Abstract
Recent studies reported that some recovered COVID‐19 patients have tested positive for virus nucleic acid again. A systematic search was performed in Web of Science, PubMed, Scopus, and Google Scholar up to March 6, 2021. The pooled estimation of reinfection, recurrence, and hospital readmission among recovered COVID‐19 patients was 3, 133, and 75 per 1000 patients, respectively. The overall estimation of reinfection among males compared to females was greater. The prevalence of recurrence in females compared to males was more common. Also, hospital readmission between sex groups was the same. There is uncertainty about long‐term immunity after SARS‐Cov‐2 infection. Thus, the possibility of reinfection and recurrence after recovery is not unexpected. In addition, there is a probability of hospital readmission due to adverse events of COVID‐19 after discharge. However, with mass vaccination of people and using the principles of prevention and appropriate management of the disease, frequent occurrence of the disease can be controlled.
Keywords: COVID‐19, hospital readmission, meta‐analysis, recurrence, reinfection, systematic review
Highlights
The pooled estimation of re‐infection rate among recovered COVID‐19 patients was 3 per 1000 patients.
The pooled estimation of recurrence rate among recovered COVID‐19 patients was 133 per 1000 patients.
Hospital readmission among recovered COVID‐19 patients was 75 per 1000 patients.
The possibility of re‐infection, recurrence and hospital readmission after recovery is not unexpected. Thus, adherence to principles of prevention besides vaccination is essential.
1. INTRODUCTION
The SARS‐Cov‐2 pandemic has recently appeared as a global threat to human health. 1 It has forced many countries to take unprecedented steps to prevent it from spreading and has imposed high costs on the health care sector of countries. 2 Up to August 9, 2021, the World Health Organization (WHO) has approved about 202 million confirmed cases, with nearly 4300 million deaths and 4 billion doses of vaccine have been administered. 3 Most COVID‐19 patients have experienced mild to moderate symptoms and recovered without requiring special treatment. Mild‐type infected individuals experience fever, cough, sore throat, diarrhea, headaches, myalgia or arthralgia, exhaustion, and loss of sense of smell and taste. 4 , 5 The symptoms of COVID‐19 pneumonia include dyspnea, loss of appetite, dizziness, pain or pressure in the chest, and fever (above 38°C). Older people and those with underlying medical problems such as cardiovascular disease, diabetes, chronic respiratory disease, and cancer will probably experience more severe complications. 6 The increase in the liver enzyme and the low number of lymphocytes with an increase in CRP (C‐reactive protein) is often present in COVID‐19 patients. 7 It can eventually lead to acute respiratory distress syndrome (ARDS) and death. 8 , 9 The COVID‐19 virus spreads mainly through saliva or sprays from the nose; the best way to prevent and reduce transmission speed is adequate awareness of the COVID‐19 viruses and how it spreads. By washing hands or using alcohol and not touching the face, one can protect himself and others against infection. Being infected with the virus does not produce permanent immunity and antibody against the virus in all people. 10 More than 90% of individuals infected with SARS‐Cov‐2 produce antibodies about a week after starting the symptoms, which lasts for at least 3 months. 11 , 12 There is an urgent need to understand better whether those who have recovered from COVID ‐19 are secure to reinfection or not. The effectiveness of vaccination strategies, concerns for herd immunity, and general modeling for the epidemic depend on the effectiveness and period of immunity versus COVID‐19. The acute respiratory syndrome of the coronavirus 2 (SARS‐Cov‐2) has already produced distinct immunity responses. However, the rate and time of immunity in infected individuals against reinfection are not clarified. 13 Immunity after infection may be created with immune responses of IgG antibodies and mediated by specialized T cells. Primary considerations when examining the immunity of postinfection include identifying protective functions, identifying measurable biological markers, and the precise definition of reinfection, recurrence, and readmission, death, or transfer to other people. 14 , 15 The exact cause of the disease recurrence of some COVID‐19 patients is unclear. It was previously mentioned that imperfect eradication of the virus from the tissues was the cause. Another notion says that inadequate antibodies or the rapid disappearance of these antibodies due to a stimulus factor are the cause. 15 Short‐period immunity against reinfection has been observed in non‐human models of SARS‐Cov‐2. 12
Reinfection and possible hospital readmission is a significant and costly problem in the pandemic. 16 In addition, the rotation and dynamics of the virus and its mutation in the countries lead to resistance to treatment and vaccinations. 17 Trying to accurately define and monitor the rate of reinfection, recurrence, readmission, and affecting factors can provide practical strategies for policy‐making to prevent reinfection, improve health care, and treat patients. According to the past reports, the frequency of readmission was reported as between 2% and 5%. Readmission is usually in the first week after clearance. It may be due to other underlying diseases except for reinfection. 18 The reinfection rate in different countries has been reported between less than 0.5% to more than 5%. 19 , 20 Thus, according to the various reports about the probability of reinfection, recurrence, or hospital readmission due to COVID‐19 in the whole world, we decided to design a study with the purpose of determining the definition and extent of reinfection, recurrence of positive nucleic acid (repositivity), and hospital readmission, which may have positive clinical and epidemiological implications for the treatment and control of the infection. This study systematically searches and summarizes the frequency and definitions of reinfection rates, recurrence, and hospital readmission following SARS‐Cov‐2 infection.
