Abstract
Chloroquine or its derivative hydroxychloroquine (HCQ) combined with or without azithromycin (AZ) have been widely investigated in observational studies as a treatment option for coronavirus 2019 (COVID‐19) infection. The network meta‐analysis aims to summarize evidence from randomized controlled trials (RCTs) to determine if AZ or HCQ is associated with improved clinical outcomes. PubMed and Embase were searched from inception to March 7, 2021. We included published RCTs that investigated the efficacy of AZ, HCQ, or its combination among hospitalized patients with COVID‐19 infection. The outcomes of interest were all‐cause mortality and the use of mechanical ventilation. The pooled odds ratio was calculated using a random‐effect model. A total of 10 RCTs were analyzed. Participant's mean age ranged from 40.4 to 66.5 years. There was no significant effect on mortality associated with AZ plus HCQ (odds ratio [OR] = 0.562 [95% confidence interval {CI}: 0.168–1.887]), AZ alone (OR = 0.965 [95% CI: 0.865–1.077]), or HCQ alone (OR = 1.122 [95% CI: 0.995–1.266]; p = 0.06). Similarly, based on pooled effect sizes derived from direct and indirect evidence, none of the treatments had a significant benefit in decreasing the use of mechanical ventilation. No heterogeneity was identified (Cochran's Q = 1.68; p = 0.95; τ 2 = 0; I 2 = 0% [95% CI: 0%–0%]). Evidence from RCTs suggests that AZ with or without HCQ was not associated with a significant effect on the mortality or mechanical ventilation rates in hospitalized patients with COVID‐19. More research is needed to explore therapeutics agents that can effectively reduce the mortality or severity of COVID‐19.
Keywords: azithromycin, chloroquine, coronavirus, coronavirus disease 2019, hydroxychloroquine, mechanical ventilation, mortality, network meta‐analysis
Abbreviations
- AZ
azithromycin
- HCQ
hydroxychloroquine
- ICU
intensive care unit
- RCT
randomized controlled trial
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the etiological agent of coronavirus disease 2019 (COVID‐19), is an enveloped virus with a positive‐sense single‐stranded ribonucleic acid (RNA) genome. This virus was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China. On March 12, 2020 this local epidemic was escalated to a pandemic by the World Health Organization (WHO) due to its rapid rate of global spread. Ever since its emergence, COVID‐19 has devastated the healthcare, socioeconomic and educational sectors of society through a ripple effect. Infected patients can have a wide range of presentations ranging from asymptomatic infection to acute respiratory distress syndrome and death. 1 Worldwide case fatality rate ranges between 2% and 3%. 2 , 3 In addition to affecting the respiratory tract, SARS‐CoV‐2 infection has been shown to establish an proinflammatory milieu capable of promoting various complications such as thrombosis, cardiac arrhythmias, exacerbations of heart failure, acute kidney injury, stroke and encephalitis. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 The medical, economical and psychological impact of social distancing, quarantining and isolation may have lingering effects in the community. 13 , 14 , 15 These challenges have driven the scientific community to seek therapies aiming at treatment and prevention of COVID‐19 in a race against time to curtail the morbidity and mortality associated with this rapidly spreading disease. 16 , 17 , 18 Various pharmacological agents have been studied over the course of the pandemic for their effectiveness on COVID‐19. Azithromycin (AZ) and hydroxychloroquine (HCQ) were extensively investigated in a series of observational studies and randomized controlled trials for their real‐world efficacy against COVID‐19, due to their availability, prior success in the treatment of inflammatory airway disease, in vitro antiviral activity, cost, and publicity. Previous meta‐analyses and systematic reviews have analyzed the evidence based on mostly observational studies and noted heterogeneous results. To validate the potential efficacy of AZ and HCQ, a network meta‐analysis including only randomized clinical trials was undertaken to estimate the treatment effect of these agents based on higher‐quality data.
2. METHODS
2.1. Search strategies and selection criteria
Systematic literature searches were performed in PubMed and Embase from inception to March 7, 2021. The selection criteria included (1) randomized clinical trials that investigated the efficacy of AZ, HCQ, or its combination; (2) studies that were published as original research articles; and (3) studies that reported the clinical outcomes at the end of follow‐up. All searches were limited to the English language. Duplicates were removed before screening references. Detailed queries are provided in Table S1.
2.2. Study outcomes and treatment comparison
In this analysis, the outcomes of interest are (1) all‐cause mortality and (2) the use of mechanical ventilation. Randomized trials that involve any of the following treatment comparisons were analyzed: (1) AZ plus HCQ (AZ + HCQ); (2) AZ; (3) HCQ; and (4) usual standard of care or placebo (control group).
