Abstract
Objective
B cell depletion is an established therapeutic principle in a wide range of autoimmune diseases. However, B cells are also critical for inducing protective immunity after infection and vaccination. We undertook this study to assess humoral and cellular immune responses after infection with or vaccination against SARS–CoV‐2 in patients with B cell depletion and controls who are B cell–competent.
Methods
Antibody responses (tested using enzyme‐linked immunosorbent assay) and T cell responses (tested using interferon‐γ enzyme‐linked immunospot assay) against the SARS–CoV‐2 spike S1 and nucleocapsid proteins were assessed in a limited number of previously infected (n = 6) and vaccinated (n = 8) autoimmune disease patients with B cell depletion, as well as previously infected (n = 30) and vaccinated (n = 30) healthy controls.
Results
As expected, B cell and T cell responses to the nucleocapsid protein were observed only after infection, while respective responses to SARS–CoV‐2 spike S1 were found after both infection and vaccination. A SARS–CoV‐2 antibody response was observed in all vaccinated controls (30 of 30 [100%]) but in none of the vaccinated patients with B cell depletion (0 of 8). In contrast, after SARS–CoV‐2 infection, both the patients with B cell depletion (spike S1, 5 of 6 [83%]; nucleocapsid, 3 of 6 [50%]) and healthy controls (spike S1, 28 of 30 [93%]; nucleocapsid, 28 of 30 [93%]) developed antibodies. T cell responses against the spike S1 and nucleocapsid proteins were found in both infected and vaccinated patients with B cell depletion and in the controls.
Conclusion
These data show that B cell depletion completely blocks humoral but not T cell SARS–CoV‐2 vaccination response. Furthermore, limited humoral immune responses are found after SARS–CoV‐2 infection in patients with B cell depletion.
Introduction
Depletion of B cells is an effective therapeutic strategy to treat severe autoimmune disease (1). Diseases with robust activation of B cells and plasma cells, such as rheumatoid arthritis (2), multiple sclerosis (3), granulomatosis with polyangiitis (4), dermatomyositis (5), IgG4‐related disease (6), pemphigus (7), and immune thrombocytopenic purpura (8) are sensitive to B cell targeting. Rituximab, a monoclonal antibody binding the B cell–specific surface molecule CD20, effectively depletes circulating B cells over a period of several months and shows widespread therapeutic efficacy in patients with autoimmune disease (9). B cell depletion, however, may also seriously impair the development of protective immunity after infection and vaccination. Of note, rituximab treatment has been associated with more severe courses of COVID‐19 (10) and impaired immune response to established vaccines (11, 12). To date, reliable data on the impact of B cell depletion on the dynamics of protective antibody responses upon infection and vaccination remain sparse (13), while protective antibody responses have been clearly documented in subjects naive to SARS–CoV‐2 infection (14) and previously infected immunocompetent subjects (15). The current SARS–CoV‐2 pandemic thus provides a unique opportunity to profile the immune response of a naive population to a defined infectious agent. In addition, it allows us to study the magnitude of a newly evolving adaptive immune response to infection and to vaccination in both healthy individuals and in patients with B cell depletion.
Patients and Methods
Ethical approval
Ethical approval (no. 157_20 B) to conduct this analysis was granted by the institutional review board of the University Clinic of Erlangen as the responsible ethics committee. Written informed consent was obtained from the study participants.
Patients and controls
Sera from rituximab‐treated patients and healthy controls were collected within the COVID‐19 study program of the Deutsche Zentrum fuer Immuntherapie (16). This study program was initiated in February 2020 and monitors anti‐SARS–CoV‐2 antibody responses in healthy controls, COVID‐19 patients, and patients with autoimmune diseases (16). Healthy controls did not have an immune‐mediated inflammatory disease nor did they receive any treatment with immunomodulatory agents. Vaccinated patients and vaccinated healthy controls did not have any history of COVID‐19 or positive COVID‐19 polymerase chain reaction results before the analysis.
