Abstract
Vaccination is a significant advancement or preventative strategy for controlling the spread of various severe infectious and noninfectious diseases. The purpose of vaccination is to stimulate or activate the immune system by injecting antigens, i.e., either whole microorganisms or using the pathogen's antigenic part or macromolecules. Over time, researchers have made tremendous efforts to reduce vaccine side effects or failure by developing different strategies combining with immunoinformatic and molecular biology. These newly designed vaccines are composed of single or several antigenic molecules derived from a pathogenic organism. Although, whole‐cell vaccines are still in use against various diseases but due to their ineffectiveness, other vaccines like DNA‐based, RNA‐based, and protein‐based vaccines, with the addition of immunostimulatory agents, are in the limelight. Despite this, many researchers escape the most common fundamental phenomenon of protein posttranslational modifications during the development of vaccines, which regulates protein functional behavior, evokes immunogenicity and stability, etc. The negligence about post translational modification (PTM) during vaccine development may affect the vaccine's efficacy and immune responses. Therefore, it becomes imperative to consider these modifications of macromolecules before finalizing the antigenic vaccine construct. Here, we have discussed different types of posttranslational/transcriptional modifications that are usually considered during vaccine construct designing: Glycosylation, Acetylation, Sulfation, Methylation, Amidation, SUMOylation, Ubiquitylation, Lipidation, Formylation, and Phosphorylation. Based on the available research information, we firmly believe that considering these modifications will generate a potential and highly immunogenic antigenic molecule against communicable and noncommunicable diseases compared to the unmodified macromolecules.
Keywords: immunogenicity, infectious diseases, noninfectious diseases, posttranslational modifications, vaccines
In this review, we have discussed different types of posttranslational/transcriptional modifications that are usually considered during vaccine construct designing: Glycosylation, Acetylation, Sulfation, Methylation, Amidation, SUMOylation, Ubiquitylation, Lipidation, Formylation, and Phosphorylation. This review specifically focuses on the relationship between the PTMs and vaccine molecules in improving the rate of protective immune responses to combat both communicable and noncommunicable diseases.
1. INTRODUCTION
Globally, diseases have been mainly categorized as communicable and noncommunicable (noncommunicable diseases [NCD]), which have become a significant public health issue. If we flashlight at communicable and NCD, as per the World Health Organization (WHO) factsheet data (https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death), among the top 10 death‐causing diseases, seven are NCD, and the total mortality rate contributed around 80%. Simultaneously, around 17 million people died every year due to various infections instigated by pathogenic microorganisms, such as viruses, bacteria, protozoa, fungi, and helminths (https://www.ncbi.nlm.nih.gov/books/NBK10757/). Communicable diseases were the leading cause of early death from now on. The infections can be transmitted through various sources, such as human to human (antroponoses), from animal to human (zoonoses), and from the abiotic environment to humans (sapronoses), which have become a significant threat for all species (Hubálek, 2003). Over centuries humanity has suffered many deadly pandemics/epidemics, notably Black Death (Plague) in the 13th century, typhus fever in the 16th century, small‐pox and cholera in the 18th century, malaria in the late 18th century, Spanish flu in 1918, H1N1 flu in 2004, Ebola in 2015, COVID‐19 in 2019, and many more (Huremović, 2019). Due to the black death, 25–75 million people lost their lives in Europe (13th century). Subsequently, smallpox also hit people in England, and the disease continued to afflict people globally for many years. Later on, these infections became significant challenges for human health around the globe. Many infections caused by viruses (e.g., COVID‐19, chikungunya, Ebola, Lassa, HIV), bacteria (e.g., meningitis), and parasites (e.g., Leishmania, malaria) are still a threat to humanity. Among them, several diseases have been declared as pandemic/epidemic by international and national health agencies. According to the International health regulations 2005 law, all the countries joined and assisted in saving and protecting public health and spread awareness about the community's vulnerability to diseases (https://www.who.int/health-topics/international-health-regulations#tab=tab_1). An unsurpassed example of the recent outbreak is Ebola hemorrhagic fever, which affected humans and primates badly between 2014 and 2016 (Feldmann & Geisbert, 2011). Recently, COVID‐19, caused by SARS‐CoV‐2, was first reported in Wuhan, China, and later declared a pandemic by WHO. The disease has frightened the whole world by spreading all over (Yi et al., 2020). Besides this, no proven preventive measures are available against these emerging death‐dealing infections. For centuries, the treatment of infectious diseases is somewhat relying on the immunization and this has contributed in eradication of many diseases. As per the WHO report of 2018, about 90% of infants globally received the DTP3 (diphtheria‐tetanus‐pertussis vaccines) vaccines in the global immunization program. However, the global estimation of immunization coverage for other infectious diseases is as follows: yellow fever 49%, rubella 69%, Rotaviruses 35%, polio 85%, pneumonia 47%, hepatitis B 42%, and Haemophilus influenzae type B has shown to be covered around 72% of the population worldwide (https://www.who.int/immunization/newsroom/Global_Immunization_Data.pdf).
However, on the other side, NCDs, such as lower respiratory infection, cancer, diabetes, coronary artery, chronic obstructive pulmonary disease (COPD), and many more are significant causes of death globally (https://www.who.int/gard/publications/The_Global_Impact_of_Respiratory_Disease.pd). They have carved a niche for themselves for years. The number of death cases by NCD is increasing day by day; this accounts for the death of 41 million people around the globe, which is approximately 71% of total death worldwide (https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-mortality). Majorly four NCD covers around 80% of the total premature death, e.g., cardiovascular diseases are responsible for 17.9 million deaths annually, and it is highest among the NCD, followed by cancers (9.0 million), respiratory diseases (3.9 million), and diabetes (1.6 million) as per WHO NCD factsheet database (https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases). CVD (cardiovascular diseases) are a family of disorders affecting the heart and blood vessels, of which heart attacks and strokes were observed to be the primary cause that blocks the blood flow to the heart or brain due to fat deposition in the inner walls of the blood vessels. Vaccines are in dire demand to treat CVD, which roughly accounts for 31% of global deaths each year (https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). COPD (Chronic obstructive pulmonary lung disease) affects the lungs and respiratory tract; globally, estimated to affect 251 million lives and accounts for 5% of global deaths by 2016 (https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)). Pneumococcal and Influenza vaccines were reported to be efficient in treating COPD due to COPD exacerbations caused by Streptococcus pneumonia and the seasonal flu viruses. Diabetes is a metabolic disorder that results in a constitutive hyperglycemic condition. Recent global estimates show that 422 million people have diabetes and 1.6 million deaths each year (https://www.who.int/health-topics/diabetes#tab=tab_1). Other NCDs include hypertension, obesity, autoimmune, inflammatory, neurodegenerative diseases, also accounts for significant cause of death globally.
