Abstract
Novel coronavirus disease (COVID‐19) first described in Wuhan, China in December 2019, has rapidly spread across the world and become a global public health emergency. Literature on the neurological manifestations of COVID‐19 is limited. We report a 24‐year‐old male, who presented with vertigo, dysarthria, and bradyphrenia 3 weeks after being diagnosed with COVID‐19 on nasopharyngeal reverse transcription polymerase chain reaction. The patient was diagnosed with acute cerebellitis based on magnetic resonance imaging features and showed improvement posttreatment with intravenous methylprednisone for 5 days. The scope of this article is to highlight the importance of early identification of neurological symptoms and timely management as the outcomes may be catastrophic.
Keywords: cerebellitis, COVID‐19, SARS‐CoV‐2
Abbreviations
- Cerebrospinal fluid
CSF
- CNS
central nervous system
- COVID‐19
coronavirus infectious disease‐2019
- IDSA/ATS
Infectious Diseases Society of America/American Thoracic Society
- IVMP
intravenous methylprednisolone
- nCov
novel coronavirus
- PNS
peripheral nervous system
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory distress syndrome coronavirus 2
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2/COVID‐19) first detected in Wuhan, China, in December 2019, is now a pandemic with over 100 million confirmed cases, as reported by the World Health Organization. 1 Although primary manifestations involve respiratory and cardiac systems, several studies have shown neurological features and complications in patients with COVID‐19 infection. The reported neurological symptoms include headache and dizziness followed by encephalopathy and delirium. 2 , 3 , 4 Neurological complications include stroke (hemorrhagic and ischemic), Guillain Barre syndrome, acute transverse myelitis, and acute encephalitis. 4 , 5 Central nervous system (CNS) involvement is usually seen with severe cases of COVID‐19. We report a unique case of acute cerebellitis post‐COVID‐19 infection with characteristic changes noted on CSF analysis and imaging.
2. CASE REPORT
A 24‐year‐old right‐handed man presented to the emergency room with a week‐long history of severe headache, dysarthria, vertigo, myalgias, fevers, vomiting, photophobia, and mild encephalopathy. The patient had tested positive for COVID‐19 (nasopharyngeal reverse transcription polymerase chain reaction [RT‐PCR]) infection 3 weeks prior to family contact exposure. He did not endorse loss of taste or smell and did not experience any respiratory symptoms at that time. Head computed tomography (CT) and intracranial CT angiography were normal. He was treated symptomatically for headache and discharged home with a follow‐up appointment with neurology for headache evaluation. During this evaluation 2 days later, the patient denied any complaints of visual disturbances, dysphagia, focal weakness of extremities or paresthesias, neck rigidity, balance issues, changes in gait or any episodes of seizures. On examination, however, the patient was found to have ataxic dysarthria with moderate bradyphrenia.