Rituximab‐treated patients and healthy controls were vaccinated with the BNT162b2 messenger RNA (mRNA) SARS–CoV‐2 vaccine at official public vaccination centers, based on occupational exposure risk, comorbidities, and age‐related risk in accordance with the recommendations of the Robert Koch Institute. Sera were collected ≥10 days after the second vaccination and ≥30 days after onset of infection in the infected participants. For all participants, we collected demographic data (e.g., age, sex) as well as disease‐specific data (e.g., type of autoimmune disease, type of treatment). The elapsed time between antibody testing and either infection (mean ± SD 3.8 ± 2.9 months [range 1–8 months]) or vaccination (mean ± SD 3.6 ± 3.0 months [range 1–8 months]) was very similar among the rituximab‐treated patients. Patients underwent a mean ± SD of 5.4 ± 4.3 rituximab infusions, administered at a dose of 1,000 mg every 6 months.
Anti‐SARS–CoV‐2 antibodies
IgG antibodies against the S1 domain of the spike protein and the nucleocapsid protein of SARS–CoV‐2 were tested by 2 Conformité Européenne commercial enzyme‐linked immunosorbent assays, according to the protocols of the manufacturers (Euroimmun; Epitope Diagnostics). Optical density (OD) was determined at 450 nm, with a reference wavelength at 630 nm. Cutoffs of <0.8 and <0.2 were considered as negative for IgG antibodies against the spike S1 protein and the nucleocapsid protein, respectively. An in‐house neutralization assay for assessment of inhibition of binding to angiotensin‐converting enzyme 2 by antibodies was used. Assays were performed in accordance with the guidelines of the German Medical Association with stipulated internal and external quality controls.
Anti‐SARS–CoV‐2 T cells
The detection of SARS–CoV‐2–specific T cells was conducted via an interferon‐γ (IFNγ) enzyme‐linked immunospot (ELISpot) assay (T‐SPOT.COVID; Oxford Immunotec). Isolation of peripheral blood mononuclear cells (PBMCs) was carried out via density‐gradient centrifugation. Leucosep tubes (Greiner Bio‐One) were filled with 15 ml Lymphoflot (Bio‐Rad) and centrifuged briefly to collect the fluid under the membrane. A maximum of 30 ml citrate blood was transferred to the tube and filled up to 50 ml with RPMI 1640 medium (Gibco) that was preheated to 37°C. Cells were centrifuged at 760g for 20 minutes, and the upper layer containing PBMCs was transferred to 50‐ml tubes and centrifuged at 610g for 10 minutes. The cell pellet was then washed with 30 ml of RPMI 1640 medium (at 37°C) at 610g for 10 minutes prior to resuspension at a concentration of 2.5 × 106/ml in AIM‐V medium (Gibco) that was preheated to 37°C.
Fifty microliters of either AIM‐V medium, Panel A, Panel B, or Positive Control were added to the wells of the precoated multi‐titer ELISpot plate (Oxford Immunotec). One hundred microliters of the cell suspension was added to each well and carefully mixed by pipetting. After an incubation period at 37°C with 7% CO2 for 16–20 hours, the wells were washed 4 times with 200 µl of phosphate buffered saline (PBS; Gibco). The conjugate reagent was diluted at 1:200 in PBS, and 50 µl of this dilution was added to each well. Following a 60‐minute incubation period at 4°C, the wells were washed 4 times with 200 µl of PBS. Fifty microliters of substrate solution was added to each well and incubated for 7 minutes. The plate was washed 3 times with H2O and then air‐dried. The spots were counted and analyzed using an ELISpot reader (AID). Results are reported as spot‐forming units (SFUs) per 2.5 × 105 cells. According to the manufacturer’s guidelines, a response was considered positive when the number of spots in the respective panel was ≥8 SFUs above the negative control. Samples with negative controls >10 SFUs were considered invalid.
