Abstract
Social restrictions during the coronavirus disease 2019 pandemic strongly affected the epidemiology of influenza and respiratory syncytial virus (RSV). As rhinovirus seemed to spread despite the restrictions, we aimed to analyze rhinovirus epidemiology in children during the pandemic. This register‐based study used data from the Finnish Infectious Disease Register. Nationwide rhinovirus findings from July 2015 to March 2021 were included and stratified by age (0–4, 5–9, and 10–14). Cumulative 14‐day incidence per 100000 children was calculated. Four thousand five hundred and seventy six positive rhinovirus findings were included, of which 3788 (82.8%) were among children aged 0–4. The highest recorded incidence was 36.2 among children aged 0–4 in October 2017. The highest recorded incidence during the pandemic period was 13.6 in November 2020. The impact of the restrictions was mostly seen among children aged 0–4 years of age in weeks 14–22 in 2020. The incidence has since remained near reference levels in all age groups. Strict restrictions temporarily interrupted the circulation of rhinovirus in spring 2020. Rhinovirus incidence returned to normal levels soon after the harsh restrictions were lifted. These looser social restrictions prevented RSV and influenza seasons but failed to prevent the spread of rhinovirus.
Keywords: epidemiology, nonpharmaceutical interventions, rhinovirus, surveillance
1. BACKGROUND
More than a year has passed since the coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was declared a pandemic on March 11, 2020, by the World Health Organization. Most countries implemented lockdowns to reduce the spread of SARS‐CoV‐2 in spring 2020. These lockdowns were effective in reducing the spread of common respiratory pathogens in spring 2020.1, 2, 3
Despite these restrictions, multiple reports have described that rhinoviruses spread normally. A study from the United Kingdom showed that the re‐opening of schools led to a rapid increase in rhinovirus detection.4 Our previous report found that rhinovirus infections started to increase during the summer holidays, and school opening seemed to have little impact on detection in children in Finland.5 A study from Austria demonstrated that the lockdown reduced rhinovirus detections at first, but as soon as the first restrictions were lifted, the spread continued.6 Reports from Japan and China have confirmed that rhinovirus has spread among school‐aged children during the restrictions and despite the use of face masks.7, 8 In Australia, rhinovirus detections first decreased after lockdown started but later exceeded the incidence of previous years, even though the restrictions were unchanged.9 Interestingly, another Australian study showed that respiratory syncytial virus (RSV) cases had a high increase after the restrictions were eased.10 However, this was not seen in Finland, where the RSV season 2020–2021 did not occur despite schools and day care remaining open.11 The social and traveling restrictions prevented the 2020–2021 influenza season in Finland.12 With these restrictions Finland has managed to keep the incidence of SARS‐CoV‐2 as the lowest in Europe during the second and third waves.
The aim of this report is to describe the epidemiology of rhinovirus infections in children during the COVID‐19 pandemic in Finland.
2. MATERIALS
Data for this retrospective register‐based surveillance study were gathered from the National Infectious Disease Register, maintained by the Finnish Institute of Health and Welfare. The register is an open‐access surveillance system updated daily in which all laboratories are mandated by the law on contagious diseases to immediately report all findings on notifiable diseases. The reporting delay is minimal, and the register provides current information. The complete list of notifiable diseases can be found in the register description (https://thl.fi/en/web/infectious-diseases-and-vaccinations/surveillance-and-registers/finnish-national-infectious-diseases-register).
For this study, all the laboratory‐confirmed rhinovirus cases from July 2015 to March 2021 in the entire Finnish pediatric population (age 0–14 years) of 0.9 million were included. The positive rhinovirus findings are presented as 14‐day cumulative incidence per 100 000 children and stratified by age, as most rhinovirus cases are detected in the youngest children. Rhinovirus is part of the multiplex polymerase chain reaction (PCR) respiratory panel, which is used in pediatric hospitals for testing and diagnosing acute respiratory illnesses. The population information was gathered from the Finnish Population Center's open‐access population statistics datasets. As rhinovirus typically spreads in the fall and late winter, the season from July 2020 to March 2021 was compared to the corresponding dates from 2015 to 2020. Due to the study's use of open‐access public data, no research permissions or ethical evaluations were needed.
The restrictions implemented in Finland were as follows:
-
–
March–May 2020: Nationwide lockdown with school and day care closures.
-
–
June–September 2020: Minimal restrictions aimed toward adults; traveling not prohibited.