The patient was admitted directly to the hospital and magnetic resonance imaging (MRI) showed nodular leptomeningeal enhancement along the bilateral cerebellar folia, more pronounced on the left. T2/FLAIR images demonstrated cortical hyperintensity in the areas of leptomeningeal enhancement. Restricted diffusion was seen on diffusion‐weighted imaging/apparent diffusion coefficient (DWI/ADC) secondary to cytotoxic edema in these same areas. Findings were concerning for cerebellitis (refer to Figure 1). CSF RT‐PCR was negative for SARS‐CoV‐2. However, CSF analysis demonstrated pleocytosis (eight nucleated cells) with lymphocytic predominance 92%, protein levels of 68 mg/dl, glucose of 171 mg/dl, and serum glucose 272 mg/dl. CSF PCR was negative for herpes simplex virus 1 (HSV‐1) and HSV‐2, cytomegalovirus, enterovirus. Lyme panel was negative. Cryptococcal antigen was negative. CSF culture showed no growth. West Nile virus, IgM, and IgG were negative. Varicella‐zoster PCR was again negative. VDRL PCR was negative. Serum and CSF paraneoplastic as well as the autoimmune panel was negative for anti‐GABA B receptor, anti AMPA, anti‐Yo, anti‐Hu, anti‐Ri, anti‐Purkinje cell cytoplasmic antibody 2, antiglutamic acid decarboxylase, antimetabotropic glutamate receptor 1 (anti‐mGluR1), anti‐CV2, antiparaneoplastic antigen Ma 2 (anti‐PNMA2), anti‐N‐methyl‐d‐aspartate receptor, anticontactin‐associated protein 2 (anti‐CASPR2), and leucine‐rich glioma‐inactivated protein 1 (LGI 1). Other investigations included elevated WBC of 12.1 × 103/µl, platelet of 518 × 103/µl, lymphocytes of 3.60 × 103/µl, C‐reactive protein (2.2 mg/L), serum ferritin (388 ng/ml), lactate dehydrogenase (404 U/L), D‐dimer (200 ng/ml). Chest X ray and subsequently CT of the chest were performed due to COVID‐19 diagnosis, which were both unremarkable.
Figure 1.
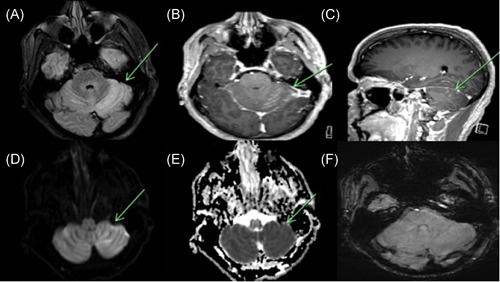
MRI Brain axial FLAIR image (A) demonstrates abnormal cortical hyperintensity in the bilateral cerebellar hemispheres diffusely, left more than right (green arrow). Axial and sagittal T1 postcontrast images (B, C) show cortical and leptomeningeal enhancement involving the bilateral cerebellar hemispheres (green arrow). DWI demonstrates hyperintensity in the region of parenchymal signal abnormality on FLAIR in the bilateral cerebellar hemispheres, left more than right (D), with the corresponding hypointensity on ADC consistent with restricted diffusion secondary to cytotoxic edema (E). SWI images (F) show no evidence of signal dropout to suggest hemorrhage. ADC, apparent diffusion coefficient; DWI, diffusion‐weighted imaging; MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging
These findings were compatible with an inflammatory CNS disorder and a diagnosis of cerebellitis was made. The patient was initially also started on acyclovir and ceftriaxone for the concern of bacterial versus viral meningoencephalitis but this was discontinued once the CSF Gram stain, culture, and HSV panel were unremarkable.
The patient was started on intravenous (IV) methylprednisolone on January 27, 2021. A total of 5 days of IV Solu‐Medrol 1 gram daily was given. He reported significant improvement in his symptoms following the 2nd day of IV Solu‐Medrol and complete resolution of symptoms at the time of discharge. At a follow‐up appointment in the clinic 2 weeks after discharge, the patient remained symptom‐free and at his cognitive baseline. The patient had nonsevere COVID‐19 based on ATS/IDSA guidelines. 6 Follow up MRI was not performed at this time as the patient's symptoms and abnormal physical exam findings had resolved.