Statistical analysis
Subject characteristics are presented as the mean ± SD for continuous data and as the number and percentage for categorical data. We used Wilcoxon’s rank sum test for pairwise between‐group comparisons of OD from the anti–spike S1 IgG and anti–nucleocapsid IgG assays. P values were adjusted for a family of 6 possible pairwise comparisons per assay using the Bonferroni‐Holm method and were considered significant when less than 0.05.
Results
To address the question of whether autoimmune patients in whom peripheral B cells have been depleted are able to develop specific humoral immunity to SARS–CoV‐2 vaccination, we screened data from an ongoing longitudinal SARS–CoV‐2 antibody study in Germany which measures IgG responses against the SARS–CoV‐2 spike S1 and nucleocapsid proteins in patients with autoimmune inflammatory diseases and healthy controls (16). We identified 8 rituximab‐treated patients who received the BNT162b2 mRNA SARS–CoV‐2 vaccine and 6 rituximab‐treated patients who had experienced a clinically symptomatic, mRNA‐confirmed infection with SARS–CoV‐2. The most frequent COVID‐19 related symptoms in the 6 rituximab‐treated patients with SARS–CoV‐2 infection were cough (n = 5), anosmia (n = 5), fever (n = 4), and dyspnea (n = 4). Three patients required hospitalization, and none of them required intensive care. The characteristics of patients and controls are summarized in Table 1.
Table 1.
Characteristics of the autoimmune disease patients with B cell depletion and healthy controls*
|
Healthy controls, previously infected (n = 30) |
Healthy controls, vaccinated (n = 30) |
Patients with B cell depletion, previously infected (n = 6) |
Patients with B cell depletion, vaccinated (n = 8) |
|
|---|---|---|---|---|
| Age, mean ± SD years | 61.0 ± 16.6 | 57.1 ± 7.5 | 62.5 ± 12.8 | 53.5 ± 7.7 |
| Female sex | 12 (40.0) | 23 (76.7) | 5 (83.3) | 5 (62.5) |
| Humoral immune response | ||||
| Anti–spike S1 IgG, mean ± SD OD | 5.4 ± 2.5 | 8.1 ± 2.5 | 2.9 ± 2.2 | 0.2 ± 0.3 |
| Anti–spike S1 IgG, OD >0.8 | 28 (93.3) | 30 (100.0) | 5 (83.3) | 0 (0) |
| Anti–nucleocapsid IgG, mean ± SD OD† | 0.31 ± 0.09 | 0.10 ± 0.04 | 0.18 ± 0.09 | 0.09 ± 0.02 |
| Anti–nucleocapsid IgG, OD >0.2† | 28 (93.3) | 0 (0) | 3 (50.0) | 0 (0) |
| Cellular immune response | ||||
| Anti–spike S1 IFNγ >3 SFUs, no. positive/no. tested (%) | 4/5 (80) | 5/5 (100.0) | 6/6 (100.0) | 6/8 (75) |
| Anti–nucleocapsid IFNγ >5 SFUs, no. positive/no. tested (%) | 5/5 (100) | 0/5 (0) | 5/6 (83.3) | 0/8 (0) |
| Disease | ||||
| Granulomatosis with polyangiitis | – | – | 2 (33.3) | 3 (37.5) |
| Rheumatoid arthritis | – | – | 3 (50.0) | 3 (37.5) |
| Multiple sclerosis | – | – | 0 (0) | 1 (12.5) |
| Dermatomyositis | – | – | 0 (0) | 1 (12.5) |
| IgG4‐related disease | 1 (16.7) | 0 (0) |
Except where indicated otherwise, values are the number (%) of subjects. IFNγ = interferon‐γ; SFUs = spot‐forming units.
Anti–nucleocapsid IgG was only measured in 6 of the 8 vaccinated patients with B cell depletion.
