Abstract
The global SARS‐CoV‐2 pandemic has contributed to more than 163 million confirmed infections and 3.3 million deaths worldwide. The severity of the pandemic has led to an unprecedented effort to develop multiple effective vaccines. Due to excellent safety and efficacy data from clinical trials, several vaccines were approved. We report a case series of postvaccinal encephalitis in temporal correlation to vaccination with ChAdOx1 nCov‐19. The diagnostic criteria for possible autoimmune encephalitis were fulfilled. Our patients responded well to immunosuppressive therapy with corticosteroids. The incidence has been estimated to be approximately 8 per 10 million vaccine doses. Complication of postvaccinal encephalitis after ChAdOx1 nCoV‐19 vaccination still appear to be very rare, but need to be diagnosed and treated adequately. Large pooled data from observational epidemiologic studies are necessary to verify causality. ANN NEUROL 2021;90:506–511
Background
The global SARS‐CoV‐2 pandemic has contributed to more than 163 million confirmed infections and 3.3 million deaths worldwide. 1 The severity of the pandemic has led to an unprecedented effort to develop multiple effective vaccines. Due to excellent safety and efficacy data from clinical trials, the ChAdOx1 nCoV‐19 vaccine (AZD1222) was approved consisting of a replication‐deficient chimpanzee adenoviral vector ChAdOx1, containing the SARS‐CoV‐2 structural surface glycoprotein antigen (spike protein; nCoV‐19) gene. 2 In addition to local and systemic side effects of the ChAdOx1 nCoV‐19 vaccine without major clinical relevance, concern emerged due to a growing number of recent reports of autoimmune induced thrombocytopenia with subsequent thrombosis. 3 , 4 , 5
In general, vaccinations can cause a strong expression of proinflammatory cytokines and a T cell response. 6 , 7 Peripheral proinflammatory cytokines expressed after vaccination are considered to be causal by reaching the brain and partly resulting in neuroinflammation after microglia activation, depending on the immunogenetic background and the innate immune memory. 7
Herein we present a case series of three patients with autoimmune encephalitis related to prior ChAdOx1 nCoV‐19 vaccination, previously undescribed.
Informed Consent
Written informed consent was obtained from all patients for publication of this case series and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Case Presentation
First Case
A 21‐year‐old female patient received the first dose of the ChAdOx1 nCov‐19 vaccine experiencing typical side effects like fever and malaise, from which she recovered the day after. Except for obesity, she had no previous somatic or psychiatric diseases and was not on any medication. The following days she developed headache and progressive neurological symptoms including attention and concentration difficulties starting on day 5 after vaccination, resulting in admission to our hospital 11 days after vaccination. Emergency brain magnetic resonance imaging (MRI) was performed with normal status of the parenchyma (Fig 1, 1C). A lumbar puncture on the day of admission revealed lymphocytic pleocytosis of 46 leukocytes/μl (Fig 1, 1B). The extensive diagnostic workup remained negative, including chest X‐ray, sonography of the abdomen, and serological examinations of serum and cerebrospinal fluid (CSF) (Table 1). Later on, the patient suffered from an epileptic seizure. Repeated EEG recordings revealed diffuse abnormally slow theta rhythms without epileptiform activity (Fig 1, 1A). The patient was consistently stuporous, showed no meningeal signs and no paresis, partially complied with simple tasks, but did not respond adequately. Diagnosing possible autoimmune encephalitis, immunosuppressive therapy with 10mg dexamethasone per day was initiated with significant improvement of the symptomatology. A follow up lumbar puncture was performed showing decrease in pleocytosis to 12 cells/μl. At discharge, normal EEG activity was detected. There was still mild cognitive slowing without functional impairment. Subsequent MRI examination 4 months later continued to reveal a normal state of the parenchyma without sequelae (Fig 1, 1D).
FIGURE 1.