2.3. Data extraction and quality assessment
Data extracted from each study included study design, patient characteristics, and the number of events (all‐cause mortality and use of mechanical ventilation). Database search, article screening, and study selection were performed independently by two investigators using a standardized approach. Disagreement in extracted data was adjudicated by a third investigator. A flow diagram depicting the process of literature search and screening is provided in Figure 1.
Figure 1.
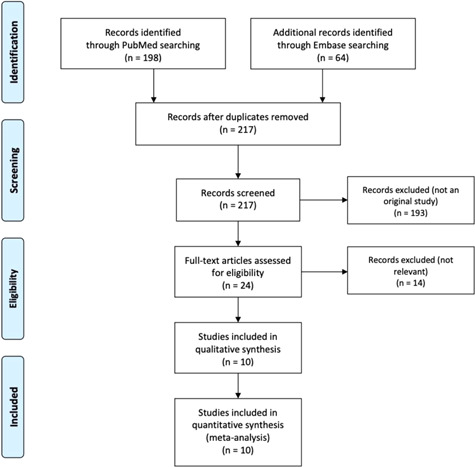
Study flow diagram
Two independent investigators assessed the quality of included studies with the revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). Disagreement in the quality assessment was resolved by discussion and consensus.
2.4. Statistical analysis
A network meta‐analysis using the netmeta package was performed to compare the treatment effect. The measure of treatment effect was expressed as odds ratio (OR) with 95% confidence interval (CI). A fixed‐effect or random‐effect model was fitted to estimate the treatment effect based on the presence of heterogeneity. Heterogeneity across the studies was evaluated using Cochran's Q test (with the threshold of p > 0.10) and Higgins's I 2 statistic (with the values of 0.25, 0.50, and 0.75 indicating a low, moderate, and a high degree of heterogeneity, respectively). Analysis was performed using R software (Version 3.5.2; The R Foundation for Statistical Computing).
3. RESULTS
3.1. Summary of included studies
A total of 10 randomized controlled trials were included in the final analysis (Figure 1). 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 The study design and patient characteristics of included studies were summarized in Table 1. Results of risk of bias assessment were presented in Figure S1. Three studies were considered to be at a high risk of bias.
Table 1.
Summary of included studies
| Study name | Design | Intervention | Dose | Follow‐up | Age, year | Male, % | White, % | BMI | Diabetes, % | Heart disease, % | Chronic lung disease, % | Severe liver disease, % | Severe kidney impairment, % | ICU admission, % | Use of corticosteroids, % | SARS‐CoV‐2 test positive, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RECOVERY 23 | Randomized, controlled, open‐label, platform trial | AZ vs. control | AZ 500 mg once per day by mouth or intravenously for 10 days or until discharge | 28 days | 65.4 (15.6) vs. 65.2 (15.7) | 62 vs. 62 | 76 vs. 77 | NR | 27 vs. 28 | 27 vs. 26 | 24 vs. 25 | 2 vs. 1 | 6 vs. 6 | NR | 61 vs. 61 | 91 vs. 92 |
| DISCOVERY 24 | Randomized, controlled, open‐label trial | HCQ vs. control | HCQ: four tablets (200 mg) at hour 0, and at hour 6, and, starting at hour 12, two tablets twice daily for 10 days. | 28 days | <50 years: 335 vs. 317; 50–69 years: 410 vs. 396; ≥70 years: 202 vs. 193 | 50.6 vs. 59.1 | 199 vs. 205 | NR | 199 vs. 205 | 193 vs. 194 | 62 vs. 66 | 15 vs. 14 | NR | NR | NR | NR |
| HAHPS 20 | Randomized Open‐Label, Active Comparator Trial | HCQ vs. AZ | AZ loading dose of 500 mg on the first day, followed by 250 mg daily for the next 4 days. HCQ orally as a loading dose of 400 mg twice on the first day followed by 200 mg twice daily for the following 4 days | 28 days | 51 (42–60) vs. 58 (43–68) | 67 vs. 54 | 60 vs. 67 | NR | 35 vs. 33 | 9 vs. 7 | NR | 0 vs. 2 | 7 vs. 12 | NR | 2 vs. 5 (before enrollment) | 100 |