2. METHODS
2.1. Search strategy
A systematic search was performed in PubMed, Web of Science, Scopus, and Google Scholar up to March 6, 2021, in the English language. Two researchers independently searched for studies. The database searches were conducted using the following keywords: “SARS‐Cov‐2,” “novel coronavirus,” “2019‐nCoV,” “COVID‐19,” “Recurrence,” “Reactivation,” “Re‐positive,” “Relapse,” “Re‐infection,” “Readmission.” The terms of search strategies were according to the following keywords: ((“novel coronavirus” OR “2019‐nCov” OR “COVID‐19” OR “SARS‐Cov‐2” [Mesh])) AND ((Recurr* [Mesh] OR Recrudescence* OR Relapse*OR Re‐positive*) OR (Reinfect*[Mesh]) OR (Readmission*[Mesh] OR “hospital readmission”)).
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the studies were published in English; (2) peer‐reviewed and published articles included desired indicators and definitions. The exclusion criteria were as follows: (1) duplicate articles that refer to publishing the same intellectual material more than once, by the author or publisher and (2) gray literature such as non‐peer‐reviewed dissertations, government reports, conference proceedings/papers, statements by professional organizations, and so forth.
2.3. Study selection and data collection
We included studies based on PRISMA guidelines for performing the standard meta‐analysis techniques. After retrieving articles from the mentioned databases and eliminating duplicate articles, two authors conducted the screening process and data collection independently based on inclusion criteria. The following information was extracted from the selected studies: the first author's name, country of origin, study design, sample size, age, sex, diagnostic criteria of 2019‐nCoV, type of index (recurrence, reinfection, readmission), frequency of indices, time of occurrence from discharge, clinical signs and symptoms, comorbidity, disease severity, and outcome of disease (recovered or death).
2.4. Quality assessment
The quality check process was performed by two reviewers that assessed the quality of data in included studies. We used the STROBE checklist to ensure the quality of selected observational studies. After a full‐text quality assessment of selected studies, studies with high and medium quality were included in the analyses and finally, the key findings were extracted.
2.5. Statistical analysis
The “metaprop” command was used to perform fixed or random‐effects meta‐analysis in STATA. Random forest was used to show the results. The between‐study heterogeneity was assessed using a χ 2‐test‐based Cochran's Q statistic test at p < 0.05 and I 2 greater than 50%. A fixed‐effect model was used based on p value ≥ 0.05 or I 2 lower than 50%, and a random‐effect model was used based on p value < 0.05 or I 2 greater than 50%, finally pooled estimates in meta‐analyses were estimated with a 95% confidence interval. In some studies, the denominator of the fraction could not be extracted to calculate the frequency of gender in patients. To avoid overestimation and to avoid ignoring studies with high sample size, based on the results of other studies, the average frequency of women with the COVID‐19 was considered as 47% and calculations of missed data were made based on this frequency (Table 1). All of the analyses were conducted with Stata packages (version 14).
Table 1.
The description of eligible studies reporting the frequency of reinfection, recurrence, and readmission among COVID‐19 patients
| Reinfection | Number of patients with index | Number of confirmed cases with Positive test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author | Type of study | Country | Samples | Index | Total | Female | Male | Total | Female | Male |
| Abu‐Raddad, L. J. | Observational | Qatar | General population | Reinfection | 54 | 7 | 47 | 13 326 | – | – |
| Panagiota Caralis | Case report | USA | General population | Reinfection | 7 | 2 | 5 | 600 | – | – |
| Alyson M. Cavanaugh | Observational | USA | Residents of a Skilled Nursing Facility (SNF) | Reinfection | 5 | 4 | 1 | 12 | – | – |
| Aodhán Seán Breathnach | Observational | UK | General population | Reinfection | 8 | 8 | 0 | 10 727 | 6400 | 4327 |
| Stefan Pilz | Retrospective | Austria | General population | Reinfection | 40 | 25 | 15 | 14 840 | – | – |
| Hui Zhu | Retrospective | China | General population | Recurrence | 17 | 12 | 5 | 98 | 66 | 32 |
| Jing Lu | Observational | China | General population | Recurrence | 87 | 42 | 45 | 619 | 190 | 200 |
| Maolu Tian | Observational | China | General population | Recurrence | 20 | 8 | 12 | 147 | – | – |
| Jianghong An | Observational | China | General population | Recurrence | 38 | 22 | 16 | 262 | 136 | 126 |
| Sheng‐Long Chen | Retrospective | China | General population | Recurrence | 189 | 86 | 103 | 1282 | 654 | 628 |
| Jiazhen Zheng | Cohort | China | General population | Recurrence | 27 | 15 | 12 | 285 | 157 | 128 |
| Bo Yuan | Cohort | China | General population | Recurrence | 20 | 13 | 7 | 182 | 98 | 84 |
| Chao Yang | Observational | China | General population | Recurrence | 93 | 57 | 36 | 479 | – | – |
| Tie‐Jun Shui | Retrospective | China | General population | Recurrence | 59 | 30 | 29 | 758 | 362 | 396 |
| Youjiang Li | Observational | China | General population | Readmission | 4 | 2 | 2 | 13 | 7 | 6 |
| Jie Chen | Cohort | China | General population | Readmission | 81 | 51 | 30 | 1087 | 635 | 452 |
| Eleftheria Atalla | Observational | USA | General population | Readmission | 19 | 7 | 12 | 279 | 148 | 191 |
| Hong Cao | Retrospective | China | General population | Readmission | 8 | 5 | 3 | 108 | – | – |
| I. Yeo | Retrospective | USA | General population | Readmission | 48 | 25 | 23 | 1062 | 430 | 632 |
| Siqin Ye | Case series | USA | General population | Readmission | 31 | 12 | 19 | 409 | 164 | 245 |
| Zhiqi Yang | Retrospective | China | General population | Readmission | 7 | 3 | 4 | 79 | 36 | 44 |
| Uyaroğlu OA | Observational | Turkey | General population | Readmission | 11 | 5 | 6 | 154 | 77 | 77 |
| Chenxi Li | Observational | China | General population | Readmission | 15 | 11 | 4 | 85 | – | – |
| Woo‐Hwi Jeon | Observational | South Korea | General population | Readmission | 328 | 158 | 170 | 7590 | 4495 | 3095 |
| Amy M. Lavery | Observational | USA | General population/SNF | Readmission | 9504 | 4599 | 4902 | 106 543 | 52 206 | 54 337 |
3. RESULTS
After assessing the quality of selected studies, 25 observational studies were included in the analysis (Figure 1). The majority of studies were conducted in China. Details of the selected studies and their characteristics have been shown in Table 1.
