Abstract
As the coronavirus disease 2019 (COVID‐19) pandemic continues to evolve, differences in epidemiological and clinical features among pediatrics have been noticed across different countries. We describe the spectrum of COVID‐19 in pediatric patients treated in tertiary health care. We conducted a retrospective chart review of pediatric patients admitted to Indus Hospital & Health care network, Korangi campus, Karachi; from April 1st, 2020 to July 31st, 2020. A total of 141 COVID‐19 cases were reported, males were 81 (57%) and the median age was 8 (0.3–17) years. Moderate and severe infections were noted in 36(26%), and 17(12%) children respectively. Fever (50%) was the most common clinical feature. The SF ratio less than 264 was significantly associated with severe disease (p < .05). Lab investigations that differed significantly across disease severity groups included IL‐6 levels (p < .01) and Prothrombin time (p < .05). Majority of children were advised home isolation 89 (63%), 29 (20.5%) were admitted while mortality was observed in 10 (7%) children. No significant difference was observed between children with and without malignancy. Pre‐existing comorbidities are significantly associated with COVID‐19 infections among children. Reduced SF ratio, elevated Prothrombin time, and interleukin‐6 levels are associated with greater disease severity.
Keywords: COVID‐19, infections, interleukin‐6, multisystem inflammatory syndrome (MIS‐C), pandemics, pediatrics, tertiary healthcare
1. INTRODUCTION
The rapid spread of coronavirus disease 2019 (COVID‐19) infection worldwide has resulted in a global pandemic and the World Health Organization has officially declared COVID‐19 a public health emergency of international concern.1 In Pakistan, the first case of COVID‐19 was reported from Karachi in February 2020.2 Recent figures show that children and adolescents younger than 20 years of age constitute 10.6% (24 625 of 231 818) of the total reported confirmed cases of COVID‐19 in Pakistan as of July 8, 2020, with a mortality rate of 0.3% for those aged 10 years or younger and 0.5% for those aged 11–20 years.3 Government of Pakistan established COVID‐19‐designated hospitals in March 2020.2 Indus Hospital and Health Care network, a tertiary care Centre situated in Korangi, Karachi was chosen as COVID‐19 treatment facility and has been actively involved in managing COVID‐19 cases.
Abundant literature is available on epidemiological and clinical features of COVID‐19, and it shows that prevalence and severity are more in adults than children. Two cohort studies of 44 672 confirmed cases in China4 and 149,082 cases in the United State5 reported COVID‐19 infection in 2% Chinese and 1.7% American children. However, there are subpopulations of children who have the chance of developing severe COVID‐19 infections. They include infants,6 children having an underlying pulmonary disease6, and immunocompromised medical conditions.7 Children may also play a significant role in community transmission. Available data suggest that children may have more nasopharyngeal carriage of the virus in the upper respiratory tract8 and prolonged fecal shedding in the stool after initial infection.9
Although most COVID‐19 infections in children are mild recently, there have been reports of severe COVID‐19 infections in children known as Multisystem inflammatory syndrome (MIS‐C). This syndrome shares common features with other pediatric inflammatory conditions, including Kawasaki Disease, and staphylococcal and streptococcal toxic shock syndromes.10 MIS‐C cases have been reported from Europe,10 Italy,11 and recently from Pakistan.3
Most of the local literature on COVID‐19 is based on the adult population, and gaps exist regarding clinical manifestations, lab investigations, and MIS‐C management in children in Pakistan. It may not be prudent to follow adult guidelines for the management of COVID‐19, as many infectious diseases affect children differently from adults. The current study is done to identify gaps in knowledge for pediatric covid infection in our population.
Therefore, the current study is undertaken to describe epidemiological and clinical features, imaging data, laboratory findings, response to treatments, and outcomes of pediatric patients with COVID‐19 in Karachi and compare the severity of illness with laboratory investigation through a retrospective chart review.
2. MATERIALS AND METHODS
2.1. Study setting and design
A retrospective study of pediatric patients admitted to Indus Hospital and Health care network, Korangi campus, Karachi from April 2020 to July 2020 with laboratory‐confirmed COVID‐19 infection.
2.2. Participants
All children between the ages of one month and 18 years, with evidence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection by a polymerase chain reaction in nasal swabs, were included. We also included patients admitted for other reasons but tested positive for COVID‐19 infection during screening. Children from the Pediatric ward, intensive care, day care, and oncology unit were included. As per institutional policy tele‐clinics were conducted for children who were advised home isolation. The outcomes noted in tele‐clinic were recorded in computerized hospital records.
