Abstract
Nonsteroidal anti‐inflammatory drugs (NSAIDs) were thought to increase the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus entrance into cells. Hence, it was suggested in the media that NSAIDs could lead to a higher risk of infection and/or disease severity. To determine the existence or absence of this association, we aimed to systematically evaluate the risk of SARS‐CoV‐2 infection and mortality and the risk of severe coronavirus disease 2019 (COVID‐19) associated with previous exposure to NSAIDs.
MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE were searched in February 2021 for controlled studies. The results were calculated through random‐effect meta‐analyses and reported in terms of odds ratios (ORs) with 95% confidence intervals (CIs). Heterogeneity was assessed with I2 test.
Eleven studies were included, comprising a total of 683 715 patients. NSAID exposure did not increase the risk of having a positive test for SARS‐CoV‐2 infection (OR, 0.97; 95%CI, 0.85‐1.11, I2 = 24%; 5 studies). The exposure to NSAIDs did not increase the risk of severe/critical COVID‐19 disease (OR, 0.92; 95%CI, 0.80‐1.05; I2 = 0%; 5 studies) nor all‐cause mortality among patients with COVID‐19 (OR, 0.86; 95%CI, 0.75‐0.99; I2 = 14%, 4 studies).
Our data did not suggest that exposure to NSAIDs increases the risk of having SARS‐CoV‐2 infection or increases the severity of COVID‐19 disease. Also, the fragility of the studies included precludes definite conclusions and highlights the need for further robust data.
Keywords: ACE‐2, COVID‐19, nonsteroidal anti‐inflammatory drugs, NSAIDs, SARS‐CoV‐2
In December 2019, the first clusters of patients with pneumonia of unknown cause occurred in Wuhan, China. It was later confirmed that it was caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 This virus is responsible for coronavirus disease 2019 (COVID‐19), a disease with a broad spectrum of clinical manifestations, of which fever is one of the main symptoms. 1 , 2 , 3 Nonsteroidal anti‐inflammatory drugs (NSAIDs) are broadly prescribed or sold over the counter to relieve fever and other inflammatory symptoms. 4 The main mechanism of action of NSAIDs is inhibition of the formation of prostaglandins (as well as prostacyclin and thromboxane) from arachidonic acid via inhibition of cyclooxygenase (COX) enzymes 1 and 2. 5 , 6
However, in March 2020, French health authorities warned against the use of ibuprofen for managing mild symptoms of COVID‐19 due to the possibility of ibuprofen increasing the expression of the angiotensin‐converting enzyme‐2 (ACE‐2) receptor, which is the target for cell penetration of the SARS‐CoV‐2 virus. 7 , 8 , 9 The World Health Organization initially subscribed to this recommendation, but rapidly advised against it, since there was no clinical evidence to support it. 10 The change in recommendations highlighted the fragility of the available evidence for NSAID use and COVID‐19 risk. This fragility could lead to doubts in clinical management. While a fever can be managed by other drugs such as acetaminophen, acute and chronic pain (osteoarticular or not) in some cases can benefit if managed with NSAIDs. The absence of solid evidence regarding the association of NSAIDs and COVID‐19 disease, either of the risk of infection or prognostic impact, may preclude unequivocal drug management in these patients in the current pandemic context.
Therefore, we sought to systematically review all published controlled studies comparing the risks of NSAID and non‐NSAID groups (irrespective of placebo use, standard of care, and no treatment/exposure arms).
Methods
This systematic review followed the reporting principles of Meta‐analyses of Observational Studies in Epidemiology 11 and Preferred Reporting Items for Systematic Reviews and Meta‐analyses. 12 The protocol of this study was developed and registered in the International Prospective Register of Systematic Reviews with the registration number CRD42020216806. The protocol was not published in any peer‐reviewed journal. Conduct and reporting followed the Preferred Reporting Items for Systematic Reviews and Meta‐analyses statement (see Supplemental Information 2).
Eligibility Criteria
We considered eligible randomized controlled trials (RCTs), cohort/nested case‐control studies, and case‐control studies with information about the risk of infection or the risk of disease complications associated with NSAIDs compared with a control group.
Drug exposure was defined as exposure to any NSAID, including COX‐2 inhibitors, at any dose (except patients treated with low‐dose acetylsalicylic acid), and we accepted duration of treatment more than 30 days before SARS‐CoV‐2 infection documented or high clinical suspicion, to minimize protopathic bias. This cutoff point was defined clinically and based on the fact that these individuals would be using NSAIDs for reasons unrelated to COVID‐19 symptoms.
Patients with a confirmed SARS‐CoV‐2 infection taking placebo, standard of care, or another analgesic drug, were considered eligible for control groups. Therefore, studies enrolling patients taking NSAIDs without a non‐NSAIDs/placebo arm or patients taking NSAIDs <30 days before SARS‐CoV‐2 documented infection, were excluded.