Figure 1.
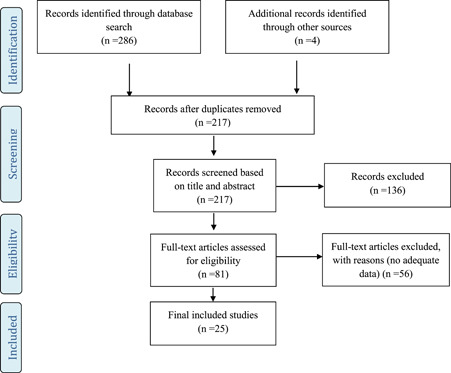
Flow diagram for the selection process of identified articles
3.1. Reinfection
Reinfection with SARS‐Cov‐2 has been reported all over the world. In definition, “re‐infection” means that a person was infected with an agent, recovered, and then become infected later. New infection can be due to a previous infectious agent or becoming infected with a new variant of the agent. 21 According to the CDC's protocol, based on clinical recovery and discharge criteria, recovered patients should have at least one negative SARS‐Cov‐2 PCR result. There are two times for epidemiological and clinical assessment of suspected reinfection cases: (1) persons with at least one detection of SARS‐Cov‐2 RNA test, more than 90 days after the first detection of SARS‐Cov‐2 RNA, whether or not symptoms were present, and (2) persons with COVID‐19‐like symptoms and detection of SARS‐Cov‐2 RNA between 45 and 89 days since first SARS‐Cov‐2 infection, with evidence of close‐contacts with a confirmed case and without evidence of another cause of infections. 22 For epidemiological confirmation of reinfection, viral genotype assays of the first and second specimens are needed. 21
The patients with reinfection ranged in age from 15 to 99 years old. 23 , 24 , 25 , 26 , 27 According to the reports of COVID‐19 reinfection cases in studies, most of them were detected based on RT‐PCR test using nasopharyngeal swab specimens. 23 , 24 , 25 , 26 , 27 However, in other studies, in addition to the PCR test, chemiluminescent microparticle immunoassay for anti‐SARS‐Cov‐2 antibodies was also used. 23 , 25 The minimum and maximum time of reinfection onset from initial infection was reported from 45 to 172 days. 23 , 26 But in another study, the most average time of reinfection onset days from initial infection was reported as 212 days (212 ± 25 days). 24 Most of the reinfected patients were asymptomatic or with mild‐to‐moderate symptoms such as respiratory or gastrointestinal symptoms that recovered 23 , 25 , 26 but in one study hospitalization at the time of reinfection was reported (about 12%). 24 However, according to the results of studies, among patients with SARS‐Cov‐2 reinfection, only two died, one woman aged about 80 years after hospitalization due to respiratory failure 27 and the other 72‐year‐old woman with a history of rhabdomyolysis and acute vascular occlusion that the main cause of her death was not due to a reinfection with SARS‐Cov‐2. 24 The underlying diseases among reinfected patients were not prevalent. 23 , 24 , 25 But the most common were diabetes and immune system deficiencies such as HIV and the use of immunosuppressive drugs due to certain diseases. 26 , 27
Based on the results of Table 2, the overall prevalence of reinfection among COVID‐19 patients was 3 (95% CI 0.8–5) per 1000 patients. In these studies, there was heterogeneity in the prevalence of reinfection (I 2 92.08%, p < 0.001) (Figure 2). After assessing the prevalence of reinfection based on sex groups, it was observed that in males compared to females, reinfection was more common (3.2 vs. 2.1 per 1000 patients).
Table 2.