3. DISCUSSION
Acute cerebellitis is an inflammatory syndrome associated with cerebellar swelling and dysfunction, hypothesized to be a result of infection (most commonly viral) and less commonly after vaccination. 7 , 8 Patients typically present with acute cerebellar dysfunction (ataxia, nystagmus, or dysmetria), headache, nausea, and altered consciousness. It is a rare pathology that has a wide range of clinical presentations. Acute cerebellitis should be suspected in any presentation with symptoms suggestive of posterior fossa involvement. Although acute cerebellitis cases are more common in children, the diagnosis should also be suspected in adults in the appropriate clinical setting as with our case. 9 , 10
Infectious agents associated with acute cerebellitis include viruses such as varicella‐zoster virus, Ebstein‐Barr virus, HSV‐1, influenza and respiratory syncytial virus, bacteria including streptococcus pneumoniae, borrelia burgdorferi, and salmonella typhi among others. 9 , 11 , 12 , 13 Autoimmune mechanisms have also been hypothesized due to the detection of autoimmune antibodies against Purkinje cells. 14
A non‐contrast head CT is often the first study performed in patients who present with acute cerebellitis. This is to assess for other etiologies for the patient's symptoms, such as infarct or hemorrhage. In the setting of acute cerebellitis, noncontrast head CT is important to assess for complications secondary to edema including herniation and hydrocephalus. Early diagnosis and intervention are essential, as more severe cases with obstructive hydrocephalus may require decompressive suboccipital craniectomy. Delay can result in complications such as cerebellar tonsillar herniation and death.
The gold standard for diagnosis is MRI and the findings are categorized into three main groups which include bi‐hemispheric cerebellitis, hemicerebellitis, and cerebellitis with encephalitic findings. 12 , 15 , 16 The most common MRI findings include abnormal cortical hyperintensity in the affected areas of the cerebellum, often bilaterally, on T2/FLAIR‐weighted images. These areas also demonstrate restricted diffusion on DWI/ADC images secondary to cytotoxic edema. T1 postcontrast images often show both leptomeningeal enhancement along the cerebellar folia and enhancement of the cortex in the affected regions. Hemorrhage is not commonly seen. Cases with severe vasogenic edema may result in compression of the fourth ventricle and resulting upstream hydrocephalus. Brainstem compression may also be present and tonsillar herniaton. 15 , 17
The main differential diagnosis of acute cerebellitis is acute infarct or neoplasm. Acute infarcts will present with restricted diffusion and T2/FLAIR signal abnormality in the affected areas. Acute cerebellitis may also present with restricted diffusion. However, leptomeningeal enhancement along the cerebellar folia is not typical in the setting of infarct and is usually seen in the setting of cerebellitis and is a helpful distinguishing factor. In addition, acute infarcts will conform to the involved vascular territories. However, FLAIR signal abnormality can become more pronounced in the setting of larger infarcts with subsequent development of vasogenic edema which can mimic cerebellitis. 12 , 15 , 18 In the rare chance of uncertain cases, interval follow‐up is recommended.
A neoplasm can usually be differentiated from cerebellitis on the initial MRI. A mass in the posterior fossa will present as a well‐defined lesion typically with surrounding edema. Mass effect is not a differentiating factor as this can occur with both disease processes. If cerebellitis is complicated by hemorrhage, as in the case of the very rare pseudotumor cerebellitis with hemorrhage, 18 interval follow‐up should be performed to assess for expected evolution and to exclude an underlying mass lesion. Other lesions in the cerebellum such as Lhermitte Duclos (dysplastic cerebellar gangliocytoma,) can typically be differentiated from acute cerebellitis as there is usually no restricted diffusion or enhancement in the former. 19
An altered level of consciousness due to brainstem compression resulting from cerebellar inflammation can mask the initial manifestations of cerebellar involvement. Patients can present with symptoms of raised intracranial pressure (RICP) including autonomic dysfunction and coma. This kind of presentation is known as fulminant acute cerebellitis and should be considered as a differential in patients presenting with symptoms of RICP of sudden onset as it is associated with severe irreversible sequelae and death. 20
There are several mechanisms by which Coronavirus can involve the nervous tissue and these include direct invasion, hypoxia, via ACE2, and autoimmunity. 3 , 21 Previous studies have shown neurotropic characteristics in the SARS‐CoV virus. Gu et al. examined autopsies of individuals affected with SARS and found viral particles and genomic sequences in nervous tissue. Similarly, Lu et al. concluded that SARS‐CoV‐2 had a genomic similarity of 79% and homogenous receptor binding structure as compared to that of SARS‐CoV. 22 , 23
In our brief literature review, we reviewed cases of cerebellitis in patients of COVID‐19. Fadakar et al. 24 reported a 47‐year‐old man who developed progressive vertigo, ataxia, and headache 10 days after presenting with viral symptoms. This patient had non‐severe COVID‐19 based on ATS/IDSA guidelines. 6 Examination revealed an ataxic gait and mild dysarthria. Brain MRI showed hyperintensities on FLAIR sequence within the bilateral cerebellar hemispheres as well as within the vermis with cortical and meningeal enhancement of the cerebellum on postcontrast imaging. Nasopharyngeal and CSF RT‐PCR were positive for SARS‐CoV‐2. CSF showed mild pleocytosis 10/mm3 with 80% lymphocyte, normal glucose, elevated protein 58 mg/dl. PCR was negative for other viruses and the paraneoplastic and autoimmune panels were unremarkable. CT chest showed no consolidation. The patient showed improvement in vertigo and ataxia after treatment with an antiviral(lopinavir/ritonavir) for 14 days.
