To the editor,
Currently, coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is still the biggest public health crisis faced by the world. In spite of the several vaccines that have been approved for emergency use, ongoing generation of various SARS‐CoV‐2 variants is challenging to the efficacy of these vaccines in preventing COVID‐19. High transmissibility of SARS‐CoV‐2 and the high proportion of presymptomatic and asymptomatic cases who are contagious, highlight the importance of early diagnosis and mass screening of COVID‐19, which enables timely and appropriate interventions to prevent further spread of the virus. 1 , 2 Due to high sensitivity and specificity, the reverse‐transcription quantitative polymerase chain reaction (RT‐qPCR) was widely used as the gold standard for diagnosing COVID‐19 with upper respiratory tract specimens. Given the challenge in detection capacity for mass screening for presymptomatic and asymptomatic cases, and the requirement for frequent testing and monitoring of high‐risk contacts during the COVID‐19 pandemic, simple, fast, sensitive, and inexpensive point‐of‐care testing (POCT) assays are largely encouraged. Rapid antigen test and viral RNA detection by isothermal amplification assays (e.g., reverse‐transcription loop‐mediated isothermal amplification [RT‐LAMP]) are two kinds of POCT approaches that are suitable for mass screening of SARS‐CoV‐2 in resource‐rich and resource‐limited settings.
Recently, seven papers published in J Med Virol evaluated the performance of nine rapid antigen testing devices on SARS‐CoV‐2 detection (Table 1). 3 , 4 , 5 , 6 , 7 , 8 , 9 Two‐thirds of these rapid antigen tests had overall sensitivities (30.8%–68.9%) below the WHO recommended standard of ≥80%. One rapid antigen test (COVID‐VIRO®) showed an excellent sensitivity of >90% even for the samples with a low viral load (>32 C t values in RT‐qPCR), 3 one (COVID‐19 Ag ECO Test) had an overall sensitivity of 82.0%, 4 and one (Panbio™ COVID‐19 Ag Rapid Test) showed variable sensitivities (75.0%–100%) in three different investigations. 4 , 5 , 6 After excluding one study with very small samples size (n=44) that showed very high sensitivity (100%), 4 we recalculated the sensitivity of the Panbio™ Ag Test using the data from two different investigations, 5 , 6 and obtained a 76.3% (216/283) (Table 1). In spite of having a significantly positive correlation of antigen testing with SARS‐CoV‐2 viral culture assays, 10 it is clear that the vast majority (6/7) of tested rapid antigen assays have substantially lower sensitivity than the WHO recommended standard, especially for asymptomatic cases. 6 , 8 The low sensitivity of these antigen testing assays is mainly due to low detection capacity for samples with low viral load (<100 000 RNA copies/ml or C t < 30). Therefore, it is of concern whether the antigen detection alone is sufficient for early diagnosis and/or mass screening of COVID‐19 in the fight against the pandemic.
Table 1.
Sensitivities and specificities of nine rapid antigen testing assays for SARS‐CoV‐2 detection
| Rapid antigen tests | Patient information/disease status | Sample size | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|
| COVID‐VIRO® | Symptomatic/asymptomatic | 248 | 96.7% | 100% | [3] |
|
Abbott Panbio™ COVID‐19 Ag Rapid Test COVID‐19 Ag ECO Test |
Symptomatic |
44 68 |
100% 82.0% |
94.0% 98.0% |
[4] |
|
Abbott Panbio™ COVID‐19 Ag Rapid Test BioSpeedia COVID19 Speed‐Antigen Test |
Symptomatic/presymptomatic/asymptomatic | 401 |
75.0% 65.5% |
NA 100% |
[5] |
| Abbott Panbio™ COVID‐19 Ag Rapid Test | Children: Symptomatic/asymptomatic | 744 | 82.4% | 100% | [6] |
|
CoV‐Ag Rapid Test Cassette (BioRad) GSD NovaGen COVID‐19 Ag Rapid Test (NovaTec) Aegle CoV‐Ag Rapid Test Cassette (LumiraDx) |
NA | 199 |
62.7% 61.9% 64.0% |
100% 85.7% 100% |
[7] |
| AgPOCT (Roche) |
Symptomatic/asymptomatic (symptomatic) (asymptomatic) |
2375 (1539) (836) |
68.9% (69.5%) (62.0%) |
99.6% (99.5%) (97.6%) |
[8] |
| COVID‐19 Ag Respi‐Strip (Coris BioConcept) | NA | 50 | 30.8% | 100% | [9] |
| Abbott Panbio™ COVID‐19 Ag Rapid Test a | Adult/children; Symptomatic/presymptomatic/asymptomatic | 1145 | 76.3% a | NA | This study |
Transmissible SARS‐CoV‐2 persists during incubation and acute phases of COVID‐19 (Figure 1), and presymptomatic and asymptomatic COVID‐19 individuals are the main source of the transmissible virus. The majority of the infections were found to be acquired via silent transmission from presymptomatic and asymptomatic individuals. 1 , 2 To find the presymptomatic individuals, as well as asymptomatic individuals whether they are infectious or not, is crucial for the containment of COVID‐19 (Figure 1). High proportion of asymptomatic and presymptomatic cases with high transmissibility of SARS‐CoV‐2 highlight the importance of mass and/or contact‐based screening for presymptomatic and asymptomatic individuals, which enables timely and appropriate interventions to prevent silent transmission.
Figure 1.