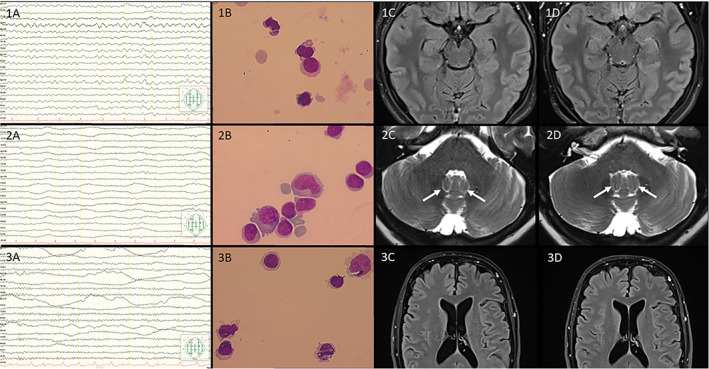
First Patient 1A‐D, Second Patient 2A‐D, Third Patient 3A‐D (1A to 3A): Representative electroencephalographic recordings from all three patients using A‐P (anterior‐posterior) longitudinal bipolar montage (sensitivity 7 mcV/mm; filter 1 to 70 Hz; interval between 2 vertical lines: 0.3 seconds). (1B to 3B): Representative images of CSF cytology demonstrating lymphocytic dominance. (1C‐D to 3 C‐D): Representative T2w (2C‐D) and FLAIR MR Images (1C‐D, 3C‐D) on admission (C) aswell as at 2‐4 months follow‐up (D). Zoomed views were chosen according to the very clinical symptoms described, e.g. showing normal appearance of the fastigial nucleus (arrows in 2C‐D). No imaging evidence of acute disseminated encephalomyelitis (ADEM) was depicted. [Color figure can be viewed at www.annalsofneurology.org]
TABLE 1.
Laboratory Investigations in Blood and Cerebrospinal Fluid (CSF) Done in All Three Cases
| Blood investigation | Standardized investigation of CSF | Advanced virus investigation of CSF | Advanced antineuronal antibody investigation of serum and CSF |
|---|---|---|---|
| Cell count, kidney and liver function, thyroid function incl. Thyroid‐Antibodies, serum protein electrophoresis, HIV, Tuberculosis (TB)‐QuantiFERON blood test, vasculitis screen (ANA, Ena‐Screen, ANCA‐Screen, RF, anti‐ds DNA, C3c, C4, IgG, IgA, IgM) | CSF aspect, White blood cell count (/μl), Red blood cell count (/μl), Monocytes (%),Lymphocytes (%),Total Proteins (mg/l), Glucose (mg/dl), Lactate (mmol/l), Isoelectrofocusing, Syphilis, Lyme disease | DNA‐PCR of Herpes Simplex Virus, Varicella‐Zoster Virus, Cytomegaly and Epstein Barr Virus. Measles IgG, Rubella IgG, Herpes Simplex Virus 1/2 IgG, Varicella Zoster Virus IgG | GAD65, NMDA‐, and GABA‐B‐receptor, IgLON5, AMPA‐R subtype 2, DPPX, LGI1, CASPR2, Glycine‐receptor, mGluR5, mGluR1, Amphiphysin, CV2/CRMP5, Ma2/Ta, Hu, Ri, Yo, Zic4, Recoverin, Sox1, Titin, DNER/Tr |
Second Case
A 63‐year‐old female had been vaccinated against COVID‐19 with ChAdOx1 nCov‐19 vaccine. Two days later, she was diagnosed with deep vein thrombosis in her left leg and treated with oral anticoagulation as an outpatient. Six days after vaccination, her gait deteriorated, she developed a vigilance disorder and a twitching all over her body, so she was admitted as an inpatient to our department on day seven after the vaccination. Subsequently, she developed a severe immobilizing opsoclonus‐myoclonus syndrome. MRI was performed with normal status of the parenchyma (Fig 1, 2C). An encephalitis was diagnosed due to the result of lumbar puncture showing a lymphocytic pleocytosis of 115 leukocytes/μl (Fig 1, 2B). EEG recording revealed diffuse abnormally slow theta and delta rhythms without epileptiform activity (Fig 1, 2A). The extensive additional diagnostic workup remained negative, including a paraneoplastic etiology based on the CSF and serum findings (Table 1) and imaging of the thorax and abdomen.
Initially, the patient was treated with a broad anti‐infective therapy without any significant effect. With the criteria fulfilled of possible autoimmune encephalitis, immunosuppressive therapy with a total of 5g methylprednisolone over 5 days was administered. This led to an immediate clinical improvement, so that the patient could mobilize on her own a few days later. The opsoclonus‐myoclonus syndrome resolved over time; at discharge, just a low‐grade tremor persisted as the only residual neurological deficit. Subsequent MRI examination after 3.5 months continued to reveal a normal state of the parenchyma (Fig 1, 2D).