2.3. Study variables
A predesigned questionnaire was used to review data obtained from computerized health records of the hospital. The questionnaire was pilot tested for accuracy before the collection of data. Information was obtained regarding age, gender, pre‐existing comorbidities, like heart disease, diabetes, immune compromise, rheumatologic disorders, and malignancy. The mode of the presentation was noted, which included contact tracing or screening for admission or day care management. Clinical features were noted along with the clinical course in terms of the presence and nature of organ failure, respiratory support (like oxygen therapy, noninvasive ventilation, high‐flow nasal cannula, or invasive ventilation) and additional organ support, like vasoactive medications and renal replacement therapy. Information was collected for lab investigations and imaging studies. Laboratory investigations included in the study consisted of complete blood count (absolute leukocyte count, absolute neutrophil count, neutrophil‐lymphocyte ratio, and hemoglobin), serum biochemistry (C‐reactive protein, Procalcitonin, lactate dehydrogenase, alanine aminotransferase, total bilirubin, creatinine, prothrombin time, partial thromboplastin time and d‐dimer), interleukin‐6 levels (IL‐6), Troponin‐I, B‐type natriuretic peptide (Pro‐BNP) levels, imaging studies (chest X‐ray and computed tomography [CT] chest, CT brain), and echocardiography.
Chest imaging (chest X‐ray and CT scan chest) was categorized in typical, indeterminate, and atypical findings based on the expert consensus statement.12 Pharmacotherapy details were noted, which included antibiotics, Steroids, Remdesivir, Tocilizumab, intravenous Immunoglobulin, and Aspirin. Absolute leukocyte count less than 1.5 × 109/L and less than 1.0 × 109/L were considered low for children less rhgan 6 years and between 6 and 18 years of age, respectively.
ANC of less than 1.5 × 109/L was considered low, and neutrophil to lymphocyte ratio greater than 3.1 was considered significant.
COVID‐19 infection was classified into mild, moderate, and severe categories based on institutional and WHO guidelines.13 Clinical features, lab investigations, and chest imaging findings were used to categorize illness. The same classification was used to categorize disease severity in children with and without malignancy. The mild/asymptomatic category included children who had mild symptoms or were asymptomatic and identified during screening or contact tracing. Moderate category consisted of at least two‐organ involvement, which included severe diarrhea, dehydration, pneumonia, motor weakness with or without loss of consciousness, hepatitis, presence of lab derangements or SpO2 > 90%. Severe disease included multiorgan dysfunction, Spo2 < 90% on five liters of oxygen pediatric acute respiratory distress syndrome (PF ratio <300 or SF ratio <264), respiratory failure, shock, central nervous system involvement, cardiac manifestations, or child requiring assisted ventilation. MIS‐C category with features of either Kawasaki or toxic shock syndrome was included in severe disease.
2.4. IRB consideration
Approval from the ethical review board of the hospital was taken before starting the study.
2.5. Outcomes
The admitted patients' clinical outcomes were noted at discharge or death while for the nonadmitted patients, the outcome was noted from tele‐clinic records. The study's main outcomes were clinical characteristics and trajectory of the disease and outcomes of children with confirmed COVID‐19 infection. Secondary outcomes included the association of clinical severity with laboratory investigations.
2.6. Statistical analysis
SPSS version 20 was used to calculate the frequency of qualitative variables, that is, gender, mode of presentation, symptoms, medication history, chest imaging, echocardiographic features, treatment received, and outcome. The mean, median, and standard deviation, interquartile range, and confidence interval were calculated for quantitative variables, such as age, duration of hospital stay, SF ratio, hematological markers, biochemistry markers, inflammatory markers, and myocardial enzymes. The χ 2 test was applied to compare differences between categorical variables. An independent sample t test and one‐way analysis of variance were calculated to find the mean differences. p < .05 was considered as significant.
3. RESULTS
A total of 165 cases were included, out of these 24 children had unknown outcomes and were excluded. The remaining 141 children were included in the final analysis, out of which 80 (57%) had malignancies while 61 (43%) children had nonmalignant conditions (Figure 1 and Table 1).
Figure 1.
