 nucleocapsid antibody (no 10‐605; ProSci), and a goat anti‐mouse IgG Alexa Fluor 647 secondary antibody along with DAPI and visualized by microscopy with the CellInsight CX7 High‐Content Screening Platform (ThermoFisher) and high‐content software.
Enzyme‐linked immunospot (ELISpot)
ELISpot plates (Human IFNγ ELISpot Plus, no. 3420‐4HPT‐2) were preseeded under sterile conditions following the recommendations of the manufacturer (Mabtech) in duplicates with 250,000 cells per well from cryopreserved peripheral blood mononuclear cells (PBMCs) isolated from each participant. Cells were incubated for 24 hours with SARS–CoV‐2 spike protein S1 (1 μg/well) (no. RP‐87681; Invitrogen) or vehicle, in the presence of anti‐CD28 (1 μg/ml) (Biolegend). Cells were removed by washing the wells with PBS + 1% FBS. Membrane was probed with a 1:1,000 dilution of the detection antibody provided by the manufacturer (1 hour at 22°C). After washing, plates were developed using tetramethylbenzidine substrate solution. Spots were imaged and counted using an ImmunoSpot S6 Analyzer (Cellular Technology Limited). For each sample, the monoclonal antibody CD3‐2 was used to capture cytokine production as a positive control.
Statistical analysis
Categorical variables were summarized by computing counts and proportions of patients. Continuous variables are expressed as the mean ± SD or the median and interquartile range (IQR) or range, as appropriate. Two‐group comparisons were performed using the chi‐square or Fisher's exact test for categorical variables and the 2‐sample t‐test or Mann–Whitney U test for continuous variables. Spearman's rank correlation coefficient was computed for the association between the ELISA and microneutralization assays. An exploratory logistic regression analysis was also conducted to identify potential independent predictors of low postvaccine ELISA antibody response (≤100 units/ml, the lowest value seen in controls). Variable selection in the final model was based on both statistical significance (P < 0.10, given limited sample size and power of the study) as well as clinical considerations. All statistical analyses were performed using SAS version 9.4.
RESULTS
Patient population
A total of 90 patients with SLE and 20 controls were included in this study. Table 1 shows the demographics of the patients and controls in addition to SLE‐specific information. Cases and controls were relatively well‐matched; however, controls were more likely to be male (P = 0.007). Whereas controls only received the BNT162b2 and mRNA‐1273 vaccines, SLE patients received all 3 vaccines currently available in the US, including Ad26.COV2.S. In addition, 12% of the SLE patients had a history of prior COVID‐19 infection compared to 10% of controls. Forty‐four percent of patients had a history of lupus nephritis (LN), 10% had secondary antiphospholipid syndrome (APS), and 5.6% had received a kidney transplant. The majority of SLE patients (79%) were receiving HCQ, and 29% were receiving systemic glucocorticoids (mean dose of 7 mg prednisone). Forty‐two percent were receiving ≥1 immunosuppressant, with MMF being the most common (21%), followed by belimumab (11%). In addition, 17% of patients were receiving a combination of immunosuppressants. Figure 1 shows the number of SLE patients included in each subsequent analysis.
Table 1.
Characteristics of the vaccinated SLE patients and healthy controls*
| Controls (n = 20) | SLE patients (n = 90) | |
|---|---|---|
| Age, mean ± SD years | 45.3 ± 14.2 | 45.5 ± 14.2 |
| Sex† | ||
| Female | 12 (60.0) | 79 (87.8) |
| Male | 8 (40.0) | 11 (12.2) |
| Race | ||
| White | 13 (65.0) | 43 (47.8) |
| Black | 2 (10.0) | 16 (17.8) |
| Asian | 4 (20.0) | 17 (18.9) |
| Other | 1 (5.0%) | 14 (15.5) |
| Ethnicity | ||
| Hispanic/Latino | 1 (5.0) | 34 (37.8) |
| COVID‐19 vaccine | ||
| BNT162b2 (Pfizer) | 17 (85.0) | 61 (67.8) |
| mRNA‐1273 (Moderna) | 3 (15.0) | 24 (26.7) |
| Ad26.COV2.S (Johnson & Johnson) | 0 (0) | 5 (5.5) |
|
Days between 2nd vaccine dose and postvaccine blood draw, mean (range) |
23 (14–31) | 24 (5–69) |
| Prior history of COVID‐19 (PCR or IgG) | 2 (10.0) | 11 (12.2) |
| SLE risk factors | ||
| History of LN | N/A | 40 (44.4) |
| Kidney transplant recipient | N/A | 5 (5.6) |
| APS | N/A | 9 (10.0) |
| Medication(s) | ||
| HCQ | – | 71 (79) |
| Dose, mean ± SD mg | – | 321.0 ± 89.7 |
| Chloroquine | – | 1 (1) |
| Dose, mg | – | 250.0 |
| Prednisone | – | 26 (29) |
| Dose, mean ± SD mg | – | 7.2 ± 7.6 |
| Immunosuppressants | – | 38 (42) |
| AZA | – | 5 (6) |
| Dose, mean ± SD mg | – | 130.0 ± 27.4 |
| MMF | – | 19 (21) |
| Dose, mean ± SD mg | – | 1,967.1 ± 731.1 |
| Mycophenolic acid | – | 2 (2) |
| Dose, mean ± SD mg | – | 900.0 ± 254.6 |
| Tacrolimus | – | 5 (6) |
| Dose, mean ± SD mg | – | 4.0 ± 2.3 |
| MTX | – | 8 (9) |
| Dose, mean ± SD mg‡ | – | 14.6 ± 6.0 |
| Belimumab | – | 10 (11) |
| Cyclophosphamide | – | 0 (0) |
| Rituximab | – | 3 (3) |
| Leflunomide | – | 1 (1) |
| Abatacept | – | 1 (1) |
| Adalimumab | – | 1 (1) |
| Obinutuzumab | – | 1 (1) |
| Eculizumab | – | 1 (1) |
| Apremilast | – | 1 (1) |
| SLE clinical trial | – | 1 (1) |
| Prednisone + immunosuppressant | – | 22 (24) |
| Combination immunosuppressants | – | 15 (17) |
Except where indicated otherwise, values are the number (%) of subjects. SLE = systemic lupus erythematosus; PCR = polymerase chain reaction; LN = lupus nephritis; APS = antiphospholipid syndrome; N/A = not applicable; HCQ = hydroxychloroquine; AZA = azathioprine; MMF = mycophenolate mofetil.