All 14 rituximab‐treated vaccinated or infected patients were tested for anti‐SARS–CoV‐2 IgG antibodies after having received their second shot of the vaccine or ≥4 weeks after the infection, respectively. For control purposes, anti‐SARS–CoV‐2 IgG antibodies were also tested in 30 healthy controls after SARS–CoV‐2 vaccination and in 30 additional healthy controls after SARS–CoV‐2 infection. The majority of SARS–CoV‐2–infected controls (93%) developed IgG antibodies against the spike S1 protein (mean ± SD 5.4 ± 2.5 at an OD of 450 nm; cutoff for positivity >0.8 at an OD of 450 nm) and all vaccinated controls (100%) developed IgG antibodies against the spike S1 protein (mean ± SD 8.1 ± 2.5) (Figure 1A). As expected, IgG antibodies against the nucleocapsid protein were observed only in previously infected controls (mean ± SD 0.31 ± 0.09; cutoff for positivity >0.2) but not in vaccinated controls (mean ± SD 0.10 ± 0.04).
Figure 1.
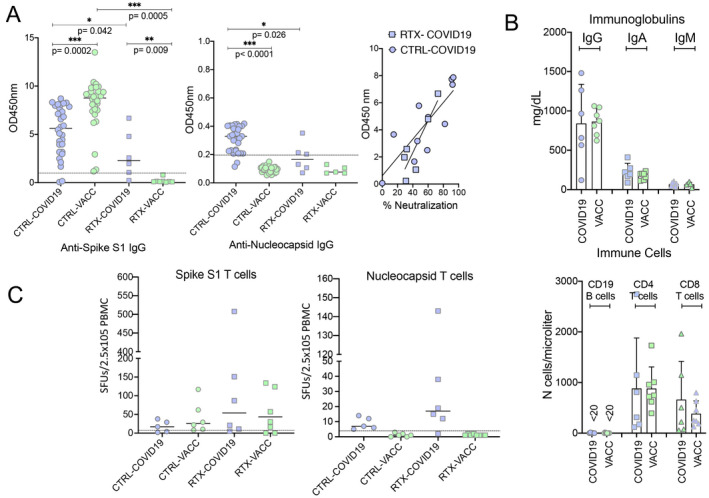
Anti‐SARS–CoV‐2 immune responses in previously infected patients and vaccinated patients who have undergone B cell depletion. A, Antibodies against the spike S1 protein of SARS–CoV‐2 (assessed by enzyme‐linked immunosorbent assay [ELISA]; Euroimmun) (left) and nucleocapsid protein of SARS–CoV‐2 (assessed by ELISA; Epitope) (middle), and correlation between neutralizing antibody activity (percent inhibition of binding of spike S1 protein–expressing cells to angiotensin‐converting enzyme 2) and the spike S1 protein antibody response (OD at 450 nm) (right). Tests were performed in 30 healthy controls after SARS–CoV‐2 infection (CTRL‐COVID‐19), 30 healthy controls after SARS–CoV‐2 mRNA vaccination (CTRL‐VACC), 6 rituximab‐treated, B cell–depleted autoimmune disease patients after SARS–CoV‐2 infection (RTX‐COVID19), and 8 rituximab‐treated, B cell–depleted autoimmune disease patients after SARS–CoV‐2 mRNA vaccination (RTX‐VACC). Symbols represent individual subjects; horizontal lines show the mean. Comparisons were conducted using Wilcoxon’s signed rank sum test. B, Serum levels of IgG, IgA, and IgM (top) and numbers of CD19 B cells, CD4 T cells, and CD8 T cells (bottom) in rituximab‐treated, B cell–depleted autoimmune disease patients after SARS–CoV‐2 infection and rituximab‐treated, B cell–depleted autoimmune disease patients after SARS–CoV‐2 mRNA vaccination. Symbols represent individual subjects; bars show the mean ± SD. C, Enzyme‐linked immunospot assay results showing T cell responses to antibodies against the spike S1 protein (left) and the nucleocapsid protein (right) in healthy controls after SARS–CoV‐2 infection, healthy controls after SARS–CoV‐2 mRNA vaccination, rituximab‐treated B cell–depleted autoimmune disease patients after SARS–CoV‐2 infection, and rituximab‐treated, B cell–depleted autoimmune disease patients after SARS–CoV‐2 mRNA vaccination. Symbols represent individual subjects; horizontal lines show the mean. SFUs = spot‐forming units; PBMC = peripheral blood mononuclear cell.