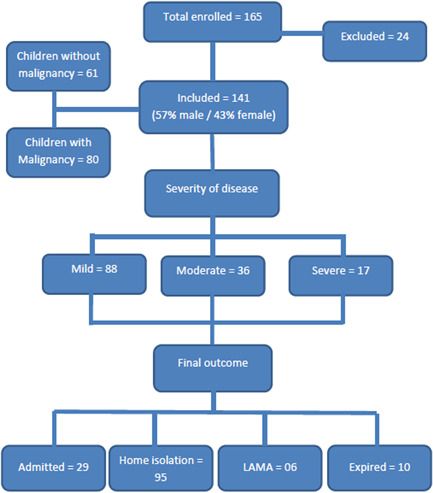
Flowchart of patient characteristics, disease severity, and final outcomes
Table 1.
Difference of demographic and clinical features in mild, moderate, and severe infection groups
| Characteristics | Mild (88) | Moderate (36) | Severe (17) | p value |
|---|---|---|---|---|
| Age (year) | ||||
| Mean ± SD | 7.9±4.7 | 7.2 ± 5.2 | 7.4 ± 4.7 | 0.705a |
| Median (IQR) | 9 (3‐12) | 7.5 (2–11.7) | 7(3.5‐11.5) | |
| 95% CI (LB‐UB) | 6.9–8.9 | 5.3–9.0 | 4.9–9.8 | |
| Gender | ||||
| Male | 53 (60%) | 19 (53%) | 9 (52%) | 0.691b |
| Female | 35 (40%) | 17 (47%) | 8 (48%) | |
| Mode of presentation | ||||
| Contact tracing | 41 (46%) | 0 | 1 (6%) | 0.000b, d |
| Admission screening | 46 (52%) | 35 (97%) | 16 (94%) | |
| Came positive during hospital stay | 1 (1%) | 1 (3%) | 0 | |
| Fever | 30 (34%) | 27 (75%) | 14 (82%) | 0.000b, d |
| Gastrointestinal symptoms | 16 (18%) | 11 (30%) | 9 (53%) | 0.008b, d |
| Neurological symptoms | 2 (2%) | 1 (3%) | 4 (23%) | 0.001b, d |
| Respiratory symptoms | 13 (15%) | 12 (33%) | 7 (41%) | 0.012b, d |
| PARDS (SF ratio <264) | 0 | 4 (11%) | 5 (29%) | 0.015b, d |
| Bleeding | 3 (4%) | 5 (14%) | 4 (23%) | 0.015b, d |
| Dehydration | 6 (7%) | 7 (19%) | 7 (41%) | 0.001b, d |
| MISC‐related clinical features | 0 | 0 | 7 (41%) | 0.000b, d |
| Comorbidities | 0 | 5 (14%) | 4 (23%) | 0.000b, d |
| Medication history | ||||
| Steroids (within last 14 days) | 5 (6%) | 5 (14%) | 1 (6%) | 0.450b |
| Chemotherapy (within last 14 days) | 15 (17%) | 12 (33%) | 3 (18%) | 0.331b |
| DMARDS | 2 (2%) | 2 (5%) | 0 | 0.698b |
| Duration of hospital stay, days | ||||
| Mean ± SD | 0 | 7.5 ± 6.7 | 10.6 ± 9.2 | 0.392c |
| Median (IQR) | 3 (1–8) | 5 (3–18) | ||
| 95% CI (LB‐UB) | 3.2–11.7 | 2.9–18.3 | ||
| Final outcome | ||||
| Recovered | 80 (91%) | 33 (91%) | 4 (23%) | 0.000b, d |
| Referred out | 4 (4.5%) | 1 (3%) | 3 (17%) | |
| LAMA | 4 (4.5%) | 1 (3%) | 1 (6%) | |
| Died | 0 | 1 (3%) | 9 (54%) |
Abbreviations: ANOVA, analysis of variance; CI, confidence interval; DMARDS, methotrexate, hydroxychloroquin, actimera; IQR, interquartile range; LB, Lower bound; MISC, multisystem inflammatory syndrome in children; PARDS, pediatric acute respiratory distress syndrome; UB, upper bound.
One‐way ANOVA.
χ2 test.
Independent t test.
Significant value, MISC‐related clinical features (oral, peripheral conjunctivitis, rash).
3.1. Demographic variables
The median age was 8 years,3, 4, 5, 6, 7, 8, 9, 10, 11, 12 males were 81 (57%) while females were 60 (43%). Contact tracing identified 42 (30%) children, 97 (69%) children tested positive on admission screening and 2 (1%) came positive during hospital stay (Table 1). The maximum number of cases were seen in June (55% 78/141) followed by May (28%; 40/141).