The overall estimation (per 1000 patients) of reinfection, recurrence, and readmission among recovered COVID‐19 patients
| Parameters | No. of studies | Estimate (per 1000) | 95% CI | p for heterogeneity | I 2 (%) | |
|---|---|---|---|---|---|---|
| Overall | 5 | 3 | 0.8–5 | <0.001 | 92.08 | |
| Reinfection | Male | 5 | 3.2 | 0.4–6 | <0.001 | 94 |
| Female | 5 | 2.1 | 0.3–4 | 0.001 | 82.42 | |
| Recurrence | Overall | 9 | 133 | 105–160 | <0.001 | 84.17 |
| Male | 9 | 132 | 96–168 | <0.001 | 79.32 | |
| Female | 9 | 149 | 112–187 | <0.001 | 81.09 | |
| Readmission | Overall | 11 | 75 | 54–96 | <0.001 | 97.41 |
| Male | 11 | 70 | 50–90 | <0.001 | 91.76 | |
| Female | 11 | 70 | 50–100 | <0.001 | 96.92 |
Figure 2.
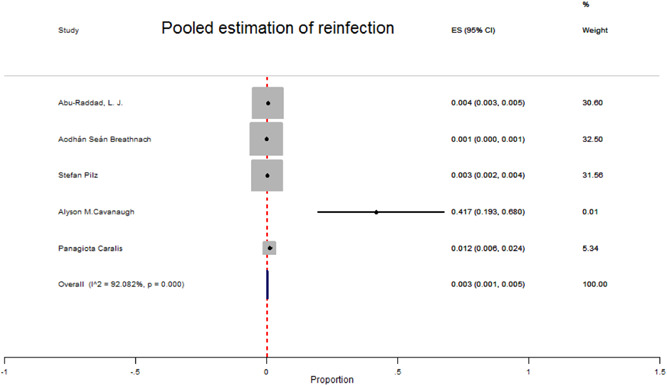
Forest plot for the pooled prevalence of reinfection due to SARS‐Cov‐2 among recovered COVID‐19 patients
3.2. Recurrence
“Recurrence” or “Recrudescence” or “Relapse” or “Reactivation” is the manifestation of symptoms after recovery. 21 In COVID‐19, based on the literature, the meaning of recurrence is referred to infection with the same species and strain of SARS‐Cov‐2 that may occur due to immunodeficiency of the host. 28 , 29 Based on another literature, COVID‐19 recurrence is defined as persons with detection of SARS‐Cov‐2 RNA within 90 days after the first detection of SARS‐Cov‐2 RNA without evidence of close‐contacts with confirmed cases. 21 In some literature, repositivity of the diagnostic test is considered similar to the concept of relapse that refers to have a positive SARS‐Cov‐2 RNA test in an asymptomatic patient up to 90 days following negative test and recovery from the initial infection. Low viral load can be a feature of repositivity in that such cases do not seem to play a significant role in disease transmission. 21
The patients with COVID‐19 recurrence evidence ranged in age from 4 to 80 years old. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Most of them were detected based on RT‐PCR tests using anal or nasopharyngeal swab specimens. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Some of them were detected using CT scan and chemiluminescent microparticle immunoassay for anti‐SARS‐Cov‐2 antibodies, too. 30 , 31 , 32 , 35 , 36 , 37 , 38 The minimum and maximum time of recurrence onset from first discharge were reported from 7 to 47 days with an average of 17.25 days. 33 Most of the COVID‐19 recurrent cases were asymptomatic or with mild‐to‐moderate symptoms that recovered. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 In contrast, in three studies, severe (15%, 4.32%, and 6%) and critical (10%, 0%, and 2%) recurrent cases were reported, 33 , 34 , 37 that all of them have recovered. 32 Comorbidity among recurrent cases was prevalent 31 , 33 , 34 , 35 , 36 that the most prevalent underlying diseases among them were hypertension, diabetes, chronic respiratory diseases, liver diseases, and cardiovascular diseases. 31 , 33 , 34 , 35 , 36 According to Table 2, the overall prevalence of recurrence among COVID‐19 patients was 133 (95% CI: 105–160) per 1000 patients (Figure 3). In these studies, there was heterogeneity (I 2 84.17%, p < 0.001). The prevalence of recurrence in females compared to males was more common (149 vs. 132) per 1000 patients.
Figure 3.