All the countries transitioning from developing to developed countries are troubled by newly emerging diseases, and the root of some new disease appearance is still unknown. Advanced technologies, available treatments, and diagnostics approach all have succumbed to these ailments. A significant rise in infectious and noninfectious diseases was observed in past decades due to technological advancement, socioeconomic changes, human behavior, microbes adaptations, pollution, stress, tobacco consumption, and mutations in strains, lifestyle changes, and other comorbidities (Dikid et al., 2013). Other than that, vaccine failures and drug resistance actively contribute to remerging infections, resulting in inadequate immune responses. The antivaccine movement is also a great factor in the declining vaccination rate among the population. In this growing world, most people prefer not to have vaccination due to safety concerns.
Further, to counteract the uprising incidences of communicable and NCDs, we need a reliable preventive measure, and vaccination proved to be a better approach than drugs. From the 19th century to date, vaccines were being extensively researched due to their beneficial impact on human health and survival. In this modern era, next‐generation vaccines are in the limelight due to their efficiency in eliciting cell‐mediated and humoral immunity. Newly adjuvanted vaccines that are DNA‐based, RNA‐based, viral vector‐based, as well as subunit multiepitopes, are in the pipeline. Apart from adjuvants and delivery systems, posttranslational modifications are also known to affect vaccine stability and immunogenicity. Nowadays, PTMs have been at the critical target for designing or obtaining the potential immunogens that can generate immense immune responses and improved antigenicity (Jefferis, 2016). Even though there are profuse pieces of literature on emerging infectious and noninfectious diseases, much more is left to be understood about the emergence of these minacious diseases, immunization, and vaccine efficacy. Here, we will summarize the importance of various posttranslational modifications (occur naturally or synthetically) essential for augmented vaccine immunogenicity.
2. A DRIVE FROM CLASSICAL VACCINES TO 21ST‐CENTURY NEXT‐GENERATION VACCINES
Immunization is essential for the prevention of any disease and upsurges the life expectancy of humans and animals. In the past century, classical vaccine formulation was in practice, which possessed the whole pathogen, either live or attenuated, to prevent many infections (Plotkin, 2005). If we throw light on the vaccination, in 1798, the first vaccine for smallpox was developed by Sir Edward Jenner, known as the originator of vaccinology. He inoculated the vaccinia virus in a 13‐year‐old boy, who proved his immunity against the disease, and a few centuries later, in the Year 1979, the disease was declared eradicated by the massive use of vaccination (Minor, 2015). However, after a certain period, this disease's adverse effect was reported, like myocarditis and encephalitis. Later on, many scientists have contributed to harmful diseases. In 1897, Louis Pasteur developed the first live attenuated and heat‐inactivated vaccines against animal‐borne diseases like rabies and anthrax. Far ahead, anthrax vaccines have shown long‐term side effects, whereas the rabies vaccine came up with postvaccination effects like encephalomyelitis in the experimental models (Jenner, 1909). The ability of viruses to survive and grow under the tissue culture condition made way for the development of attenuated vaccines against measles (1950s) and poliomyelitis (1960s) (McGettigan, 2010). Afterward, a series of vaccine development against the influenza virus, rotavirus, typhoid, and tuberculosis was continued by following the same strategy. These attenuated vaccines, in comparison to heat‐killed vaccines, have shown strong immune responses during immunization. However, the live attenuated vaccines have a higher chance of mutation and contain the live organism, leading to the disease expansion in nonimmunized models (Wilson, 1963). For example, the oral polio vaccine, which composed of live attenuated poliovirus, has shown paralysis in about 2 million population after vaccination. As time passed by, the classical type of vaccines came up with many disadvantages, as these vaccination strategies were based on isolation of the pathogens, that is, whole‐cell preparation; these preparations have a high chance of bacterial and other toxic contaminations (Yadav et al., 2014). Some vaccines are still in use despite everything, like the Bacillus Calmette Guerin (BCG) vaccine against tuberculosis. BCG vaccine was developed in the late 1900s and is currently administered to young ones in many parts of the world (Luca & Mihaescu, 2013). The vaccine efficacy and safety are the two affirmative parameters that make any vaccine successful, but in terms of classical vaccines they ended up together. Later on, to overcome the disadvantages of classical vaccine formulation, the new generation vaccines, such as peptide vaccine, polysaccharide vaccine, messenger RNA (mRNA) vaccines, DNA vaccines, viral vector‐based vaccines, and subunit vaccines have come out with a new silver lining.
The recent and versatile reverse vaccinology approach employs various bioinformatic tools and parameters for the development of suitable and potential recombinant protein (vaccine candidate) that focused on the way to utilize the explicit components or pathogenic proteins of pathogens that is responsible for the generation of potential cell‐mediated as well as humoral immunity (Ojha et al., 2020, 2020). Rino Rappuoli first proclaimed this approach, which has made a remarkable contribution and is currently being used to prevent Serogroup B meningococcus (Rappuoli, 2000; Rappuoli et al., 2018). However, the new generation vaccine strategies are developed to target the leading cause of infection against communicable and NCDs. The classic example is of meningococcal disease caused by Neisseria meningitides serogroup B (MenB). The genomic sequence of the pathogen was analyzed using immunoinformatic approaches.