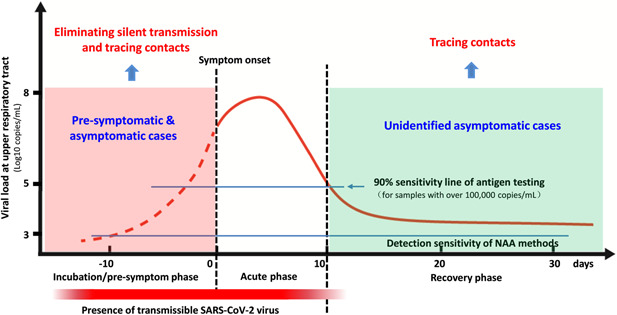
SARS‐CoV‐2 viral load dynamics at upper respiratory tract during COVID‐19. SARS‐CoV‐2 viral load at the upper respiratory tract varies largely during the course of COVID‐19, and peaks within about 4 days after symptom onset (or about 2 weeks after initial infection in asymptomatic cases). Symptomatic and asymptomatic individuals share similar viral load dynamics during the course of COVID‐19, which is divided into three phases, incubation/pre‐symptom, acute, and recovery phases. SARS‐CoV‐2 viral load is substantially lower during incubation/pre‐symptom and recovery phases than the acute phase, and transmissible SARS‐CoV‐2 virus persists from initial infection up to about 10 days after symptom onset or two to three weeks since initial infection in asymptomatic cases
The incubation phase represents the early stage of infection, during which the rapidly replicating virus is highly transmissible, but the viral load (or antigen level) might be relatively low (Figure 1). The major challenge for rapid antigen testing is that its low detection sensitivity for low viral load samples will miss a large proportion of presymptomatic and asymptomatic COVID‐19 individuals during the incubation phase (Figure 1), and these missed SARS‐CoV‐2 carriers will enlarge the silent transmission chain. 1 , 2 Therefore, the vast majority of the rapid antigen testing alone should be cautiously recommended for early diagnosis and mass screening of COVID‐19 because of its low sensitivity.
As a promising POCT technique, RT‐LAMP has comparable detection sensitivity with RT‐qPCR, but significantly shorter sample‐to‐result time (about 30 min vs. about 4 h for RT‐qPCR), easier operation, and less dependent on sophisticated equipment. 11 In particular, we and other groups developed direct probe‐based SARS‐CoV‐2 RT‐LAMP assays that can detect clinical samples at a level of over 1000 copies/ml (unpublished data), 12 enabling the finding of most presymptomatic and asymptomatic COVID‐19 individuals (Figure 1). Although RT‐qPCR is considered as the golden standard for SARS‐CoV‐2 detection, high dependence on molecular laboratory (sophisticated equipment with professionals) and long sample‐to‐result time (about 4 h) limit its capacity in mass screening or community‐based testing of SARS‐CoV‐2. As a good alternative, direct probe‐based RT‐LAMP assay or other nucleic acid amplification (NAA)‐based POCT strategies should be recommended to use alone or together with rapid antigen test in mass screening or community‐based testing of SARS‐CoV‐2.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Chiyu Zhang: conceptualization, supervision, validation, visualization, writing – original draft, writing – review and editing. Zhenzhou Wan: conceptualization, investigation, funding acquisition, writing – original draft. Yongjuan Zhao: investigation, visualization, writing – original draft. Renfei Lu: investigation. Yajuan Dong: investigation. All authors have accessed verified the underlying data.
ACKNOWLEDGMENT
This study was supported by grants from the Health Commission of Jiangsu Province, China (Z2019038).
Zhenzhou Wan and Yongjuan Zhao contributed equally to this paper.
REFERENCES
- 1. Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID‐19 outbreaks. Proc Natl Acad Sci U S A. 2020;117:17513‐17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26:672‐675. [DOI] [PubMed] [Google Scholar]
- 3. Courtellemont L, Guinard J, Guillaume C, et al. High performance of a novel antigen detection test on nasopharyngeal specimens for diagnosing SARS‐CoV‐2 infection. J Med Virol. 2021;93:3152‐3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuda EM, de Campos IB, de Oliveira IP, Colpas DR, Carmo A, Brígido L. Field evaluation of COVID‐19 antigen tests versus RNA based detection: potential lower sensitivity compensated by immediate results, technical simplicity, and low cost. J Med Virol. 2021;93:4405‐4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soleimani R, Deckers C, Huang TD, et al. Rapid COVID‐19 antigenic tests: usefulness of a modified method for diagnosis. J Med Virol. 2021;93:5655‐5659. 10.1002/jmv.27094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eleftheriou I, Dasoula F, Dimopoulou D, et al. Real‐life evaluation of a COVID‐19 rapid antigen detection test in hospitalized children. J Med Virol. 2021. 10.1002/jmv.27149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blairon L, Cupaiolo R, Thomas I, et al. Efficacy comparison of three rapid antigen tests for SARS‐CoV‐2 and how viral load impact their performance. J Med Virol. 2021. 10.1002/jmv.27108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holzner C, Pabst D, Anastasiou OE, et al. SARS‐CoV‐2 rapid antigen test: fast‐safe or dangerous? An analysis in the emergency department of an university hospital. J Med Virol. 2021;93:5323‐5327. 10.1002/jmv.27033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciotti M, Maurici M, Pieri M, Andreoni M, Bernardini S. Performance of a rapid antigen test in the diagnosis of SARS‐CoV‐2 infection. J Med Virol. 2021;93:2988‐2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pekosz A, Parvu V, Li M, et al. Antigen‐based testing but not real‐time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis. 2021. 10.1093/cid/ciaa1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Y, Wan Z, Yang S, et al. A mismatch‐tolerant reverse transcription loop‐mediated isothermal amplification method and its application on simultaneous detection of all four serotype of dengue viruses. Front Microbiol. 2019;10:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sherrill‐Mix S, Hwang Y, Roche AM, et al. Detection of SARS‐CoV‐2 RNA using RT‐LAMP and molecular beacons. Genome Biol. 2021;22:169. [DOI] [PMC free article] [PubMed] [Google Scholar]


