Abstract
Cardiovascular (CV) engagement in coronavirus disease 2019 (COVID‐19) is a huge determinant of prognosis during the acute phase of the disease. However, little is known about the potential chronic implications of the late phase of COVID‐19 and about the appropriate approach to these patients. Heart failure, type 1 and type 2 myocardial infarction, arrhythmias, myocarditis, pulmonary fibrosis, and thrombosis have been shown to be related to severe acute respiratory syndrome coronavirus 2 infection, and a ‘long COVID‐19’ illness has been recognized with fatigue, chest pain, and dyspnoea among the most frequent symptoms reported after discharge from hospital. This paper focuses on some open questions that cardiologists are going to face during the next months in a general cardiology outpatient clinic, in particular how to evaluate a ‘post‐COVID’ patient during follow‐up of CV complications of the acute phase and how to manage new CV symptoms that could be the consequence, at least in part, of heart/vessels and/or lung involvement of the previous virus infection. Present symptoms and signs, history of previous CV disease (both preceding COVID‐19 and occurring during viral infection), and specific laboratory and imaging measurements during the acute phase may be of interest in focusing on how to approach the clinical evaluation of a post‐COVID patient and how to integrate in our standard of care the new information on COVID‐19, possibly in a multidisciplinary view. Dealing with the increased COVID‐associated CV risk burden and becoming acquainted with potential new e‐cardiology approaches aimed at integrating the cardiology practice are relevant new challenges brought by severe acute respiratory syndrome coronavirus 2 infection and its sequelae.
Keywords: SARS‐CoV‐2, COVID‐19, Late phase, Post‐COVID, ‘Long COVID‐19’ illness, Pandemic, General cardiology, Pneumonia, Myocardial injury, Digital health, Telemedicine, Wearables
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing coronavirus disease 2019 (COVID‐19) has reached pandemic levels since March 2020. SARS‐CoV‐2 not only causes viral pneumonia but also has major implications on various systems. Different clinical aspects of general cardiology and internal medicine, including lung, kidney, liver, and cerebral complications mimicking a cardiac, vascular, or complex multisystem pathology and unusual presentations, have been shown to be related to SARS‐CoV‐2 infection. 1 , 2 , 3 , 4
Intricate interactions among systems may occur upon virus infection. Immunological disorders and inappropriate immune activation with during systemic inflammatory response may lead to altered peripheral resistance and microvascular dysfunction, endothelial injury, abnormal thrombo‐embolic balance, plaque instability, and myocardial damage. 5 , 6 Acute lung parenchymal injury, lung inflammation, leading to micro‐vessel thrombosis and dysfunction, and hypoxemia 7 , 8 entail an increased cardiac workload. This deleterious impact on cardiac function, especially in patients with pre‐existing clinical or subclinical heart failure (HF), may further impair peripheral tissues and renal perfusion. 7
Myocarditis due to the generalized inflammatory reaction plays an important role in patients with acute HF aside from arterial and venous thrombotic complications presenting as acute coronary syndromes (both myocardial infarction type 1 and type 2), venous thrombo‐embolism (VTE), and pulmonary embolism. 7 , 9 , 10 Other expressions of cardiac injury may be a takotsubo syndrome or a Kawasaki‐like syndrome, whereas a direct virus involvement in cardiomyocyte damage has not yet been reported. 11 , 12 , 13 , 14 A wide range of arrhythmias has been reported to complicate the course of COVID‐19 including potential pro‐arrhythmic effects of medical treatment targeted at COVID‐19 used during the first months of pandemic. 10
Patients with cardiovascular (CV) risk factors including male sex, advanced age, diabetes, hypertension, and obesity and patients with established CV and cerebrovascular disease have been identified as particularly vulnerable populations with increased morbidity and mortality, 7 , 15 mainly in‐hospital mortality.
Although COVID‐19 has a direct involvement with the CV system, an apparent worldwide reduction of acute coronary events during the COVID pandemic has been observed. Various studies have found that the incidence of hospitalization for acute myocardial infarction and admissions for HF have decreased during the pandemic. 16 , 17 , 18 , 19 Possible underestimation of symptoms in community and/or prevailing urgencies associated with overwhelming pandemic in hospital prevent patients from optimal treatment of acute CV disease. Possible explanations for the decreased hospitalization rate include patient fear of being infected if hospitalized (avoidance of medical care) and a redistribution of health care.
Late phase of coronavirus disease 2019: how to approach the clinical evaluation of a post‐coronavirus disease 2019 patient
In the late post‐COVID‐19 phase, cardiologists might be interested in surveillance of previously detected abnormalities (during the acute phase) to follow‐up the potential recovery or progression of disease. Continuous control and surveillance strategies in these patients should be based on the severity of clinical presentation, as well as on completeness of pre‐discharge clinical and imaging workout. Possibly, a follow‐up should be planned before discharge from hospital, focusing on a cardiological follow‐up in the patients with cardiac diseases diagnosed before or during the acute COVID‐related hospital stay. Evidence is growing that many patients have long‐lasting effects of SARS‐CoV‐2 infection and a ‘long COVID‐19’ illness has been recognized, 20 , 21 , 22 , 23 , 24 , 25 and studies involving several countries are ongoing to focus on late COVID sequelae. 26
In patients without CV involvement during the acute phase, cardiologists may be called to assess subacute and chronic clinical pictures of COVID‐19‐related CV disease. These may include more specific, concerning symptoms (i.e. new signs of HF, typical chest pain, and palpitations) or vague, non‐specific complains such as fatigue, weakness, shortness of breath, or cough. Fatigue, chest pain, and dyspnoea together with sleep difficulties and anxiety/depression are among the most reported symptoms weeks after discharge from hospital after an acute phase of COVID‐19. 20 , 21 , 22 , 27 Acute disease severity was the most relevant predictor of symptoms at 6 months after COVID onset, and therefore, patients who are more severely ill during their hospital stay seem to be the main target for a tight follow‐up after discharge. 27
Therefore, post‐COVID‐19 patients will be referred to the general cardiologist office because of (i) follow‐up of CV involvement diagnosed during the acute phase or (ii) CV complains occurring in the late phase or long‐lasting symptoms.
Patients with acute cardiovascular complications during acute coronavirus disease 2019 follow‐up
Patients with acute COVID‐19‐associated CV complications should be followed and checked with a complete clinical evaluation and managed as patients with acute non‐COVID 19‐associated CV events with some specific suggestions listed in Table 1 .
Table 1.