Further, three recombinant antigens Neisserial Heparin Binding Antigen or NHBA, Factor H binding protein or fHbp, and Neisseria Adhesin A or NadA, were selected for vaccine designing. Along with this, the outer membrane vesicle component of another epidemic strain (from New Zealand [OMVNz] was added as a fourth constituent, and the formulated vaccine was named 4CMenB; Carter, 2013). A series of clinical trials were conducted to validate vaccine efficacy. After the completion of clinical trials, in the Year 2013, 4CMenB (Bexsero) was first approved in Europe (in 12 member states—AT, BE, CZ, ES, FR, DE, GR, IE, IT, LU, NO, and UK.), and later on, introduced into UK National Immunization Programme in September 2015 and at Ireland in 2016. In this program, the vaccine was administered to all newborn children at 2, 4, and 12 months scheduled (Serruto et al., 2012). Another example is of respiratory syncytial virus subunit vaccine, which is safe in use and immunogenic, and has not shown any conflicts with the humoral response to the influenza vaccine. (Dudas & Karron, 1998).
Besides using next‐generation vaccine technologies in communicable diseases, the same has also been used to develop vaccines against NCDs. Alike medications, NCD immunizations at present being developed, are essentially envisioned for patients already experiencing the disease. For example, hepatitis B virus and human papillomavirus (HPV), which are significant causes of liver and cervical cancer. HPV is also active in cancer etiology in a variety of other areas, including the pharynx. Widespread implementation of successful viral vaccines will play an essential role in reducing the rising cancer burden in developed countries. However, many examples present the transient success of the anticancerous vaccine in some patients. Despondently, only one anticancer vaccine has obtained a license in the United States, i.e, Provenge, a prostatic cancer vaccine that targets the enzyme prostatic acid phosphatase (Anassi & Ndefo, 2011). There are currently no Food and Drug Administration (FDA)‐approved prophylactic vaccines against NCDs; many promising candidates are still under clinical trials, for example, the vaccines for Alzheimer's and Parkinson's disease. These vaccines avert the alpha‐synuclein or amyloid‐beta proteins accumulation, which is responsible for disease progression (Darrow & Kesselheim, 2015).
The next generation of vaccines utilizes adjuvant and various delivery vehicles to enhance vaccine immunogenicity. One of the fundamental approaches is the PTMs (either transcriptional or translational modification) of vaccine candidates of protein, peptide, mRNA, DNA or polysaccharides etc. There are several types of PTMs reported; a few of them are glycosylation (addition of carbohydrate), acetylation (addition of acetyl group), sulphation (addition of sulfate group), and methylation (methyl group addition). These modifications most commonly occur in the natural environment, but they can be synthesized chemically. Naturally, PTM modification can occur during the recombinant protein or native protein expression using the mammalian expression systems. Further, these modifications can be assessed through various techniques, whereas in in vitro conditions, PTMs can be artificially induced (chemically synthesized) in the vaccine macromolecules to enhance their immunogenicity and stability.
3. POSTTRANSLATIONAL MODIFICATIONS IN VACCINE CANDIDATES
Protein synthesis or translation of protein is a biological process performed by ribosomal mRNA. After the polypeptide chain synthesis, the protein undergoes posttranslational modifications to generate a mature protein product. Almost all proteins undergo this modification at either the N or C terminal or the side chains of amino acids. These modifications are generally responsible for alteration in a protein's physical and chemical properties, which defines the protein functions in enzymatic activity, proteolytic cleavage, transportation, stability, folding, etc. Several modifications occur with different recurrence frequency and roles; for instance, glycosylation is responsible for estimating the half‐life of protein and cell‐cell interactions, whereas phosphorylation and ubiquitinylation are accountable for signal transduction and proteolytic degradation of the protein, respectively. In the Year 2011, Khoury et al. (2011) has developed a server named PTM structural database (http://selene.princeton.edu/PTMCuration) to keep the record and statistics of the frequency of putative and experimental posttranslational modification that occur across the proteome. Every month, the server's web page gets automatically updated to maintain the statistics of the PTM confined in the Swiss‐Prot database. In all the complex organisms, PTM occurs but prokaryotic organisms may lack this feature. The role of PTM (naturally or synthetically) has been known to be directly or inversely associated with vaccine immunogenicity, and for obtaining the modified recombinant proteins, many eukaryotic protein‐expression systems are existing for obtaining the essential vaccine preparations, an example of the same is shown in Figure 1. The host can identify the nature of self‐molecules (antigens), but the non‐self‐modifications in the antigens possess the ability to incite an immune response by developing antipathogen antibodies (Figure 2). However, the alterations in recombinant proteins through PTMs and their variances in efficacy in provoking a tremendous immune response are not yet well understood. Here, in this section, we will summarize the essential and most frequently occurred PTMs, unambiguously focused on the studies related to vaccines' efficacy after and before PTM in the field of infectious and noninfectious diseases.
Figure 1.
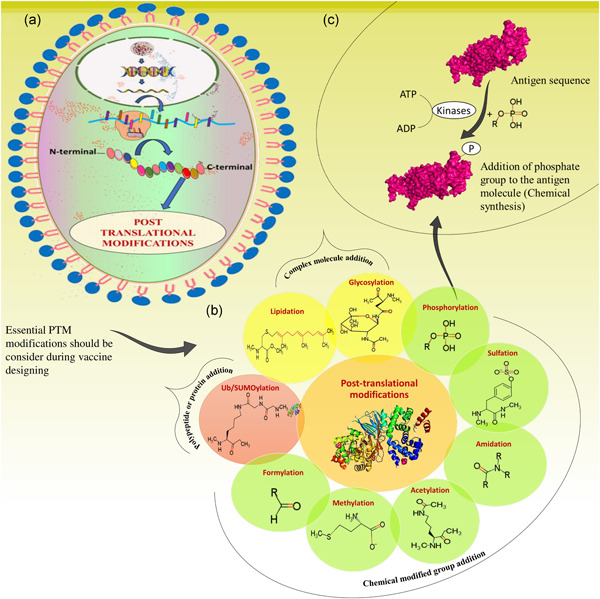
Pictorial representation of posttranslational modifications in a cell, through natural process or synthesized chemically by various enzymatic reactions. (A) Cell (where protein biosynthesis occurs via ribosomes). (B) Types of posttranslational modifications essentially considered for vaccine designing (enzymatic/chemical modifications of proteins). (C) Example of phosphorylation (enzymatic reaction of PTM)
Figure 2.