P = 0.007.
Includes 1 patient with an unknown dose of methotrexate (MTX), prescribed at an outside institution.
Figure 1.
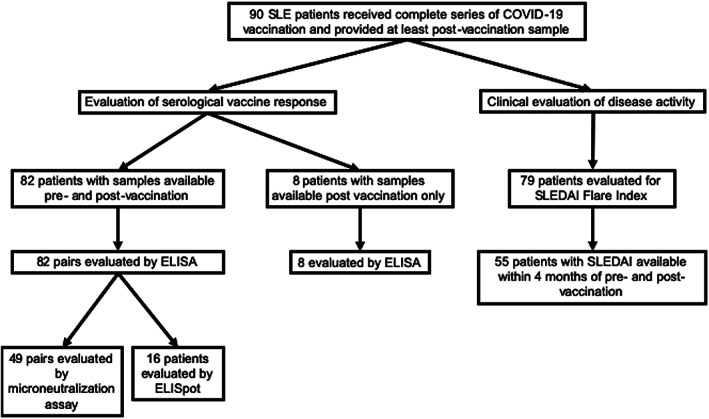
Flow diagram of the systemic lupus erythematosus (SLE) patients included in each analysis. ELISA = enzyme‐linked immunosorbent assay; ELISpot = enzyme‐linked immunospot; SLEDAI = SLE Disease Activity Index.
Decreased COVID‐19 antibody responses in SLE patients compared to controls
Ninety SLE patients (82 with data from pre‐ and postvaccination analyses) and 20 healthy controls (all with data from pre‐ and postvaccination analyses) were evaluated for IgG antibody levels against the RBD of SARS–CoV‐2 spike protein (anti‐RBD) (Figures 2A–D). Overall, prevaccine levels in patients with SLE were significantly lower (median 9.1 [IQR 2.8–23.9]) than in controls (median 34.5 [IQR 11.2–74.0]; P = 0.001), as were the postvaccine levels (median 235.2 [IQR 75.9–531.4] versus median 435.7 [IQR 269.0–768.6], respectively; P = 0.01). Postvaccine antibody levels in 26 SLE patients (28.8%) fell below the lowest level of the controls (≤100 units/ml), which is shown in Figure 2D.
Figure 2.