Clinical suggestions for patients who presented acute CV complication during acute COVID‐19
| Cardiovascular complications diagnosed during acute COVID‐19 | Specific suggestions that should integrate a complete clinical evaluation |
|---|---|
| If a clear diagnosis in relation with myocardial injury has been performed in the acute phase | |
| Acute myocardial infarction type 1 |
Coronary angiography if not performed in the acute phase as soon as possible Check for CV risk factors and treat accordingly TTE |
| Acute myocardial infarction type 2 |
CCTA Identify and treat underlying causes Check for CV risk factors and treat accordingly TTE |
| Myocarditis |
TTE at 3 months in clinically stable patients CRM if available at baseline or for differential diagnosis |
| Heart failure |
TTE at 3 months in clinically stable patients Check for CV risk factors and treat accordingly |
| Arrhythmias |
Check for correctable risk factors (e.g. drug‐associated prolonged QTc, such as hydroxychloroquine, and electrolyte disorders) Holter ECG and TTE as soon as possible |
| In persisting unexplained sinus tachycardia | Consider TTE |
| Pulmonary embolism |
Check for temporary and permanent major and minor risk factor Check for thrombophilia in young patients or concomitant unusual site thrombosis TTE: check for pulmonary hypertension at 3 months or prior if symptoms |
CCTA, coronary computed tomography angiography; COVID‐19, coronavirus disease 2019; CMR, cardiovascular magnetic resonance; CV, cardiovascular; ECG, electrocardiogram; TTE, transthoracic echocardiography.
Myocardial injury in acute COVID‐19 may be diagnosed by elevated serum biomarkers, in particular high‐sensitivity cardiac troponin T/I (Tn) concentrations, and cardiac imaging. 7 , 9 , 28 , 29 In early studies of the COVID‐19 pandemic, 20–35% of hospitalized COVID‐19 patients had elevated cardiac biomarker levels (i.e. Tn and natriuretic peptides: B‐type natriuretic peptide and N‐terminal pro‐B‐type natriuretic peptide). These alterations have been associated with increased mortality rate. 9 , 28 , 30 Very high Tn levels seem useful to distinguish specific cardiac disease during acute COVID from myocardial cell involvement associated to viral injury added on top of previous cardiac disease (Tn levels up to three times above the upper limit of the normal value; see figure 11 of the European Society of Cardiology document on acute COVID‐19 referenced as number 7). 7 , 31
Cardiac abnormalities were observed in half of all COVID‐19 patients undergoing echocardiographic studies, which have demonstrated primarily left ventricular (LV) preserved ejection fraction and LV diastolic dysfunction associated with right ventricular (RV) abnormalities as common abnormalities. 28 , 32 , 33 In a prospective international multicentre study enrolling 1216 patients with presumed COVID‐19, 667 patients (55%) had an abnormal echocardiogram. Severe cardiac disease (severe ventricular dysfunction or tamponade) was observed in 182 patients (15%). In 901 patients without pre‐existing cardiac disease, the echocardiogram was abnormal in 46%, and 13% had severe disease. Independent predictors of LV and RV abnormalities were distinct, including elevated natriuretic peptides for the former and severity of COVID‐19 symptoms for the latter. 33 , 34 Abnormal increase in ascending aorta and left atrial enlargement were found to be associated with severe inflammation and cardiac injury. 35
When assessing patients with acute COVID‐19‐associated acute CV complications, it should be kept in mind that there may be long‐term cardiac effects of COVID‐19 infection. 9 , 28 First, it is possible that an acute cardiac event may be the precipitating factor of a pre‐existing, but previously asymptomatic, subclinical HF to a symptomatic HF. Second, it could be theoretically possible that a persistent myocardial damage caused by SARS‐CoV2 infection may increase the future risk of an overt decompensation. Finally, many survivors of severe acute COVID‐19 will be at risk for chronic right HF, pulmonary hypertension, and diastolic dysfunction, secondary to myocardial impairment during the acute phase and as a result of chronic pulmonary disease. 9 , 28 , 36 , 37 , 38 Moreover, considering the high prevalence of pre‐existing hypertension in COVID‐19 patients, it is possible that a proportion of the population exhibited pre‐existing LV hypertrophy associated with diastolic dysfunction. 39 The occurrence of arrhythmias, in particular atrial fibrillation, during the acute phase is a frequent observation. 10 New‐onset atrial fibrillation in sepsis is associated with increased stroke and mortality. 40 It could be advisable to perform a Holter electrocardiogram (ECG) monitoring and transthoracic echocardiography (TTE) as soon as possible in patients with arrhythmias in the acute phase, together with laboratory evaluation of electrolyte and hormonal disorders.
Taking into account safety issues, any imaging strategy should be preceded by a comprehensive clinical evaluation including clinical history of the time of hospitalization and contemporary clinical history, physical examination, 12‐lead ECG, and blood tests possibly including cardiac biomarkers. If diagnostic workout protocols including imaging had been terminated during the in‐hospital phase, patients should be followed as suggested by current clinical guidelines. Otherwise, the same guidelines should be used to complete missing diagnostic steps. For myocarditis, TTE is a first‐line imaging modality, followed by CV magnetic resonance (CMR) when needed and with appropriate protocols, and including more advanced imaging modalities (e.g. positron emission tomography). 41 , 42 Coronary computed tomography angiography may be useful if coronary anatomy has to be assessed in suspected coronary artery disease. 43 If the functional evaluation of coronary stenosis is clinically appropriate, particularly if prevalence of active COVID‐19 in the community is supposed to be moderate to high, pharmacological stress is preferred over exercise, because of safety concerns. Associated imaging modality should be used based on local expertise and also on the individual safety considerations. 36 , 37 , 38
Possible cardiovascular symptoms and signs occurring in the late phase
If new CV symptoms and/or persistence of symptoms after recovery from the acute phase (both resting and upon‐effort dyspnoea, shortness of breath, chest pain, tachycardia, and fatigue) or signs (peripheral oedema) occur weeks/months after acute COVID‐19, the suggestion is to integrate the usual patient workout with the evaluation detailed in Table 2 .
Table 2.
Clinical evaluation of patients with CV symptoms and/or signs in the late phase of COVID‐19
| Patient evaluation | Check for |
|---|---|
| Clinical history |
‐ Severity of acute COVID‐19 and related complications ‐ Tn levels and biomarker levels in the acute phase ‐ Imaging parameters at TTE, LUS, and lung CT scan in the acute phase ‐ Treatment for acute COVID‐19 and related complications, in particular antithrombotic drugs, antiviral agents, hydroxychloroquine, steroid, and other anti‐inflammatory agents |
| Physical examination |
‐ Prevalent symptoms and signs ‐ Cardiac rhythm, respiratory sounds, and peripheral oedema ‐ Oxygen saturation |
| Echocardiography |
Left ventricle ‐ LV dimensions ‐ LV septal thickness (transient septal pseudohypertrophy) ‐ LV ejection fraction ‐ Regional wall motion abnormalities (coronary or non‐coronary pattern) ‐ LV strain if available ‐ LV diastolic function (comprehensive assessment) ‐ Valve morphology and function Right ventricle ‐ RV size (EDD and RV area) ‐ RV geometry (e.g. sphericity index) ‐ RV systolic function global (RV strain if available) and regional (McConnell's sign) ‐ Tricuspid regurgitant pulmonary gradient ‐ Pulmonary flow patterns (AcT and notching) Pericardial evaluation and myopericardial brightness |
| Lung ultrasound |
‐ Interstitial syndrome (BLUE protocol) ‐ Integrative assessment of congestion in heart failure |
| Lung computed tomography |
‐ Lung parenchyma ‐ Patency of pulmonary arteries ‐ Patency of coronary arteries ‐ Myocardial damage |
| Cardiovascular magnetic resonance |
‐ Accurate assessment of chamber size and function ‐ Detection of ischaemia/myocardial infarction ‐ Assessment of myocarditis and stress cardiomyopathy |
COVID‐19, coronavirus disease 2019; CT, computed tomography; CV, cardiovascular; EDD, end‐diastolic diameter; LUS, lung ultrasound; LV, left ventricular; RV, right ventricular; Tn, high‐sensitivity cardiac troponin T/I; TTE, transthoracic echocardiography.