Povlow et al. reported a 30‐year‐old man who presented with stroke‐like symptoms, acute onset of nausea, vomiting, slurred speech, and difficulty with walking due to issues with balance along with the viral respiratory symptoms. He tested positive for COVID‐19 (nasopharyngeal RT‐PCR, CSF RT‐PCR was not performed) and was categorized under the non‐severe category according to the ATS/IDSA classification. 6 On examination, the patient had dysarthria, incoordination, imbalance, and trouble reaching for items. Contrast‐enhanced MRI brain was unremarkable. CSF showed pleocytosis 7/mm3 with lymphocytic predominance, normal protein, and was negative for other viruses. 25 This patient did not fulfill strict criteria for cerebellitis 12 , 15 and the diagnosis was made based on the clinical presentation.
4. CONCLUSION
We illustrate a rare case of post‐COVID‐19 related acute cerebellitis. This case highlights the importance of maintaining a high clinical suspicion in the appropriate patient population (children and/or those with recent viral illness or vaccination) given that our patient was evaluated multiple times before making a diagnosis. Prompt diagnosis with MRI and early treatment with corticosteroids may reduce the morbidity and mortality associated with cerebellitis. Acute cerebellitis is a rare condition, which despite its unique clinical presentation and radiological findings, is considered a part of acute postinfectious cerebellar ataxia. It can also have a fulminant course, which can result in permanent irreversible damage or even death.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Patient consent available.
AUTHOR CONTRIBUTIONS
Conceptualization: Shitiz Sriwastava, Parissa Feizi, Muhamad Alvi, and Amelia Adcock. Drafting the manuscript: Shitiz Sriwastava, Parissa Feizi, Muhammad Alvi, and Amelia Adcock. Data abstraction and data analysis: Maria Moreno‐Escobar, Sanjiti Podury, Medha Tandon, and Badria Munir. Editing and final draft: Shitiz Sriwastava.