| TEACH 27 | Double‐blind, placebo‐controlled, randomized clinical trial | HCQ vs. control | Dosing of both HCQ and calcium citrate was 400 mg (2 tablets) by mouth 2 times per day (day 1) and 200 mg (1 tablet) by mouth 2 times per day (days 2–5); | 30 days | 66.5 (16.4) vs. 65.8 (16.0) | 67.2 vs. 50.8 | 34.3 vs. 29.5 | BMI < 30: 74.7 vs. 52.4; BMI ≥ 30: 36.0 vs. 47.7 | 28.4 vs. 36.1 | 31.3 vs. 21.3 | 7.5 vs. 6.6 | NR | 10.4 vs. 4.9 | 13.4 vs. 8.2 | 10.4 vs. 9.8 | 100 |
| ORCHID 26 | Randomized, blinded, placebo‐controlled trial | HCQ vs. control | HC 400 mg twice daily for 2 doses, then 200 mg twice daily for 8 doses | 28 days | 58 (45–69) vs. 57 (43–68) | 55.8 vs. 55.7 | 31.0 vs. 28.6 | 31.3 (26.4–37.2) 31.1 (27.2–36.5) | 36.4 vs. 32.9 | 7.9 vs. 9.7 | 7.4 vs. 8.9 | NR | 11.6 vs. 5.9 | NR | 18.4 of the participants | 100 |
| RECOVERY 28 | Randomized, controlled, open‐label platform trial | HCQ vs. control | hydroxychloroquine sulfate (in the form of a 200‐mg tablet) in a loading dose of four tablets (total dose, 800 mg) at baseline and at 6 h, which was followed by two tablets (total dose, 400 mg) starting at 12 h after the initial dose and then every 12 h for the next 9 days or until discharge, whichever occurred earlier | 28 days | 65.2 ± 15.2 vs. 65.4 ± 15.4 | 61.5 vs. 62.6 | 75.7 vs. 72.8 | NR | 27.4 vs. 27.1 | 27 vs. 25 | 21.4 vs. 22.6 | 1.2 vs. 1.5 | 7.1 vs. 8.3 | 16.7 vs. 16.9 | NR | 89.6 vs. 90.9 |
| COALITION‐II 22 | Open‐label, randomized clinical trial | AZ vs. control | 500 mg via oral, nasogastric, or intravenous administration once daily for 10 days | 30 days | 59.4 (49.3–70.0) vs. 60·2 (52.0–70.1) | 65 vs. 67 | NR | 26.4 (23.5–31.8) vs. 27·2 (23.7–31.7) | 38 vs. 39 | 7 vs. 5 | 6 vs. 7 | NR | 12 vs. 10 | NR | 21 vs. 15 | 100 |
| Sekhavati 25 | Open‐label, randomized controlled trial | AZ + HCQ vs. HCQ | oral AZ 500 mg daily, oral LPV/r 400/100 mg twice daily and oral HCQ 400 mg daily for 5 days | 30 days after discharge | 54.38 ± 15.92 vs. 59.89 ± 15.55 | 50.00 vs. 41.82 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 100 |
| Abd‐Elsalam 19 | Open‐label, randomized controlled trial | HCQ vs. control | HCQ 400 mg twice daily (in day 1) followed by 200 mg tablets twice daily | 4 weeks | 40.35 ± 18.65 vs. 41.09 ± 20.07 | 57.7 vs. 59.8 | NR | BMI < 30: 37.1 vs. 39.2; BMI≥ 62.8 vs. 62.8 | NR | NR | NR | 0.0 vs. 2.1 | 2.1 vs. 4.1 | 11.3 vs. 13.4 | NR | 100 |
| COALITION‐I 21 | Randomized, open‐label, three‐group, controlled trial | AZ + HCQ vs. HCQ vs. control | HC 400 mg twice daily vs. HC 400 mg twice daily plus AZ 500 mg once daily for 7 days | 15 days | 49.6 ± 14.2 vs. 51.3 ± 14.5 vs. 49.9 ± 15.1 | 56.7 vs. 64.3 vs. 54.2 | NR | NR | 18.4 vs. 21.3 vs. 17.6 | 1.8 vs. 1.4 vs. 1.3 | 1.8 vs. 1.8 vs. 1.8 | NR | 0.9 vs. 0.5 vs. 0.9 | 13.8 vs. 14.5 vs. 13.2 | 1.8 vs. 0.5 vs. 1.3 | 79.3 vs. 71.9 vs. 76.2 |
Abbreviations: AZ, azithromycin; BMI, body mass index; HCQ, hydroxychloroquine; ICU, intensive care unit; NR, not reported.