The clinical history of acute COVID‐19 manifestations to be considered in an ambulatory CV setting is focused on the severity of acute COVID‐19 stages, Tn levels, acute phase imaging data, and treatments for acute COVID‐19, in order to have insight on the potential myocardial injury and sequelae, lung involvement, and cytokine/thrombo‐embolic activation. 7
The complete clinical examination is performed as usual, with focus on the prevalent symptoms and on signs such as heart rhythm, murmurs, abnormal breathing crepitus/noises including velcro‐like crackles (associated with interstitial lung disease), peripheral oedema, and peripheral O2 saturation. If available, elevated concentrations of B‐type natriuretic peptide/N‐terminal pro‐B‐type natriuretic peptide in a dyspnoeic post‐COVID‐19 patient might be helpful to assess cardiac dysfunction or clinical HF, together with other clinical signs, and justify the request for TTE as a first‐line imaging modality. However, to date, the utility of serum biomarkers is undefined in the chronic phase. 36 , 37 , 38
A 12‐lead ECG should be always repeated and compared with ECG of the acute phase if available. In an outpatient setting, the echocardiogram has a central value in post‐COVID‐19 cardiac and lung signs/symptoms evaluation, to make differential diagnosis of heart vs. lung involvement or mixed implication (Figure 1 ). Once the prevalent lung involvement in determining symptoms has been evidenced, a referral to a specialist in lung diseases is indicated.
Figure 1.
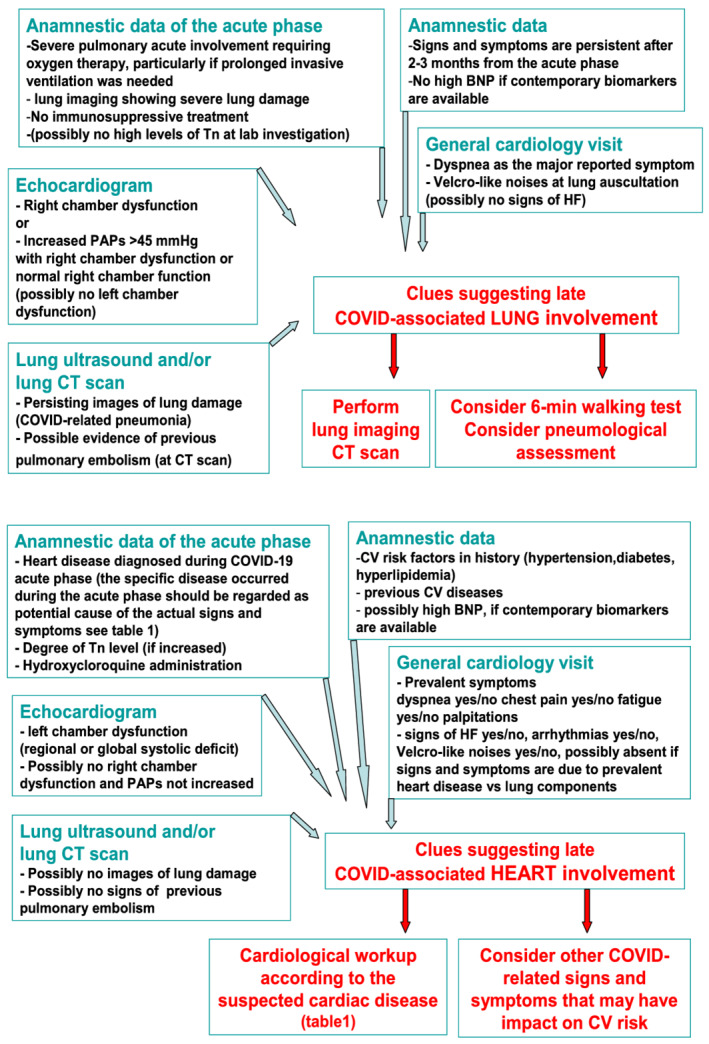
Signs and symptoms associated with chronic lung or heart involvement (prevalent cause or mixed components). COVID‐19, coronavirus disease 2019; CT, computed tomography; CV, cardiovascular; HF, heart failure; PAPs, pulmonary artery pressures; Tn, high‐sensitivity cardiac troponin T/I.
A possible role of exercise testing and cardiopulmonary exercise testing in post‐COVID patients may be considered for the evaluation of maximal exercise capacity and cardiopulmonary involvement 44 , 45 provided the acute myocarditis has been ruled out. Moreover, if a patient is not aware of a previous COVID‐19 despite heart/lung involvement that may suggest a previous SARS‐CoV‐2 infection, a serological evaluation aimed at confirming or excluding a previous infection, together with referral to the general practitioner, may be advised.
Heart and lung imaging
Echocardiogram
Left atrial and LV size and function should be carefully assessed as well as regional wall motion abnormalities. If regional wall motion abnormalities are detected, it should be mentioned in the report whether they are consistent with coronary anatomy or diffuse. 36 , 37 LV diastolic function should be also evaluated along with the valvular apparatus. 46 , 47 RV size and function, including a comprehensive estimate of the probability of pulmonary hypertension, are an integral part of any TTE. 46 , 48 Abnormalities in pulmonary haemodynamic should be viewed and interpreted in the context of LV dysfunction and valvular abnormalities and also in the perspective of lung disease (both parenchymal and vascular). In particular, integration of measurement of LV systolic and diastolic function, of RV size, morphology, and function, is needed to attribute increased pulmonary artery pressure to either parenchymal or vascular lung disease. 49
Lung ultrasound
The COVID‐19 pandemic has created a huge interest in lung ultrasound. Its use is rational during the acute course of the disease provided that a standardized approach is used, such as the BLUE protocol. 50 , 51 The role of lung ultrasound in ruling out chronic lung outcomes of COVID‐19 is currently debatable. 52 Moreover, it is useful when assessing HF, haemodynamic severity, and guide management. 53 , 54 Nevertheless, there are no robust data on its accuracy as a diagnostic tool, its real prognostic relevance, and cut‐off values to be used.