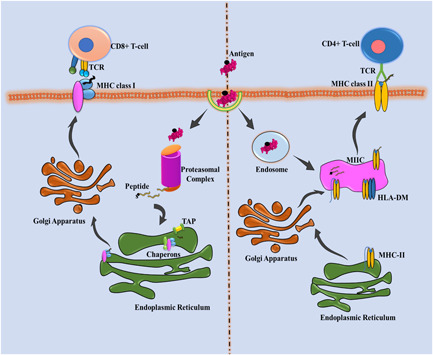
Antigenic representation of vaccine candidate through MHC complexes. The internalized antigenic protein with posttranslational modification is processed into the peptides and presented through the MHC complex on APCs' surface, further recognized through respective CD4+ and CD8+ T‐cells. APC,(Antigen‐presenting cell); HLA, human leukocyte antigen; MHC, major histocompatibility complex; MIIC, MHC Class II compartment; TCR, T‐lymphocyte cell receptor
3.1. Glycosylation
Glycosylation is a chemical modification of macromolecules; the carbohydrate moieties covalently attached to the lipids and proteins molecules at the N or C terminal, and hence refereed as N‐linked and O‐linked glycosylation. This kind of modification is necessary for cell adhesion, proper functioning, and folding of the protein (Shental‐Bechor & Levy, 2008). In the N‐linked glycosylation, an oligosaccharide attachment occurs at the Asn residue of the glycosylation motif, composed of Asn‐X‐Ser/Thr (X‐ amino acid except for proline) (Breitling & Aebi, 2013). O‐linked glycosylation ensues on Ser and Thr residues, but this modification has no defined glycosylation sites (due to several glycosyltransferase enzymes; sometimes, the addition of sugar/carbohydrate can occur at different amino acid residue like Pro instead of Ser/Thr) (Brockhausen & Stanley, 2015). Bacteria generally exhibit the carbohydrate moieties on their surface, which are unknown to the host, while viruses represent their carbohydrate moieties by taking over the host machinery. These carbohydrate moieties and carrier proteins have been proved to be very immunogenic and have shown a promising role in vaccine preparation (Watanabe et al., 2019). The connection between vaccine efficacy and glycosylation is still not much known. Some of the glycan vaccines, which have been reported against bacterial infections and are known to influence vaccine candidates' immunogenicity, are Streptococcus pneumonia, Haemophilus influenza Type b, and Neisseria meningitides vaccines; for other infectious and noninfectious diseases, it is still in the process of development (Kim et al., 2020; Mettu et al., 2020). The previous studies result showed that the glycosylated protein expressed in mammalian system has shown higher immunogenicity when compared to vaccine candidate expressed in bacterial expression systems (Nascimento & Leite, 2012). Nuttall et al. (2006), in the Year 2006 had developed a glycan protein‐conjugated vaccine against the tick‐borne disease. They have expressed the recombinant protein Bm86 and Bm95 proteins in Pichia pastoris, and the resultant protein was found to be more immunogenic compared to the nonglycosylated one, and hence proved as potential immunogens (de La Fuente et al., 2006; Nuttall et al., 2006).
Further, Cuccui et al. (2013) had developed a recombinant glycoconjugate vaccine using protein glycan coupling technology. They have demonstrated the Francisella tularensis O‐antigen's conjugation to the carrier protein exotoxin A of Pseudomonas aeruginosa, by utilizing Campylobacter jejuni PglB oligo‐saccharyl transferase, followed by the expression of recombinant glycoconjugate vaccine in Escherichia coli. The vaccination of BALB/c mice with the purified glycoconjugate vaccine showed boosted immunoglobulin G (IgG) levels. Next, in 2016, Li and his coworkers observed that the vaccine candidate (sE2 protein) against hepatitis C virus, expressed using mammalian expression system, has altered glycosylation patterns and was responsible for the augmentation of immunogenicity by raising the antibody titers in mice model. Further, when mice got immunized with the same vaccine candidate by removing the N‐glycans in the sE2, they have observed the reduced antibody titers. With this, it could be concluded that glycosylated proteins will be very beneficial for vaccine designing compared to nonglycosylated ones to enhance mainly humoral immune responses (Li et al., 2016). Later, Faburay et al. (2017), in the Year 2017, had given an account on the subunit vaccine against Ehrlichia ruminantium, which causes the infection in sheep. In this study, they have used the glycosylated major antigenic protein1 protein of Ehrlichia ruminantium and expressed the same in a baculovirus expression system. They have found that the designed vaccine has generated a strong humoral and cell‐mediated immune response against E. ruminantium.
After bacterial and viral infections, the prominence of this modification has been reported in malaria, a parasitic disease. RTS, S/A01, the first vaccine approved against malaria, is composed of O and C‐linked glycans present on the surface proteins, CRP, and TRAP of Plasmodium and therefore used as the primary vaccine candidates (Goddard‐Borger & Boddey, 2018; Swearingen et al., 2016). In 2017, Lopaticki et al. (2017) and coworkers reported that the protein POFUT2 (O‐fucosyltransferase) of Plasmodium falciparum is responsible for O‐glycosylation of circumsporozoite protein (CSP) and TRAP, and the modification in these proteins enhance their endocytosis. Further, the major histocompatibility complex (MHC) II represented these proteins to get recognized by the T‐cell receptor, leading to B‐cell activation to enhance antibody production. In contrast, the only glycosylated CSP protein is responsible for the CD8+ cell‐mediated immune response. This data suggested that glycosylated vaccine candidates are responsible for introducing both potential cell‐mediated and humoral immune responses.
Tumor cells possess a high frequency for the occurrence of O‐linked glycosylation on their surface. Tn, sTn, and T antigens have been synthesized with the help of T‐synthase (gluconyltransferase), and this T synthase folds appropriately with the aid of Cosmc, an essential molecular chaperon. Overexpression of these tumor‐associated carbohydrate antigens (TACA; Tn, Sn, T) having aberrant O‐linked glycosylation leads to adverse or poor condition in cancer patients by promoting metastasis (Fu et al., 2016). Nowadays, these TACA has been targeted for the development of anticancerous vaccines. Concerning this, in 2012, Abdel‐Aal et al. (2012) have designed a synthetic Tn‐based anticancer vaccine. The designed construct comprises LAA (Lipoamino acid; TLR‐2 stimulant), Tn antigen B‐cell epitopes, CD4+ T cell epitope from poliovirus, and CD8+ T‐cell epitope from ovalbumin. After this, the immunogenicity of the designed construct was checked in BALB/c mice, and it was found that the vaccine candidates without any immunostimulatory agent were able to induce a tremendous immune response.