Third Case
A 63‐year‐old male patient presented with isolated aphasia and fever 8 days after being vaccinated with ChAdOx1 nCov‐19. With the suspected diagnosis of herpes encephalitis, he was initially treated with intravenous acyclovir. MRI of the brain was normal, in particular no evidence of ischemia or herpes encephalitis (Fig 1, 3C). A lumbar puncture was performed showing a pleocytosis of 7 leukocytes/μl (Fig 1, 3B). A viral infection was excluded by extensive testing for neurotropic viruses in CSF and serum (Table 1). Normal EEG activity was detected (Fig 1, 3A). On a test‐psychological level, a slow down in the area of intrinsic readiness to react (alertness) was additionally observed. Assuming possible autoimmune encephalitis, immunosuppressive therapy with steroids should be administered, but was rejected by the patient. In a test‐psychological follow‐up 2 weeks later, further improvement could be observed. MRI follow‐up 2.5 months later showed no evidence of structural lesions (Fig 1, 3D).
Discussion
The presented case series is characterized by onset of symptoms of encephalitis within 7 to 11 days after ChAdOx1 nCoV‐19 vaccination. In all three cases, the criteria for possible autoimmune encephalitis defined by Graus were fulfilled as follows 8 : (1) subacute onset of working memory impairment, impaired mental status, or psychiatric symptoms, (2) CSF pleocytosis, and in one case, seizures without prior epilepsy, and (3) exclusion of alternative causes. Consequently, the diagnosis of possible autoimmune encephalitis was made and immunosuppressive treatment was initiated. Fortunately, all three patients responded to therapy and exhibited a benign course of the disease with almost complete recovery from neurological symptoms.
The second case is particularly interesting, because a severe opsoclonus‐myoclonus syndrome (OMS) occurred after vaccination. OMS is a rare condition with dyskinesia of eye movements and myoclonic movements of the trunk and limbs. OMS has been described previously as a consequence of flu vaccination, 9 after vaccination to human papilloma virus, 10 and after anti‐rubella vaccination. 11 Similar to our second reported case, OMS syndrome occurred within 2 weeks after vaccination.
After exclusion of pathogen‐induced encephalitis (Table 1), two of the three patients in our case series were treated with immunosuppressive therapy with rapid clinical improvement. The third patient rejected immunosuppressive treatment, but improved spontaneously. No severe sequelae occurred in all three cases. Immunotherapy including steroids or even plasmapheresis for encephalitis after vaccination has been reported before in cases after vaccination against hepatitis A‐, hepatitis B‐, poliovirus, diphtheria and tetanus. 12
In general, vaccinations can cause a strong expression of proinflammatory cytokines and a T cell response. This was also demonstrated for ChAdOx1 nCoV‐19 vaccine. 13 After vaccination, antigens are recognized as potential pathogens by both conserved pathogen‐ and damage‐associated molecular patterns as well as pattern‐recognition receptors that are found on local or peripheral circulating immune cells (eg monocytes and macrophages) and on resident stromal cells. 7 Induction and transcription of many target genes occurs, resulting in synthesis and release of pyrogenic cytokines (ie interleukin [IL]‐1, IL‐6, tumor necrosis factor‐alpha [TNF‐α], and prostaglandin‐E2) into the bloodstream that mimic the response to natural infection. Subsequent to stimulation, the immune system initiates a complex series of innate immune events including phagocytosis, release of inflammatory mediators including chemokines and cytokines, activation of complement, and cellular recruitment. Mediators and products of inflammation in the circulation can affect other body systems to cause systemic side‐effects, and ultimately can cause neuroinflammation in some subjects after microglia activation, depending on the immunogenetic background and the innate immune memory. 6 , 7 We cannot verify this with our presented cases; however, this could be an explanation for a possible association between vaccination and possible autoimmune encephalitis. Due to the lack of antibody detection, the diagnosis of a definite autoimmune encephalitis cannot be made. Based on the diagnostic constellation, a postvaccinal encephalitis could be discussed.
In a survey over 20 years in the United States, 14 1,396 cases of encephalitis occurred after vaccination. Responsible vaccinations were hepatitis B (354 cases), influenza vaccination (208 cases), vaccination for measles, mumps and rubella‐MMR (208 cases), and vaccination for Haemophilus influenza type B (120 cases). The onset of encephalitis within 2 weeks after vaccination was reported in 708 patients (50.7%). Thus, the temporal association between vaccination and symptom onset is well in line with our cases.
Public institutions collect the occurrence of adverse reactions for the different COVID‐19 vaccines. Recent data from the National Institute of Public Health of Québec (INSPQ) show that 67 reports of unusual clinical manifestations were made in Quebec for each 100,000 doses administered, all vaccines combined. In the case of AstraZeneca, this proportion rises to 182.5 reports per 100,000 doses. The vast majority of unusual clinical manifestations listed by the INSPQ are considered as “without gravity”. 15
The occurrence of encephalitis after vaccination with ChAdOx1 nCoV‐19 (AZD1222) is reported in several publicly available databases (Table 2). A total of 79 cases of encephalitis worldwide were identified after ChAdOx1 nCov‐19 vaccination. By the reported periods and vaccinations given in this period, an estimation of the incidence can be made, which is about 8 per 10 million vaccination doses.