Chest computed tomography scan
Chest computer tomography (CT) may be used as a comprehensive, non‐invasive imaging modality, which allows for the evaluation of lung parenchyma, and pulmonary and coronary arteries both in the acute and subacute setting. 43 Various imaging findings on chest CT have been reported at initial presentation. 55 Moreover, multiple studies categorized COVID‐19 CT findings into several stages based on time since the onset of symptoms. These were classified into four main successive stages: early, intermediate, and late and a fourth restoration stage. 56 , 57 Dual‐energy CT may be useful not only for the evaluation of lung perfusion abnormalities in COVID‐19 patients in the acute setting but also to monitor lung sequelae in follow‐up scans. 58
The role of CT in the assessment of both long‐term parenchymal and vascular pulmonary disease is well consolidated. 59 , 60 , 61 Some studies showed that imaging of myocardial fibrosis by CT is feasible with good agreement with CMR. However, further larger studies are warranted before its implementation in the clinical arena. Coronary computed tomography angiography is used considering its diagnostic accuracy, particularly its excellent negative predictive value. 43
Cardiovascular magnetic resonance
The indications for CMR in the assessment of the late clinical phase of COVID‐19 include accurate assessment of chamber size and function, and detection of ischaemia, myocardial infarction, myocarditis, and stress cardiomyopathy. Referrals for CMR in this setting should be thus guided by its potential impact on patient management. 41 While clinical indications to CMR in the late COVID‐19 phase, to date, are not different from those previously recommended, specific technical and safety suggestions have been proposed as guidance in the acute (as well as in the convalescent phase). 42
Long‐term coronavirus disease 2019‐related increased cardiovascular risk
Besides the direct effects of COVID‐19 on heart, vessels, lung, and other several tissues, the SARS‐CoV‐2 infection and its sequelae may have an impact on other various risk factors leading, in turn, to late symptoms and increased CV risk (Figure 2 ). Prolonged inflammatory state, hypercoagulability, dyslipidaemia induced or worsened by immunosuppressive treatment, depression, and post‐COVID‐19 sarcopenia, which may induce or worsen frailty, are adjunct risk factors for CV in post‐COVID‐19 patients.
Figure 2.
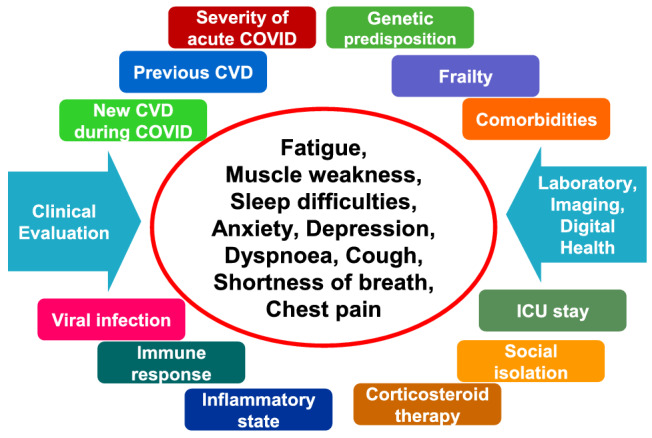
Late covid associated symptoms. CVD, cardiovascular disease; ICU, intensive care unit.
Micro‐inflammatory state and altered immune activation are closely related to atherosclerosis formation and vascular events. 62 Neutrophil‐mediated necroinflammation, endothelial injury, and the shift of intracellular and intra‐tissues pathways towards inflammation‐associated vascular pathology are key step in COVID‐19 tissue damage, and it has been postulated that COVID‐19‐related persistence of symptoms could be in relationship with systemic coagulopathies and vascular wall deterioration, resulting in dysregulated neutrophil extracellular trap biology. 63 , 64 , 65 , 66
Indeed, COVID‐19‐linked multi‐morbidity such as HF, renal failure, diarrhoea, neurological symptoms, and/or sarcopenia due to malnutrition and inactivity during the acute phase, particularly if a hospitalization was required, together with social isolation, loneliness, and depression are frailty‐related conditions. 67 Effectively, depression, anxiety, and sleep disturbances are frequent in COVID‐19 patients. 68 , 69
Frailty confers increased risk for CV disease. 70 The frail status, being a dynamic phenomenon, may become overt or worsen during COVID‐19, and therefore, the post‐COVID‐19 period may expose further these patients to increased CV risk burden.
Currently, there is no evidence available of potential influences of these transient or permanent adjunct risk factors on CV events. However, careful attention should be paid to consider and treat the various faceted daily life changes conveyed by COVID‐19. Moreover, due to the complex clinical characteristics of the disease, the involvement on various organs and systems, and different social implications, a multidisciplinary approach to COVID‐19 sequelae is warranted. A widespread vaccination is warranted to prevent CV events in both the CV diseased population and in healthy subjects.
Pharmacological cardiovascular treatments in the late phase of coronavirus disease 2019
In the late phase of COVID‐19, both CV treatments for heart and vessels diseases diagnosed prior to acute COVID‐19 or occurring during the acute phase should be continued as in usual care. 7 However, some pharmacological treatments have been highlighted as potentially harmful in COVID‐19 patients, whereas the need and timing of anticoagulation treatment is debated.
The first warning was focused on angiotensin‐converting enzyme (ACE) 2 as a potential entry receptor for SARS‐CoV‐2 infection. ACE2 is known to degrade angiotensin (Ang) II and to generate Ang‐(1–7), which antagonizes the effects of Ang II action, which is part of the beneficial effects of these drugs. Because renin–angiotensin–aldosterone system blockade by ACE inhibitors, Ang II type 1 receptor antagonists, and mineralocorticoid antagonists, as well as statins, enhance ACE2, these treatments have been regarded during the first phase of pandemic as potentially able to increase susceptibility to SARS‐CoV‐2 infection and severity of the disease. 71 , 72 A delicate balance between ACE2, metalloproteinase domain 17, and transmembrane protease serine 2 interactions in each specific pathophysiological condition appears as a critical key factor in the interplay with SARS‐CoV‐2. 73 However, experimental data have promptly overcome these alerts, and several scientific societies suggested avoiding drug changes or discontinuation in patients assuming renin–angiotensin–aldosterone system blocking drugs. 74 , 75 , 76 , 77
Life‐threatening arrhythmias occurrence in COVID‐19 may be due to specific drugs and to drug‐to‐drug interactions added on top of myocardial damage and electrolyte/volume imbalance. These stimuli are more relevant in the acute phase of the disease. 78 , 79
According to the last edition of the European Society of Cardiology guidelines for pulmonary embolism, all patients with pulmonary embolism should be treated with anticoagulants for at least 3 months. 80 Beyond this period, the balance between the risk of VTE recurrence and that of bleeding should be carefully assessed. To optimally estimate VTE recurrence risk, each patient should be assessed for the presence of major permanent (e.g. active cancer or antiphospholipid syndrome) or transient major (e.g. recent major surgery) and/minor risk factors, checked for pulmonary hypertension, and stratified according to the severity of acute COVID‐19, in particular if thrombotic VTE occurred associated to an acute hyperinflammatory syndrome (e.g. high levels of neutrophil/lymphocyte ratio, markers of cytokinaemia, such as C‐reactive protein, interleukin‐6, hyperferritinaemia, and signs of hepatic injury). 81 Additionally, young patients or patients with concomitant unusual site thrombosis (such as splanchnic vein thrombosis) should be tested for thrombophilia.
In case of patients with a permanent risk factor, such as active cancer, anticoagulation should be prolonged indefinitely, independently of the severity of the acute COVID‐19. However, patients with severe acute COVID‐19 without concomitant major or minor risk factors and admitted to intensive care unit with an acute hyperinflammatory syndrome may stop anticoagulation after 3–6 months if pulmonary hypertension has been excluded.