Although anti‐SARS–CoV‐2 S1 IgG levels were lower than those in healthy controls, surprisingly, 5 of 6 rituximab‐treated SARS–CoV‐2–infected patients (83.3%) developed IgG antibodies (mean ± SD 2.9 ± 2.2) (Figure 1A). These antibodies in rituximab‐treated infected patients also had a similar neutralizing capacity as those in infected controls. In contrast, none of the 8 vaccinated rituximab‐treated patients developed anti‐SARS–CoV‐2 IgG antibodies (mean ± SD 0.2 ± 0.3). The mean elapsed time to antibody testing was similar among rituximab‐treated patients who had been previously infected (mean ± SD 3.8 ± 2.9 months [range 1–8 months]) and those who had been vaccinated (mean ± SD 3.6 ± 3.0 months [range 1–8 months]). In addition, the time interval between the last rituximab infusion and infection/vaccination was comparable (infection, mean ± SD 2.9 ± 3.8 months; vaccination, mean ± SD 3.1 ± 3.7 months).
Peripheral B cells were undetectable or lower than 15 cells/μl in all infected and vaccinated patients. Peripheral CD4 and CD8 T cell counts as well as serum levels of IgG, IgA, and IgM did not differ between rituximab‐treated patients who previously had a SARS–CoV‐2 infection and those who had been vaccinated (Figure 1B). We were also able to assess SARS–CoV‐2–specific T cell responses using an IFNγ ELISpot assay in a limited subset of the patients from each group (Figure 1C). T cell responses against both the spike S1 and nucleocapsid proteins were found after SARS–CoV‐2 infection in healthy controls and patients with B cell depletion. Furthermore, the majority of vaccinated patients, including those depleted of B cells, developed a T cell response against the spike S1 protein, but (as expected) no T cell responses against the nucleocapsid protein were found in the vaccinated patients.
Discussion
These data provide interesting and unexpected new insights into the immune response to infection and vaccination in patients with B cell depletion. First, they show that SARS–CoV‐2 vaccination fails to trigger significant humoral immune responses in patients with B cell depletion. This finding may suggest that vaccination should preferentially take place before a B cell–depleting treatment is started in order to mount a significant humoral immune response.
Surprisingly, and in contrast to vaccination, infection with SARS–CoV‐2 triggered specific antibody responses, despite the absence of circulating B cells. Although these antibody responses were lower than those in healthy controls, this finding sheds light on the differences between vaccination and infection. While local antigen presentation and T cell and B cell activation may predominate in vaccinated individuals, infection may trigger a much more systemic adaptive immune response. Therefore, residual tissue B cells (e.g., in the bone marrow), which may escape rituximab treatment, could be sufficient to induce a humoral immune response after SARS–CoV‐2 infection. Previous biopsy data in rituximab‐treated patients, who were depleted of circulating peripheral B cells, have shown that tissue B cells can escape depletion (17).
Second, our data indicate that SARS–CoV‐2 vaccination, like infection, can trigger specific T cell–mediated immune responses even in the absence of peripheral B cells. Such T cell responses may explain why SARS–CoV‐2 infection can still be controlled in patients with B cell depletion. In addition, the data suggest that potentially protective T cell–mediated immunity may develop after the vaccination in the absence of B cells.
A limitation of this study is the small number of patients with B cell depletion who were exposed to SARS–CoV‐2 infection or vaccination. Although results were highly consistent even in this small sample, further studies will be required. Furthermore, only the BNT162b2 mRNA SARS–CoV‐2 vaccine was assessed in this data set, requiring the collection of additional data from other (i.e., vector‐based) vaccines. Despite these limitations, the data very consistently showed that T cell responses against SARS–CoV‐2 can develop in both vaccinated and previously infected patients with B cell depletion. The data also indicate that SARS–CoV‐2 infection, but not vaccination, can in principle trigger limited humoral immune responses against the virus in patients with B cell depletion, indicating that infection can reach and activate residual tissue B cells.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Schett had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Simon, Tascilar, Krönke, Schett.