Eight of the studies were open‐label. The majority of the studies had two treatment arms and a follow‐up duration of 1 month, except for COALITION‐I that had a three‐arm design and 15 days of follow‐up. The mean age of enrolled patients ranges from 40.4 to 66.5 years. Men are slightly more predominant than women in all of the included studies. The prevalence of comorbidities and the proportions of corticosteroid usage vary widely across the studies.
3.2. Mortality
The network graph was provided in Figure S2. Compared to the control group (i.e., patients who received usual care or placebo), there was no significant effect on mortality associated with AZ + HCQ (OR = 0.562 [95% CI: 0.168–1.887]; p = 0.35), AZ alone (OR = 0.965 [95% CI: 0.865–1.077]; p = 0.52), or HCQ alone (OR = 1.122 [95% CI: 0.995–1.266]; p = 0.06) (Figure 2).
Figure 2.

Pooled treatment effect on mortality. AZ, azithromycin; HCQ, hydroxychloroquine
The effect on mortality of all treatment comparisons was summarized in Table 2. Based on pooled effect sizes derived from direct evidence (dark shaded area), none of the pairwise comparisons demonstrated a significant difference in mortality. Similarly, based on pooled effect sizes derived from direct and indirect evidence (light shaded area), none of the treatments had a significant benefit in reducing mortality.
Table 2.
Network league table for mortality
| Treatment | Control | AZ | AZ + HCQ | HCQ |
|---|---|---|---|---|
| Control | – | 1.031 (0.924; 1.151) | 1.677 (0.394; 7.127) | 0.896 (0.794; 1.011) |
| AZ | 1.036 (0.929; 1.156) | – | . | 0.143 (0.016; 1.243) |
| AZ + HCQ | 1.778 (0.530; 5.963) | 1.715 (0.509; 5.783) | – | 0.500 (0.133; 1.873) |
| HCQ | 0.891 (0.790; 1.005) | 0.860 (0.731; 1.012) | 0.501 (0.150; 1.680) | – |
Abbreviations: AZ, azithromycin; HCQ, hydroxychloroquine.
No significant heterogeneity was identified (Cochran's Q = 3.62; p = 0.89; t 2 = 0; I 2 = 0% [95% CI: 0%–22.2%]). In the design‐by‐treatment interaction model, no significant inconsistency was detected between designs (Q = 3.62; p = 0.43). Additionally, there was no significant inconsistency between direct and indirect evidence (Figure 3).
Figure 3.
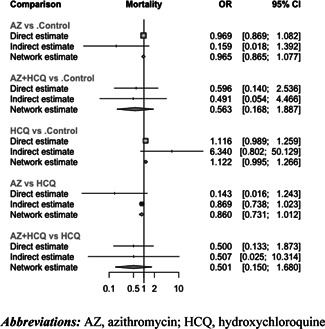
Comparing direct and indirect evidence on mortality. AZ, azithromycin; HCQ, hydroxychloroquine
3.3. Use of mechanical ventilation
The network graph was provided in Figure S3. Compared to the control group (i.e., patients who received usual care or placebo), there was no significant effect on the use of mechanical ventilation associated with AZ + HCQ (OR = 1.689 [95% CI: 0.892–3.198]; p = 0.11), AZ alone (OR = 0.930 [95% CI: 0.788–1.097]; p = 0.39), or HCQ alone (OR = 1.125 [95% CI: 0.942–1.345]; p = 0.19) (Figure 4).
Figure 4.

Pooled treatment effect on the use of mechanical ventilation. AZ, azithromycin; HCQ, hydroxychloroquine
The effect on the use of mechanical ventilation of all treatment comparisons was summarized in Table 3. Based on pooled effect sizes derived from direct evidence (dark shaded area), none of the pairwise comparisons demonstrated a significant difference. Similarly, based on pooled effect sizes derived from direct and indirect evidence (light shaded area), none of the treatments had a significant benefit in decreasing the use of mechanical ventilation.
Table 3.
Network league table for the use of mechanical ventilation
| Treatment | Control | AZ | AZ + HCQ | HCQ |
|---|---|---|---|---|
| Control | – | 1.076 (0.912; 1.269) | 0.600 (0.282; 1.278) | 0.889 (0.744; 1.062) |
| AZ | 1.076 (0.912; 1.269) | – | . | . |
| AZ + HCQ | 0.592 (0.313; 1.121) | 0.550 (0.285; 1.065) | – | 1.521 (0.713; 3.244) |
| HCQ | 0.889 (0.744; 1.062) | 0.826 (0.648; 1.053) | 1.501 (0.792; 2.842) | – |
Abbreviations: AZ, azithromycin; HCQ, hydroxychloroquine.