Decision to stop or continue indefinitely anticoagulation in other clinical scenario should be carefully balanced individually with the bleeding risk and decision‐making process should be made in individual‐case basis, 82 and prospective randomized trials are still needed to demonstrate potential benefits of thromboprophylaxis intensification in post‐COVID patients.
Role of e‐cardiology in the evaluation and management of chronic coronavirus disease 2019‐linked clinical manifestations
During the current COVID‐19 pandemic, e‐cardiology became extremely relevant in the field of cardiology.
Telemedicine and m‐health were the most relevant e‐health facets, 83 which were quickly adapted by healthcare providers to tackle the challenge to assure healthcare service under pandemic condition, even for COVID‐19‐related chronic manifestations.
The most prominent telemedical approach for general cardiologists was the rapid dissemination of online video consultations in the outpatient setting. During March and April 2020, the number of online video consultations increased by 600% in Germany compared with the same time frame in 2019. 84 The implementation of certificated video conference systems is easy to handle and needs only a short user instruction. The patient needs only two technical requirements: (i) an end device with front camera and (ii) an e‐mail account. The online video consultation represents an ideal tool for the follow‐up of a well‐known patient and should only be used as an ‘add‐on’ to reduce the number of regular on‐site visits, rather than to substitute all on‐site visits, in particular for new patients.
First experiences showed that patient's willingness to participate in online consultation is about 45% with increasing tendency, even in older patients. However, if this trend to online video consultation will be a sustainable development towards digital health is unclear. 85 One‐third of the cardiological outpatients still prefer on‐site visits only, even under pandemic conditions. Moreover, the pandemic has led to a worldwide implementation of remote monitoring of cardiac implantable electronic devices, in order to reduce the in‐office device control and reduce the risk of contagion. 86 , 87
Another e‐cardiology approach represents telemonitoring with a daily transfer of vital parameter. This remote patient management model was established for recently hospitalized high‐risk HF patients and is very beneficial under current pandemic conditions to these patients who are also at high risk for COVID‐19. One example is the TELEMED5000‐COVID‐19 study (German clinical trial register number: DRKS00022244), which is an ongoing observational trial including 100 patients, which survived an inpatient stay of COVID‐19. In addition to the home equipment used in telemedicine for HF patients, all study patients will receive a tablet with a voice‐analysing app. The voice recording will be analysed using methods of artificial intelligence and is assumed to be a new vital parameter to quantify fatigue and/or pulmonary congestion. Another app should support repeated 6 min walking test in the patient's surroundings (Figure 3 ). This vital parameter could be used as a surrogate for changing exercise capacity during the recovery period. The follow‐up period of this trial will be 1 year, and the first results will be available in 2021.
Figure 3.

Example of a telemonitoring home equipment set to follow‐up coronavirus disease 2019 patients: a tablet with a voice‐analysing app using methods of artificial intelligence and an app for recording of a repeated 6 min walk test. Further devices are an electrocardiogram, a blood pressure device, a pulse oximeter, and a body scale. © Charité—Universitätsmedizin Berlin.
Wearables should be envisaged in chronic cardiac patients to continue regular monitoring, to reduce risk of transmission due to their increased risk of infection with worse outcome, and to diagnose COVID‐19 infection promptly. 88 , 89 , 90 , 91 Wearables certified as medical device use allow monitoring of vital signs such as oxygen saturation, respiratory rate, blood pressure, body temperature, ECG (to measure QT intervals also), and also position and movement assessment, pulmonary auscultation, and cough monitoring. 92 The choice to use wearables for remote care should be shared by the patient. In conclusion, e‐health technologies were rapidly adapted during the onset of the pandemic, but the hypothesis of a beneficial effect for management of chronic COVID‐19‐related clinical manifestations needs to be proven (Figure 2 ).
Conflict of interest
None declared.
Author contributions
D.R. and L.G. recognize a similar authorship. D.R., L.G., R.A., and M.F. gave particular contribution in the conception, design, and coordination of the work. F.K., A.S., S.N., R.C., F.D., and G.G. contributed to the conception of the work, drafted the manuscript, and revised critically the manuscript. All authors gave important intellectual contribution to the entire manuscript, gave the final approval, and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Richter, D. , Guasti, L. , Koehler, F. , Squizzato, A. , Nistri, S. , Christodorescu, R. , Dievart, F. , Gaudio, G. , Asteggiano, R. , and Ferrini, M. (2021) Late phase of COVID‐19 pandemic in General Cardiology. A position paper of the ESC Council for Cardiology Practice. ESC Heart Failure, 8: 3483–3494. 10.1002/ehf2.13466.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maresca A, Mongiardi C, Montalbetti L, Dentali F, Ageno W, Mauri M, Baj A, Grandi AM, Grossi P, Dalla Gasperina D, Zerba F, Sessa A, Guasti L. Unusual presentation of COVID‐19: encephalitis and syndrome of inappropriate anti‐diuretic hormone secretion. Int J Clin Med 2020; 11: 559–564. [Google Scholar]
- 4. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID‐19. Nat Med 2020; 26: 1017–1032. [DOI] [PubMed] [Google Scholar]
- 5. Siddiqi HK, Libby P, Ridker PM. COVID‐19—a vascular disease. Trends Cardiovasc Med 2020: S1050–S1738 30128–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dupont T, Caillat‐Zucman S, Fremeaux‐Bacchi V, Morin F, Lengliné E, Darmon M, Peffault de Latour R, Zafrani L, Azoulay E, Dumas G. Identification of distinct immunophenotypes in critically ill coronavirus disease 2019 patients. Chest 2020: S0012–S3692 35351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ESC guidance for the diagnosis and management of CV disease during the COVID‐19 pandemic. https://www.escardio.org/Education/COVID‐19‐and‐Cardiology/ESC‐COVID‐19‐Guidance (Accessed May, 2021).
