Abstract
Reverse transcription fluorescence resonance energy transfer‐polymerase chain reaction (FRET‐PCRs) were designed against the two most common mutations in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) (A23403G in the spike protein; C14408T in the RNA‐dependent RNA polymerase). Based on high‐resolution melting curve analysis, the reverse transcription (RT) FRET‐PCRs identified the mutations in american type culture collection control viruses, and feline and human clinical samples. All major makes of PCR machines can perform melting curve analysis and thus further specifically designed FRET‐PCRs could enable active surveillance for mutations and variants in countries where genome sequencing is not readily available.
Keywords: FRET‐PCR, high‐resolution melting curve analysis, mutations, SARS‐CoV‐2
Highlights
A23403G in the spike protein and C14408T in the RNA‐dependent RNA polymerase are identified as two most common mutations in the SARS‐CoV‐2 variants. Reverse transcription FRET‐PCRs identified the mutations while no DNA sequencing required. Designed FRET‐PCRs could enable active surveillance for mutations and variants in countries where genome sequencing is not readily available.
1. INTRODUCTION
Genetic mutations giving rise to variants of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) continue to emerge and circulate worldwide during the coronavirus disease 2019 (COVID‐19) pandemic. These mutations enable SARS‐CoV‐2 variants to be categorized into eight clades1 and six major lineages.2 Some emerging SARS‐CoV‐2 variants may have increased potential for transmissibility and virulence and lowered protection from vaccines.3, 4, 5, 6, 7, 8 Surveillance for variants and their spread is important in understanding the dynamics of the COVID‐19 pandemic and in developing effective control policies. Screening for variants, however, is generally infrequent as it requires genome sequencing which is expensive, time‐consuming, and not readily available in most countries.9 Comparison of sequences in global initiative on sharing avian influenza data (GISAID) and reported cases from the six countries most affected by COVID‐19 reveals genotyping was only performed on under 1% of cases in the United States, Brazil, India, France, Russia, Italy, and South Africa, and 8.3% in the UK (Table S1).
As it is practically impossible for even the most advanced countries to sequence all positive samples, it would be very useful if tests were available which could be readily used by laboratories around the world to identify mutations and thereby greatly facilitate the detection and tracking of SARS‐CoV‐2 variants. To test this concept, we developed reverse transcription (RT) fluorescence resonance energy transfer‐polymerase chain reaction (FRET‐PCRs) against two of the commonest mutations found worldwide and used them to test clinical samples. Our results show that RT FRET‐PCRs can be developed against mutations in the SARS‐CoV‐2. Development of similar RT FRET‐PCRs against other mutations of interest will enable general diagnostic laboratories around the world to monitor variants rapidly and conveniently, and thereby implement more targeted and appropriate control programs.
2. MATERIALS AND METHODS
2.1. Identifying common mutations in SARS‐CoV‐2 variants
The A23403G and the C14408T mutations are the most common mutations from the original Wuhan strain that persists in almost all variants today.9 They are present in all variants of interest (VOI) and variants of concern (VOC) determined by the CDC10 and reported to be the most common in the United States and globally.11 We confirmed this by analyzing all available high‐quality SARS‐CoV‐2 sequences from GISAID (https://www.gisaid.org/—accessed on April 28, 2021) which revealed the A23403G and the C14408T occurred in over 99.85% (250,568/250,945) of the five major variants recognized today (Table 1). These variants were originally found in the United Kingdom (20I/501Y.V1, VOC 202012/01, or B.1.1.7), South Africa (20H/501Y.V2 or B.1.351), Brazil (P.1), Denmark (Cluster 5), and recently in the United States (CAL.20 C).
Table 1.
Prevalence of A23403G and C14408T mutations in the different SARS‐CoV‐2 clades and variants
| Clades/variants | Number of submitted sequencesa | With A23403G mutation | With C14408T mutation |
|---|---|---|---|
| Clade | |||
| L | 3686 | 0, 0.00% | 34, 0.92% |
| S | 7902 | 266, 3.37% | 12, 0.15% |
| V | 4264 | 9, 0.21% | 3, 0.07% |
| Total | 15,852 | 275, 1.70% | 49, 0.31% |
| G | 85,109 | 85,070, 99.95% | 84,455, 99.23% |
| GH | 166,783 | 166,724, 99.96% | 165,623, 99.30% |
| GR | 145,342 | 145,320, 99.98% | 145,035, 99.79% |
| GRY | 221,434 | 221,345, 99.96% | 221,213, 99.90% |
| GV | 111,023 | 111,009, 99.99% | 110,922, 99.91% |
| Total | 729,691 | 729468, 99.97% | 727248, 99.67% |
| Variant | |||
| VUI202012/01 (B.1.1.7) | 230,771 | 230,702; 99.97% | 230,683; 99.96% |
| 501Y.v2 (B.1.351) | 4489 | 4,489; 100% | 4,219; 93.99% |
| 501Y.V3 (P.1) | 1565 | 1,543; 98.59% | 1,556; 99.42% |
| 452 R.V1 (B.1.429 + B.1.427) | 13,774 | 13,771; 99.98% | 13,764; 99.93% |
| 484 K.V3 (B.1.525) | 346 | 346; 100% | 346; 100% |
| Total | 250,945 | 250851; 99.96% | 250568; 99.85% |
Abbreviations: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
The high‐quality SARS‐CoV‐2 sequences were obtained from GISAID on April 28, 2021.