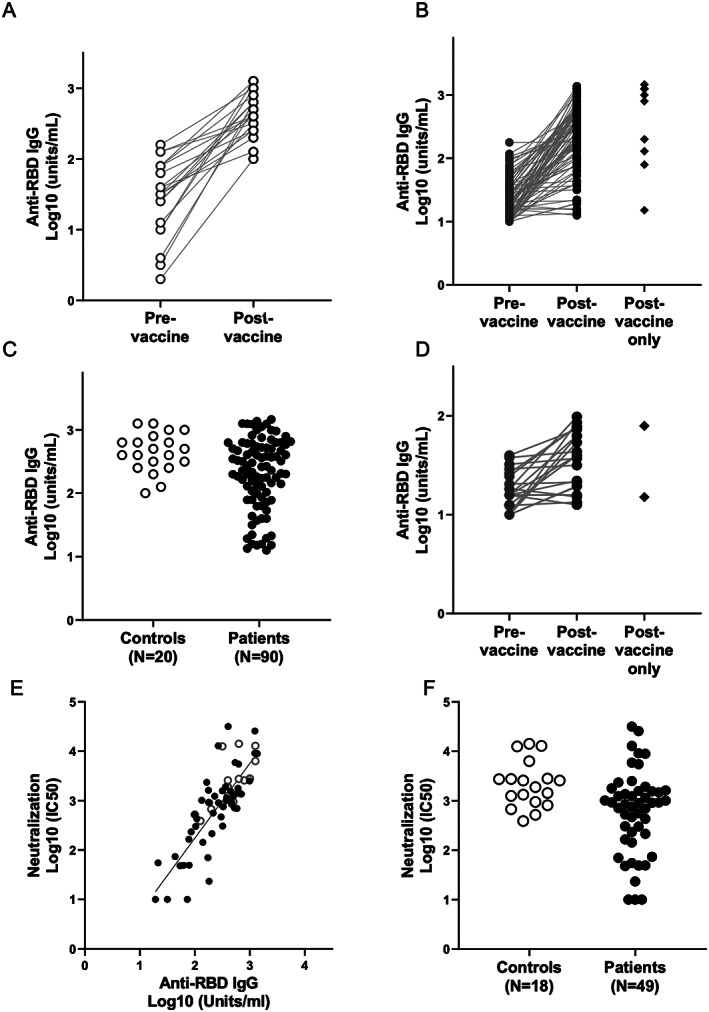
Antibody response to COVID‐19 vaccine in SLE patients. Throughout, open circles represent controls, and solid circles represent SLE patients. Serum IgG titers against the SARS–CoV‐2 spike protein receptor‐binding domain (RBD) were obtained by direct ELISA. Binding of human IgG to recombinant SARS–CoV‐2 RBD was performed as described in Patients and Methods. A, IgG titers of 20 controls pre‐ and postvaccination. B, IgG titers of 81 SLE patients pre‐ and postvaccination and 9 SLE patients with only postvaccination data available. C, Comparison of IgG titers postvaccination between the 2 groups, with controls showing significantly higher titers than SLE patients. D, IgG titers of a subgroup of patients (n = 24) from B with anti‐RBD IgG ≤100 units/ml (the lowest response in controls). E, Correlation of SARS–CoV‐2 virus neutralization titers in the sera of vaccinated subjects with IgG titers. Each dot represents the serum evaluation of a participant with binding of human IgG to recombinant SARS–CoV‐2 RBD (x‐axis) versus live virus neutralization at 50% inhibition concentration (IC50; y‐axis). There is a strong correlation between spike RBD IgG and live virus neutralization (R = 0.76, P < 0.0001). F, Log10 postvaccination neutralization titers for controls compared to SLE patients. See Figure 1 for other definitions.
To address the functionality of the antibody responses assessed by ELISA, pre‐ and postvaccine samples from 49 SLE patients and 18 controls were also evaluated by the SARS–CoV‐2 live microneutralization assay. As shown in Figure 2E, there was a strong correlation between the 2 assays (R = 0.76, P < 0.0001), suggesting that the ELISA is a good end point assay to evaluate the immune response to the vaccines. Similar to what we observed with the ELISA results, postvaccine microneutralization titers were significantly lower in SLE patients compared to controls (P = 0.0075) (Figure 2F).
A comparison of clinical and laboratory factors in the 64 SLE patients who generated responses to COVID‐19 vaccine that were compatible with controls and the 26 patients with low responses who had ELISA results ≤100 units/ml is shown in Table 2. In unadjusted analyses, subjects with low responses were more likely to be receiving prednisone, MMF or mycophenolic acid, a combination of prednisone and ≥1 immunosuppressant, or ≥2 immunosuppressants, while those with high responses were more likely to only be receiving antimalarials or receiving no medication. In addition, low responders were more likely to have received Ad26.COV2.S, although sample sizes were limited, and to have had a normal anti–double‐stranded DNA (anti‐dsDNA) antibody level prior to vaccination (Table 2). Logistic regression analysis yielded a final model that included the following 4 independent predictors of low ELISA response among SLE patients: receiving any immunosuppressive therapy other than antimalarials (adjusted odds ratio [OR] 15.14 [95% CI 2.80–82.03] compared to antimalarials/no medications; P = 0.002), normal anti‐dsDNA antibody level prior to vaccination (OR 14.50 [95% CI 2.20–95.66] compared to abnormal anti‐dsDNA antibody level; P = 0.006), lower platelet count (OR 1.55 [95% CI 0.96–2.51] per 50 × 109 cells/liter decrease; P = 0.07), and normal C3 level (OR 4.95 [95% CI 0.91–26.0] compared to low C3 level; P = 0.06). A separate subgroup analysis including only those patients who were receiving any immunosuppressants confirmed the association of a normal anti‐dsDNA antibody level with poor antibody response (OR 8.98 [95%CI 1.89–42.6]; P = 0.006 after adjustment for platelet and C3). Further details about the patients with lower responses are provided in Table 3.
Table 2.