The O‐glycosylated peptides or proteins are also known to play a very crucial role in MHC‐based immune responses (CD4+ or CD8+) in cancer. MUC1 (epithelial Type 1 transmembrane mucin), the promising marker for breast cancer diagnosis, is currently being used as an active target for immunotherapies. In comparison to the non‐glycosylated, the mucin glycoforms are positively associated with the antibody‐generated immune responses. For addressing the same issue, an experimental setup was done to measure the proteolytic degradation of glycosylated mucin by cathepsin L present in antigen‐presenting cells. Cathepsin L is a lysosomal proteinase that helps in the processing of antigens, regulates B‐cell production, and other physiological processes, such as apoptosis, remodeling of cell‐matrix, growth regulation, and generation of immune responses, especially MHC Class II (Gomes et al., 2020). The results obtained with this study using T‐hybridomas CD4+ technology reveal that the O‐glycosylated peptides recognized explicitly by T cell receptors get presented on the surface of antigen‐presenting cells for the generation of CD4+ T cell immune response (Stepensky et al., 2006). Comparable methodologies are also being continued in the MHC Class I pathway, which focuses on recognizing immunogenic glycopeptides produced by immunoproteasomes.
With the abovementioned information, it could be concluded that the protein or peptide‐based glycosylated vaccine candidates evoke strong humoral and cellular immune responses. Nevertheless, in the case of nucleic acid vaccine designing, the same cannot be concluded. A recent study done by Ozdilek et al., 2020 suggested that the glycosylated Ag85A (a surface protein of Mycobacterium tuberculosis), when expressed in a mammalian expression system, does not prompt a potential humoral immune response in mice, in comparison to the native protein expressed in a bacterial expression system (Ozdilek et al., 2020). This shows that the mammalian expression system‐based glycosylated proteins may affect the nucleic acid vaccine candidate's antigenicity and immunogenicity.
3.2. Phosphorylation
The addition of the phosphate group to the macromolecules is referred to as phosphorylation. This type of modification helps to maintain and regulate cell signaling, cell cycle, cell growth, and programmed cell death functioning. The frequency of occurrence of this modification is higher in eukaryotes in comparison to other PTMs. Serine and Threonine are significant sites that undergo phosphorylation. The phosphopeptides are transported from cytosol to the endoplasmic reticulum with the help of a TAP transporter. Phosphorylated peptides or epitopes (synthetic or natural) are known to better recognized by the cytotoxic T lymphocytes, i.e., presented by MHC Class I molecules, hence, directly involved in the generation of specific immune responses (Zarling et al., 2000). One of the comparative studies of phosphorylated and non‐phosphorylated peptides, performed by Zarling et al. (2006), has shown that the cytotoxic T lymphocytes (CTLs) can recognize the phosphopeptides compared to non‐phosphorylated ones and hence involved in the induction of immense immune responses. In Alzheimer's disease, phosphorylation plays a very notable role in the development of vaccine designing. Richter et al. (2014), in the Year 2014, reported a doubly phosphorylated tau neuropeptide vaccine against Alzheimer's disease. They have designed this peptide vaccine by utilizing the B cell neoepitopes from the tau protein (at phosphorylated sites‐ Tau199–208[pS202/pT205], Tau209–217[pT212/pS214], and Tau229–237[pT231/pS235]) and fused these epitopes with the Clostridium tetani (tetanus) toxin or the Ag85B antigen from Mtb and transgenic mice P301S was immunized with these doubly phosphorylated peptides. After immunization with these three phosphorylated peptides, it was found that there was a substantial enhancement in the paralysis and survival rates of mouse P301S (Doi et al., 2007; Richter et al., 2014).
Further, the vaccine construct was formulated with the aluminum hydroxide to achieve the enhanced immune response against Alzheimer's disease in the transgenic Alu‐GelS (Bungener et al., 2008) and injected in mice to efficiently obtain the protective humoral response. In Alzheimer's disease, the tau proteins get hyperphosphorylated, which leads to protein aggregation. To reduce this hyperphosphorylation, Kontsekova et al. (2014a, 2014b) had come up with the tau immunotherapy concept. This study has developed a tau peptide vaccine to prevent or minimize the oligomerization and degeneration of tau and neurofibrils, respectively. Immunization of mice with the AADvac1 vaccine results in effective blockage of hyperphosphorylation and oligomerization of the tau protein, leading to an improved animal neurobehavioral parameter model. The immunological effect was seen due to the presence of a carrier protein (KLH) joined with the tau peptide (294KDNIKHVPGGGS305) and formulated with an adjuvant that was responsible for the induction of a strong humoral immune response in the mice model (Kontsekova et al., 2014a, 2014b). These studies conclude that the peptides exhibiting phosphorylation give the impression to be a capable treatment option for other communicable and NCDs.