The outcomes of interest were the following: (1) SARS‐CoV‐2 infection documented by reverse transcription polymerase chain reaction; clinical and imaging features along with other laboratory testing (eg, SARS‐CoV‐2antigen detection rapid diagnostic test) or reported by authors as having high clinical suspicion of SARS‐CoV‐2 infection; (2) critical disease outcome admitted in the analysis included hospital admission and progression to the intensive care unit or admission directly to the intensive care unit, which was defined according to the World Health Organization's interim guidance 13 ; (3) all‐cause mortality. Furthermore, we aimed to undertake additional analyses to evaluate the association between NSAIDs and associated outcomes, such as the risk of hospitalization, risk of mechanical ventilation, or risk of ECMO. We also focused on NSAID safety, particularly cardiovascular risk.
Case series, case reports, commentaries, and reviews were excluded.
Search Methods
The reviewers performed an electronic database search using MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE to identify relevant studies (search strategy in Table S1, Supplemental Information 1). We performed a sensitive search strategy for MEDLINE and EMBASE, which rely on MESH terms: “anti‐inflammatory Agents,” “non‐steroidal,” “cyclooxygenase 2 inhibitors,” and “nonsteroid anti‐inflammatory agent.” In addition, a search was performed for additional articles by screening the references of potentially included studies for further review. The search was performed on February 11, 2021.
Study Selection and Data Collection Process
Three of the authors (C.S., L.P., R.B.) independently screened the search results for inclusion by assessing titles and abstracts. Discrepancies were resolved by consensus‐based discussion or by a third reviewer (D.C.). Chance‐corrected agreement between reviewers was assessed using the Cohen's kappa statistic, a measure of the agreement between 2 raters (reviewers) who each classified items into mutually exclusive categories. 14 The studies that were not excluded went to the full‐text assessment phase. We excluded studies that provided no adjusted estimation and editorials or narrative reviews without original data. The motives for exclusion were recorded at this stage.
The risk of bias was independently evaluated by 3 authors (C.S., L.P., R.B.) using the Cochrane Risk of Bias Tool for RCTs and the ROBINS‐I tool for observational studies. 15 We also assessed chance‐corrected agreement between reviewers using the Cohen's kappa statistic. 14 We also used the Downs and Black tool 16 to assess the methodological quality of the included studies by 3 reviewers (C.S., L.P., R.B.). Once again, all disagreements were resolved via third‐party adjudication performed by a third author (D.C.).
Statistical Analysis and Pooled Data Evaluation
We used RevMan (version 5.3) software for statistical analysis (Nordic Cochrane Centre, Cochrane Collaboration, Oxford, England) and to derive forest plots showing the results of individual studies and pooled analysis, if feasible. A random‐effects meta‐analysis was performed by weighting by the inverse‐variance method to estimate pooled odds ratio (OR) and 95% confidence intervals (95%CIs), irrespective of the statistical heterogeneity assessed through the I² statistic. We used the hazard ratio when the OR was not available or not possible to calculate. Additional analysis according to the study design was performed. Publication bias assessment was performed through funnel plot examination and an Egger test ensuring that a sufficient number of studies were included. A P value ≤.05 was considered significant.
Results
Included Studies
The search returned 1372 records, resulting in 1180 records after removing duplicates. Following abstract screening and evaluation of full‐text eligibility, 76 articles were assessed for full‐text screening; of these, 11 were included for qualitative and quantitative syntheses (Figure 1; details of excluded studies at Table S2, Supplemental Information 1). The kappa for an interrater agreement was 0.53. The strength of agreement was moderate.
Figure 1.
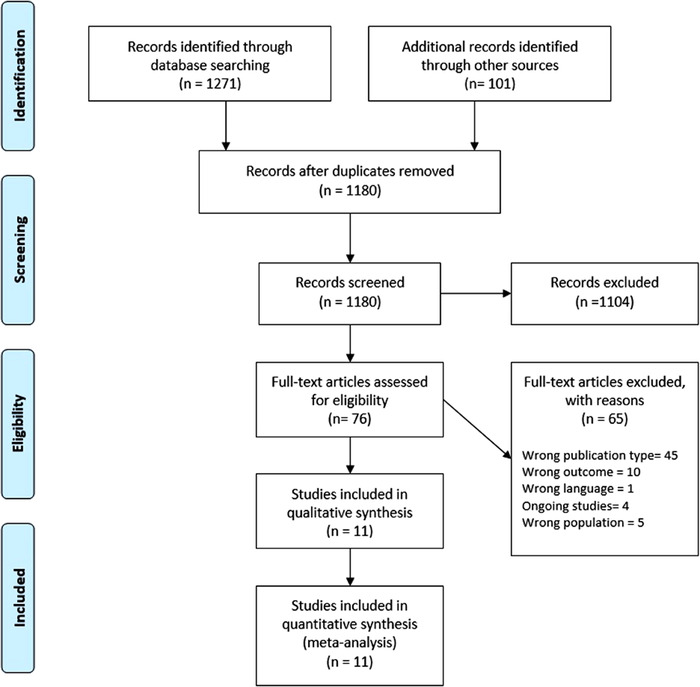
Flowchart of study selection process.