Moreno‐Escobar MC, Feizi P, Podury S, et al. Acute cerebellitis following SARS‐CoV‐2 infection: A case report and review of the literature. J Med Virol. 2021;93:6818‐6821. 10.1002/jmv.27232
REFERENCES
- 1. WHO . WHO coronavirus disease (COVID‐19) dashboard. https://covid19.who.int/?gclid=Cj0KCQiA6t6ABhDMARIsAONIYywpPDg3Hu-cQtbypR3MmCf9RzstxhVodEx73VZCfpbDuLfpNoy-73caAvp_EALw_wcB
- 2. Mao L, Jin H, Wang M, et al. Neurological manifestations of hospitalised patients with COVID‐19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iadecola C, Anrather J, Kamel H. Effects of COVID‐19 on the nervous system. Cell. 2020;183:16‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sriwastava S, Kataria S, Tandon M, et al. Guillain Barré syndrome and its variants as a manifestation of COVID‐19: a systematic review of case reports and case series. J Neurol Sci. 2021;420:117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreno‐Escobar MC, Kataria S, Khan E, et al. Acute transverse myelitis with Dysautonomia following SARS‐CoV‐2 infection: a case report and review of literature. J Neuroimmunol. 2021;353:577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nussinovitch M, Prais DF, Volovitz B, Shapiro R, Amir J. Post‐infectious acute cerebellar ataxia in children. Clin Pediatr. 2003;42(7):581‐584. [DOI] [PubMed] [Google Scholar]
- 8. Lancella L, Esposito S, Galli ML, et al. Acute cerebellitis in children: an eleven year retrospective multicentric study in Italy. Ital J Pediatr. 2017;43(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bozzola E, Bozzola M, Tozzi AE, et al. Acute cerebellitis in varicella: a ten year case series and systematic review of the literature. Ital J Pediatr. 2014;40:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Samkar A, Poulsen MNF, Bienfait HP, Van Leeuwen RB. Acute cerebellitis in adults: a case report and review of the literature. BMC Res Notes. 2017;10(1):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Shokri SD, Karumannil SA, Mohammed SS, Sadek MS. Post‐Epstein‐Barr virus acute cerebellitis in an adult. Am J Case Rep. 2020;21:e918567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takanashi J, Miyamoto T, Ando N, et al. Clinical and radiological features of rotavirus cerebellitis. AJNR Am J Neuroradiol. 2010;31(9):1591‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahajan SK, Sharma S, Kaushik M, et al. Scrub typhus presenting as acute cerebellitis. J Assoc Physicians India. 2016;64(2):69‐70. [PubMed] [Google Scholar]
- 14. Mitoma H, Manto M, Hampe CS. Immune‐mediated cerebellar ataxias: practical guidelines and therapeutic challenges. Curr Neuropharmacol. 2019;17(1):33‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Bruecker Y, Claus F, Demaerel P, et al. MRI findings in acute cerebellitis. Eur Radiol. 2004;14(8):1478‐1483. [DOI] [PubMed] [Google Scholar]
- 16. Carceller Lechón F, Duat Rodríguez A, Sirvent Cerdá SI, et al. Hemicerebellitis: report of three paediatric cases and review of the literature. Eur J Paediatr Neurol. 2014;18(3):273‐281. [DOI] [PubMed] [Google Scholar]
- 17. Yildirim MA‐O, Gocmen R, Konuskan B, Parlak SA‐O, Yalnizoglu D, Anlar B. Acute cerebellitis or postinfectious cerebellar ataxia? Clinical and imaging features in acute cerebellitis. J Child Neurol. 2020;35(6):380‐388. [DOI] [PubMed] [Google Scholar]
- 18. Singh P, Bhandal SK, Saggar K, Pooni PA, Jaswal RS. Pseudotumoral hemicerebellitis with hemorrhage. J Pediatr Neurosci. 2012;7(1):49‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joo G, Doumanian J. Radiographic findings of dysplastic cerebellar gangliocytoma (Lhermitte‐Duclos disease) in a woman with cowden syndrome: a case study and literature review. J Radiol Case Rep. 2020;14(3):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy EI, Harris AE, Omalu BI, Hamilton RL, Branstetter BF 4th, Pollack IF. Sudden death from fulminant acute cerebellitis. Pediatr Neurosurg. 2001;35(1):24‐28. [DOI] [PubMed] [Google Scholar]
- 21. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fadakar N, Ghaemmaghami S, Masoompour SM, et al. A first case of acute cerebellitis associated with coronavirus disease (COVID‐19): a case report and literature review. Cerebellum. 2020;19(6):911‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Povlow A, Auerbach AJ. Acute cerebellar ataxia in COVID‐19 infection: a case report. J Emerg Med. 2021;60(1):73‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]