2.2. SARS‐CoV‐2 Reverse‐Transcription FRET‐PCRs
Representative sequences around the mutations were aligned, and upstream and downstream primers and probes were designed to amplify and detect all SARS‐CoV‐2. The 6‐carboxyfluorescein (6‐FAM)‐labeled probes were further designed to contain the unique A23403G or C14408T mutation (Table 2). The 6‐FAM probe was 3ʹ labeled as the FRET energy donor probe excited by 488 nm light. The LC Red 640 probe was 5ʹ‐labeled and 3ʹ‐phosphorylated as the acceptor probe.
Table 2.
The oligonucleotides used in this study
| Target of PCR | Primer/Probe | Sequences (5ʹ–3ʹ) |
|---|---|---|
| A23403G | Upstream primer | TGTTCTTTTGGTGGTGTCAGT |
| Downstream primer | TAGAATAAACACGCCAAGTAGGAGT | |
| 6‐FAM‐probe | TTCTTTATCAGGATGTTAACTGCACAGAA‐6FAM | |
| LCRed 640 probe | LCR640‐TCCCTGTTGCTATTCATGCAGATCA‐PHOSPATE | |
| C14408T | Upstream primer | TTAAATATTGGGATCAGACATACC |
| Downstream primer | GAAGTGGTATCCAGTTGAAACT | |
| 6‐FAM‐probe | AAAACTTGTAAGTGGGAACACTGT ‐6FAM | |
| LCRed 640 probe | LCR640‐ GAGAATAAAACATTAAAGTTTGCA‐phosphate |
Abbreviations: ATCC, american type culture collection; 6‐FAM, 6‐carboxyfluorescein; PCR, polymerase chain reaction.
Each 20 μl PCR reaction contained 2.0 U Platinum Taq DNA polymerase (Invitrogen) and 0.0213 U ThermoScript™ reverse transcriptase (Invitrogen). Primers were used at 1 μM, the LCRed 640 probe at 0.2 μM, and the 6‐FAM probe at 0.1 μM. PCR was performed on a Roche Light Cycler 480 II system (Roche Molecular Biochemicals). Thermal cycling was preceded by a 10‐min reverse transcription reaction at 55°C followed by a 5 min denaturation at 95°C, and 40 cycles of 10 s @ 95°C, 10 s @ 55°C, and 10 s @ 72°C.
Genomic RNA of two SARS‐CoV‐2 viruses from american type culture collection (ATCC) served as controls and as quantitative standards: 2019‐nCOV/USA‐WA1/2020 which does not contain the A23403G and the C14408T mutations, and 201/501Y.V1 which contains both mutations. To generate quantitative standards, PCR products of the two control viruses were purified by 4% MetaPhor agarose gel electrophoresis and quantified by PicoGreen DNA fluorescence assays (Molecular Probes).
The melting curve which assesses the dissociation of the PCR products and labeled probes was determined by monitoring the fluorescence from 35°C to 75°C with a temperature transition rate of 0.2°C per second. The first derivatives of F2/F1 were evaluated to determine the T m of the probe (Figures 1 and 2). Nucleotide mismatches between the 6‐FAM‐probes and the SARS‐CoV‐2 variants result in distinct T m values.
Figure 1.