The main characteristics of the included studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 are depicted in Table 1. The sample sizes ranged from 268 to 561 037 patients, with a total of 683 715 patients included in our review. The majority of included studies were retrospective cohort studies (7), 1 prospective study, and 3 case‐control studies (Table 1). No RCTs fulfilled our eligibility criteria. Two of the studies were conducted in patients with osteoarthritis and rheumatoid arthritis. 19 , 27 Two studies were performed in Spain, 2 in the entire United Kingdom, 2 in Denmark, 1 in Scotland, 1 in Saudi Arabia, 1 in South Korea, 1 in France, and 1 in Italy. Almost all the included studies failed to report the type or the dose of NSAIDs used. In 10 of the studies included in the meta‐analysis, the authors used matching methods to adjust the groups for confounding factors: 5 studies used Cox regression, 4 used logistic regression, 2 studies used the propensity score matching method, and 1 study used Poisson regression. The other study adjusted for confounding factors but did not report a matching method.
Table 1.
Main Characteristics of Included Studies
| Cohort/Nested Case‐Control Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study Year | Design | Region | Population | NSAIDs | Control | Mean‐Median Age/% Women | Comorbidities | Outcome Adjustments |
| Chandan et al (2020) | Retrospective cohort study | United Kingdom |
|
|
|
|
|
|
| Wong et al (2021) | Retrospective cohort study | United Kingdom |
|
|
|
|
|
|
| Blanch‐Rubió et al (2020) | Retrospective cohort study | Spain |
|
|
|
66.4/80.5% |
|
Age, sex, comorbidities (Poisson regression) |
| Kragholm et al (2020) | Retrospective cohort study | Denmark |
|
|
|
|
|
Age, comorbidities (Cox regression) |
| Lund et al (2020) | Retrospective cohort study | Denmark |
|
|
|
50/58% |
|
Age, sex, comorbidities, phase of the outbreak and use of prescription drugs (Propensity score matching) |
| Abu Esba et al (2020) | Prospective cohort study | Saudi Arabia |
|
Group 4 (acute and chronic NSAID users a combined): 146 | Non‐NSAIDs: 357 |
|
|
|
| Liabeuf et al (2020) | Retrospective cohort study | France |
|
NSAIDs a : 9 | Non‐NSAIDs: 259 | 73/ 42% |
|
|
| Vila‐Corcoles et al (2020) | Retrospective cohort study | Spain |
|
|
Non‐NSAIDs: 33 286 | 70.9/51.9% |
|
|
| Huh et al (2021) | Population‐based case‐control study | South Korea |
|
|
|
49.4/52.6% |
|
Age, sex, coverage for low household income, CCI, and comorbidities (Logistic regression) |
| Mancia et al (2020) | Population‐based case‐control study | Italy |
|
68/37% | NR |
|
||
| McKeigue et al (2021) | Population‐based case‐control study | Scotland |
|
|
|
|
NR |
|
BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COX‐2, cyclooxygenase enzyme 2; CVD, cardiovascular disease; DMARDs, disease‐modifying antirhreumatic drugs; ICD‐10, International Classification of Diseases, Tenth Revision; IHD, ischemic heart disease; NR, not reported; NSAIDs, nonsteroidal anti‐inflammatory drugs; RASI, renin‐angiotensin system inhibitor; RT‐PCR, reverse transcriptase‐polymerase chain reaction; THIN, The Health Improvement Network database.
Type of NSAIDs not specified.
Chandan et al, NSAIDs included: aceclofenac, celecoxib, dexibuprofen, dexketoprofen, diclofenac potassium, diclofenac sodium, etodolac, etoricoxib, flurbiprofen, ibuprofen, indometacin, ketoprofen, ketorolac trometamol, mefenamic acid, meloxicam, nabumetone, naproxen, parecoxib piroxicam sulindac, tenoxicam, tiaprofenic acid, and tolfenamic acid.
Huh et al, NSAIDs included: aceclofenac, dexibuprofen, diclofenac potassium, etodolac, rofecoxib, ketorolac, loxoprofen, meloxicam, talniflumate, zaltoprofen, celecoxib, nalnumeton, lornoxicam, morniflumate. sulindac, aclofenac, dexibuprofen, diclofenac sodium, and loxoprofen sodium.
Risk of Bias
The risk of bias of the included studies assessed through ROBINS‐I tool 15 was serious in 3 studies and moderate in the rest. All observational studies had a risk of bias due to confounding. The kappa for interrater agreement was 0.54. The strength of agreement was considered moderate. See Supplemental Information 1, Table S3, for more information regarding the quality assessment of the included studies.
The 11 included studies in this review scored from 12 to 14 (of 28) on the Modified Downs and Black tool. 16 Table S4 (see Supplemental Information 1) summarizes the results of the risk of bias assessment using the Downs and Black assessment tool.