3.2. Clinical features
Asymptomatic children were 88 (62%), while moderate and severe disease were seen in 36 (26%) and 17(12%) children, respectively. Overall the most common presenting symptoms were fever 71/141 (50%), gastrointestinal complaints 36/141 (25%), and respiratory tract symptoms 32/141 (22%) (Table 1). Neurological complications were less commonly seen (7/141; 5%). Among seven children with neurological complications, seizures and neuromuscular weakness were the common symptoms observed. One child developed vision loss due to the development of posterior reversible encephalopathy syndrome (PRES).
Severe disease was seen in 17/141 (12%) children, out of these, 7 children had features of MISC while 10 children had other severe complications of COVID‐19. Among children who had MIS‐C, 5 children had multiorgan dysfunction while 2 had myocarditis. Mortality was observed in 10/141 (7%) children, out of which 4 children had MIS‐C (Table 1).
3.3. Disease management
Oxygen therapy was given to 16 (11%) children while 6 (4%) required invasive ventilation. The mean duration of oxygen therapy in the severe group was 6.3 ± 6.6 days. Inotropic support was given to 6/16 (37%) children in the severe group (Table 2).
Table 2.
Difference of laboratory investigations and final outcome in mild, moderate, and severe infection groups
| Mild (88) | Moderate (36) | Severe (17) | p value | |
|---|---|---|---|---|
| Clinical parameters (mean ± SD) | ||||
| SF ratio <264 | 475 ± 61.0 | 461.2 ± 75.9 | 362.7 ± 149.2 | 0.000a, c |
| 95% CI (LB‐UB) | 457.15–494.71 | 432.31–490.11 | 283.23–442.27 | |
| Hematological markers (mean ± SD) | ||||
| Hemoglobin | 9.78 ± 2.26 | 8.50 ± 2.54 | 9.36 ± 2.56 | 0.296a |
| 95% CI (LB‐UB) | 9.09–10.48 | 7.54–9.47 | 8.0–10.73 | |
| Total lymphocyte count | 15.08 ± 21.6 | 38.19 ± 95.3 | 23.9 ± 50.0 | 0.239a |
| 95% CI (LB‐UB) | 8.93–21.24 | 4.38–72.0 | −1.82–49.68 | |
| Absolute neutrophil count | 6.56 ± 10.1 | 5.29 ± 7.92 | 6.2 ± 6.7 | 0.853a |
| 95% CI (LB‐UB) | 3.458–9.675 | 2.27–8.30 | 2.58–9.82 | |
| Absolute leukocyte count | 3.49 ± 4.62 | 5.69 ± 8.56 | 5.5 ± 7.8 | 0.350a |
| 95% CI (LB‐UB) | 2.07–4.91 | 2.43–8.95 | 1.34–9.68 | |
| Neutrophil–lymphocyte ratio | 1.75 ± 1.88 | 1.5 ± 1.6 | 1.1 ± 1.4 | 0.429a |
| 95% CI (LB‐UB) | 1.17–2.33 | 0.92–2.19 | 0.38–1.89 | |
| Platelet count | 252.8 ± 230.8 | 206.3 ± 218.2 | 218.2 ± 227.7 | 0.247a |
| 95% CI (LB‐UB) | 181.7–323.8 | 123.30–289.32 | 96.8–339.63 | |
| Biochemistry markers (mean ± SD) | ||||
| Alanine aminotransferase | 43.2 ± 44.8 | 29.8 ± 20.8 | 45.7 ± 52.8 | 0.517a |
| 95% CI (LB‐UB) | 24.77–61.7 | 18.75‐40.96 | 5.13–86.42 | |
| Sodium | 135.6 ± 3.80 | 175.4 ± 197.6 | 134.3 ± 4.0 | 0.353a |
| 95% CI (LB‐UB) | 134.3–136.9 | 93.8–257.0 | 132.1–136.4 | |
| Potassium | 4.6 ± 4.6 | 3.5 ± 0.64 | 3.7 ± 0.6 | 0.428a |