Bivariate analysis of predictors for poor postvaccine ELISA antibody response (≤100 units/ml) among the SLE patients*
| Postvaccine ELISA antibody response | P | ||
|---|---|---|---|
| ≤100 units/ml(n = 26) | >100 units/ml (n = 64) | ||
| Age, mean ± SD years | 47.7 ± 13.3 | 44.6 ± 14.5 | 0.36 |
| Sex | 0.29 | ||
| Female | 21 (80.8) | 58 (90.6) | |
| Male | 5 (19.2) | 6 (9.4) | |
| Race | 0.46 | ||
| White | 11 (42.3) | 32 (50.0) | |
| Black | 3 (11.5) | 13 (20.3) | |
| Asian | 7 (26.9) | 10 (15.6) | |
| Other | 5 (19.2) | 9 (14.1) | |
| Ethnicity | 0.93 | ||
| Hispanic | 10 (38.5) | 24 (37.5) | |
| Non‐Hispanic | 16 (61.5) | 40 (62.5) | |
|
Days between 2nd vaccine dose and postvaccine blood draw, median (IQR) |
19.5 (14.0–44.0) | 17.0 (12.0–26.0) | 0.11 |
| Vaccine type | 0.039 | ||
| BNT162b2 | 15 (57.7) | 46 (71.9) | |
| mRNA‐1273 | 7 (26.9) | 17 (26.6) | |
| Ad26.COV2.S | 4 (15.4) | 1 (1.6) | |
| Prior history of COVID‐19 (PCR or IgG) | 4 (18.2) | 7 (13.7) | 0.72 |
| History of LN | 15 (57.7) | 25 (39.7) | 0.12 |
| Kidney transplant recipient | 3 (11.5) | 2 (3.1) | 0.14 |
| APS | 0 (0) | 9 (14.1) | 0.055 |
| Prednisone + ≥1 immunosuppressant | 10 (38.5) | 12 (18.8) | 0.049 |
| Combination immunosuppressants | 9 (34.6) | 6 (9.4) | 0.01 |
|
Only antimalarials (HCQ + chloroquine) among those receiving medication |
3 (12.0) | 33 (56.9) | 0.0002 |
| Any MMF (MMF + mycophenolic acid) | 12 (46.2) | 9 (14.1) | 0.001 |
| Any prednisone | 12 (46.2) | 14 (21.9) | 0.021 |
| Any belimumab | 4 (15.4) | 6 (9.4) | 0.47 |
| No immunosuppressants | 1 (3.8) | 6 (9.4) | 0.67 |
| Only HCQ or no medications | 4 (15.4) | 39 (60.9) | <0.0001 |
| Prevaccine anti‐dsDNA antibody level† | 0.023 | ||
| Normal | 14 (87.5) | 28 (56.0) | |
| High | 2 (12.5) | 22 (44.0) | |
| C3 level | 0.35 | ||
| Low | 3 (18.8) | 17 (34.0) | |
| Normal | 13 (81.3) | 33 (66.0) | |
| C4 level | 1.00 | ||
| Low | 4 (25.0) | 12 (24.0) | |
| Normal | 12 (75.0) | 38 (76.0) | |
| SLEDAI score, mean ± SD† | 2.00 ± 2.34 | 3.46 ± 4.16 | 0.085 |
| Platelet count, mean ± SD × 103/μl† | 202.81 ± 78.37 | 243.74 ± 85.60 | 0.095 |
|
Urine protein:creatinine ratio, mean ± SD† |
0.17 ± 0.18 | 0.28 ± 0.58 | 0.26 |
| Lymphocyte count, mean ± SD × 103/μl† | 1.19 ± 0.65 | 1.36 ± 0.97 | 0.43 |
Except where indicated otherwise, values are the number (%) of subjects. ELISA = enzyme‐linked immunosorbent assay; SLE = systemic lupus erythematosus; IQR = interquartile range; PCR = polymerase chain reaction; LN = lupus nephritis; APS = antiphospholipid syndrome; HCQ = hydroxychloroquine; MMF = mycophenolate mofetil; anti‐dsDNA = anti–double‐stranded DNA (see Table 1 for other definitions).
Laboratory measures and SLE Disease Activity Index (SLEDAI) scores were based on patients with prevaccine data available within 4 months of vaccine (n = 66).
Table 3.