Risk of SARS‐CoV‐2 Infection (Positive Test) Associated With NSAIDs
Five studies had information about COVID‐19 infection (positive test) and NSAIDs, and they were not associated with an increased risk of having a positive test for COVID‐19 infection (OR, 0.97; 95%CI, 0.85‐1.11; I2 = 24%; 5 studies) (Figure 2). The analysis excluding population case‐control studies 20 , 24 showed similar results (OR, 0.87; 95%CI, 0.68‐1.11; 3 studies) without statistical heterogeneity (I2 = 0%) (Figure 3).
Figure 2.
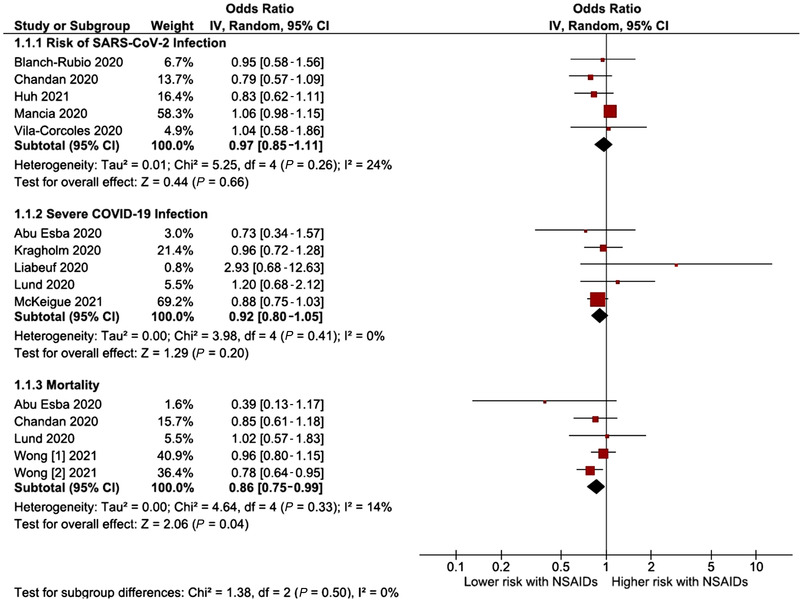
Forest plot of the pooled analysis evaluating the effect of NSAIDs on risk of SARS‐CoV‐2 infection, risk of severe SARS‐CoV‐2 infection, and all‐cause mortality. NSAIDs, nonsteroidal anti‐inflammatory drugs; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Figure 3.
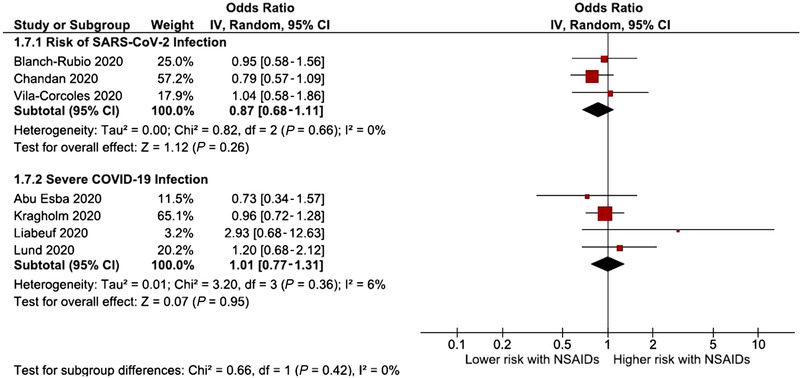
Forest plot of the pooled analysis evaluating the effect of NSAIDs on risk of SARS‐CoV‐2 infection and risk of severe SARS‐CoV‐2 infection, only with cohort studies. A sensitivity analysis was not performed for the mortality outcome since all the studies that assessed mortality were cohorts. NSAIDs, nonsteroidal anti‐inflammatory drugs; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Risk of Severe Disease Associated With NSAIDs Among Patients With COVID‐19 Disease
The risk of severe COVID‐19 disease was evaluated in 5 studies. The risk of severe COVID‐19 disease associated with NSAIDs was not significantly increased or decreased (OR, 0.92; 95%CI, 0.80‐1.05; 5 studies) (Figure 2). There was no significant heterogeneity in the analysis. When excluding the population case‐control study 25 the estimate stood at OR 1.01 (95%CI, 0.77‐1.31; I2 = 6%; 4 studies) (Figure 3).