| 95% CI (LB‐UB) | 3.00–6.19 | 3.29–3.82 | 3.46–4.12 | |
| Creatinine | 0.49 ± 0.19 | 0.45 ± 0.136 | 0.52 ± 0.18 | 0.481a |
| 95% CI (LB‐UB) | 0.42–0.56 | 0.39‐‐0.51 | 0.420–‐0.62 | |
| Lactate dehydrogenase | 380.6 ± 269.9 | 411.2 ± 294.5 | 551.6 ± 595.9 | 0.734a |
| 95% CI (LB‐UB) | 97.3–663.9 | 138.9‐683.6 | 0.536–1102.8 | |
| C‐reactive protein | 25.2.1 ± 19.0 | 39.8 ± 50.8 | 74.0 ± 80.5 | 0.322a |
| 95% CI (LB‐UB) | 1.6–48.9 | 6.5‐86.1 | 16.4–131.6 | |
| Albumin | 2.51 ± 1.02 | 3.38 ± 0.60 | 2.8 ± 0.87 | 0.262a |
| 95% CI (LB‐UB) | 1.23–3.78 | 2.68–4.17 | 1.93–3.77 | |
| Inflammatory markers (mean ± SD) | ||||
| Ferritin | 365.6 ± 363.6 | 587.6 ± 732.5 | 990.6 ± 1688.0 | 0.662a |
| 95% CI (LB‐UB) | −213.1–944.3 | −24.8–1200 | −781.19–2762.5 | |
| Interleukin‐6 | 8.60 ± 3.7 | 15.6 ± 2.59 | 26.1 ± 3.2 | 0.000a, c |
| 95% CI (LB‐UB) | 4.74–12.46 | 11.56–19.81 | 20.99–31.21 | |
| Prothrombin time | 11.5 ± 1.15 | 12.2 ± 0.78 | 22.30 ± 22.0 | 0.034a, c |
| 95% CI (LB‐UB) | 11.03–12.08 | 11.7–12.8 | 3.88–40.71 | |
| d‐dimer | 0.52 ± 0.43 | 2.2 ± 1.3 | 1.39 ± 0.86 | 0.071a |
| 95% CI (LB‐UB) | −0.16–1.21 | 0.59–3.87 | 0.31–2.47 | |
| Myocardial enzymes (mean ± SD) | ||||
| Troponin‐ I | 19.6 ± 6.9 | 143.5 ± 201.5 | 376.7 ± 553.9 | 0.360a |
| 95% CI (LB‐UB) | 10.9–28.2 | 102.1–299.1 | 135.64‐889.0 | |
| B‐type natriuretic peptide | 11.0 ± 5.83 | 3852.5 ± 5418.5 | 4463.1 ± 3000.7 | 0.057a |
| 95% CI (LB‐UB) | 3.79–18.2 | 249.3–574.3 | 1314.1–7612.2 | |
| Chest Imaging findings X‐ray/CT chest) | 0.069b | |||
| Normal | 21 (23%) | 13 (36%) | 6 (35%) | |
| Atypical | 7 (8%) | 7 (19%) | 3 (17%) | |
| Typical | 2 (2%) | 0 | 3 (17%) | |
| Indeterminate | 1(1%) | 0 | 0 | |
| Echocardiographic features | 0.199b | |||
| Normal | 2 (2%) | 0 | 1 (6%) | |
| LV dysfunction | 0 | 0 | 1 (16%) | |
| Ejection fraction <65 | 0 | 2 (5%) | 2 (11%) | |
Abbreviations: ANOVA, analysis for variance; CI, confidence interval; CT, computed tomography; LB, lower bound; LV, left ventricle; UB, upper bound.
One‐way ANOVA.
χ2 test.
Significant value.
3.4. Difference of clinical features, lab investigations, and treatment among disease severity groups
The SpO2/FiO2 (SF) ratio was the only clinical feature that differentiated between mild and severe cases, (median [IQR], 490 [457‐494] vs. 461 [283‐442]), respectively, with the significant p < 0.005 (Table 2).
Among the laboratory parameters, a significant difference was observed in IL‐6 among mild, moderate, and severe groups (median [IQR], 10.3 [4.7–12.4], 15.5 (11.5–19.8), and 26 [20.9–31.2]). Prothrombin time also showed significant difference among the three groups (median [IQR], 11.5 [11–12], 12.2 [11.7–12.8], and 14 [3.8–40.7]) with p < 0.005 (Table 2).
Thirteen (8%) and 10 (59%) children received injectable steroids while Remdesivir and IVIG were given to three children (Table 3).
Table 3.