No significant heterogeneity was identified (Cochran's Q = 1.68; p = 0.95; t 2 = 0; I 2 = 0% [95% CI: 0%–0%]). In the design‐by‐treatment interaction model, no significant inconsistency was detected between designs (Q = 0.00; p = 0.95). Additionally, there was no significant inconsistency between direct and indirect evidence (Figure 5).
Figure 5.
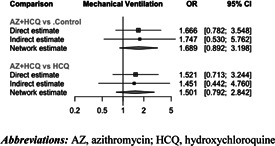
Comparing direct and indirect evidence on the use of mechanical ventilation. AZ, azithromycin; HCQ, hydroxychloroquine
4. DISCUSSION
This systematic review and network meta‐analysis summarized the current evidence on the efficacy of chloroquine or its derivative HCQ combined with or without AZ on the COVID‐19 infection. Based on this data, none of the examined agents either alone or in combination were effective in the reduction of mortality or mechanical ventilation rate.
Following observational studies, several randomized clinical trials were conducted to validate the findings regarding the efficacy of AZ and HCQ. A randomized clinical trial conducted in the early pandemic failed to support the efficacy of HCQ in symptom severity reduction. 29 The COALITION I and COALITION II trials showed that for patients hospitalized with mild to moderate COVID‐19, management with AZ and HCQ was not associated with any improvement in mortality, duration of hospital stay, or clinical status in terms of invasive and noninvasive ventilation as assessed using an ordinal outcome scale. 21 , 22 The RECOVERY trial included 7763 participants to study the benefit of the provision of AZ only to patients hospitalized with COVID‐19. 23 Information on laboratory markers of viral load, inflammatory status, immune response, coexistent bacterial infection, or use of nonmacrolide antibiotics was not collected, nor was information on radiological or physiological outcomes. The RECOVERY trial also compared HCQ with control among 4716 patients and showed no difference in mortality between the HCQ group and the usual‐care group. 28 Of note, patients in the HCQ group were less likely to be discharged from the hospital alive within 28 days than those in the usual‐care group. With respect to adverse events, there was no difference in the incidence of new major cardiac arrhythmia among the patients who received HCQ. The TEACH trial was a double‐blind randomized controlled trial designed to evaluate the efficacy of a 5‐day course of HCQ. 27 The results demonstrated that the use of HCQ was not associated with improved mortality or any improvement in markers of clinical severity, such as the use of invasive and noninvasive ventilation. Furthermore, patient enrolled in the HCQ arm had longer hospital stays owing to abnormalities in QT intervals and d‐dimer levels which required inpatient management. The ORCHID trial assessed improvement in clinical status in patients hospitalized with COVID‐19 treated with HCQ using a WHO‐approved 7‐category respiratory status ordinal scale (COVID Outcomes Score). 26 The ORCHID study found no benefit of HCQ use. The HAHPS trial compared HCQ with AZ in 85 patients, and showed no benefit of HCQ over AZT; moreover, patients treated with HCQ had a higher rate of acute kidney injury. 20 In the open‐label DISCOVERY trial, 11 330 patients were randomly assigned to HCQ, remdesivir, lopinavir, interferon, or control. 24 In this trial conducted across 30 countries, none of the treatments were successful in reducing in‐hospital mortality, initiation of ventilation, and hospitalization duration. Of these, 947 were randomized to receive HCQ and 906 patients were included in the control group. HCQ was not associated with a significant difference in mortality compared to the control group.