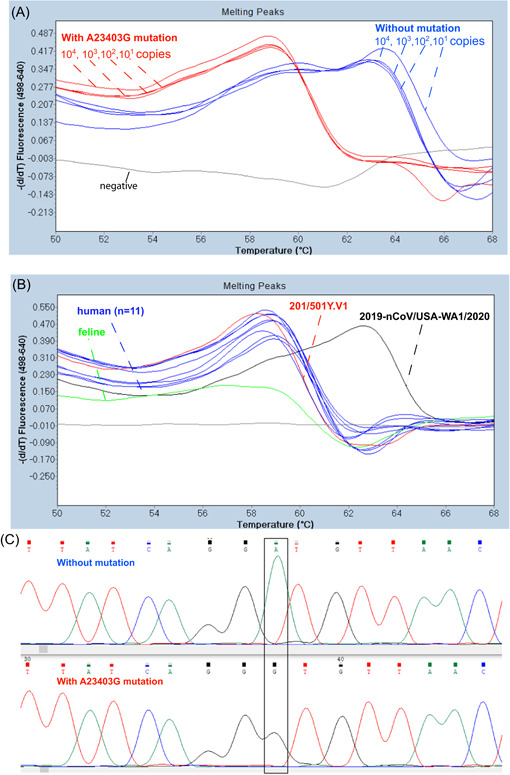
Melting temperature (T m) analysis of SARS‐CoV‐2 controls and feline and human isolates with an RT‐FRET‐PCR for the A23403G mutation. The 6‐FAM probe designed to match exactly with the SARS‐CoV‐2 control without the mutation (2019‐nCOV/USA‐WA1/2020) had a T m of 63.1°C. This was irrespective of copy number. With the SARS‐CoV‐2 control that had the mutation (201/501Y.V1) there was an A to G mismatch with the probe (chromas graph C) that resulted in a lower T m of 58.2°C. (B) RT FRET‐PCRs of the clinical samples from a cat and people all had a T m of around 58.2°C indicating the presence of the A23403G mutation. 6‐FAM, 6‐carboxyfluorescein; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
Figure 2.
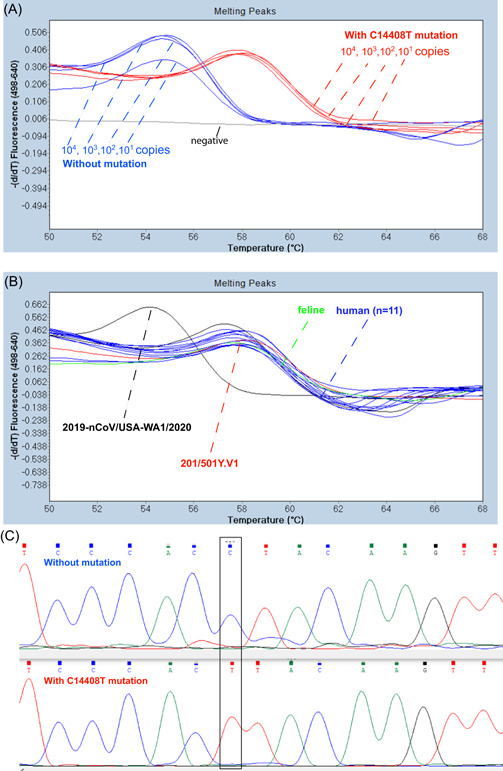
Melting temperature (T m) analysis of SARS‐CoV‐2 controls and feline and human isolates with an RT‐FRET‐PCR for the C14408T mutation. The 6‐FAM probe designed to match exactly with the SARS‐CoV‐2 control without the mutation (201/501Y.V1) had a T m of 57.7°C. This was irrespective of copy number. With the SARS‐CoV‐2 control that had the mutation (201/501Y.V1) there was an A to C mismatch with the probe (chromas graph C) that resulted in a lower T m of 54.3°C. (B) RT FRET‐PCRs of the clinical samples from a cat and people all had a T m of around 54.3°C with this RT FRET‐PCR indicating the presence of the C14408T mutation. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
2.3. Test samples
RT FRET‐PCRs were performed on ATCC controls without (2019‐nCOV/USA‐WA1/2020) and with the mutations (201/501Y.V1) and convenience samples of genomic RNA from the trachea of a SARS‐CoV‐2 positive cat (provided by Alabama Thompson Bishop Sparks State Diagnostic Laboratory), and 11 SARS‐CoV‐2‐positive samples from human nasal swabs (provided by Kansas State Veterinary Diagnostic Laboratory, College of Veterinary Medicine, Kansas State University, USA). All positive samples had C t values between 15 and 18. The Kansas lab also provided RNA from human nasal swabs found negative for SARS‐CoV‐2 which acted as negative controls. The PCR products of all tested samples and controls were sent to Elim Biopharmaceuticals for DNA sequencing.
3. RESULTS
The RT FRET‐PCRs we developed were very sensitive, all detecting as few as 10 copies of the gene target in a reaction (Figures S1 and S2).