- 8. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation 2020; 142: 68–78. [DOI] [PubMed] [Google Scholar]
- 9. Bonow RO, O'Gara PT, Yancy CW. Cardiology and COVID‐19. JAMA 2020; 324: 1131–1132. [DOI] [PubMed] [Google Scholar]
- 10. Giustino G, Pinney SP, Lala A, Reddy VY, Johnston‐Cox HA, Mechanick JI, Halperin JL, Fuster V. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC Focus Seminar. J Am Coll Cardiol 2020; 76: 2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh S, Desai R, Gandhi Z, Fong HK, Doreswamy S, Desai V, Chockalingam A, Mehta PK, Sachdeva R, Kumar G. Takotsubo syndrome in patients with COVID‐19: a systematic review of published cases. SN Compr Clin Med 2020; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burgi Vieira C, Ferreira AT, Botelho Cardoso F, Pelicano Paulos J, Germano N. Kawasaki‐like syndrome as an emerging complication of SARS‐CoV‐2 infection in young adults. Eur J Case Rep Intern Med 2020; 7: 001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P, Chalumeau M, Casanova JL, Cohen JF, Allali S. Kawasaki‐like multisystem inflammatory syndrome in children during the covid‐19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369: m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mele D, Flamigni F, Rapezzi C, Ferrari R. Myocarditis in COVID‐19 patients: current problems. Intern Emerg Med 2021; 23: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID‐19: a systematic review and meta‐analysis. PLoS One 2020; 15: e0241265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid‐19 pandemic and the incidence of acute myocardial infarction. N Engl J Med 2020; 383: 691–693. [DOI] [PubMed] [Google Scholar]
- 17. de Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Perrone Filardi P, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R, Sinagra G, Indolfi C, Società Italiana di Cardiologia and the CCU Academy investigators group . Reduction of hospitalizations for myocardial infarction in Italy in the COVID‐19 era. Eur Heart J 2020; 41: 2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Chris Roebuck C, Gale CP, Mamas MA, Deanfield JE, de Belder MA, Luescher TF, Denwood T, Landray MJ, Emberson JR, Collins R, Morris EJA, Casadei B, Baigent C. COVID‐19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020; 396: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Severino P, D'Amato A, Saglietto A, D'Ascenzo F, Marini C, Schiavone M, Ghionzoli N, Pirrotta F, Troiano F, Cannillo M, Mennuni M, Rognoni A, Rametta F, Galluzzo A, Agnes G, Infusino F, Pucci M, Lavalle C, Cacciotti L, Mather PJ, Grosso Marra W, Ugo F, Forleo G, Viecca M, Morici N, Patti G, de Ferrari GM, Palazzuoli A, Mancone M, Fedele F. Reduction in heart failure hospitalization rate during coronavirus disease 19 pandemic outbreak. ESC Heart Fail 2020; 23: 4182–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carfì A, Bernabei R, Landi F, Gemelli Against COVID‐19 Post‐Acute Care Study Group . Persistent symptoms in patients after acute COVID‐19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosales‐Castillo A, García de Los Ríos C, Mediavilla García JD. Persistent symptoms after acute COVID‐19 infection: importance of follow‐up. Med Clin (Barc) 2020: S0025–S7753 30661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahase E. Covid‐19: what do we know about “long covid”? BMJ 2020; 370: m2815. [DOI] [PubMed] [Google Scholar]
- 23. del Rio C, Collins LF, Malani P. Long‐term health consequences of COVID‐19. JAMA 2020; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinney SP, Giustino G, Halperin JL, Mechanick JI, Neibart E, Olin JW, Rosenson RS, Fuster V. Coronavirus historical perspective, disease mechanisms, and clinical outcomes: JACC Focus Seminar. J Am Coll Cardiol 2020; 76: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiong TY, Redwood S, Prendergast B, Mao CM. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J 2020; 41: 1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigfrid L, Cevik M, Jesudason E, Lim WS, Rello J, Amuasi J, Bozza F, Palmieri C, Munblit D, Holter JC, Kildal AB, Reyes LF, Russell CD, Ho A, Turtle L, Drake TM, Beltrame A, Hann K, Bangura IR, Fowler R, Lakoh S, Berry C, Lowe DJ, Mcpeake J, Hashmi M, Dyrhol‐Riise AM, Donohue C, Plotkin D, Hardwick H, Elkheir N, Lone NI, Docherty A, Harrison E, Baille JK, Carson G, Semple MG, Scott JT. What is the recovery rate and risk of long‐term consequences following a diagnosis of COVID‐19? A harmonised, global longitudinal observational study protocol. BMJ Open 2021; 11: e043887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freaney PM, Shah SJ, Khan SS. COVID‐19 and heart failure with preserved ejection fraction. JAMA 2020; 30. [DOI] [PubMed] [Google Scholar]
- 29. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID‐19‐associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020: 3827–3835, ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toraih EA, Elshazli RM, Hussein MH, Elgaml A, Amin M, El‐Mowafy M, El‐Mesery M, Ellythy A, Duchesne J, Killackey MT, Ferdinand KC, Kandil E, Fawzy MS. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID‐19 patients: a meta‐regression and decision tree analysis. J Med Virol 2020: 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, van Vleck T, Vaid A, Chaudhry F, de Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount Sinai COVID Informatics Center . Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol 2020; 76: 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, Arbel Y, Topilsky Y. Spectrum of cardiac manifestations in COVID‐19: a systematic echocardiographic study. Circulation 2020; 142: 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, White A, Salvo GD, Sade LE, Pearce K, Newby DE, Popescu BA, Donal E, Cosyns B, Edvardsen T, Mills NL, Haugaa K. Global evaluation of echocardiography in patients with COVID‐19. Eur Heart J Cardiovasc Imaging 2020; 21: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim J, Volodarskiy A, Sultana R, Pollie MP, Yum B, Nambiar L, Tafreshi R, Mitlak HW, RoyChoudhury A, Horn EM, Hriljac I, Narula N, Kim S, Ndhlovu L, Goyal P, Safford MM, Shaw L, Devereux RB, Weinsaft JW. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID‐19. J Am Coll Cardiol 2020; 76: 1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song L, Zhao S, Wang L, Yang K, Xiao W, Clifford SP, Huang J, Chen X. Cardiovascular changes in patients with COVID‐19 from Wuhan, China. Front Cardiovasc Med 2020; 7: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zoghbi WA, DiCarli MF, Blankstein R, Choi AD, Dilsizian V, Flachskampf FA, Geske JB, Grayburn PA, Jaffer FA, Kwong RY, Leipsic JA, Marwick TH, Nagel E, Nieman K, Raman SV, Salerno M, Sengupta PP, Shaw LJ, Chandrashekhar YS, ACC Imaging Council . Multimodality cardiovascular imaging in the midst of the COVID‐19 pandemic: ramping up safely to a new normal. JACC Cardiovasc Imaging 2020; 13: 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudski L, Januzzi JL, Rigolin VH, Bohula EA, Blankstein R, Patel AR, Bucciarelli‐Ducci C, Vorovich E, Mukherjee M, Rao SV, Beanlands R, Villines TC, Di Carli MF, Expert Panel From the ACC Cardiovascular Imaging Leadership Council . Multimodality imaging in evaluation of cardiovascular complications in patients with COVID‐19: JACC Scientific Expert Panel. J Am Coll Cardiol 2020; 76: 1345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, Almeida AG, Schulz‐Menger