Demographic information and medications of the SLE patients with lower vaccine responses (n = 26)*
| Patient | Age | Sex | Vaccine type | Medication(s) |
|---|---|---|---|---|
| 1 | 39 | Male | BNT162b2 | HCQ, MMF, obinutuzumab |
| 2 | 71 | Female | BNT162b2 | MTX, abatacept |
| 3 | 57 | Female | BNT162b2 | HCQ, belimumab |
| 4 | 57 | Female | BNT162b2 | Prednisone (4 mg), HCQ, MTX, adalimumab |
| 5 | 61 | Female | BNT162b2 | HCQ, MMF |
| 6 | 42 | Female | BNT162b2 | HCQ, tacrolimus, rituximab |
| 7 | 38 | Female | BNT162b2 | Prednisone (5 mg), mycophenolic acid, tacrolimus |
| 8 | 59 | Female | BNT162b2 | Prednisone (5 mg), HCQ |
| 9 | 62 | Male | BNT162b2 | HCQ, belimumab |
| 10 | 54 | Female | BNT162b2 | HCQ |
| 11 | 49 | Female | BNT162b2 | Prednisone (3 mg), MMF, belimumab |
| 12 | 53 | Female | BNT162b2 | MMF |
| 13 | 47 | Female | BNT162b2 | Prednisone (10 mg), HCQ, MMF |
| 14 | 40 | Male | BNT162b2 | Prednisone (5 mg), HCQ, MMF |
| 15 | 29 | Female | BNT162b2 | Prednisone (2.5 mg), HCQ, MMF |
| 16 | 39 | Male | mRNA‐1273 | HCQ, rituximab |
| 17 | 46 | Female | mRNA‐1273 | Prednisone (5 mg), HCQ, MMF |
| 18 | 35 | Female | mRNA‐1273 | HCQ, AZA, belimumab |
| 19 | 66 | Female | mRNA‐1273 | Prednisone (5 mg) |
| 20 | 28 | Female | mRNA‐1273 | HCQ |
| 21 | 44 | Female | mRNA‐1273 | HCQ |
| 22 | 41 | Female | mRNA‐1273 | Prednisone (20 mg), MMF |
| 23 | 55 | Female | Ad26.COV2.S | Prednisone (5 mg), HCQ, mycophenolic acid |
| 24 | 72 | Female | Ad26.COV2.S | None |
| 25 | 27 | Female | Ad26.COV2.S | HCQ, MMF |
| 26 | 28 | Male | Ad26.COV2.S | Prednisone (10 mg), MTX |
SLE = systemic lupus erythematosus; HCQ = hydroxychloroquine; MMF = mycophenolate mofetil; MTX = methotrexate; AZA = azathioprine.
Evaluation of IFNγ secretion in response to SARS–CoV‐2 spike protein S1
Sixteen SLE patients (of whom 4 had pre‐ and postvaccine data available) and 2 controls (both with pre‐ and postvaccine data available) were further evaluated addressing T cell reactivity, which was operationally reported by the release of IFNγ in response to challenge of PBMCs in the absence and presence of the full‐length S1 protein (COVID‐19 antigen), as described in Patients and Methods. Individuals were chosen to represent a range of responses to the SARS–CoV‐2 spike protein and SARS–CoV‐2 microneutralization assay but with a particular focus on the patients with low seroreactivity on both assays. As shown in Supplementary Figure 1 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41937/abstract), there was a correlation between the postvaccine ELISpot number and ELISA evaluations (R = 0.57, P = 0.0135). In a subset of patients with poor antibody responses, IFNγ production was likewise diminished (Supplementary Figure 1).
Stable disease activity in the majority of SLE patients after COVID‐19 vaccination
Of the 90 patients evaluated, 55 patients had completed a SLEDAI assessment within 4 months of their first vaccine dose, which was then compared to their postvaccine SLEDAI assessment that was completed an average of 23.6 days (range 5–70) after the final vaccine dose (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.41937/abstract). Overall, there was no meaningful difference in SLEDAI score between pre‐ and postvaccine visits (3.2 versus 2.9). There were no changes in the percentages of patients with abnormal anti‐dsDNA antibodies and/or abnormal complement levels; likewise, the levels of C3 and C4 were similar pre‐ and postvaccination (Supplementary Table 1). Nine of the 79 patients (11.4%) experienced a postvaccination flare, with all but 1 considered to be mild/moderate (2 in new organ systems: arthritis with no treatment and pericarditis treated with naproxen). The severe flare was characterized by arthritis and treated with MTX; the patient had discontinued HCQ due to maculopathy several years prior to vaccination. Further details of the flares are provided in Table 4.
Table 4.
SLE flares postvaccination*
| Flare severity | Flare type | Flare details | Timing of flare | Vaccine type | Treatment |
|---|---|---|---|---|---|
| Mild/moderate | Pleuritis | Recurrent mild pleuritis | After 1st dose | BNT162b2 | No treatment |
| Mild/moderate | Arthritis |
Recurrent mild joint pain and swelling |
After 2nd dose | BNT162b2 | No treatment |
| Mild/moderate | Renal |
Recurrent proteinuria; urine protein:creatinine ratio increased from 0.8 to 1.4 |
After 2nd dose | BNT162b2 |
Rituximab/tacrolimus changed to voclosporin |
| Mild/moderate | Oral ulcers |
Recurrent oral ulcers; patient had been off of belimumab for 3 months |
After 2nd dose | mRNA‐1273 | No treatment |
| Mild/moderate | Pericarditis |
New presumed pericarditis, EKG negative; resolved with naproxen |
After 2nd dose (2 weeks) |
BNT162b2 | Naproxen |
| Severe | Arthritis | Recurrent arthritis | After 2nd dose | mRNA‐1273 | Methotrexate |
| Mild/moderate | Thrombocytopenia |
Recurrent thrombocytopenia within patient's range |
After 2nd dose | BNT162b2 | No treatment |
| Mild/moderate | Arthritis |
New mild joint pain and swelling |
After 2nd dose | BNT162b2 | No treatment |
| Mild/moderate† | Thrombocytopenia | Recurrent thrombocytopenia | After 2nd dose | BNT162b2 | No treatment |
|
Non–SLE‐related event |
COPD/asthma flare | After 2nd dose | BNT162b2 |
Treated in emergency room with steroids, then released |
EKG = electrocardiogram; COPD = chronic obstructive pulmonary disease.