3.3. Methylation
Methylation refers to the addition of methyl group to the genetic material DNA (Cytosine C5 5‐methylcytosine with DNA methyltransferase; DNMTs; can also be methylated on the C4 position (James et al., 2013), and due to this chemical epigenetic modification, the activity of the molecule changes without affecting its DNA sequence. This epigenetic modification is essential for regulating gene expression, X‐chromosome inactivation, genomic imprinting, and many more (Jin et al., 2011). The mutation or defects in DNMTs can lead to tumor development; hence this epigenetic modification is a prominent hallmark in many diseases (Suzuki & Bird, 2008). The palindromic CpG nucleotide sequence, present favorably in bacterial DNA or plasmid, is the leading site for the occurrence of methylation and possesses the immunostimulatory activity, hence act as an adjuvant in DNA vaccines. These CpG motifs recognize the Toll‐like receptor 9 (receptor on ‐Antigen Presenting Cells [APCs]) and result in the activation of TLR‐9 associated signaling and, in response, trigger the production of interleukin (IL)‐12, Type 1 interferon, which leads to cell‐mediated immune responses (Abdulhaqq & Weiner, 2008). The vaccination of bovine herpesvirus‐1 glycoprotein D with immunostimulatory CpG motifs has shown the augmentation of cellular immune responses in cattle compared to the without CpG motifs (Ioannou et al., 2002). Although nucleic acid vaccines have shown promising results, approaching the peptides for vaccination would be of great importance in cancer and other disorders or infectious diseases. The programmed cell death PDL‐1 and PDL‐2 immunogenic peptides, expressed by tumorigenic cells, macrophages, and myeloid‐derived suppressor cells, can be used for vaccination purposes (Munir Ahmad et al., 2016). The prominence of PTM can be based on having or not having the modification; sometimes, by blocking the modification virtuous immune response can be generated. Here is one example in which, by blocking DNMT, hematological malignancies can be prevented (Klausen et al., 2018). The methylation can be prevented using azacitidine (binds to cytidine nucleotides), a DNMT inhibitor. This inhibition leads to the gene transcription of cancer‐testis antigens, whose expression is mainly regulated by DNA methylation. These tumor antigens are then processed and presented on MHC molecules' surface, which is why it is being used as an effective cancer immunotherapy option. Again, it was also found that the DNMT inhibitors were involved in enhancing the expression of APCs and presentation molecules, CALR, CD58, B2M, PSMB8, PSMB9 at the transcription and translational level in ovarian and colon cancer (Siebenkäs et al., 2017).
Apart from DNA vaccines, in vitro transcribed (IVT) mRNA, vaccines are also an alluring strategy in next‐generation vaccines against infectious and noninfectious diseases. The most commonly used modifications for the generation of IVT mRNA vaccine are 5′ m7G hat, poly(A) tail, 5′ and 3′‐untranslated region, and pseudonucleotide modification to improve or enhance stability and translation efficiency, which are ultimately responsible for the elevated immune response (Wadhwa et al., 2020). The IVT mRNA vaccines after immunization must be targeted to the cell cytoplasm. Although many vehicles are available for delivering mRNA vaccines, nonviral approaches are the most preferred ones and are in use because of their low cost, ease of manufacture, and better protection. In the case of the ZIKA virus, researchers have formulated the potent anti‐ZIKV mRNA vaccine by using prM‐E glycoproteins of the ZIKV H/PF/2013 strain. This vaccine contains 1‐methylpseudouridine (m1ψ) modified nucleoside responsible for the stability, prevention from degradation, and efficient translation of the mRNA in in vivo conditions. For the longer protein expression, the vaccine was encased in lipid nanoparticles (Pardi et al., 2017). Apart from infectious diseases, mRNA vaccines are also in practice against NCDs. CV9103, an mRNA vaccine formulated as full‐length, self‐adjuvanted mRNAs against prostate cancer. This vaccine is designed in such a way that it can target quadrat antigens‐prostate‐specific antigen, prostate‐specific cell antigen, prostate‐specific membrane antigen, and six‐transmembrane epithelial antigen of the prostate 1 (Kübler et al., 2015) overexpressed during prostate cancer. The results of the Phase I/II CV9103 clinical trial have shown promising effects against prostate cancer. The PSC mRNA vaccine constructs resulted in elevated antigen‐specific T‐cells and are the first vaccine approved by the FDA against prostate cancer. Recently, Nance and the group have proposed modifications in mRNA vaccines developed against COVID‐19 (Nance & Meier, 2021). In these mRNA vaccines, the uridine nucleobase suggested being replaced with N1‐methylpseudouridine (m1Ψ) to augment the efficacy of these mRNA vaccines. This motive behind this chemical modification is (1) to control the unrestrained immune responses, which ultimately directs to allergic reactions in the host body; (2) this modification enhances the protein translation initiation process and slows down the elongation process, which helps to maintain the half‐life and stability of mRNA. This type of chemical modification in RNA makes it an approaching therapeutic option in the upcoming times.
3.4. Acetylation
Acetylation refers to the addition of the acetyl group to the macromolecules with acetyltransferases. The acetylation occurs either at the N‐terminus of the α‐amino group of proteins (on almost 85% of mammalian proteins) (Polevoda & Sherman, 2002) or the ε‐amino group of proteins (mainly lysine residue of transcription and nuclear factors (Roth et al., 2001), histone proteins (Imhof et al., 1997), and α‐tubulin (MacRae, 1997). This kind of modification is associated with various cellular processes and enzymatic activities, such as providing stability to DNA, protein–protein interactions, and DNA binding.
Due to the intensifying antibiotic resistance in microorganisms, the glycoconjugate vaccines pave the new road to obliterate these infections. The utilization of acetylated capsular polysaccharides (CPS) and lipopolysaccharides (LPS) from bacteria are well known for generating glycoconjugate vaccines against various bacterial infections, such as meningitis, pneumonia, and sepsis. CPS, teichoic acids, and LPS represent the bacterial leading defense mechanism against complement and bacteriophages (Campos et al., 2004). The carbohydrate portion of this glycoconjugate contains the major antigenic determinants that distinguish various serotypes of bacteria. The bacterial polysaccharide capsule is known to be a potential vaccine candidate due to O‐acetyl, sialic acid, and phosphate groups. Several species of bacteria, such as S. pneumoniae 9V and N. meningitidis serogroup A, Staphylococcus aureus, possess the polysaccharide capsule O‐acetyl group, which helps them to escape from host‐mediated immune responses (Scully et al., 2018).
O‐antigens' utilization for vaccine designing is also of great importance, and attempts have also been made to understand the influence of O‐acetylation patterns in antigenic peptides or proteins. By taking this modification into account, a conjugate vaccine against Sh. sonnei shigellosis was designed in which the O‐antigen of Sh. sonnei was fused with recombinant exoprotein A of P. aeruginosa. The vaccine was then tested in Israeli military recruits; after vaccination, it was found that the vaccine can confer protection against the infection and eliciting high Ab titers (Cohen et al., 1997).