Mortality Risk Associated With NSAIDs Among Patients With COVID‐19 Disease
The association between NSAIDs and mortality risk in patients with COVID‐19 was evaluated in 4 studies. However, Wong et al, 27 assessing primary care records (OpenSAFELY platform), identified and assessed 2 cohorts (people who had never used NSAIDs in the past 3 years from the general population and people with rheumatoid arthritis/osteoarthritis). Regarding all‐cause mortality, NSAIDs were associated with neither an increase nor a reduction in the risk of this outcome (OR, 0.86; 95%CI, 0.75‐0.99; I2 = 14%; 4 studies) (Figure 2). We performed a subsequent exploratory subgroup analysis for all types of NSAIDs, where we did not apply the time of exposure eligibility criterion. However, only 2 authors (Drake et al 28 and Wong et al 27 ) had data available for ibuprofen. This subgroup analysis did not significantly increase or reduce the risk of COVID‐19 deaths (OR, 0.98; 95%CI, 0.78‐1.22; 2 studies) (see Figure S1, Supplemental Information 1).
Additional Analysis
We performed an additional analysis where we included studies where we did not apply the time of exposure eligibility criterion. Thereby, we observed that the risk of severe COVID‐19 infection was not significantly increased (OR, 1.05; 95%CI, 0.88‐1.25; 7 studies [Drake et al 28 and Jeong et al 29 were included]) with a significant heterogeneity (I2 = 54%). Regarding mortality, NSAIDs were also not associated with either an increase or a reduction in the risk of this outcome (OR, 0.85; 95%CI, 0.74‐0.96; I2 = 42%; Bruce et al, 30 Drake et al, 28 and Imam et al 31 were included) (Figure S2, Supplemental Information 1).
About the risk of mechanical ventilation in patients with COVID‐19, only 2 authors had data available. This outcome was not significantly increased or decreased (OR, 0.97; 95%CI, 0.82‐1.16; 2 studies). There was no significant heterogeneity in the analysis (Figure S3, Supplemental Content). Data were not available for other associated outcomes.
We were unable to perform an analysis of cardiovascular safety outcomes since data were not available for these outcomes.
Additionally, the Egger test was not statistically significant for the risk of having COVID‐19 infection (P = .17), risk of mortality among those COVID‐19 symptomatic (P = .50), and risk of severe disease among those with COVID‐19 (P = .02). The funnel plots are depicted in Figure S4, Supplemental Information 1.
Discussion
Our main findings were (1) NSAIDs were not associated with an increased risk of being infected with SARS‐CoV‐2; and (2) among patients with COVID‐19, the previous exposure to NSAIDs did not increase the risk of severe disease or mortality.
Concerns about the use of NSAIDs in patients with COVID‐19 have been raised on the basis of unpublished data. Theoretically, it was claimed that drugs such as ACE inhibitors, angiotensin receptor blockers, and ibuprofen could upregulate the ACE‐2, which might mediate the entrance of SARS‐CoV‐2 in epithelial cells, increasing the chance of having COVID‐19 and worsening a patient's prognosis. Also, NSAIDs might delay the diagnosis of COVID‐19 by masking inflammation and fever. Such claims led to alarmism in the scientific and nonscientific community, since NSAIDs are highly used in various conditions, and they are broadly prescribed or sold over the counter. Since then, several studies have attempted to understand the effects of different drug classes in ACE‐2 expression and their effects on the stability of ACE‐2 and viral receptor‐binding protein complexes. A recent review shows that when NSAIDs are taken continuously, with consequent inhibition of COX, the glomerular filtration rate and renal perfusion are reduced. This increases angiotensin II levels and may initiate a compensatory mechanism, with subsequent upregulation of ACE‐2 expression to counteract angiotensin II. This could make larger amounts of ACE‐2 available for SARS‐CoV‐2 entry into the cell. 32 Contrarily, a study in computational modeling found that some NSAIDs, such as ibuprofen, aspirin, and acetaminophen, can alter the stability between the viral receptor‐binding protein and ACE‐2. 33 Another study, in asthmatic patients, found that daily aspirin in an anti‐inflammatory dose was not associated with increased transcription of ACE‐2 or angiotensin‐derived peptides. 34 These findings do not directly point to a beneficial effect of NSAIDs in SARS‐CoV‐2 infection, but they do imply that there is still much to learn about the interaction between the virus and its entry‐gate enzyme.
In some non–COVID‐19 preclinical studies, NSAIDs were associated with suppression of interleukin (IL)‐6 production and suppression of prostaglandin E2, which upregulates the production of IL‐6 and IL‐8. 35 These proinflammatory mediators are also associated with more severe COVID‐19, 36 , 37 since hyperinflammatory responses are the basis of the pathology of severe cases of this disease, such as acute respiratory distress syndrome. 38 Additionally, the growing body of evidence points to the importance of anti‐inflammatory drugs, such as corticosteroids or drugs that inhibit the production of IL‐6, in severe cases. However, it is hypothesized that, in early phases of COVID‐19, this concomitant immunosuppression could promote SARS‐CoV‐2 replication. 32
Other studies observed that different NSAID types could have different capabilities to suppress enzymatic activities of COX‐1 and COX‐2, 39 , 40 which implies a variety of effects that influence the study results. Unfortunately, data were scarce, and we were unable to categorize NSAIDs. Therefore, more research is needed to evaluate if NSAID type can increase or decrease the risk of poorer outcomes in patients with COVID‐19.