TABLE 2.
Overview of Encephalitis as Adverse Event after COVID‐19 Vaccination from Publicly Available Databases and Estimation of an Incidence
| Institution | Time period | Number of vaccine doses | Observed number of encephalitis | Reference |
|---|---|---|---|---|
| Gov.uk | 04/01/21 to 16/06/21 | ChAdOx1 nCov‐19 (AZD1222): | 32 Encephalitis | https://www.gov.uk/ |
| 24.5 million first doses | (7 Encephalitis viral) | |||
| 19.6 million second doses | 1 Bickerstaff's encephalitis | |||
| 1 Limbic encephalitis | ||||
| 4 Non‐infective encephalitis | ||||
| Pfizer‐Biontech mRNA vaccine (BNT162b2): | ||||
| 16.8 million first doses | 12 Encephalitis | |||
| 10.9 million second doses | (3 Encephalitis viral) | |||
| 1 Encephalitis autoimmune | ||||
| 1 Limbic encephalitis | ||||
| 1 Non‐infective encephalitis | ||||
| European Medicines Agency (EMA) | 29/01/21 to 10/06/21 | ChAdOx1 nCov‐19 (AZD1222): almost 46 million doses |
33 Encephalitis 10 ADEM |
https://www.ema.europa.eu |
| Paul‐Ehrlich‐Institute | 27/12/20 to 31/05/21 | ChAdOx1 nCov‐19 (AZD1222): | 8 Encephalitis | https://www.pei.de |
| Germany | 9.2 million doses | |||
| Pfizer‐Biontech mRNA vaccine(BNT162b2): | 5 Encephalitis | |||
| 36.9 million doses | ||||
| COVID‐19 Vaccine Moderna (mRNA‐1273): | ‐ | |||
| 4.0 million doses |
ChAdOx1 nCov‐19 (AZD1222): A total of 79 cases in 99.3 million doses with a resulting incidence of almost 0.08 per 100,000. Pfizer‐Biontech mRNA vaccine (BNT162b2): A total of 20 cases in 110.6 million doses with a resulting incidence of almost 0.02 per 100,000.
The same analysis yielded 20 reported cases of unexplained encephalitis following vaccination with the Pfizer‐Biontech mRNA vaccine (BNT162b2), for a total of more than 110 million doses of vaccine. The estimated incidence is thus 2 in 10 million, and thus only one‐quarter of case numbers as compared to ChAdOx1 nCov‐19.
A large survey of 24 hospitals in a region of 5 million people in the United Kingdom revealed a number of 75 cases with encephalitis of unexplained etiology in a 2‐year period. 16 The Paul Ehrlich Institute in Germany reported the occurrence of encephalitis within a period of up to 91 days after vaccination with ChAdOx1 nCov‐19. According to the determined incidence, 16 approximately 19 cases with encephalitis of unexplained etiology could be expected per 10 million doses during this period of 91 days. In the survey of 1,396 encephalitis cases over 20 years in the United States, 14 an onset within 2 weeks after vaccination is quite common (50.7%)—as also seen in our cases. Therefore, within 2 weeks, 2.9 cases of unexplained encephalitis could be expected to occur per 10 million doses of vaccination according to the large UK survey. 16 Assuming that 50.7% of incident encephalitis cases occur within 2 weeks, this would correspond to an incidence of 4 per 10 million doses in our incidence estimation, thus, a higher rate than the expected spontaneously occurring encephalitis cases. However, the exact duration of symptom onset could unfortunately not be obtained with certainty from the publicly available databases. Additionally, one could expect that not all cases were reported and that the actual number of postvaccinal encephalitis are most likely higher. A systematic survey is therefore of great relevance for the examination of a causal relationship.
Moreover, the significant difference in accumulation of cases after ChAdOx1 nCov‐19 vaccination compared to vaccination with the Pfizer‐Biontech mRNA vaccine (ChAdOx1 nCoV‐19: 79 cases in 99.3 million doses; Pfizer‐Biontech mRNA vaccine: 20 cases in 110.6 million doses (Table 2); Pearson's χ2 = 41.923, p < 0.001) and the lack of reports after vaccination with other COVID‐19 vaccines does suggest a causal relationship. Certainly, the temporal relationship between vaccination and encephalitis cannot prove causality. This is also demonstrated by the report of the occurrence of Guillain‐Barré syndrome in both the placebo arm and the active arm of a COVID‐19 vaccination trial. 17 Proof of causality is only possible with large pooled data from observational epidemiologic studies that ultimately demonstrate a higher incidence of encephalitis compared with spontaneous occurrence of encephalitis by chance after vaccination has taken place. Although we are aware, that proof of causality will not be possible in individual cases, we believe the remarkable temporal association between ChAdOx1 nCov‐19 vaccination and the presentation of encephalitis in three cases is noteworthy.