Salmonella typhi is known to cause typhoid fever, and due to the presence of Virulence Antigen (Vi), a CPS (Bystricky & Szu, 1994), it can escape from host machinery. This capsular Vi protein is a homopolymer of GalNAcA repeating units and is O‐acetylated at the C‐3 specific position. The O‐acetylated Vi‐conjugated protein, when given in an adequate amount (>52% as per WHO) as a vaccine candidate against enteric disease, is known to provide protective immunity in comparison to partially or deacetylated Vi protein in clinical studies (Szu et al., 1991, 2013). This summarizes that the amount of O‐acetylated proteins is also an essential aspect of measuring vaccine candidate effectiveness or potency (Hitri et al., 2019). A comparative study done by Scully et al. (2018) has reported that the O‐acetylated CP5‐CRM197 glycoconjugate vaccine (with adjuvant AIPO4) upon immunization against the Staphylococcus aureus infection generated the potential anticapsular antibody responses in the murine pyelonephritis model rather than the de‐O‐Acetylated vaccine.
Apart from the polysaccharide acetylation modification, peptide modification with N‐terminal acetylation is also in practice to improve the cyclization of an epitope that may be responsible for the confirmational epitope assembly, prevent the enzymatic peptide degradation, enhance the immunogenicity of peptide vaccine (Purcell et al., 2007); e.g., MART‐1(27–35) immunogenic peptide use for development of a vaccine against the metastatic melanoma (Tarhini et al., 2012). Like in cancer, the modified immune peptides have also played a significant role in neuro‐related disorders (Campbell et al., 1995). The angiotensin and bradykinin immunogens or neuropeptides have been in the picture for many years and involved antisera production of broad specificity. One of the studies done by Nusserberg et al. (1983) revealed, N‐acetylated (at Asn and Val) angiotensin peptides at the N terminus, coupled with the carrier protein, immunization has shown higher immunogenicity in comparison to the nonacetylated one. Aiming or incorporating the O‐acetyl group can result in elicited antibodies and efficacious functional and mechanistic immune responses.
3.5. Sulfation
One another type of posttranslational modification is sulfation, which is primarily linked with membrane and secretory proteins. In sulfation, sulfotransferases catalyze the transfer of a sulfate (SO3–) group to an oxygen atom of Tyr, Ser, or Thr residues in the trans‐Golgi network. Likewise, for the production of N‐glycan sulfation of glycoproteins, which occurs in Golgi, sulfotransferases catalyze the transfer of a sulfate group from 3′‐phosphoadenosine‐5′‐phosphosulfate (PAPS) to a glycoprotein substrate. These N‐glycans are usually sulfated at the C‐3 position of Gal, C‐6 position of GlcNAc (N‐acetylglucosamine), C‐4 position of GalNAc (N‐acetylgalactosamine) of terminal Galactose‐N‐acetylglucosamine and N‐acetylgalactosamine residues (She et al., 2019). Sulfation in polysaccharides is known to play a role in enhancing humoral and cell‐mediated immune responses. For instance, a sulfated polysaccharide vaccine candidate against the Newcastle disease (ND), an infectious disease of birds caused by the Newcastle disease virus (NDV), was reported by Zhang et al. (2013). In this study, the broiler chicks were vaccinated with sulfated Agrocybe chaxingu polysaccharide (sACP) and nonsulfated ACP (nACP) to evaluate the immune responses. The presence of sulfate on polysaccharides was analyzed by performing 3‐(4,5‐dimethythiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide assay. Modified sACP1.32 and sACP1.79 were tested in vivo in the broiler chicks, using the nACP as a control. They found that the sACP (sACP1‐32 and sACP1.79) vaccinated group, at 21 days, showed slight enhancement in the relative thymus and spleen weight compared to nACP group. Apart from this, the serum ND antibody titers were also shown to be increased at the age of 16, 22, 28, 34, and 42, along with splenic lymphocytes (T and B‐cells) proliferation at age of 22 and 28 days. With this comparative study, it was summarized that sulfated sACP1.79 has shown the enhanced interferon‐gamma (IFN‐γ) and IL‐6 expression level in comparison to sACP1.32, and further it could be served as an immunopotentiator molecule.
Apart from the vaccines, sulfated peptides have also shown a promising role as potent antiviral inhibitors. The same has been reported in HIV‐1; the virus owns an outer membrane glycoprotein (gp120), which contains a V2 loop that is essential for viral entry (Cimbro et al., 2016; Ran et al., 2017). This V2 loop has sulfated tyrosine residues (Tys173 and Tys177) and is involved in sustaining the envelope from antibody protection. After that, it was found that the V2 peptide mimics the morphology and function of CCR5 (critical cellular coreceptor) that maintains the interaction of HIV‐1. This study found that the V2 (tyrosine‐sulfated) peptide act as a potential antiviral inhibitor by blocking the CCR5 interaction site in the gp120 (Cimbro et al., 2016). This novel approach can be studied further in other diseases to design new peptide‐based inhibitors and vaccine candidates.
Another study performed by Yi‐Min She et al. (2019), by applying extensive glycoproteomics followed by LC‐MS/MS analysis, was performed to determine the ideal sulfated N‐glycan peptides in influenza vaccines. In this study, a large number of sulfated glycopeptides having sulfated complex and hybrid N‐glycan were identified. After resolving the isobaric N‐glycan structure, it was found that around 60% of sulfated glycans were present, and majorly three types of N‐glycans were dominant, namely sulfated‐3‐Gal, sulfated‐4‐GalNAc, and sulfated‐6‐GlcNAc. Moreover, this also revealed sulfated N‐glycans' topological location, which helped explain the various functions performed by sulfated N‐glycans, such as receptor binding, enzyme activity, and viral infection. This study concluded that the sulfation on the influenza virus's glycoproteins evokes antigenicity and innate immunity, ultimately paving the way for newly modified vaccines against the infections.