-
–
October 2020–March 2021: Stepwise restrictions on gatherings based on regional COVID‐19 incidence; face masks if aged ≥15; day care and elementary schools remained open; traveling restrictions.
3. RESULTS
A total of 4 576 positive rhinovirus findings were included in this study. Of these, 3788 (82.8%) were among children aged 0–4, 552 (12.1%) among children aged 5–9, and 236 (5.1%) among children aged 10–‐14. The highest recorded peak in 14‐day incidence during the study period was 36.2 per 100 000 children in the youngest age group in October 2017. The highest recorded 14‐day incidence during the pandemic period was 13.6 per 100 000 children aged 0–4 in November 2020. The overall incidence in children aged 0–14 has remained stable since January 2019, and a clear decrease was only seen immediately after the lockdown was declared in Finland in March 2020 (Figure 1). After the lockdown measures were lifted at the beginning of June 2020, the rates of rhinovirus findings returned to normal levels and later remained stable. The impact of the restrictions was mostly seen among children aged 0–4 years of age in weeks 14 to 22 in 2020. Since then, the incidence has been stable and near the reference levels (Figure 2A). In the older children (5–9 years of age), the incidences of rhinovirus detections were lower in season 2020–2021 compared to previous seasons (Figure 2B). In the oldest age group (10–14 years of age) the incidence remained at a normal level throughout the pandemic (Figure 2C).
Figure 1.
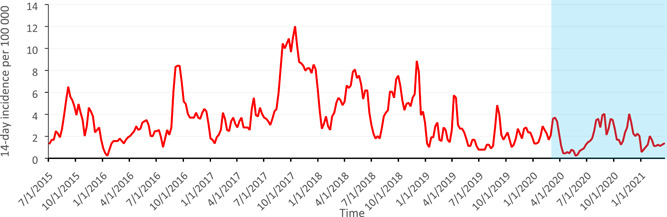
Fourteen‐day cumulative incidences of laboratory‐confirmed rhinovirus infections per 1,00,000 children for children aged 0–14 from July 2015 to March 2021 in Finland. Light blue box indicates the pandemic period
Figure 2.
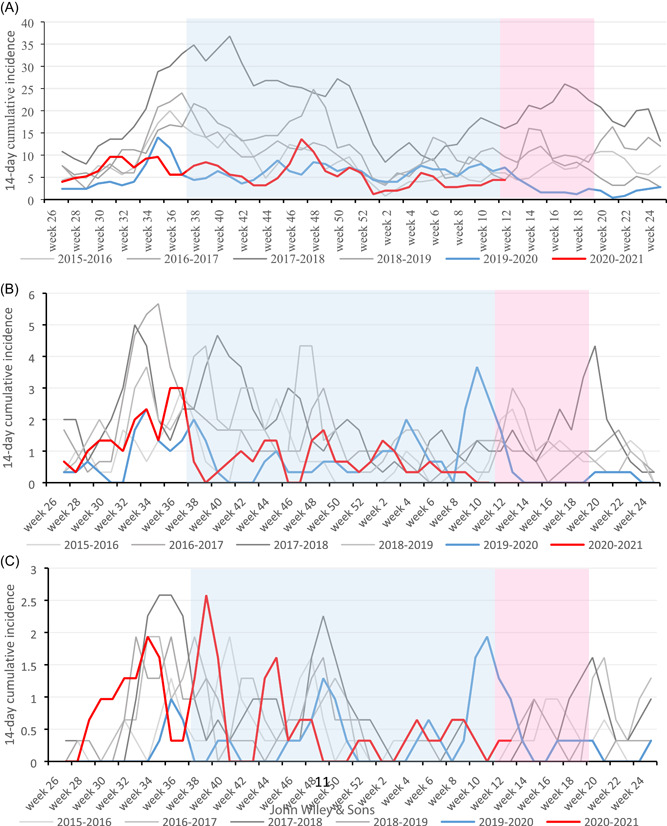
Age‐stratified comparison of rhinovirus seasons based on laboratory‐confirmed findings from July 2015 to March 2021 presented as 14‐day cumulative incidence per 1,00,000 children in each age group. (A) Children aged 0–4. (B) Children aged 5–9, and (C) Children aged 10–14. Light pink box indicates the lockdown with school and day care closures in weeks 12–20 in spring 2020. Light blue box indicates the period of regional restrictions without school and day care closures from September 2020 to March 2021
4. DISCUSSION
Our results show that the current non‐pharmaceutical interventions were not effective in preventing the spread of rhinovirus infections in children, although these interventions were effective against seasonal influenza and RSV. Rhinovirus detections decreased during the initial lockdown with school closures, but as soon as the restrictions were eased, detections returned to a near‐normal level. Our results are in line with recent reports from South Korea,13 Australia,14 Italy,15 and USA,16 which all describe the unchanged rhinovirus detection rates during social restrictions.