The control 2019‐nCOV/USA‐WA1/2020 without the A23403G mutation had a T m of 63.1°C in the RT FRET‐PCRs for the A23403G mutation (Figure 1). This was irrespective of copy number. This T m of 63.1°C was clearly distinguished from the T m of 58.2°C obtained with the control 201/501Y.V1 that had the A23403G mutation.
Similarly, there was a marked difference in the T m of the control 2019‐nCOV/USA‐WA1/2020 with no C14408T mutation (54.3°C) and that of the T m obtained with the control 201/501Y.V1 that had the C14408T mutation (57.7°C).
The feline and human samples all had very similar T m (around 58°C) in both RT FRET‐PCRs indicating all samples carried both mutations.
Sequencing of the DNA of the PCR products further confirmed the presence of the mutations in the control sample and that the feline and human samples were variants containing both mutations.
4. DISCUSSION
The RT FRET‐PCRs we designed to establish if high‐resolution melting curve analysis could detect mutations in the SARS‐CoV‐2 virus showed that the technique can rapidly and conveniently detect mutations in both control and clinical samples. RT FRET‐PCRs can be performed in under 2 h and the examples we developed are able to not only demonstrate if a sample is positive for SARS‐CoV‐2 but also if the mutations we targeted were present. Although we used a Roche 480 II platform, all major brands of PCR machines can perform melting curve analysis with dual‐labeled probes, and thus RT FRET‐PCRs can be readily used for active surveillance and screening for mutations and variants, thereby reducing requirements for sequencing. It can be also used for large‐scale retrospective molecular epidemiology studies of SARS‐CoV‐2 and its variants worldwide.
In conclusion, we have shown highly sensitive RT FRET‐PCRs can be developed to detect SARS‐CoV‐2 infections and to determine whether specific mutations are present. This highly specific and readily available platform should be able to be readily and rapidly adapted to monitor the presence of other mutations and associated variants that are of concern in countries around the world. This technique will greatly facilitate the monitoring of the origins and spread of mutations in variants in the COVID‐19 pandemic and more readily provide data that can be used for public health intervention programs.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Chengming Wang, Subarna Barua, Monirul Hoque, and Patrick J. Kelly designed the experiment. Subarna Barua, Monirul Hoque, Patrick J. Kelly, Jianfa Bai, Gregg Hanzlicek, Lance Noll, Heather Walz, Calvin Johnson, and Constantinos Kyriakis collected the samples and performed the experiment. Chengming Wang, Subarna Barua, Monirul Hoque, and Patrick J. Kelly analyzed the data and wrote the manuscript. All authors have read and approved the manuscript.
Supporting information
Supporting information.
Supporting information.
Barua S, Hoque M, Kelly PJ, et al. High‐resolution melting curve FRET‐PCR rapidly identifies SARS‐CoV‐2 mutations. J Med Virol. 2021;93:5588‐5593. 10.1002/jmv.27139
Subarna Barua and Monirul Hoque contributed equally to this study.
REFERENCES
- 1.GISAID , Global phylogeny, updated by Nextstrain. 2021.
- 2.Pango team. Lineage descriptions; 2021. cov-lineages.org.
- 3.Tang JW, Tambyah PA, Hui DS. Emergence of a new SARS‐CoV‐2 variant in the UK. J Infect. 2021;82(4):e27‐e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) lineage with multiple spike mutations in South Africa; 2020 (www.medrxiv.org/content/10.1101/2020.12.21.20248640v1). Accessed December 22, 2020.
- 5.Cele S, Gazy I, Jackson L, et al. A. Escape of SARS‐CoV‐2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142‐146. 10.1038/s41586-021-03471-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faria NR, et al. Genomic characterisation of an emergent SARS‐CoV‐2 lineage in Manaus: preliminary findings. https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586
- 7.Larsen HD, Fonager J, Lomholt FK, et al. Preliminary report of an outbreak of SARS‐CoV‐2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Euro Surveill. 2021;26(5):2100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Davis BD, Chen SS, Sincuir Martinez JM, Plummer JT, Vail E. Emergence of a novel SARS‐CoV‐2 variant in Southern California. JAMA. 2021;325(13):1324‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyranoski D. Alarming COVID variants show vital role of genomic surveillance. Nature. 2021;589(7842):337‐338. [DOI] [PubMed] [Google Scholar]
- 10.CDC . SARS‐CoV‐2 variant classifications and definitions. www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html
- 11.Wang R, Chen J, Gao K, Hozumi Y, Yin C, Wei GW. Analysis of SARS‐CoV‐2 mutations in the United States suggests presence of four substrains and novel variants. Commun Biol. 2021;4(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