A patient with systemic lupus erythematosus (SLE), antiphospholipid syndrome, and end‐stage renal disease was admitted 13 days after the second vaccine dose. The patient had anticoagulation therapy temporarily withheld for a procedure, presented with shortness of breath, and was found to have superior vena cava syndrome. The patient had a prolonged hospital course, complicated by bleeding and sepsis, and was transitioned to hospice care and died 50 days after the second dose.
DISCUSSION
To our knowledge, this is the first reported study focused on patients with SLE who received full regimens of a COVID‐19 vaccine, and overall IgG antibody responses against the SARS–CoV‐2 spike protein RBD were significantly decreased compared to vaccinated controls, with 28.8% of patients generating responses falling below the lowest level observed in the healthy controls. Receiving any immunosuppressive agent other than antimalarials and having a normal anti‐dsDNA antibody level prior to vaccination were identified as independent predictors for poor response to the COVID‐19 vaccine. Seroreactivity to the SARS–CoV‐2 spike RBD strongly correlated with the functional SARS–CoV‐2 microneutralization assay and correlated with the ELISpot assay. Overall, there was no change in SLEDAI score pre‐ and postvaccination, with 11.4% of patients having a flare and 1.3% of those flares being severe, supporting the relative safety of the vaccination in SLE patients.
The finding of anti‐dsDNA antibodies positively correlating with higher responses to COVID‐19 vaccination was initially unexpected, especially given that this finding persisted even after controlling for medication use. Moreover, disease activity per se was not associated with more effective seroreactivity. It could be hypothesized that the presence of anti‐dsDNA antibodies is a proxy of elevated type I IFN activity in these patients. Indeed, studies have shown that high IFNα activity in patients with SLE is associated with the presence of disease‐specific autoantibodies, such as anti‐dsDNA (18). These autoantibodies can form immune complexes, further stimulating type I IFN production (19). Besides their potent antiviral properties, type I IFNs induce the maturation and activation of myeloid dendritic cells, and promote B cell survival and differentiation into antibody‐producing cells (20, 21). These considerations support the hypothesis that those with stronger responses to the COVID‐19 vaccines could have higher baseline type I IFN activity, due to its potential to enhance antibody responses to foreign antigens. Thus, patients with anti‐dsDNA antibodies, despite receiving immunosuppressive therapy, may be more likely to develop a strong humoral response to the COVID‐19 vaccines. Alternatively, these analyses did not account for patient adherence to medication or the possibility that elevated dsDNA antibodies reflects inefficacy of immunosuppression, which might account for these findings. These potential insights merit further investigation.
Given the exclusion of patients receiving immunosuppressants from the regulatory vaccine studies, several groups have already explored the influence of immunosuppressive medications on the response to vaccination. Boyarsky et al evaluated patients with organ transplants and reported that antimetabolite maintenance immunosuppression was associated with an absent or reduced anti‐RBD spike response after the first dose of the vaccine (22). A follow‐up study from the same group in 658 transplant recipients who received the second dose of the SARS–CoV‐2 mRNA vaccine showed an increase in seroreactivity in response to the second dose; however, poor responses were associated with antimetabolite immunosuppressive treatment (23).
Concordant with our results, several studies have shown decreased vaccine‐induced seroreactivity in patients with rheumatic diseases. In 123 such patients, including 24 with SLE, those receiving MMF or rituximab were less likely to develop an antibody response to the spike protein after the first dose of the SARS–CoV‐2 mRNA vaccine; these findings were confirmed in a larger study of 404 patients, including 87 with SLE, after the second dose (24, 25). In an analysis of 26 patients with chronic inflammatory diseases (CIDs) that included 2 patients with SLE who were receiving HCQ, SARS–CoV‐2 antibodies were significantly lower in patients, compared to controls, after both doses of BNT162b2 or mRNA‐1273. No patients experienced a disease flare after both doses of the vaccine (26). A large study of 133 patients with CIDs, including 15 patients with SLE, who received an mRNA vaccine showed that patients with CIDs had a 3‐fold reduction in anti–spike protein IgG response with B cell depletion, glucocorticoids, and antimetabolites (27). A subsequent analysis of 89 patients that included 10 patients with SLE showed that rituximab was associated with impaired serologic response to the SARS–CoV‐2 vaccine (28). Haberman et al demonstrated that MTX adversely affected both the humoral and cellular immune responses to COVID‐19 mRNA vaccines in patients with immune‐mediated inflammatory diseases (29). A large study from Furer et al that included 101 patients with SLE showed that older age and treatment with glucocorticoids, rituximab, MMF, and abatacept were associated with reduced immunogenicity as measured by serum IgG antibody levels against SARS–CoV‐2 spike S1/S2 proteins 2–6 weeks after vaccination (30). Our study showed that receiving any non‐antimalarial immunosuppressive therapy was independently associated with decreased response to COVID‐19 vaccines in patients with SLE.