J, Dweck MR, Pontone G, Sade LE, Gerber B, Maurovich‐Horvat P, Bharucha T, Cameli M, Magne J, Westwood M, Maurer G, Edvardsen T. COVID‐19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020; 21: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doyen D, Dupland P, Morand L, Fourrier E, Saccheri C, Buscot M, Hyvernat H, Ferrari E, Bernardin G, Cariou A, Mira JP, Jamme M, Dellamonica J, Jozwiak M. Characteristics of cardiac injury in critically ill patients with coronavirus disease 2019. Chest 2020: S0012–S3692 35109–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new‐onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011; 306: 2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han Y, Chen T, Bryant J, Bucciarelli‐Ducci C, Dyke C, Elliott MD, Ferrari VA, Friedrich MG, Lawton C, Manning WJ, Ordovas K, Plein S, Powell AJ, Raman SV, Carr J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID‐19 pandemic. J Cardiovasc Magn Reson 2020; 22: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kelle S, Bucciarelli‐Ducci C, Judd RM, Kwong RY, Simonetti O, Plein S, Raimondi F, Weinsaft JW, Wong TC, Carr J. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID‐19 infection. J Cardiovasc Magn Reson 2020; 22: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pontone G, Scafuri S, Mancini ME, Agalbato C, Guglielmo M, Baggiano A, Muscogiuri G, Fusini L, Andreini D, Mushtaq S, Conte E, Annoni A, Formenti A, Gennari AG, Guaricci AI, Rabbat MR, Pompilio G, Pepi M, Rossi A. Role of computed tomography in COVID‐19. J Cardiovasc Comput Tomogr 2020: S1934–S5925 30436–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brawner CA, Ehrman JK, Bole S, Kerrigan DJ, Parikh SS, Lewis BK, Gindi RM, Keteyian C, Abdul‐Nour K, Keteyian SJ. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc 2021; 96: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao Y, Chen R, Geng Q, Mo X, Zhan C, Jian W, Li S, Zheng J. Cardiopulmonary exercise test might be helpful for insight interpretation of impaired pulmonary function on recovered COVID‐19 patients. Eur Respir J 2020: 2004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cameli M, Pastore MC, Soliman Aboumarie H, Mandoli GE, D'Ascenzi F, Cameli P, Bigio E, Franchi F, Mondillo S, Valente S. Usefulness of echocardiography to detect cardiac involvement in COVID‐19 patients. Echocardiography 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shah BN, Schlosshan D, McConkey HZR, Buch MH, Marshall AJ, Cartwright N, Dobson LE, Allen C, Campbell B, Khan P, Savill PJ, Briffa NP, Chambers JB. Outpatient management of heart valve disease following the COVID‐19 pandemic: implications for present and future care. Heart 2020; 106: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 48. Harjola VP, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo‐Leiro MG, Falk V, Filippatos G, Gibbs S, Leite‐Moreira A, Lassus J, Masip J, Mueller C, Mullens W, Naeije R, Nordegraaf AV, Parissis J, Riley JP, Ristic A, Rosano G, Rudiger A, Ruschitzka F, Seferovic P, Sztrymf B, Vieillard‐Baron A, Yilmaz MB, Konstantinides S. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail 2016; 18: 226–241. [DOI] [PubMed] [Google Scholar]
- 49. D'Alto M, Romeo E, Argiento P, Pavelescu A, Mélot C, D'Andrea A, Correra A, Bossone E, Calabrò R, Russo MG, Naeije R. Echocardiographic prediction of pre‐ versus postcapillary pulmonary hypertension. J Am Soc Echocardiogr 2015; 28: 108–115. [DOI] [PubMed] [Google Scholar]
- 50. Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheff) 2017; 13: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gargani L, Soliman‐Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID‐19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging 2020; 21: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray JJV. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short‐ and long‐term outcomes. JACC Heart Fail. 2019; 7: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, Kenizou D, Maillier B, Nazeyrollas P, Roul G, Fillieux L, Abraham WT, Januzzi J Jr, Sebbag L, Zannad F, Mebazaa A, Rossignol P, INI‐CRCT, Great Network, and the EF‐HF Group . Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail 2018; 6: 273–285. [DOI] [PubMed] [Google Scholar]
- 55. el Homsi M, Chung M, Bernheim A, Jacobi A, King MJ, Lewis S, Taouli B. Review of chest CT manifestations of COVID‐19 infection. Eur J Radiol Open 2020; 7: 100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT findings in coronavirus disease‐19 (COVID‐19): relationship to duration of infection. Radiology 2020; 295: 200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID‐19). Radiology 2020; 295: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Som A, Lang M, Little B. Pulmonary vascular pathology in Covid‐19. N Engl J Med 2020; 383: 887. [DOI] [PubMed] [Google Scholar]
- 59. Bankier AA, Dennie C. Modern diagnosis in the evaluation of pulmonary vascular disease. In Hodler J., Kubik‐Huch R. A., von Schulthess G. K., eds. Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging [Internet]. Cham (CH): Springer; 2019. Feb 20. p 2019 Chapter 17. [Google Scholar]
- 60. Ostridge K, Wilkinson TM. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur Respir J 2016; 48: 216–228. [DOI] [PubMed] [Google Scholar]
- 61. Kiely DG, Levin D, Hassoun P, Ivy DD, Jone PN, Bwika J, Kawut SM, Lordan J, Lungu A, Mazurek J, Moledina S, Olschewski H, Peacock A, Puri GD, Rahaghi F, Schafer M, Schiebler M, Screaton N, Tawhai M, van Beek EJ, Vonk‐Noordegraaf A, Vanderpool RR, Wort J, Zhao L, Wild J, Vogel‐Claussen J, Swift AJ. EXPRESS: statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ 2019; 9: 2045894019841990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 2015; 116: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thierry AR, Roch B. Neutrophil extracellular traps and by‐products play a key role in COVID‐19: pathogenesis, risk factors, and therapy. J Clin Med 2020; 9: E2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin L, Luo S, Qin R, Yang M, Wang X, Yang Q, Zhang Y, Wang Q, Zhu R, Fan H, Wang H, Hu Y, Wang L, Hu D. Long‐term infection of SARS‐CoV‐2 changed the body's immune status. Clin Immunol 2020; 218: 108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Busch MH, Timmermans SAMEG, Nagy M, Visser M, Huckriede J, Aendekerk JP, de Vries F, Potjewijd J, Jallah B, Ysermans R, Oude Lashof AML, Breedveld PH, van de Poll MCG, van der Horst ICC, van Bussel BCT, Theunissen ROMFIH, Spronk HMH, Damoiseaux JGMC, Ten Cate H, Nicolaes GAF, Reutelingsperger CP, van Paassen P. Neutrophils and contact activation of coagulation as potential drivers of COVID‐19. Circulation 2020; 18: 1787–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Parackova Z, Zentsova I, Bloomfield M, Vrabcova P, Smetanova J, Klocperk A, Mesežnikov G, Casas Mendez LF, Vymazal T, Sediva A. Disharmonic inflammatory signatures in COVID‐19: augmented neutrophils' but impaired monocytes' and dendritic cells' responsiveness. Cells 2020; 9: E2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maltese G, Corsonello A, di Rosa M, Soraci L, Vitale C, Corica F, Lattanzio F. Frailty and COVID‐19: a systematic scoping review. J Clin Med 2020; 9: 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, Huang E, Zuo QK. The prevalence of depression, anxiety, and sleep disturbances in COVID‐19 patients: a meta‐analysis. Ann N Y Acad Sci 2020; 2: 90–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janiri D, Kotzalidis GD, Giuseppin G, Molinaro M, Modica M, Montanari S, Terenzi B, Carfì A, Landi F, Sani G, Gemelli Against COVID‐19 Post‐acute Care Study Group . Psychological distress after Covid‐19 recovery: reciprocal effects with temperament and emotional dysregulation. An exploratory study of patients over 60 years of age assessed in a post‐acute care service. Front Psychiatry 2020; 11: 590135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, Savelieva I, Fumagalli S, Bo M, Rocca A, Jensen MT, Pierard L, Sudano I, Aboyans V, Asteggiano R. Frailty in cardiology: definition, assessment and clinical implications for general cardiology: a consensus document of the Council for Cardiology Practice (CCP), Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Primary Care Cardiology Society (EPCCS), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio‐Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e‐Cardiology, WG Thrombosis, of the European Society of Cardiology. Eur J Prevent Cardiol 2021. in press. [DOI] [PubMed] [Google Scholar]