The aerosol spread of SARS‐CoV‐2 has now been recognized widely by World Health Organization and Centers for Disease Control and Prevention. Rhinovirus is known to transmit by direct contact17 as the virus can survive in fingers and direct evidence supports the hypothesis that RV colds are transmitted by accidental self‐inoculation of the nose or conjunctiva following inadvertent contamination of the fingers with the virus.18 However, a previous study compared viral detection in exhaled particles with and without surgical face masks in symptomatic patients, finding that face masks did not reduce the transmission of droplets and aerosols containing rhinoviruses.19 Therefore, it is interesting that the improved hygiene measures have not reduced the transmission of rhinovirus and only strict lockdown measures reduced the incidence.
These results of the transmission dynamics of rhinovirus infections are important, as the knowledge of the clinical importance of rhinovirus infections in children has increased in recent years. Rhinovirus is the most common cause of acute respiratory infections in children.20 A great part of the rhinovirus infections are asymptomatic and therefore the spreading is difficult to control as asymptomatic carriers are able to spread the virus.21 Rhinovirus typically causes a mild disease for adults, but for children, it is a common cause of wheezing and asthma exacerbations.22 Wheezing episodes typically occur for children under 2 years of age, and rhinovirus type C is associated with severe wheezing and febrile infections.23 Furthermore, rhinovirus as the cause of first severe wheezing has been shown to predict later asthma in children.24
The strengths of this study are the nationwide infectious disease register, which collects current information on notifiable diseases and is updated daily. All the previous studies that focused on rhinovirus detections during the COVID‐19 pandemic have been local or regional studies; thus, we are among the first to present nationwide surveillance results. Furthermore, we were able to analyze age‐stratified incidence. The main limitation is the lack of testing numbers, as the results might be due to limited testing, and testing rates would enable a test‐negativity analysis. However, rhinovirus is tested only as part of respiratory panels, which are used only in pediatric hospitals and emergency departments, and multiplex PCR testing is typically used for patients needing inpatient admission. Therefore, we feel that the current rates are the best possible prediction and present the real incidence of rhinovirus infections evaluated at pediatric hospitals in Finland. Another limitation is that due to the register design we have no information whether the children who tested positive for rhinovirus had any co‐infections.
Strict social restrictions temporarily interrupted the circulation of almost all respiratory pathogens in spring 2020. Rhinovirus incidence returned to normal levels soon after the harsh restrictions were lifted. Based on our results, the rhinovirus has a higher spreading potential in children than RSV and influenza. These results should be noted when considering prevention measures in further epidemics to optimize the level of social restrictions needed to prevent the spreading of droplet pathogens and aerosol‐transmitting pathogens.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Kuitunen I, Artama M, Haapanen M, Renko M.Rhinovirus spread in children during the COVID‐19 pandemic despite social restrictions—A nationwide register study in Finland. J Med Virol. 2021;93:6063‐6067. 10.1002/jmv.27180
REFERENCES
- 1.Fricke LM, Glöckner S, Dreier M, Lange B. Impact of non‐pharmaceutical interventions targeted at COVID‐19 pandemic on influenza burden—a systematic review. J Infect. 2021;82(1):1‐35. 10.1016/j.jinf.2020.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trenholme A, Webb R, Lawrence S, et al. COVID‐19 and infant hospitalizations for seasonal respiratory virus infections, New Zealand, 2020. Emerg Infect Dis. 2021;27(2):641‐643. 10.3201/eid2702.204041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen‐Kosma T, Renko M. Effect of social distancing due to the COVID‐19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39(12):e423‐e427. 10.1097/INF.0000000000002845 [DOI] [PubMed] [Google Scholar]