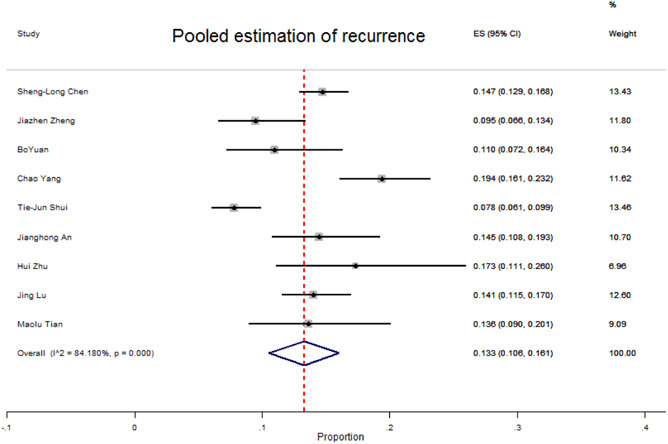
Forest plot of pooled prevalence of recurrence due to SARS‐Cov‐2 infection among recovered COVID‐19 patients
3.3. Readmission
Hospital readmission among large groups of COVID‐19 patients from first discharge has been reported. There is no standard definition to describe hospital readmission. According to the literature, readmission in COVID‐19 was defined as patients with prior hospitalization due to COVID‐19 who were readmitted within 30 days from the index hospitalization discharge date. 39 , 40 However, in another study, readmission is defined as rehospitalization within 2 months of discharge due to COVID‐19 adverse events or other health complications. 41 After discharge, most of the readmission causes were hypoxic, respiratory distress, thromboembolism, sepsis, psychiatric disorder, and repositivity of COVID‐19 tests. 20 , 39 , 40 , 42 The hospital readmitted COVID‐19 patients ranged in age from 23 to 90 years old. 20 , 43 , 44 Totally, the maximum average time of hospital readmission after previous discharge was 17 days (17 ± 6.7 days). 44 The maximum median length of hospital stay due to hospital readmission was reported as 12 days (median 12 days, interquartile range: 7–17). 45 Among readmitted patients, some of them have been admitted to the ICU. 39 , 41 , 45 In addition, most of the readmitted patients recovered, 20 , 40 , 41 , 42 , 43 , 45 , 46 , 47 but some of them died in hospital. 39 , 48 Most of the readmitted patients had a comorbidity in that the prevalent diseases among them were hypertension, diabetes, chronic kidney disease, coronary artery disease, hyperlipidemia, and obesity. 20 , 39 , 40 , 41 , 42 , 43 , 45 , 46 , 48 The overall prevalence of readmission after first discharge among COVID‐19 patients was 75 (95% CI: 54–96) per 1000 patients. There was heterogeneity in the prevalence of readmission (I 2 97.41%, p < 0.001) (Figure 4). After comparing the readmission between sex, groups were observed in that readmission among males and females was the same (70 in 1000 patients) (Table 2).
Figure 4.
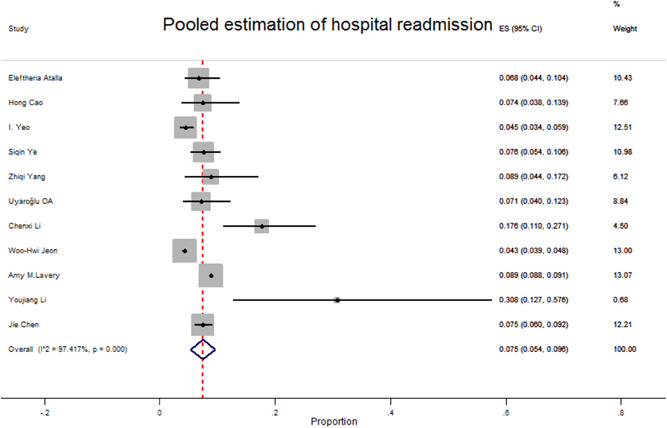
Forest plot of pooled prevalence of readmission due to SARS‐Cov‐2 infection among recovered COVID‐19 patients
4. DISCUSSION
In this systematic review and meta‐analysis, we have described the epidemiological characteristics of cases with reinfection, recurrence, or readmission due to COVID‐19. Of all patients recovering from COVID‐19 and being discharged, the overall estimation of reinfection, recurrence, and readmission was 3 (95% CI: 0.8–5), 133 (95% CI: 105– 160), and 75 (95% CI: 54–96) per 1000 patients, respectively. After comparing the present study's results with the results of past meta‐analysis studies around the world, it was observed that the prevalence of these three indicators was very different. For example, concordant with the current study, the prevalence of reinfection in a study of Arafkas et al. 13 was zero. Also, in a study of Ren et al., 49 repositivity was reported as 12% that the results are similar to the current study but in a systematic review that Piri et al. 50 performed, the recurrence rate was reported between 2.3% and 21.4%. The essential question is, whether the reported reinfected, recurrent, and hospital readmitted cases are really in line with the standard criteria? Moreover, is it possible that, for some reason, these values are overestimated or underestimated? Are the reinfected, recurrent, or readmitted patients are really infected or not? It seems that many factors need to be considered about the reported indices, especially in developing countries. One explanation for the difference between rates of indices in different studies is that diverse periods of time are considered in the definition of indices by different authors. Given that the Centers for Disease Control and Prevention (CDC) has provided criteria for identifying reinfected and recurrent patients, many researchers have not used standard criteria to describe cases. 22 Therefore, one of the essential criteria for case finding is the use of the standard definition. According to the World Health Organization (WHO) guidelines, a patient can be discharged from the hospital after two consecutive negative results at least 24 h apart. 51 Due to the lack of adequate health facilities in hospitals, maybe patients are discharged from the hospital before full recovery. Some cases may have a false negative at the time of discharge, or patients may not have completed discharge criteria. Thus, the mentioned hypotheses may affect the prevalence of reinfection, recurrence, or hospital readmission. However, we should not forget that reinfection is possible because some studies have shown that humoral immunity weakens over time. 52 Determination of recurrent cases can be due to false negatives, which according to the meta‐analysis of 957 hospitalized patients, varies from 2% to 29%. 53 The false negative can be due to the source of the samples, the sampling method, sample collection, the sensitivity and specificity of the test kit, and the variance of technicians in different labs. Basically, nasopharyngeal swab specimens are commonly used for RT‐PCR testing. The sampling operation depends a lot on the operator's experience, and the location of the samples may not be accurate. 54 In addition, it may be necessary to consider prolonged shedding of SARS‐Cov‐2 in asymptomatic or mild cases and recurrence of viral shedding, 55 related to the severity of inflammation and the immune response. 56 According to the past reports, data from 68 patients showed that the duration of viral shedding from sputum samples was significantly longer (34 days) than pharyngeal swabs (19 days). 57 Finally, it should be noted that to distinguish between reinfected from recurrent cases, the strain of the virus must be identified. 22 In all studies, no evidence of SARS‐Cov‐2 strain segregation has been reported. Thus, this issue can affect the estimated rate of reinfection and recurrence.