Conclusion
We report here the first three cases of postvaccinal encephalitis fulfilling the criteria of possible autoimmune encephalitis, defined by Graus et al and in temporal correlation shortly after vaccination with ChAdOx1 nCov‐19. The diagnosis was supported by: (1) the temporal association between vaccination and symptom onset, (2) the characteristic symptoms previously described in the OMS as a vaccine reaction in further vaccinations, (3) the extensive exclusion of other etiologies (Table 1), and (4) the response to immunosuppressive therapy with corticosteroids. In addition, we can reassuringly mention that our cases of encephalitis exhibited benign courses without sequelae. Nevertheless, a detailed diagnosis to exclude other possible causes should be performed urgently. Obviously, the complication of autoimmune encephalitis after ChAdOx1 nCoV‐19 vaccination appears to be very rare. Clearly, the benefit of vaccination outweigh the risks.
Author Contributions
F.Z., A.R. and W.R.S. contributed to the conception and design of the case series; F.Z., T.G. and A.R. contributed to the acquisition and analysis of data; F.Z., R.K., W.R.S. and A.R. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interests
The authors declare that they have no conflict of interest.
Acknowledgement
The authors received no financial support for the research, authorship and publication of this article. Open Access funding enabled and organized by Projekt DEAL.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. COVID‐19 Map . Johns Hopkins Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/map.html. Accessed May 17, 2021.
- 2. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graf T, Thiele T, Klingebiel R, Greinacher A, Schäbitz W‐R, Greeve I. Immediate high‐dose intravenous immunoglobulins followed by direct thrombin‐inhibitor treatment is crucial for survival in Sars‐Covid‐19‐adenoviral vector vaccine‐induced immune thrombotic thrombocytopenia VITT with cerebral sinus venous and portal vein thrombosis. Journal of Neurology. 2021. 10.1007/s00415-021-10599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giannotta G, Giannotta N. Vaccines and neuroinflammation. Int J Pub Heal Safe 2018;3:1000163. [Google Scholar]
- 7. Hervé C, Laupèze B, Del Giudice G, et al. The how's and what's of vaccine reactogenicity. npj Vaccines 2019;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piquet AL, Kothari M, Ermak D, Ahmed A. Opsoclonus‐Myoclonus Syndrome Post‐Vaccination and Viral Illness. International Journal of Clinical Medicine. 2012;03:304–306. 10.4236/ijcm.2012.34060. [DOI] [Google Scholar]
- 10. McCarthy JE, Filiano J. Opsoclonus myoclonus after human papilloma virus vaccine in a pediatric patient. Parkinsonism Relat Disord 2009;15:792–794. [DOI] [PubMed] [Google Scholar]
- 11. Lapenna F, Lochi L, De Mari M, et al. Post‐vaccinic opsoclonus‐myoclonus syndrome: a case report. Parkinsonism Relat Disord 2000;6:241–242. [DOI] [PubMed] [Google Scholar]
- 12. Rogalewski A, Kraus J, Hasselblatt M, et al. Improvement of advanced postvaccinal demyelinating encephalitis due to plasmapheresis. Neuropsychiatr Dis Treat 2007;3:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ewer KJ, Barrett JR, Belij‐Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV‐19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med 2021;27:270–278. [DOI] [PubMed] [Google Scholar]
- 14. Al Qudah Z, Abukwaik W, Patel H, Souayah N. Encephalitis after vaccination in United States. A report from the CDC/FDA vaccine adverse event reporting system. [1990–2010] (P03.151). Neurology 2012;78:P03.151. [Google Scholar]
- 15. Données de vaccination contre la COVID‐19 au Québec | INSPQ. Available at: https://www.inspq.qc.ca/covid-19/donnees/vaccination. Accessed June 29, 2021.
- 16. Granerod J, Ambrose H, Davies N, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population‐based prospective study. Lancet Infect Dis 2010;10:835–844. [DOI] [PubMed] [Google Scholar]
- 17. Márquez Loza AM, Holroyd KB, Johnson SA, Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain‐Barré Syndrome in the Placebo and Active Arms of a COVID‐19 Vaccine Clinical Trial. Neurology. 2021;96(22):1052–1054. 10.1212/wnl.0000000000011881. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