Previously reported data indicating the efficacy of chloroquine or HCQ against coronavirus infection were mostly observational or from in vitro experiments. 30 , 31 This led to its testing in randomized clinical trials. HCQ is an aminoquinoline derivative primarily used as an antimalarial and immunomodulatory agent. 32 The compound also has antiautophagic activity, which has driven widespread testing of its antiviral activity. As a lysosomotropic agent, HCQ alkalizes intralysosomal pH, leading to an impairment of autophagic protein degradation, a step required for successful viral uncoating. It has also been shown that HCQ‐induced accumulation of inefficient autophagosomes may result in cell death in tumor cells dependent on autophagy for survival. 33 Macrolides are commonly used to treat bacterial infections of the lower respiratory tract because of their activity against Gram‐positive bacteria and atypical pathogens such as Mycoplasma pneumoniae and Legionella spp, as well as their excellent tissue penetration. 34 , 35 More than 75% of patients with COVID‐19 who were admitted to hospital in the UK during 2020 were prescribed antibiotics and the widespread clinical use of macrolides in COVID‐19 is likely to stem from concerns of bacterial superinfection rather than previously proposed immunomodulatory action. Prior studies suggesting the efficacy of these agents against Middle East respiratory syndrome and severe acute respiratory syndrome in vitro, were the first pieces of evidence. Later, studies also reported the in vitro efficacy of these agents against SARS‐CoV‐2. 36 Another report also demonstrated that a combination of HCQ and AZ were able to inhibit the replication of SARS‐CoV‐1 and 2 viruses. 37 Interestingly, a study showed that the combination of these two antimicrobial agents possesses synergistic interactions that not only neutralize the viral shedding but also inhibit host lysosomal enzyme activity and cease viral entry to other cells. 38 , 39 This study explained that both AZ and HCQ are weak bases and are more prone to accumulate in the endosomal vesicles and lysosomes, increasing their pH and making them dysfunctional. This property was believed to block viral shedding from lysosomes and uncoating of enveloped viruses. 40 However, in vitro activity does not necessarily confer clinical efficacy. Another important point to consider is the avoidance of adding harm to the patients while using unnecessary treatments based on low‐quality evidence. A recent meta‐analysis with 11 932 participants showed that the combination of AZ and HCQ can increase mortality among patients with COVID‐19 infection. 36 One possible mechanism for this observation is that this combination was associated with an increase in life‐threatening cardiovascular events. Both HCQ and AZ are associated with prolongation of QT interval and provide a substrate for abnormal arrhythmogenic activity. 41 , 42 Furthermore, the combination of these two agents has been observed to prolong the QT interval to a greater extent than each agent alone. 43 , 44 In line with this finding, another systematic review of 14 studies reported a higher rate of mortality in patients receiving both HCQ and AZ. This study also reported an increase in the duration of hospital stay among patients receiving these two agents. 45 Of note, both of these studies included mostly observational studies and the heterogeneity was high among the included studies. Based on a large‐sized network meta‐analysis with more than 49 000 participants, HCQ reduced neither mortality rate nor disease progression. In addition, a high dose of HCQ (>600 mg/day) was associated with a slight increase in noncardiac side effects. This study shared concerns regarding the cardiac safety of the combination of HCQ and AZ especially among patients with preexisting cardiovascular disease. Based on the data from this meta‐analysis, in the context of using HCQ combined with AZ, the pooled incidence of malignant cardiac arrhythmias, such as torsade de points and severe ventricular arrhythmia, was 12.27% in patients with the cardiac disease while in a healthy population with the normal cardiac function it was 0.01%. 46 In an attempt to explain the inefficacy of HCQ, one in vitro study revealed that chloroquine was incapable of preventing viral shedding and it does not prevent the virus from entry to the lung cell. 47 Contrary to our data, one systematic review of 43 reports claimed that HCQ use was consistently associated with better outcomes among patients with Coronavirus 2019, especially when used early in the course of the disease. 48
Given that the COVID‐19 infection‐associated acute respiratory distress syndrome is the important constituent of its case fatality in the ICU setting, we also investigated whether the use of HCQ with or without AZ can reduce the need for mechanical ventilation. Our finding showed that HCQ combined with or without AZ was unable to reduce this risk. This finding was in agreement with previous reports. 49 , 50 Contrary to our results, there is some evidence suggesting the efficacy of HCQ in preventing the disease progression and namely the need for invasive ventilation. However, the studies either had a small sample size or an observational design and therefore must be interpreted cautiously. 51 , 52
5. LIMITATIONS
As with any other study, this meta‐analysis has some limitations that need to be discussed. First, 8 out of 10 studies were open‐labeled, which may have introduced selection bias. Second, the included studies were different in terms of patients’ characteristics, comorbidities, and corticosteroid usage. Third, in one of the included trials, the patients were followed for 15 days rather than a month, which may cause an underestimation of the mortality rate. Fourth, individual patient‐level data were not available for this analysis. Fifth, due to exclusion of patients at risk of prolonged QT syndrome in some of the included trials, the safety of off‐label use of HCQ and AZ may be overestimated across these trials. Sixth, due to large heterogeneity among the trials in terms of studied endpoints, we only used two important outcomes and did not investigate the efficacy of the applied treatment strategies on other endpoints such as hospital stay or symptom severity. Seventh, there was some inconsistency among the studies in terms of the duration and dosage of studied medications. Finally, the results of this study cannot be generalized to patients with mild COVID‐19 infection or prolonged QT interval.