After comparing the basic characteristics of patients between indices, the rate of reinfection in females was lower than in males. In contrast, the rate of recurrence in males was lower than in females. In addition, the result of the systematic review by Piri et al. 50 indicates that among all patients that had a recurrence, 47.7% were male, and the others were female. Also, hospital readmission based on sex difference was the same. The relationship between COVID‐19 and its complications with gender is complex and can be due to differences in comorbidities, behavioral factors, workplace, lifestyles, and biological differences (the difference in immune response due to hormonal differences) which needs further investigation. 58
Based on the current study, most COVID‐19 reinfected, recurrent, and readmitted cases were asymptomatic or were with mild to moderate symptoms. However, some patients may experience severe infections in the second episode of the disease. Therefore, the severity of the disease can vary according to the patient's health condition, demographic situations, and immune system development. 59 , 60 In conclusion, information on the prevalence, risk factors, and probability of reinfection or relapse and hospital readmission can affect both clinical practice and the healthcare system. Also, more information about different aspects of COVID‐19 can help to prioritize the health services, healthcare planning, adequate resource allocation for caring, the importance of having active surveillance, case finding, and vaccination especially in patients with comorbidities or considering patients that have a higher risk of reinfections such as middle‐aged patients and health care workers.
Our study has specific strengths and limitations. One of the strengths of the current study is that this is the first study that considers the impact of definitions in the estimates of reinfections in patients with SARS‐Cov‐2. This can make a better viewpoint in health care decisions. One important limitation is that only peer‐reviewed articles in the English language were included in the current study, which can make a bias in the interpretation of results. Also, all of the understudied populations in the articles were COVID‐19 patients who were monitored for indices after hospitalization and discharge. Thus, mild cases and outpatients during initial infection have not been studied actively for probable reinfection, recurrence, or hospital readmission. Therefore, it seems that the results of the current meta‐analysis can be generalized to inpatients and it cannot depict the status of the general population.
5. CONCLUSION
Considering that there is uncertainty about long‐term immunity after SARS‐Cov‐2 infection, the possibility of reinfection and recurrence after recovery is not unexpected. In addition, there is a probability of hospital readmission due to adverse events of COVID‐19 after discharge. It is better to prevent underestimation or false overestimation by active surveillance and case finding, creating proper definitions, and using high‐accuracy diagnostic tests. Moreover, these indices should be reported more carefully.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHORS CONTRIBUTIONS
Sahar Sotoodeh Ghorbani, Sahar Bayat, and Hadis Ghajari wrote the manuscript and participated in the literature review. Niloufar Taherpour analyzed the data, wrote the manuscript, and participated in literature review. Parisa Mohseni participated in the literature review. Seyed Saeed Hashemi Nazari supervised, designed, and checked the quality of the study. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by the School of Public Health and Safety, Shahid Beheshti University of Medical Sciences grant number 27960. The funding agency did not play any role in the planning, conduct, and reporting or in the decision to submit the paper for publication. The authors would like to thank the School of Public Health and Safety research affairs of Shahid‐Beheshti University of Medical Sciences for technically supporting this study.
Sotoodeh Ghorbani S, Taherpour N, Bayat S, Ghajari H, Mohseni P, Hashemi Nazari SH. Epidemiologic characteristics of cases with reinfection, recurrence, and hospital readmission due to COVID‐19: A systematic review and meta‐analysis. J Med Virol. 2021;94:44‐53. 10.1002/jmv.27281
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tang JW, Tambyah PA, Hui DSC. The emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J Infect. 2020;80(3):350‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perlman S. Another decade. Another Coronavirus. 2020;382(8):760‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus disease (COVID‐19). 2021. Accessed August, 2021. https://covid19.who.int/
- 4. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case‐control study from the United States. Gastroenterology. 2020;159(1):373‐5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadr S, SeyedAlinaghi S, Ghiasvand F, et al. Isolated severe thrombocytopenia in a patient with COVID‐19: a case report. IDCases. 2020;21:e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected. Pneumonia. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahmoudi S, Mehdizadeh M, Badv RS, et al. The coronavirus disease 2019 (COVID‐19) in children: a study in an Iranian Children's Referral Hospital. Infect Drug Resist. 2020;13:2649‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID‐19 induced hepatitis B virus reactivation: a novel case from the United Arab Emirates. Cureus. 2020;12(6):8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghiasvand F, Ghadimi M, Ghadimi F, Safarpour S, Hosseinzadeh R, SeyedAlinaghi S. Symmetrical polyneuropathy in coronavirus disease 2019 (COVID‐19). IDCases. 2020;21:e00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee IK, Wang CC, Lin MC, Kung CT, Lan KC, Lee CT. Effective strategies to prevent coronavirus disease‐2019 (COVID‐19) outbreak in hospital. J Hosp Infect. 2020;105(1):102‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370(6521):1227‐1230. 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS‐CoV‐2 in the Icelandic Population. N Engl J Med. 2020;382(24):2302‐2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arafkas M, Khosrawipour T, Kocbach P, et al. Current meta‐analysis does not support the possibility of COVID‐19 re‐infections. J Med Virol. 2021;93(3):1599‐1604. [DOI] [PubMed] [Google Scholar]