In addition to concerns regarding inefficient immune responses to COVID vaccination, it may be the case that vaccination induces increased autoantibody production and disease activity. As speculated by Tang et al, delivery of mRNA encoding S protein via the vaccine, likely degraded by normal cellular processes, could interact with a number of cytoplasmic RNA‐binding proteins involved in the posttranscriptional regulation of inflammation and result in worsening SLE (5). Similarly, RNA vaccines may trigger Toll‐like receptors, generating further production of type I IFN, already well‐recognized to be elevated in most SLE patients (19). It has been reported that influenza vaccines triggered a transient increase in several autoantibody specificities in 72 SLE patients, with a flare rate of 19.4% within 6 weeks postvaccination; 10 (13.9%) were mild/moderate and 4 (5.6%) were severe (31). In a study evaluating SLE flares after immunization against poliomyelitis, only 4 of 73 patients (5%) experienced flares (32). In aggregate, despite apprehensions, the data presented herein did not support significantly increased anti‐dsDNA antibody production or flares postvaccination. These results are consistent with a recent study which showed that the majority of vaccinated SLE patients had no change or decrease in disease activity after COVID‐19 vaccination as measured by the SLEDAI (30).
Our study has several limitations. Similar to other studies evaluating potential surrogate markers for vaccine efficacy, it is premature to assign a threshold level of protection based on either the IgG response to the anti‐RBD of SARS–CoV‐2 spike protein or the microneutralization assay given the number of controls. There was vaccine hesitancy among patients in the NYU Lupus Cohort, in large part due to concern regarding the potential effect on lupus activity, and thus the patients in this study may not be fully representative of the patients seen in our cohort. While known prior COVID‐19 infection was accounted for in all patients, it remains possible that asymptomatic or mild infection occurred between prevaccine blood draw and vaccination, which could influence subsequent seroreactivity. While this study included 90 patients with SLE, the number of patients receiving individual medications was too small to draw any definitive conclusions about their effects on vaccine response in SLE patients, and in our analyses, receiving any non‐antimalarial immunosuppressive agent was ultimately the strongest predictor of a poor antibody response to the COVID‐19 vaccines. Another limitation of the work is the absence of a direct comparator of SLE flare rates over the same time period. It also remains possible that a perceived SLE flare could have been a vaccine side effect.
This study has several strengths. In contrast to previous reports, the focus was limited to patients with SLE and assessed the COVID‐19 vaccines’ effects on lupus‐specific disease activity with availability of a validated disease index pre‐ and postvaccination in the majority of patients. Flares were rare, with only 1.3% being severe. These data are reassuring and support the notion that vaccines do not exacerbate disease activity, a finding that should hopefully alleviate vaccine hesitancy. Our study assessed 2 surrogate markers for B cell reactivity and a surrogate for T cell–mediated responses. Although the latter was limited to fewer patients, it was particularly applied to evaluate those with lower humoral responses and reinforced the concern about vaccine efficacy in a subset of these individuals.
In summary, in a multiracial/multiethnic study of SLE patients receiving a complete COVID‐19 vaccine regimen, nearly 30% had a low response. Having a normal anti‐dsDNA antibody level and taking any immunosuppressive medication other than antimalarials were independently associated with a decreased vaccine response. While minimal protective antibody levels remain unknown, these results, supported by other studies, raise concerns for our lupus patients, many of whom rely on medications to maintain low disease activity. Accordingly, the next phase of scientific inquiry and advance should focus on protocols addressing additional vaccination. Reassuringly, severe disease flares are infrequent, which should encourage patients to consider vaccination.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Izmirly had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Izmirly, Kim, Samanovic, Fernandez‐Ruiz, Haberman, Scher, Mulligan, Clancy, Buyon.
Acquisition of data
Izmirly, Samanovic, Fernandez‐Ruiz, Ohana, Deonaraine, Engel, Masson, Cornelius, Herati, Guttmann, Blank, Plotz, Haj‐Ali, Banbury, Stream, Hasan, Ho, Rackoff, Blazer, Tseng, Belmont, Saxena, Mulligan, Clancy, Buyon.
Analysis and interpretation of data
Izmirly, Kim, Samanovic, Fernandez‐Ruiz, Xie, Belmont, Saxena, Mulligan, Clancy, Buyon.
Supporting information
Disclosure Form
Supplemental Table 1 SLEDAI
Supplemental Figure 1 IFN‐γ secretion from peripheral blood mononuclear cells (PBMCs) in response to SARS‐CoV‐2 spike Protein S1. PBMCs obtained from 16 SLE patients and two controls were plated in enzyme‐linked immunospot (ELISpot) plates and stimulated by Spike Protein S1. The numbers of interferon‐γ (IFN‐γ)–producing cells were then detected by ELISpot. PBMCs were shown to release IFN‐γ in response to antigen (Panel A). There is a correlation between spike‐RBD IgG in sera with IFN‐γ secretion by PBMCs (Panel B). In Panel B, gray circles represent ≤100 units/ml and black circles represent >100 units/ml on ELISA. Open circles represent controls.