6. CONCLUSION
Evidence from this systematic review and network meta‐analysis suggests that chloroquine or its derivative, HCQ, combined with or without AZ did not change the mortality or mechanical ventilation rates in hospitalized patients with COVID‐19. Current evidence does not support using either of these agents alone or in combination in the management of hospitalized patients with COVID‐19 infection. Pharmacological agents used as therapeutics and postexposure prophylaxis of hospitalized patients with COVID‐19 demand further exploration and research. Despite the successful development of various vaccinations for primary prophylaxis of COVID‐19 such as BNT162b2, messenger RNA‐1273, Ad26.COV2.S, ChAdOx1 nCoV‐19, widespread global availability of such vaccines is limited due to numerous economical and logistical factors. 53 , 54 , 55 , 56 Modulation of viral glycoprotein components, receptors, suppression of cytokine storm using interleukin (IL)‐1, IL‐6, and JAK/STAT signaling pathways, RNA‐dependent RNA polymerase inhibition are being studied to reduce disease load. 57 , 58 , 59 , 60 , 61 , 62 The success of dexamethasone in reducing in‐hospital COVID‐19 associated mortality suggests immunomodulation may be an attractive therapeutic target in this patient population. 63 , 64 , 65 , 66 , 67 , 68 , 69
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information.
Chi G, Memar Montazerin S, Lee JJ, et al. Effect of azithromycin and hydroxychloroquine in patients hospitalized with COVID‐19: network meta‐analysis of randomized controlled trials. J Med Virol. 2021;93:6737‐6749. 10.1002/jmv.27259
REFERENCES
- 1. Li L, Huang T, Wang, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020;92(6):577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID‐19 case fatality rate. Lancet Infect Dis. 2020;20(7):776‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghayda RA, Lee KH, Han YJ, et al. Estimation of global case fatality rate of coronavirus disease 2019 (COVID‐19) using meta‐analyses: Comparison between calendar date and days since the outbreak of the first confirmed case. Int J Infect Dis. 2020;100:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asadi‐Pooya AA, Simani L. Central nervous system manifestations of COVID‐19: a systematic review. J Neurol Sci. 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of covid‐19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M. Inhibition of SARS‐coronavirus infection in vitro by S‐nitroso‐N‐acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 2004;8(4):223‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID‐19. Am J Emerg Med. 2020;38(7):1504‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181(5):1036‐1045 e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou X, Cheng Z, Luo L, et al. Incidence and impact of disseminated intravascular coagulation in COVID‐19 a systematic review and meta‐analysis. Thromb Res. 2021;201:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coopersmith CM, Antonelli M, Bauer SR, et al. The surviving sepsis campaign: research priorities for coronavirus disease 2019 in critical illness. Crit Care Med. 2021;49(4):598‐622. [DOI] [PubMed] [Google Scholar]
- 17. Martinez‐Rojas MA, Vega‐Vega O, Bobadilla NA. Is the kidney a target of SARS‐CoV‐2? Am J Physiol Renal Physiol. 2020;318(6):F1454‐F1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaafarani HMA, El Moheb M, Hwabejire JO, et al. Gastrointestinal Complications in critically Ill patients with COVID‐19. Ann Surg. 2020;272(2):e61‐e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abd‐Elsalam S, Esmail ES, Khalaf M, et al. Hydroxychloroquine in the treatment of COVID‐19: a multicenter randomized controlled study. Am J Trop Med Hyg. 2020;103(4):1635‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Brown SM, Peltan ID, Webb B, et al. Hydroxychloroquine vs. azithromycin for hospitalized patients with COVID‐19 (HAHPS): results of a randomized, active comparator trial. Ann Am Thorac Soc. 2020;17:1008‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate covid‐19. N Engl J Med. 2020;383(21):2041‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furtado R, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID‐19 in Brazil (COALITION II): a randomised clinical trial. The Lancet. 2020;396(10256):959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abaleke E, Abbas M, Abbasi S, et al. Azithromycin in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. The Lancet. 2021;397(10274):605‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Consortium WST . Repurposed antiviral drugs for COVID‐19—interim WHO SOLIDARITY trial results. N Engl J Med. 2021;384(6):497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sekhavati E, Jafari F, SeyedAlinaghi S, et al. Safety and effectiveness of azithromycin in patients with COVID‐19: an open‐label randomised trial. Int J Antimicro Ag. 2020;56(4):106143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324(21):2165‐2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ulrich RJ, Troxel AB, Carmody E, et al. Treating COVID‐19 With Hydroxychloroquine (TEACH): A Multicenter, Double‐Blind Randomized Controlled Trial in Hospitalized Patients. Paper presented at: Open forum infectious diseases 2020. [DOI] [PMC free article] [PubMed]