- 14. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti–SARS‐CoV‐2 antibodies in persons with mild covid‐19. N Engl J Med. 2020;383(11):1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long Q‐X, Tang X‐J, Shi Q‐L, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nature Med. 2020;26(8):1200‐1204. [DOI] [PubMed] [Google Scholar]
- 16. Felix HC, Seaberg B, Bursac Z, Thostenson J, Stewart MK. Why do patients keep coming back? Results of a re‐admitted patient survey. Soc Work Health Care. 2015;54(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McAuley AJ, Kuiper MJ, Durr PA, et al. Experimental and in silico evidence suggests vaccines are unlikely to be affected by D614G mutation in SARS‐CoV‐2 spike protein. NPJ Vaccines. 2020;5(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parra LM, Cantero M, Morrás I, et al. Hospital readmissions of discharged patients with COVID‐19. Int J Gen Med. 2020;13:1359‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall VJ, Foulkes S, Charlett A, et al. SARS‐CoV‐2 infection rates of antibody‐positive compared with antibody‐negative health‐care workers in England: a large, multicentre, prospective cohort study (SIREN). The Lancet. 2021;397(10283):1459‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J, Xu X, Hu J, et al. Clinical course and risk factors for recurrence of positive SARS‐CoV‐2 RNA: a retrospective cohort study from Wuhan, China. Aging. 2020;12(17):16675‐16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yahav D, Yelin D, Eckerle I, et al. Definitions for coronavirus disease 2019 re‐infection, relapse and PCR re‐positivity. Clin Microbiol Infect. 2021;27(3):315‐318. 10.1016/j.cmi.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Investigative criteria for suspected cases of SARS‐CoV‐2 reinfection (ICR).2020. Accessed April, 2021. https://www.cdc.gov/coronavirus/2019-ncov/php/invest-criteria.html
- 23. Abu‐Raddad LJ, Chemaitelly H, Malek JA, et al. Assessment of the risk of SARS‐CoV‐2 re‐infection in an intense re‐exposure setting. Clin Infect Dis. 2020:ciaa1846. 10.1093/cid/ciaa1846 [DOI] [Google Scholar]
- 24. Pilz S, Chakeri A, Ioannidis JP, et al. SARS‐CoV‐2 re‐infection risk in Austria. Eur J Clin Invest. 2021;51(4):e13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Breathnach AS, Riley PA, Cotter MP, Houston AC, Habibi MS, Planche TD. Prior COVID‐19 significantly reduces the risk of subsequent infection, but re‐infections are seen after eight months. J Infect. 2021;82(4):e11‐e12. 10.1016/j.jinf.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caralis P. Case Reports of COVID 19 Recurrence. J Prim Care Community Health. 2021;12:2150132720982752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavanaugh AM. Suspected recurrent SARS‐CoV‐2 infections among residents of a skilled nursing facility during a second COVID‐19 outbreak—Kentucky, July–November 2020. MMWR Morb Mortal Wkly Rep. 2021;70:273‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das P, Satter SM, Ross AG, et al. A case series describing the recurrence of COVID‐19 in patients who recovered from initial illness in Bangladesh. Tropical Med Infect Dis. 2021;6(2):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao G, Zhu Z, Fan L, et al. Absent immune response to SARS‐CoV‐2 in a 3‐month recurrence of coronavirus disease 2019 (COVID‐19) case. Infection. 2020;49:1‐5. 10.1007/s15010-020-01485-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. An J, Liao X, Xiao T, et al. Clinical characteristics of recovered COVID‐19 patients with re‐detectable positive RNA test. Ann Transl Med. 2020;8(17):1084. 10.21037/atm-20-5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu H, Fu L, Jin Y, et al. Clinical features of COVID‐19 convalescent patients with re‐positive nucleic acid detection. J Clin Lab Anal. 2020;34(7):e23392. 10.1002/jcla.23392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu J, Peng J, Xiong Q, et al. immunological and virological characterization of COVID‐19 patients that test re‐positive for SARS‐CoV‐2 by RT‐PCR. EBioMedicine. 2020;59:102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian M, Long Y, Hong Y, Zhang X, Zha Y. The treatment and follow‐up of ‘recurrence' with discharged COVID‐19 patients: data from Guizhou, China. Environ Microbiol. 2020;22(8):3588‐3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen SL, Xu H, Feng HY, et al. Epidemiological and clinical findings of short‐term recurrence of severe acute respiratory syndrome coronavirus 2 ribonucleic acid polymerase chain reaction positivity in 1282 discharged coronavirus disease 2019 cases: a multicenter, retrospective, observational study. Open Forum Infect Dis. 2020;7:ofaa432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng J, Zhou R, Chen F, et al. Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID‐19 patients in Guangzhou, China: a prospective cohort study. PLoS Neglected Trop Dis. 2020;14(8):e0008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan B, Liu HQ, Yang ZR, et al. Recurrence of positive SARS‐CoV‐2 viral RNA in recovered COVID‐19 patients during medical isolation observation. Sci Rep. 2020;10(1):11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang C, Jiang M, Wang X, et al. Viral RNA level, serum antibody responses, and transmission risk in recovered COVID‐19 patients with recurrent positive SARS‐CoV‐2 RNA test results: a population‐based observational cohort study. Emerg Microbes Infect. 2020;9(1):2368‐2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shui TJ, Li C, Liu HB, Chen X, Zhang BK. Characteristics of recovered COVID‐19 patients with recurrent positive RT‐PCR findings in Wuhan, China: a retrospective study. BMC Infect Dis. 2020;20(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atalla E, Kalligeros M, Giampaolo G, Mylona EK, Shehadeh F, Mylonakis E. Readmissions among patients with COVID‐19. Int J Clin Pract. 2021;75(3):e13700. [DOI] [PubMed] [Google Scholar]
- 40. Yeo I, Baek S, Kim J, et al. assessment of thirty‐day readmission rate, timing, causes and predictors after hospitalization with COVID‐19. J Intern Med. 2021;290:157‐165. 10.1111/joim.13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lavery AM, Preston LE, Ko JY, et al. Characteristics of hospitalized COVID‐19 patients discharged and experiencing same‐hospital readmission—United States, March–August 2020. Morb Mortal Wkly Rep. 2020;69(45):1695‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Y, Hu Y, Yu Y, et al. Positive result of Sars‐Cov‐2 in faeces and sputum from discharged patients with COVID‐19 in Yiwu, China. J Med Virol. 2020;92(10):1938‐1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cao H, Ruan L, Liu J, Liao W. The clinical characteristic of eight patients of COVID‐19 with positive RT‐PCR test after discharge. J Med Virol. 2020;92(10):2159‐2164. 10.1002/jmv.26017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li C, Luo F, Xie L, et al. study of fifteen COVID‐19 patients with positive RT‐PCR retest results after discharge. Quant Imaging Med Surg. 2020;10(6):1318‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeon WH, Seon JY, Park SY, Oh IH. Analysis of risk factors on readmission cases of COVID‐19 in the Republic of Korea: using nationwide health claims data. Int J Environ Res Public Health. 2020;17(16):5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. UyaroĞlu OA, BaŞaran NÇ, ÖziŞik L. et al. Thirty‐day readmission rate of COVID‐19 patients discharged from a tertiary care university hospital in Turkey: an observational, single‐center study. Int J Qual Health Care. 2021.33(1):mzaa144. 10.1093/intqhc/mzaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang Z, Chen X, Huang R, et al. Atypical presentations of coronavirus disease 2019 (COVID‐19) from onset to readmission. BMC Infect Dis. 2021;21(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ye S, Hiura G, Fleck E, et al. Hospital readmissions after implementation of a discharge care program for patients with COVID‐19 illness. J Gen Intern Med. 2021;36(3):722‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ren X, Ren X, Lou J, et al. A systematic review and meta‐analysis of discharged COVID‐19 patients retesting positive for RT‐PCR. EClinicalMedicine. 2021;34:100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Piri SM, Edalatfar M, Shool S, Jalalian MN, Tavakolpour S. A systematic review on the recurrence of SARS‐CoV‐2 virus: frequency, risk factors, and possible explanations. Infect Dis. 2021;53(5):315‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. SeyedAlinaghi S, Oliaei S, Kianzad S, et al. Reinfection risk of novel coronavirus (CoVID‐19): a systematic review of current evidence. World J Virol. 2020;9(5):79‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nebehay S WHO is investigating reports of recovered COVID patients testing positive again; 2020. Accessed June, 2021. https://www.reuters.com/article/us-health-coronavirus-who/who-is-investigating-reports-of-recoveredcovid-patients-testing-positive-again-idUSKCN21T0F1
- 53. Arevalo‐Rodriguez I, Buitrago‐Garcia D, Simancas‐Racines D, et al. False‐negative results of initial RT‐PCR assays for COVID‐19: a systematic review. PLoS One. 2020;15(12):e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS‐CoV‐2 in patients recovered from COVID‐19. J Med Virol. 2020;92(11):2366‐2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miyamae Y, Hayashi T, Yonezawa H, et al. Duration of viral shedding in asymptomatic or mild cases of novel coronavirus disease 2019 (COVID‐19) from a cruise ship: A single‐hospital experience in Tokyo, Japan. Int J Infect Dis. 2020;97:293‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou M, Yu FF, Tan L, et al. Clinical characteristics associated with long‐term viral shedding in patients with coronavirus disease 2019. Am J Transl Res. 2020;12(10):6954‐6964. [PMC free article] [PubMed] [Google Scholar]
- 57. Wang K, Zhang X, Sun J, et al. Differences of severe acute respiratory syndrome coronavirus 2 shedding duration in sputum and nasopharyngeal swab specimens among adult inpatients with coronavirus disease 2019. Chest. 2020;158(5):1876‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Falahi S, Kenarkoohi A. Sex and gender differences in the outcome of patients with COVID‐19. J Med Virol. 2021;93(1):151‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Azam M, Sulistiana R, Ratnawati M, et al. Recurrent SARS‐CoV‐2 RNA positivity after COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2020;10(1):20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chakravarty D, Nair SS, Hammouda N, et al. Sex differences in SARS‐CoV‐2 infection rates and the potential link to prostate cancer. Commun Biol. 2020;3(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