Details of treatment and association with the disease severity
| Treatment received | Mild (88) | Moderate (36) | Severe (17) | p value |
|---|---|---|---|---|
| Intravenous fluid | 8 (9%) | 20 (55%) | 16 (94%) | 0.000b, c |
| Remdesivir | 0 | 1 (3%) | 2 (12%) | 0.003b, c |
| Steroids | 6 (7%) | 13 (8%) | 10 (59%) | 0.000b, c |
| IVIG | 0 | 1 (3%) | 2 (12%) | 0.000b, c |
| Antibiotics | 14 (15%) | 23 (63%) | 13 (76%) | 0.000b, c |
| Tocilizumab | 0 | 1 (3%) | 1 (6%) | 0.003b, c |
| Oxygen therapy | 0 | 2 (5%) | 14 (82%) | 0.000b, c |
| Duration of oxygen support, days | ||||
| Mean ± SD | 0 | 6.50 ± 7.7 | 6.3 ± 6.6 | 0.392a |
| 95% CI (LB‐UB) | 3.20–11.7 | 2.92–18.3 | ||
| Invasive ventilation | 0 | 0 | 6 (35%) | 0.000b, c |
| Inotropes | 0 | 2 (5%) | 6 (35%) | 0.000b, c |
| Transfusions | 6 (7%) | 9 (25%) | 4 (23%) | 0.012b, c |
Abbreviations: ANOVA, analysis of variance; CI, confidence interval; LB, lower bound; LV, left ventricle; UB, upper bound.
One‐way ANOVA.
χ2 test.
Significant value.
3.5. Difference in clinical features and lab investigations between children with and without malignancy
No difference was noted in clinical features and demographic features between the two groups. B‐type natriuretic peptide (p = 0.040) and d‐dimer (p = 0.07) were statistically significant between the two groups (Table 4).
Table 4.
Difference of clinical features and laboratory investigations between children with and without malignancy
| Characteristics | Malignant (80) | Non‐malignant (61) | p value |
|---|---|---|---|
| Age (year) | |||
| Mean ± SD | 7.9 ± 4.9 | 7.7 ± 4.5 | 0.831a |
| Median (IQR) | 8 (3–12) | 8 (3–12) | |
| 95% CI (LB‐UB) | 6.82–9.01 | 6.57–8.92 | |
| Gender | |||
| Male | 46 (57.5%) | 35 (57.4%) | 0.988b |
| Female | 34 (42.5%) | 26 (42.6%) | |
| Symptoms | |||
| Fever | 49 (61.3%) | 22 (36.1%) | 0.003b, c |
| Gastrointestinal symptoms | 21 (26.3%) | 15 (24.6%) | 0.823b |
| Neurological symptoms | 5 (6.3%) | 2 (3.3%) | 0.421b |
| PARDS (SF ratio <264) | 5 (6.7%) | 4 (10.5%) | 0.474b |
| MISC‐ related clinical features | 2 (2.5%) | 5 (8.2%) | 0.123b |
| Investigation | |||
| Bacterial infection | 3 (3.75%) | 2 (3.27%) | 0.222b |
| Chest Imaging findings (X‐ray/CT chest) | |||
| Normal | 33 (44%) | 7 (22.6%) | 0.290b |
| Typical | 3 (4.0%) | 2 (6.5%) | |
| Atypical | 11 (14.7%) | 6 (19.4%) | |
| Indeterminate | 1 (1.3%) | 0 | |
| Duration of hospital stay, days | |||
| Mean ± SD | 5.6 ± 6.5 | 6.8 ± 7.8 | 0.638a |
| Median (IQR) | 4 (3–5) | 4 (1–10) | |
| 95% CI (LB‐UB) | 2.79–8.42 | 1.55–12.08 | |
| Hematological markers (mean ± SD) | |||
| Hemoglobin | 8.85 ± 2.34 | 10.2 ± 2.53 | 0.015a, c |
| 95% CI (LB‐UB) | 8.3–9.51 | 9.1–11.46 | |
| Total leukocyte count | 31.17 ± 72.47 | 11.65 ± 8.44 | 0.175a |
| 95% CI (LB‐UB) | 13.87–49.98 | 7.84–15.45 | |
| Absolute neutrophil count | 5.94 ± 9.2 | 6.0 ± 5.22 | 0.853a |
| 95% CI (LB‐UB) | 4.2–8.9 | 3.6–8.3 | |
| Absolute lymphocyte count | 4.70 ± 7.25 | 3.83 ± 2.87 | 0.975a |
| 95% CI (LB‐UB) | 3.31–7.02 | 2.6–5.28 | |
| Neutrophil‐lymphocyte ratio | 1.67 ± 2.16 | 1.76 ± 1.44 | 0.429a |
| 95% CI (LB‐UB) | 1.22–2.3 | 1.03–2.23 | |
| Myocardial enzymes (mean ± SD) | |||
| Troponin‐ I | 20.7 ± 7.5 | 253.8 ± 441.9 | 0.320a |
| 95% CI (LB‐UB) | 8.81–32.68 | −26.96–534.6 | |
| B‐type natriuretic peptide | 20.36 ± 18.85 | 3040.2 ± 3194.8 | 0.040a, c |
| 95% CI (LB‐UB) | 0.57–40.1 | 584.4–5496.0 | |
| Inflammatory markers (mean ± SD) | |||
| d‐dimer | 0.40 ± 0.43 | 1.73 ± 1.11 | 0.072a |
| 95% CI (LB‐UB) | −0.68–1.48 | 0.98–2.48 | |
| Severity | 06 (7.5%) | 11 (18.0%) | 0.057b |
| Final outcome | 0.655b | ||
| Alive | 75 (93.8%) | 56 (91.8%) | |
| Expired | 5 (6.3%) | 5 (8.2%) | |
Abbreviations: CI, confidence interval; CT, computed tomography; IQR, interquartile range; LB, Lower bound; MISC, multisystem inflammatory syndrome in children; PARDS, pediatric acute respiratory distress syndrome; UB, upper bound.