- 28. Group RC. Effect of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med. 2020;383(21):2030‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID‐19: a randomized trial. Ann Intern Med. 2020;173(8):623‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(15):732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ben‐Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicro Ag. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hara K, Suyama N, Yamaguchi K, Kohno S, Saito A. Activity of macrolides against organisms responsible for respiratory infection with emphasis on Mycoplasma and Legionella. J Antimicrob Chemother. 1987;20(Suppl B):75‐80. [DOI] [PubMed] [Google Scholar]
- 35. Blasi F. Atypical pathogens and respiratory tract infections. Eur Respir J. 2004;24(1):171‐181. [DOI] [PubMed] [Google Scholar]
- 36. Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer‐Smadja N, Mahamat‐Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID‐19) patients: a systematic review and meta‐analysis. Clin Microbiol Infect. 2021;27(1):19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andreani J, Le Bideau M, Duflot I, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS‐CoV‐2 shows synergistic effect. Microb Pathog. 2020;145:104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(2):281‐292 e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al‐Nasser AD. SARS‐CoV‐2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scherrmann J. Intracellular ABCB1 as a possible mechanism to explain the synergistic effect of hydroxychloroquine‐azithromycin combination in COVID‐19 therapy. AAPS J. 2020;22(4):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(9):1036‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choi BJ, Koo Y, Kim TY, et al. Risk of QT prolongation through drug interactions between hydroxychloroquine and concomitant drugs prescribed in real world practice. Sci Rep. 2021;11(1):6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA cardiology. 2020;5(9):1036‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID‐19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17(9):1425‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghazy RM, Almaghraby A, Shaaban R, et al. A systematic review and meta‐analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID‐19 treatment. Sci Rep. 2020;10(1):22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID‐19: a systematic review and network meta‐analysis. PLoS Med. 2020;17(12):e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoffmann M, Mösbauer K, Hofmann‐Winkler H, et al. Chloroquine does not inhibit infection of human lung cells with SARS‐CoV‐2. Nature. 2020;585(7826):588‐590. [DOI] [PubMed] [Google Scholar]
- 48. Prodromos C, Rumschlag T. Hydroxychloroquine is effective, and consistently so when provided early, for COVID‐19: a systematic review. New Microbes New Infect. 2020;38:100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid‐19: living systematic review and network meta‐analysis. BMJ (Clinical Research Ed). 2020;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ayele Mega T, Feyissa TM, Dessalegn Bosho D, Kumela Goro K, Zeleke, Negera G. The outcome of hydroxychloroquine in patients treated for COVID‐19: systematic review and meta‐analysis. Can Respir J. 2020;2020:4312519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Y, Pang M, Du C, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medrxiv. 2020;23:57‐64. [Google Scholar]
- 52. Yang TH, Chou CY, Yang YF, et al. Systematic review and meta‐analysis of the effectiveness and safety of hydroxychloroquine in treating COVID‐19 patients. JCMA. 2021;84(2):233‐241. [DOI] [PubMed] [Google Scholar]
- 53. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384:2187‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dinarello CA, Conti P, Mier JW. Effects of human interleukin‐1 on natural killer cell activity: is fever a host defense mechanism for tumor killing? Yale J Biol Med. 1986;59(2):97‐106. [PMC free article] [PubMed] [Google Scholar]
- 58. Conti P, Gallenga CE, Tetè G, et al. How to reduce the likelihood of coronavirus‐19 (CoV‐19 or SARS‐CoV‐2) infection and lung inflammation mediated by IL‐1. J Biol Regul Homeost Agents. 2020;34(2):333‐338. [DOI] [PubMed] [Google Scholar]
- 59. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19‐a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. Lancet. 2020;395(10223):e30‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325‐e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gottlieb RL, Nirula A, Chen P, et al. Effect of Bamlanivimab as Monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID‐19: a randomized clinical trial. JAMA. 2021;325(7):632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta‐1a (SNG001) for treatment of SARS‐CoV‐2 infection: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Respir Med. 2021;9(2):196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti‐IL‐6 receptor antibody, to treat COVID‐19‐related respiratory failure: a case report. Ann Oncol. 2020;31(7):961‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with covid‐19. N Engl J Med. 2021;384(9):795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Berlin DA, Gulick RM, Martinez FJ. Severe covid‐19. N Engl J Med. 2020;383(25):2451‐2460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