ACKNOWLEDGMENTS
The authors would like to thank the patients who participated in the study. They would also like to acknowledge Ranit Shriky and Rebecca Cohen for their assistance with regulatory matters and Benjamin Wainwright for his contributions to the manuscript.
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant P50‐AR‐07059), the National Institute of Allergy and Infectious Diseases, NIH (grant AI‐148574), and a Bloomberg Philanthropies COVID‐19 Response Initiative grant.
Drs. Izmirly, Kim, Samanovic, and Fernandez‐Ruiz contributed equally to this work. Drs. Mulligan, Clancy, and Buyon contributed equally to this work.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.41937&file=art41937‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Fernandez‐Ruiz R, Masson M, Kim MY, Myers B, Haberman RH, Castillo R, et al. Leveraging the United States epicenter to provide insights on COVID‐19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020;72:1971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez‐Ruiz R, Paredes JL, Niewold TB. COVID‐19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease [review]. Transl Res 2021;232:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson‐Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang W, Askanase AD, Khalili L, Merrill JT. SARS–CoV‐2 vaccines in patients with SLE. Lupus Sci Med 2021;8:e000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saxena A, Guttmann A, Masson M, Kim MY, Haberman RH, Castillo R, et al. Evaluation of SARS–CoV‐2 IgG antibody reactivity in patients with systemic lupus erythematosus: analysis of a multi‐racial and multi‐ethnic cohort. Lancet Rheumatol 2021;3:e585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. New Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS–CoV‐2 vaccine. New Engl J Med 2020;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. New Engl J Med 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boekel L, Hooijberg F, van Kempen ZL, Vogelzang EH, Tas SW, Killestein J, et al. Perspective of patients with autoimmune diseases on COVID‐19 vaccination. Lancet Rheumatol 2021;3:e241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725–34. [DOI] [PubMed] [Google Scholar]
- 12. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey‐Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 15. Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med 2005;142:953–62. [DOI] [PubMed] [Google Scholar]
- 16. Touma Z, Gladman DD, Ibanez D, Urowitz MB. Development and initial validation of the systemic lupus erythematosus disease activity index 2000 responder index 50. J Rheumatol 2011;38:275–84. [DOI] [PubMed] [Google Scholar]
- 17. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology guidance for COVID‐19 vaccination in patients with rheumatic and musculoskeletal diseases: version 2. Arthritis Rheumatol 2021;73:e30–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN‐α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 2007;8:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Postal M, Vivaldo JF, Fernandez‐Ruiz R, Paredes JL, Appenzeller S, Niewold TB. Type I interferon in the pathogenesis of systemic lupus erythematosus [review]. Curr Opin Immunol 2020;67:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. López P, Rodríguez‐Carrio J, Caminal‐Montero L, Mozo L, Suárez A. A pathogenic IFNα, BLyS and IL‐17 axis in systemic lupus erythematosus patients. Sci Rep 2016;6:20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Psarras A, Emery P, Vital EM. Type I interferon‐mediated autoimmune diseases: pathogenesis, diagnosis and targeted therapy [review]. Rheumatology (Oxford) 2017;56:1662–75. [DOI] [PubMed] [Google Scholar]
- 22. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS–CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Antibody response to 2‐Dose SARS–CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;21:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik‐Wang JM, et al. Antibody response to a single dose of SARS–CoV‐2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220289. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher‐Stine L, Garonzik‐Wang J, et al. High antibody response to two‐dose SARS–CoV‐2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021. doi: 0.1136/annrheumdis‐2021‐220656. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti‐SARS–CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220272. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El‐Qunni AA, et al. Glucocorticoids and B Cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS–CoV‐2 [preprint]. medRxiv 2021. doi: 10.1101/2021.04.05.21254656. E‐pub ahead of print. [DOI] [Google Scholar]
- 28. Spiera R, Jinich S, Jannat‐Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS‐ CoV‐2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220604. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29. Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID‐19 vaccine in immune‐mediated inflammatory disease. Ann Rheum Dis 2021. doi: 10.1101/2021.05.11.21256917. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220647. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31. Crowe SR, Merrill JT, Vista ES, Dedeke AB, Thompson DM, Stewart S, et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum 2011;63:2396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schattner A, Ben‐Chetrit E, Schmilovitz H. Poliovaccines and the course of systemic lupus erythematosus—a retrospective study of 73 patients. Vaccine 1992;10:98–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplemental Table 1 SLEDAI
Supplemental Figure 1 IFN‐γ secretion from peripheral blood mononuclear cells (PBMCs) in response to SARS‐CoV‐2 spike Protein S1. PBMCs obtained from 16 SLE patients and two controls were plated in enzyme‐linked immunospot (ELISpot) plates and stimulated by Spike Protein S1. The numbers of interferon‐γ (IFN‐γ)–producing cells were then detected by ELISpot. PBMCs were shown to release IFN‐γ in response to antigen (Panel A). There is a correlation between spike‐RBD IgG in sera with IFN‐γ secretion by PBMCs (Panel B). In Panel B, gray circles represent ≤100 units/ml and black circles represent >100 units/ml on ELISA. Open circles represent controls.