Regarding clinical studies, several authors observed that NSAID use does not appear to increase the risk of poorer outcomes in patients with COVID‐19. Drake et al 28 included a large number of patients admitted to a hospital with COVID‐19 (n = 72 179; the authors used data from the ISARIC Clinical Characterization Protocol UK cohort), across 255 health care facilities in England, Scotland, and Wales. This study aimed to analyze the association between NSAIDs and severe COVID‐19 outcomes, including mortality, critical care admission, need for invasive ventilation, need for oxygen, and acute kidney injury. Drake et al 28 observed that NSAID use was not associated with higher mortality or increased severity of COVID‐19. However, data on dosages and treatment duration were not available. Therefore, the authors were unable to assess whether there is a potentially harmful effect or not associated with drug dosages or treatment duration.
Although there is still no pharmacodynamically clear answer for this question, the data from our systematic review did not show an association between NSAIDs and an increased risk of infection or disease complications. The relevance of these data is of the utmost importance for patients who are treated chronically with these drugs as safety is being assured. In the symptomatic relief of fever, other options exist, such as acetaminophen, but our data show that NSAIDs can be added to the therapeutic options available.
In this context, the judgment of media reports and observational studies is always difficult, as data are frequently prone to bias. For example, social media reported 4 cases of younger patients with COVID‐19 whose condition worsened after taking ibuprofen for symptomatic relief. 41 This report and other studies might be at high risk of residual confounding and selective reporting bias which precludes definite conclusions. Another example relies on a systematic review of observational studies that reported a positive association between NSAIDs and pneumonia complications. 42 To decrease the risk of bias we were conservative in our analysis since we extracted data reporting only adjusted measures and respecting the directionality and significance of the results.
Despite the concerns regarding NSAIDs, the anti‐inflammatory features of corticosteroids have been demonstrated to decrease mortality in patients requiring oxygen or mechanical ventilation. 43 The inflammatory pathways in this infection still require further research so that we can understand the exact mechanisms by which these drugs might influence health outcomes.
We were unable to assess NSAID safety since there were no available data. However, we have to be aware that NSAIDs are also associated with gastrointestinal complications (like peptic ulcer disease and gastrointestinal bleeding) and the risk of cardiovascular adverse events. 44 Therefore, the choice of drug to treat anti‐inflammatory symptoms associated with COVID‐19 should be based on a benefit‐risk assessment for known side effects, and NSAIDs should be used at the lowest effective dose for the shortest possible period.
Strengths and Limitations
Our review has limitations inherent to the included studies themselves. Due to our inclusion criteria, in our search, we retrieved only observational studies with their inherent bias. Also, all the observational studies included had a high risk of bias, leading to low confidence in the results. By default, our data were subjected to a high protopathic and confounding bias. To avoid that, we restricted our search to the use of NSAIDs for at least 30 days before documented or highly suspected SARS‐CoV‐2 infection thereby, decreasing bias associated with the use of these drugs for symptomatic relief at the beginning of the clinical setting of COVID‐19. Pooling data from studies with patients with different baseline morbidities and heterogeneous risk for SARS‐CoV‐2 infection (membership bias) should also be considered as a limitation to our conclusions.
The OR was the effect measurement estimate chosen since relative estimates are more similar across studies with different designs, populations, and lengths of follow‐up than absolute effects. 45 Additionally, we selected the OR that was best suited for the chronic use analysis of NSAIDs. Therefore, in Lund et al, 23 Abu Esba et al, 17 and Huh et al, 20 we selected effect measures either evaluated by a sensitivity analysis or that gathered most events in our group of interest. Furthermore, to decrease the confounding bias in our data, we used only adjusted results reported by the authors.
Most of the studies included were unable to provide data on NSAID type, dosage, and total treatment duration. Therefore, we could not assess whether there was a potentially harmful effect of NSAIDs masked by variables. Future studies should be aware of details regarding the use of NSAIDs, including the effects of continuation or discontinuation after hospital admission, type, dosage, and treatment duration.
When we assessed the outcomes of interest, low statistical heterogeneity was observed, which is a good indicator of the robustness of the result, despite the baseline heterogeneity of the population. Also, when studies with less robust designs were excluded, the estimates kept their neutrality in terms of significance without statistical heterogeneity.
Our study analyzed the best available evidence, and it is relevant to inform all stakeholders about the safety of using NSAIDs and the risk of COVID‐19 disease.
Conclusions
NSAID use was not associated with increased risk of SARS‐CoV‐2 infection or with severe disease or increased mortality. The results are weakened by the risk of bias of the individual studies. Thus, more robust studies are needed since the quality of data included is very important to confirm the safety of NSAID use in this context.