Acquisition of data
Simon, Tascilar, Schmidt, Sokolova, Bucci, K. Manger, Schuch, Ronneberger, Hueber, Steffen, Kleyer, Schett.
Analysis and interpretation of data
Simon, Tascilar, B. Manger, Weckwerth, Fagni, Mielenz, Herrmann, Harrer, Krönke, Schett.
Supporting information
Supplementary Material
Supported by the DFG (grant FOR2886 PANDORA and the CRC1181 Checkpoints for Resolution of Inflammation), the BMBF project MASCARA, the Bavarian State Ministry for Science and Art, the European Research Council Synergy grant 4D Nanoscope, the Innovated Medicines Initiative projects RTCure and Hippocrates, the Emerging Fields Initiative MIRACLE of the Friedrich‐Alexander University Erlangen‐Nuremberg, and the Else Kröner Memorial Scholarship (grant 2019_EKMS.27 to Dr. Simon).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.41914&file=art41914-sup-0001-Disclosureform.pdf.
Drs. Simon and Tascilar contributed equally to this work.
References
- 1. Arnagai M. Modulating immunity to treat autoimmune disease. N Engl J Med 2016;375:1487–9. [DOI] [PubMed] [Google Scholar]
- 2. Edwards JC, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close DR, et al. Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81. [DOI] [PubMed] [Google Scholar]
- 3. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B‐cell depletion with rituximab in relapsing‐remitting multiple sclerosis. N Engl J Med 2008;358:676–88. [DOI] [PubMed] [Google Scholar]
- 4. Stone J, Merkel PE, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo‐phase trial. Arthritis Rheum 2013;65:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4‐related systemic disease. Arthritis Rheum 2010;62:1755–62. [DOI] [PubMed] [Google Scholar]
- 7. Joly P, Mouquet H, Roujeau JC, D’Incan M, Gilbert D, Jacquot S, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007;357:545–52. [DOI] [PubMed] [Google Scholar]
- 8. Braendstrup P, Bjerrum OW, Nielsen OJ, Jensen BA, Clausen NT, Hansen PB, et al. Rituximab chimeric anti‐CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol 2005;78:275–80. [DOI] [PubMed] [Google Scholar]
- 9. Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo‐Lopez A, et al. Phase I clinical trial using escalating single‐dose infusion of chimeric anti‐CD20 monoclonal antibody (IDEC‐C2B8) in patients with recurrent B‐cell lymphoma. Blood 1994;84:2457–66. [PubMed] [Google Scholar]
- 10. Avouac J, Drumez E, Hachulla E, Seror R, Georgin‐Lavialle S, El Mahou S, et al. Covid‐19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nived P, Jönsson G, Settergren B, Einarsson J, Olofsson T, Jørgensen CS, et al. Prime‐boost vaccination strategy enhances immunogenicity compared to single pneumococcal conjugate vaccination in patients receiving conventional DMARDs, to some extent in abatacept but not in rituximab‐treated patients. Arthritis Res Ther 2020;22:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013. 12;122:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D´Silva KM, Serling‐Boyd N, Hsu T, Sparks JA, Wallace ZS. SARS‐CoV‐2 antibody response after COVID‐19 in patients with rheumatic disease. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2020-219808. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krammer F, Komal S, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med 2021;384:1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebinger JE, Fert‐Bober J, Printsev I, Wu M, Sun N, Prosko J, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med 2021;27:981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon D, Tascilar K, Krönke G, Kleyer A, Zaiss MM, Heppt F, et al. Patients with immune‐mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS‐CoV‐2 seroconversion. Nat Commun 2020;11:3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramwadhdoebe TH, Van Baarsen LG, Boumans MJ, Bruijnen ST, Safy M, Berger FH, et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology (Oxford) 2019;58:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