Independent t test.
χ2 test.
Significant value, MISC‐related clinical features (oral, peripheral conjunctivitis, rash).
4. DISCUSSION
We observed a median age of 8 (0.3–17) years, which is consistent with other studies that report the ages ranging from 6 to 11 years.14 Slight male preponderance was noted in our cohort, 81 (57%). World‐wide data reports the mixed‐gender distribution of COVID‐19,15, 16 which suggests that regional variations of demographic variables may exist among children with COVID‐19.
The clinical course of the disease was mild in most of the children. The majority of the children 88/141 (62%) were asymptomatic or had mild clinical manifestations. Similar findings have been reported in other parts of the world.4, 8, 14 There is evidence that ACE‐2 proteins serve as receptors for both SARS‐CoV and SARS‐CoV‐2. These proteins are immature in children, thereby allowing less virus adherence and milder infection in children.17 Presence of a robust immunity in children also protects them from viral replication18 and decreases the severity of COVID‐19 infection.
In our cohort, comorbidities present in children included diabetic ketoacidosis, congenital heart disease, juvenile rheumatoid arthritis, and tuberculosis. Several studies have also reported an association of chronic illnesses with COVID‐19 infection.5, 19, 20 The association of chronic infection and COVID‐19 is linked to impaired immunity and iatrogenic effects of medicines like corticosteroids.21
Neurological complications were seen in 7 (5%) children. One case of Guillen–Barre syndrome (GBS) and posterior reversible encephalopathy (PRES) was noted. There are few reported cases of GBS associated with COVID‐1922 while PRES has been reported in adults only. Neurological sequelae of COVID‐19 are postulated to be secondary to hypoxia or autoimmune response. However, the direct neural invasion has not been demonstrated as CSF is mostly negative for SARS‐CoV.23
In our cohort, increased levels of d‐dimer, pro‐BNP, IL‐6 levels, and Prothrombin time differentiated mild from severe disease. Elevated Pro‐BNP and IL‐6 point toward impaired cardiac function. Cardiac involvement has also been reported in a cohort of European children.24 Evidence shows that hypoxia and electrolyte imbalance in the acute phase of the disease is linked to cardiac arrhythmias,25 while raised inflammatory markers like IL‐1β, IFN‐γ, and IL‐6 are associated with myocardial injury.24 These factors may have caused impaired cardiac function in our children.
We found a significant association of raised Prothrombin time and d‐dimer with disease severity, which has been observed in other children with severe COVID‐19.26 Both of these lab investigations indicate an increased tendency toward coagulopathy. Therefore, we strongly emphasize the importance of monitoring coagulopathy in children with MIS‐C or severe COVID‐19 infection. The decision to start anticoagulation therapy in such children may avert adverse outcomes.
Comparison among children with and without malignancy showed no significant difference except for low hemoglobin in children with malignancy. This was expected as the majority of children had hematological malignancies. Surprisingly, none of the groups had neutropenia, leucopenia, and raised neutrophil‐lymphocyte ratio, which are considered prognostic markers for severe disease in adults.4 On the contrary, our findings showed lymphocytosis in severe disease. Kainth et al.27 also reported lymphocytosis in a cohort of 65 children in the United State.28 These differences in hematological parameters suggest that it may not be prudent to draw parallels between adults and children in terms of complete blood count. Instead, leukocytosis and lymphocytosis in pediatric COVID‐19 infections should alert physicians for the possibility of severe disease, and may be used as a prognostic marker.
4.1. Limitations
The participants of our study were children admitted to the hospital. Therefore, our results are not representative of children with COVID‐19 in ambulatory settings. The study site was limited to a single tertiary care hospital and the results may not be generalizable to the whole population.