Conflicts of Interest
In the past 3 years, D.C. has participated in educational conferences/congresses (including travel, accommodation, and/or hospitality) and has received speaker/consultant fees from Daiichi Sankyo, Menarini, Roche, and Merck‐Serono. J.J.F. has received speaker and consultant fees from Grünenthal, Fundação MSD (Portugal), TEVA, MSD, Allergan, Medtronic, GlaxoSmithKline, Novartis, Lundbeck, Solvay, BIAL, Merck‐Serono, Merz, Ipsen, Biogen, Acadia, Allergan, Abbvie, and Sunovion‐Pharmaceuticals.
Author Contributions
Conceptualization: D.C. Data curation: D.C., C.D.S., L.P., and R.A.B. Formal analysis: D.C. Investigation: D.C., C.D.S., L.P., and R.A.B. Methodology: D.C., C.D.S., L.P., R.A.B., J.C., and J.J.F. Project administration: D.C. Software: D.C. Supervision: D.C., J.C., and J.J.F. Validation: D.C., C.D.S., L.P., R.A.B., J.C., and J. J.F. Visualization: D.C., J.C., and J.J.F. Writing— original draft: D.C., C.D.S., L.P., and R.A.B. Writing—review and editing: D.C., C.D.S., L.P., R.A.B., J.C., and J.J.F.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supporting information
Supporting Information
Supporting Information
Figure S1. Forest plot of the pooled analysis evaluating the effect of ibuprofen and risk of death in patients with COVID‐19, excluding time of exposure criterion.
Figure S2. Forest plot of the pooled analysis evaluating the effect of NSAIDs on risk of severe SARS‐CoV‐2 infection and all‐cause mortality, excluding time of exposure criterion. NSAIDs, nonsteroidal anti‐inflammatory drugs; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Figure S3. Forest plot of the pooled analysis evaluating the effect of NSAIDs and risk of mechanical ventilation in patients with COVID‐19, excluding time of exposure criterion. COVID‐19, coronavirus disease 2019; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Figure S4. Funnel plots for grouped NSAIDs evaluations and respective outcomes.
Luísa Prada and Catarina D. Santos share co‐first authorship (both the authors contributed equally to this study).
Systematic review registration number: CRD42020216806
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplemental Information files).
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet North Am Ed. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Y‐C, Chen C‐S, Chan Y‐J. The outbreak of COVID‐19: an overview. J Chin Med Assoc. 2020;83(3):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siordia JA Jr. Epidemiology and clinical features of COVID‐19: a review of current literature. J Clin Virol. 2020:104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SY, Chang YJ, Cho HM, Hwang YW, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database Systematic Rev. 2015;2015(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruno A, Tacconelli S, Patrignani P. Variability in the response to non‐steroidal anti‐inflammatory drugs: mechanisms and perspectives. Basic Clin Pharmacol Toxicol. 2014;114(1):56‐63. [DOI] [PubMed] [Google Scholar]
- 6. Fitzgerald PGC. The Coxibs, selective inhibitors of cyclooxygenase‐2. N Engl J Med. 2001;345(6):433‐442. [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21‐e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. The use of non‐steroidal anti‐inflammatory drugs (NSAIDs) in patients with COVID‐19: scientific brief. https://apps.who.int/iris/handle/10665/331796. Published April 19, 2020. Accessed April 30, 2021.
- 11. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. London: British Medical Journal Publishing Group; 2009:332‐336. [PMC free article] [PubMed] [Google Scholar]
- 13. WHO . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected. Interim guidance. Interim Guidance. 2020;16(1):9‐26. [Google Scholar]
- 14. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977:159‐174. [PubMed] [Google Scholar]
- 15. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ (Online). 2016;355:4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52(6):377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abu Esba LC, Alqahtani RA, Thomas A, Shamas N, Alswaidan L, Mardawi G. Ibuprofen and NSAID use in COVID‐19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2021;10(1):253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blanch‐Rubio J, Soldevila‐Domenech N, Tio L, et al. Influence of anti‐osteoporosis treatments on the incidence of COVID‐19 in patients with non‐inflammatory rheumatic conditions. Aging. 2020;12(20):19923‐19937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandan JS, Zemedikun DT, Thayakaran R, et al. Non‐steroidal anti‐inflammatory drugs and susceptibility to COVID‐19. Arthritis Rheumatol. 2021;73(5):731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huh K, Ji W, Kang M, et al. Association of prescribed medications with the risk of COVID‐19 infection and severity among adults in South Korea. Int J Infect Dis. 2021;104:7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kragholm K, Gerds TA, Fosbøl E, et al. Association between prescribed ibuprofen and severe COVID‐19 infection: a nationwide register‐based cohort study. Clin Translat Sci. 2020;13(6):1103‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liabeuf S, Moragny J, Bennis Y, et al. Association between renin‐angiotensin system inhibitors and COVID‐19 complications [published online ahead of print June 12, 2020]. Eur Heart J‐ Cardiovasc Pharmacother. 