- 4.Poole S, Brendish NJ, Tanner AR, Clark TW. Physical distancing in schools for SARS‐CoV‐2 and the resurgence of rhinovirus. Lancet Respir Med. 2020;8:92. 10.1016/S2213-2600(20)30502-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haapanen M, Renko M, Artama M, Kuitunen I. The impact of the lockdown and the re‐opening of schools and day cares on the epidemiology of SARS‐CoV‐2 and other respiratory infections in children—a nationwide register study in Finland. EClinicalMedicine. 2021;34:100807. 10.1016/j.eclinm.2021.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redlberger‐Fritz M, Kundi M, Aberle SW, Puchhammer‐Stöckl E. Significant impact of nationwide SARS‐CoV‐2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol. 2021;137:104795. 10.1016/j.jcv.2021.104795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu D, Lu J, Sun Z, et al. Rhinovirus remains prevalent in school teenagers during fight against COVID‐19 pandemic. Immun Inflamm Dis. 2021;9(1):76‐79. 10.1002/iid3.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takashita E, Kawakami C, Momoki T, et al. Increased risk of rhinovirus infection in children during the coronavirus disease‐19 pandemic. Influenza Other Respir Viruses. 2021;15:488‐494. 10.1111/irv.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan SG, Carlson S, Cheng AC, et al. Where has all the influenza gone? The impact of COVID‐19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25(47):2001847. 10.2807/1560-7917.ES.2020.25.47.2001847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley DA, Yeoh DK, Minney‐Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019‐related public health measures. Clin Infect Dis. 2021. 10.1093/cid/ciaa1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuitunen I, Renko M. Lessons to learn from the current pandemic for future non‐pharmaceutical interventions against the respiratory syncytial virus ‐ nationwide register‐study in Finland. Infect Dis. 2021;53(6):476‐478. 10.1080/23744235.2021.1894351 [DOI] [PubMed] [Google Scholar]
- 12.Kuitunen I. Influenza season 2020‐2021 did not begin in Finland despite the looser social restrictions during the second wave of COVID‐19: a nationwide register study. J Med Virol. 2021. 10.1002/jmv.27048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, Michelow IC, Choe YJ. Shifting patterns of respiratory virus activity following social distancing measures for COVID‐19 in South Korea. J Infect Dis. 2021. 10.1093/infdis/jiab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marriott D, Beresford R, Mirdad F, et al. Concomitant marked decline in prevalence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and other respiratory viruses among symptomatic patients following Public Health Interventions in Australia: data from St Vincent's Hospital and Associated Screening Clinics, Sydney, NSW. Clin Infect Dis. 2021;72(10):e649‐e651. 10.1093/cid/ciaa1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amaddeo A, Cason C, Cozzi G, Ronfani L, Comar M. Social distancing measures for COVID‐19 are changing winter season. Arch Dis Child. 2021. 10.1136/archdischild-2021-322004 [DOI] [PubMed] [Google Scholar]
- 16.Rodgers L, Sheppard M, Smith A, et al. Changes in Seasonal Respiratory Illnesses in the United States During the COVID‐19 Pandemic. Clin Infect Dis. 2021. 10.1093/cid/ciab311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L'Huillier AG, Tapparel C, Turin L, Boquete‐Suter P, Thomas Y, Kaiser L. Survival of rhinoviruses on human fingers. Clin Microbiol Infect. 2015;21(4):381‐385. 10.1016/j.cmi.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WM, Gern JE. Rhinovirus. In: Richman DD, Whitley RJ, Hayden FJ (eds.). Clinical Virology. fourth edn. Wiley; 2016:1143‐1164. [Google Scholar]
- 19.NHL Leung, DKW Chu, EYC Shiu, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O. Viral etiology of common cold in children, Finland. Emerg Infect Dis. 2009;15(2):344‐346. 10.3201/eid1502.081468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaman J, Morita H, Birger R, et al. Asymptomatic summertime shedding of respiratory viruses. J Infect Dis. 2018;217(7):1074‐1077. 10.1093/infdis/jix685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T, Mäkelä MJ, Vanto T, Ruuskanen O. The link between bronchiolitis and asthma. Infect Dis Clin North Am. 2005;19(3):667‐689. 10.1016/j.idc.2005.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erkkola R, Turunen R, Räisänen K, et al. Rhinovirus C is associated with severe Wheezing and Febrile respiratory illness in young children. Pediatr Infect Dis J. 2020;39(4):283‐286. 10.1097/INF.0000000000002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukkarinen M, Koistinen A, Turunen R, Lehtinen P, Vuorinen T, Jartti T. Rhinovirus‐induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140(4):988‐995. 10.1016/j.jaci.2016.12.991 [DOI] [PMC free article] [PubMed] [Google Scholar]