5. CONCLUSION
This study adds valuable information to the body of literature on COVID‐19 infections in children. Males are more prone to higher mortality compared to females. There was no significant difference among children with or without malignancy. Elevated Prothrombin time, d‐dimer, B‐type natriuretic peptide, and IL‐6 levels are shown to be associated with disease severity. They may be studied further for consideration as prognostic markers in children with severe COVID‐19 disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Saba Shahid and Mohammad Raza conceived the idea of the study and participated in study design and write‐up. Sidra Maqsood, Saba Shahid, Mohammad Raza, and Samina Junejo carried out data collection. Saba Shahid and Sidra Maqsood assisted with statistical analysis. All authors were involved in the coordination of the study, drafting the manuscript, and approving the final version.
ACKNOWLEDGMENTS
The authors acknowledge the patients who were enrolled in this report and the healthcare providers who treated them.
Shahid S, Raza M, Junejo S, Maqsood S. Clinical features and outcome of COVID‐19 positive children from a tertiary healthcare Hospital in Karachi. J Med Virol. 2021;93:5988‐5997. 10.1002/jmv.27178
DATA AVAILABILITY STATEMENT
The research data are confidential.
REFERENCES
- 1.Mahase E. China coronavirus: WHO declares international emergency as death toll exceeds 200. BMJ. 2020;368:m408. [DOI] [PubMed] [Google Scholar]
- 2.Waris A, Atta U, Ali M, Asmat A, Baset A. COVID‐19 outbreak: current scenario of Pakistan. New Microbes New Infect. 2020;35:100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadiq M, Aziz O, Kazmi U. Multisystem inflammatory syndrome associated with COVID‐19 in children in Pakistan. Lancet Child Adolesc Health. 2020;4(10):36‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 5.Bialek S, Gierke R, Hughes M, McNamara L, Pilishvili T, Skoff T. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimdal I, Moe N, Krokstad S. Human coronavirus in hospitalized children with respiratory tract infections: a 9‐year population‐based study from Norway. J Infect Dis. 2018;219(8):1198‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Ped Infect Diss Soc. 2019;8(1):21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6). [DOI] [PubMed] [Google Scholar]
- 9.Jiehao C, Jin X, Daojiong L, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71(6):1547‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395(10237):1607‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foust AM, Phillips GS, Chu WC, et al. International expert consensus statement on chest imaging in pediatric COVID‐19 patient management: imaging findings, imaging study reporting, and imaging study recommendations. Radiol Cardiothorac Imaging. 2020;2:e200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization WH. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID‐19: Scientific Brief, 15 May 2020. World Health Organization; 2020. [Google Scholar]
- 14.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145(6):e20200702.32179660 [Google Scholar]
- 15.Bhopal SS, Bhopal R. Sex differential in COVID‐19 mortality varies markedly by age. Lancet. 2020;396:532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehingia N, Raj A. Sex differences in COVID‐19 case fatality: do we know enough? The Lancet. Glob Health. 2020;9(1):14‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cristiani L, Mancino E, Matera L, et al. Will children reveal their secret? The coronavirus dilemma. Eur Respiratory Soc. 2020;55:2000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parisi GF, Indolfi C, Decimo F, Leonardi S, Del Giudice MM. COVID‐19 pneumonia in children: from etiology to management. Front Pediatr. 2020;8:616622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciuca IM. COVID‐19 in children: an ample review. Risk ManagHealthc Policy. 2020;13:661‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID‐19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev. 2020;19(5):102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadria M, Layton AT. Use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers during the COVID‐19 pandemic: a modeling analysis. PLOS Comput Biol. 2020;16(10):e1008235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalifa M, Zakaria F, Ragab Y, et al. Guillain‐Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Ped InfectDisSoc. 2020;9(4):510‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohli U, Meinert E, Chong G, Tesher M, Jani P. Fulminant myocarditis and atrial fibrillation in child with acute COVID‐19. J Electrocardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, Marcialis MA. Children's heart and COVID‐19: up‐to‐date evidence in the form of a systematic review. Europ J Ped. 2020;179:1079‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the COVID‐19 pandemic from the Heart Rhythm Society COVID‐19 Task Force; electrophysiology section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;141(21):e823‐e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China. JAMA Netw Open. 2020;3(6):e2010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kainth MK, Goenka PK, Williamson KA, et al. Early Experience of COVID‐19 in a US Children's Hospital. Pediatrics. 2020;146(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data are confidential.