10.1093/ehjcvp/pvaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lund LC, Kristensen KB, Reilev M, et al. Adverse outcomes and mortality in users of non‐steroidal anti‐inflammatory drugs who tested positive for SARS‐CoV‐2: a Danish nationwide cohort study. PLoS Med. 2020;17(9):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382(25):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKeigue PM, Kennedy S, Weir A, et al. Relation of severe COVID‐19 to polypharmacy and prescribing of psychotropic drugs : the REACT‐SCOT case‐control study Relation of severe COVID‐19 to polypharmacy and prescribing of psychotropic drugs: the REACT‐SCOT case‐control study Goldberg, David. BMC Med. 2021;19:51:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vila‐Corcoles A, Satue‐Gracia E, Ochoa‐Gondar O, et al. Use of distinct anti‐hypertensive drugs and risk for COVID‐19 among hypertensive people: a population‐based cohort study in Southern Catalonia, Spain. J Clin Hypertens. 2020;22(8):1379‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong AYS, MacKenna B, Morton CE, et al. Use of non‐steroidal anti‐inflammatory drugs and risk of death from COVID‐19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80(7):943‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drake TM, Fairfield CJ, Pius R, et al. Non‐steroidal anti‐inflammatory drug use and outcomes of COVID‐19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021;3(7):e498‐e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeong HE, Lee H, Shin HJ, Choe YJ, Filion KB, Shin JY. Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID‐19 in South Korea: a nationwide study [published online ahead of print July 27, 2020]. Clin Infect Dis. 10.1093/cid/ciaa1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruce E, Barlow‐Pay F, Short R, et al. Prior routine use of non‐steroidal anti‐inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID‐19. J Clin Med. 2020;9(8):2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. J Intern Med. 2020;288(4):469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cabbab ILN, Manalo RVM. Anti‐inflammatory drugs and the renin‐angiotensin‐aldosterone system: current knowledge and potential effects on early SARS‐CoV‐2 infection. Virus Res. 2020:198190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. González‐Paz LA, Lossada CA, Fernández‐Materán FV, Paz J, Vera‐Villalobos J, Alvarado YJ. Can non‐steroidal anti‐inflammatory drugs affect the interaction between receptor binding domain of SARS‐COV‐2 spike and the human ACE2 receptor? A computational biophysical study. Front Phys. 2020;8:587606. [Google Scholar]
- 34. Buchheit KM, Hacker J, Gakpo D, Mullur J, Sohail A, Laidlaw TM. Influence of daily aspirin therapy on ACE2 expression and function—implications for SARS‐CoV2 and patients with aspirin‐exacerbated respiratory disease. Clin Experiment Allergy. 2021;51(7):967‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho JC, Tipoe G, Zheng L, et al. In vitro study of regulation of IL‐6 production in bronchiectasis. Respir Med. 2004;98(4):334‐341. [DOI] [PubMed] [Google Scholar]
- 36. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL‐6 and CRP predict the need for mechanical ventilation in COVID‐19. J Allergy Clin Immunol. 2020;146(1):128‐136. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y, Wang J, Liu C, et al. IP‐10 and MCP‐1 as biomarkers associated with disease severity of COVID‐19. Mol Med. 2020;26(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID‐19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pairet M, Engelhardt G. Distinct isoforms (COX‐1 and COX‐2) of cyclooxygenase: possible physiological and therapeutic implications. Fund Clin Pharmacology. 1996;10(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 40. Gerbino PP. Emerging evidence in NSAID pharmacology: important considerations for product selection. Am J Manag Care. 2015;21:S139‐S147. [PubMed] [Google Scholar]
- 41. Yousefifard M, Zali A, Zarghi A, Madani Neishaboori A, Hosseini M, Safari S. Non‐steroidal anti‐inflammatory drugs in management of COVID‐19: a systematic review on current evidence. Int J Clin Pract. 2020;74(9):e13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sodhi M, Khosrow‐Khavar F, FitzGerald JM, Etminan M. Non‐steroidal anti‐inflammatory drugs and the risk of pneumonia complications: a systematic review. Pharmacotherapy. 2020;40(9):970‐977. [DOI] [PubMed] [Google Scholar]
- 43. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324(13):1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matthews ML. The role of dose reduction with NSAID use. Am J Manag Care. 2013;19(14 suppl):s273‐s277. [PubMed] [Google Scholar]
- 45. Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575‐1600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Figure S1. Forest plot of the pooled analysis evaluating the effect of ibuprofen and risk of death in patients with COVID‐19, excluding time of exposure criterion.
Figure S2. Forest plot of the pooled analysis evaluating the effect of NSAIDs on risk of severe SARS‐CoV‐2 infection and all‐cause mortality, excluding time of exposure criterion. NSAIDs, nonsteroidal anti‐inflammatory drugs; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Figure S3. Forest plot of the pooled analysis evaluating the effect of NSAIDs and risk of mechanical ventilation in patients with COVID‐19, excluding time of exposure criterion. COVID‐19, coronavirus disease 2019; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Figure S4. Funnel plots for grouped NSAIDs evaluations and respective outcomes.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplemental Information files).