- 71. South AM, Diz DI, Chappell MC. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020; 318: H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gul R, Uh‐Hyun K, Alfadda AA. Renin angiotensin system at the interface of COVID‐19 infection. Eur J Pharmacol 2020; 18: 173656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zipeto D, Palmeira JDF, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID‐19. Front Immunol 2020; 11: 576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang JG, Burnier M. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19: European Society of Hypertension COVID‐19 Task Force Review of Evidence. Cardiovasc Res 2020; 116: 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu J, Teng Y, Shang L, Gu X, Fan G, Chen Y, Tian R, Zhang S, Cao B. The effect of prior ACEI/ARB treatment on COVID‐19 susceptibility and outcome: a systematic review and meta‐analysis. Clin Infect Dis 2020: ciaa1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, Cauda R, Gialluisi A, Guaraldi G, Menicanti L, Mennuni M, Mussinelli R, My I, Parruti G, Patti G, Perlini S, Santilli F, Signorelli C, Stefanini GG, Vergori A, Abete P, Ageno W, Agostoni P, Aiello L, al Moghazi S, Arboretti R, Aucella F, Barbieri G, Barchitta M, Bartoloni A, Bonfanti P, Cacciatore F, Caiano L, Carrozzi L, Cascio A, Castiglione G, Cianfrone S, Ciccullo A, Cingolani A, Cipollone F, Colomba C, Colombo C, Cozzi O, Crisetti A, Crosta F, Danzi GB, D'Ardes D, de Gaetano Donati K, di Gennaro F, di Tano G, D'offizi G, Fusco FM, Gentile I, Graziani E, Guarnieri G, Larizza G, Leone A, Lio V, Lucia MB, Maccagni G, Madaro F, Maitan S, Mancarella S, Manuele R, Mapelli M, Maragna R, Marcucci R, Maresca G, Marongiu S, Marotta C, Marra L, Mastroianni F, Mazzitelli M, Mengozzi A, Menichetti F, Meschiari M, Milic J, Minutolo F, Molena B, Mussini C, Musso M, Odone A, Olivieri M, Palimodde A, Pasi E, Pesavento R, Petri F, Pinchera B, Pivato CA, Poletti V, Ravaglia C, Rossato M, Rossi M, Sabena A, Salinaro F, Sangiovanni V, Sanrocco C, Scoppettuolo G, Scorzolini L, Sgariglia R, Simeone PG, Trecarichi EM, Vettor R, Vianello A, Vinceti M, Virano A, Vocciante L, De Caterina R, Iacoviello L. RAAS inhibitors are not associated with mortality in COVID‐19 patients: findings from an observational multicenter study in Italy and a meta‐analysis of 19 studies. Vascul Pharmacol 2020: 106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Song SL, Hays SB, Panton CE, Mylona EK, Kalligeros M, Shehadeh F, Mylonakis E. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID‐19 patients: a preliminary study. Pathogens 2020; 9: 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, Tedrow U. Arrhythmias and COVID‐19: a review. JACC Clin Electrophysiol 2020; 6: 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Santoro F, Monitillo F, Raimondo P, Lopizzo A, Brindicci G, Gilio M, Musaico F, Mazzola M, Vestito D, di Benedetto R, Abumayyaleh M, El‐Battrawy I, Santoro CR, di Martino LFM, Akin I, de Stefano G, Fiorilli R, Cannone M, Saracino A, Angarano S, Carbonara S, Grasso S, di Biase L, Brunetti ND. QTc interval prolongation and life‐threatening arrhythmias during hospitalization in patients with COVID‐19. Results from a multi‐center prospective registry. Clin Infect Dis 2020: ciaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, van Belle E, Zamorano JL, ESC Scientific Document Group . 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543–603. [DOI] [PubMed] [Google Scholar]
- 81. Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, Starr N, Buckel W, Grisel N, Hummel E, Snow G, Morris D, Stenehjem E, Srivastava R, Brown SM. Clinical criteria for COVID‐19‐associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol 2020; 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gąsecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, Chiva‐Blanch G. Thrombotic complications in patients with COVID‐19: pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc Drugs Ther 2020. Oct; 19: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cowie MR, Bax J, Bruining N, Cleland JGF, Koehler F, Malik M, Pinto F, van der Velde E, Vardas P. e‐Health: a position statement of the European Society of Cardiology. Eur Heart J 2016; 37: 63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. ÄrzteZeitung.de . 600 Prozent Plus bei Videosprechstunde in Berlin! https://www.aerztezeitung.de/Wirtschaft/600‐Prozent‐Plus‐bei‐Videosprechstunde‐in‐Berlin‐410364.html (Accessed May, 2021).
- 85. Bitkom . Digital health—report of Bitkom Research 2020, July 9, 2020.
- 86. Piro A, Magnocavallo M, Della Rocca DG, Neccia M, Manzi G, Mariani MV, Straito M, Bernardini A, Severino P, Iannucci G, Giunta G, Chimenti C, Natale A, Fedele F, Lavalle C. Management of cardiac implantable electronic device follow‐up in COVID‐19 pandemic: lessons learned during Italian lockdown. J Cardiovasc Electrophysiol 2020; 31: 2814–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miller JC, Skoll D, Saxon LA. Home monitoring of cardiac devices in the era of COVID‐19. Curr Cardiol Rep 2020; 23: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Treskes RW, van Winden LAM, van Keulen N, van der Velde ET, Beeres SLMA, Atsma DE, Schalij MJ. Effect of smartphone‐enabled health monitoring devices vs regular follow‐up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Netw Open 2020; 3: e202165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016; 375: 154–161. [DOI] [PubMed] [Google Scholar]
- 90. Yang C, Yang J, Zhang J, Yang J. More clinical warning indicators should be explored for 911 monitoring COVID‐19 patients' condition. Int J Cardiol 2020; 310: 169–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Silven AV, Petrus AHJ, Villalobos‐Quesada M, Dirikgil E, Oerlemans CR, Landstra CP, Boosman H, van Os HJA, Blanker MH, Treskes RW, Bonten TN, Chavannes NH, Atsma DE, Teng YKO. Telemonitoring for patients with COVID‐19: recommendations for design and implementation. J Med Internet Res 2020; 22: e20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ding XR, Clifton D, Ji N, Lovell NH, Bonato P, Chen W, Yu X, Xue Z, Xiang T, Long X, Xu K, Jiang X, Wang Q, Yin B, Feng G, Zhang Y. Wearable sensing and telehealth technology with potential applications in the coronavirus pandemic. IEEE Rev Biomed Eng 2020: 48–70. [DOI] [PubMed] [Google Scholar]