3.6. SUMOylation and ubiquitylation
Ubiquitylation refers to the addition of ubiquitin (a small protein; 76 amino acids) to a protein, whereas SUMOylation is the addition of a small ubiquitin‐like modifier (SUMO) to the proteins. The former directs the proteins for degradation and involves several biological processes, such as cell signaling, programmed cell death, stress response, and DNA repair mechanism (Park et al., 2020). Whereas SUMOylation does not mean protein degradation and regulates the function of various proteins; both modifications are reversible. Apart from these functions, the discussed modifications are involved in the antigen presentation of peptides with MHC's help Class I molecules. Initially, the ubiquitin molecule attaches to the target molecules' lysine group by generating an isopeptide bond between the Ub (C terminal) and the target's q‐lysine amino group protein. The ubiquitylation process occurs chronologically; the initial step involved the E1 enzyme, which activates the Ub enzymes (ATP‐dependent activation), and then in the second step, the Ub gets transfers to the E2 (ubiquitin‐conjugating enzyme) enzyme. Last, the E3 enzyme (ubiquitin ligase) transports Ub molecules to the target protein and formulate poly‐Ub chains. The entire polyubiquitylated protein is subjected to 26S proteasomal degradation, where the protein gets chopped into small peptide molecules with the help of Ub‐specific hydrolases. These peptides are then taken up by the ER (endoplasmic reticulum) and ultimately recognized by the CD8+ T lymphocytes to generate immune responses (Stewart et al., 2016). It is known that proteosome is generally responsible for the degradation of proteins (e.g., Tumor antigens) to generate Class I MHC ligands. The polyubiquitin chains can covalently attach to the vaccine molecule by enzymatic reactions to attain protective immunity and enhance the epitopes' proteasome processing. Rodriguez et al. (1997) in the Year 1997 has reported that the fusion of ubiquitin molecule with lymphocytic choriomeningitis virus nucleoprotein at N‐terminus helps in the generation of CTL epitopes and is capable of providing protective immunity against viral infection. With this study, they have concluded that ubiquitination may improve the DNA immunization practice. In relation to this, Zhang et al. (2005) have performed a study for designing a DNA vaccine against melanoma cancer. Here, they have covalently fused the tyrosinase‐related protein 2 (melanocyte lineage differentiation antigen) with the ubiquitin moiety (self‐antigen) N‐terminus. When injected in mice C57BL/6, they found improvement in metastasis and survival rate due to the induction of protective immunity. They have concluded that targeting the self‐antigens expressed by tumors can generate the CTL epitopes (MHC‐I ligands), processed through the ubiquitin‐proteasome pathway could be a great strategy against various carcinomas.
The proteasomal targeting/Ub targeting of proteins has been pragmatically studied in several mammalian vaccine designing to achieve enhanced epitope processing and potential T cell (CD8+) immune responses. However, in various gene‐based vaccine studies, the Ub targeting had failed to target the proteasome and raise the immune response, but on the other side, mammalian vaccines expressed in the yeast system have shown robust immune responses. Nevertheless, to prove this, Andersson and Barry (2004) in the Year 2004 have reported the aspects of antigen targeting to the proteasome to maximize the immune response via gene‐based vaccines. They have performed a comparative study between the Ub constructs expressed in yeast and mammalian system. To achieve the results, they have green fluorescent protein tagged several Ub genes to detect the enhanced CD8+ T cell response and have found that the response was not due to the translation of proteins but it was because of proteasome targeting. But they have also observed that some of the Ub constructs expressed in the yeast system had the poor ability to target the mammalian cell proteasome and hence, failed to achieve the CD8+ response in mice. Whereas the Ub constructs expressed in the mammalian system had the ability to target the nucleoprotein of the flu virus. Overall, this study's data suggested that Ub fusion/targeting has the efficiency to augment CD8+ responses against both dominant and subdominant antigenic epitopes.
Apart from gene‐based vaccines, virus‐like particles (VLPs) have proved to be better vaccine candidates in many diseases and are also used as carriers for the exogenous antigenic molecules. VLPs are similar to viruses but lack the genetic material and nucleus and formed assemblages of capsid and envelop protein of several viruses together. The capsid and envelop proteins of viruses are mainly susceptible to posttranslational modifications, and one of the common modifications is the SUMO. The SUMOylation mechanism gets activated by the E1 enzyme and transferred to enzyme E2 and then ligated to target protein with or without enzyme E3. SUMOylation modification was seen to be very useful in producing foot and mouth disease VLPs, by applying a SUMO fusion protein approach in the bacterial expression system of E. coli. In 2009, Lee et al. (2009) have shown that the fusion of three SUMO‐modified FMDV capsid proteins V0, V1, and V3 was able to form a stable heterotrimeric complex, preventing inappropriate aggregation and embracing water solubility. This study showed that the addition of SUMO fractions was able to prevent the aggregation of capsid protein; however, on removing these fractions, the capsid proteins reassemble into their native form. Following the same approach, in 2013, Guo et al. (2013) reported the FMDV VLPs generated by SUMO fusion protein in the bacterial expression system of E. coli. In this study, they have generated VLPs SUMO fusion proteins (V0, V1, V3) corresponding to the study done by Lee et al. (2009). Although the study done by Lee et al. (2009) has not shown the in vivo testing of the SUMO modified VLPs; so, has been done by Guo et al. (2013) group. They immunized guinea pigs, cattle, and swine with FMD VLP (SUMO fusion proteins) through an intramuscular immunization mode. It was found that FMD VLPs were able to generate the B‐cell (specific antibody response) and T cell immune responses with the secretion of IFN‐γ, leading to the stimulation of both adaptive and innate immunity. This data suggested that SUMOylated FMD VLPs confers complete protection in many model organisms and hence, upholds the property of VLPs as promising vaccine candidates.
3.7. Lipidation/palmitoylation
This kind of posttranslational modification is the covalent attachment of fatty acid/lipid moiety to the cysteine residue and is referred to as S palmitoylation. In contrast, fatty acid attachment to the Ser and Thr is acknowledged as O‐palmitoylation (rare palmitoylation). Lipidation has an indispensable role in regulating the cell signaling, moderates the target protein's binding affinity with the lipid membrane by changing the subcellular protein localizations, and improving the function of proteins related to intrinsic and extrinsic signals. One more promising role of palmitoylation is enhancing protein stability and protecting it from proteasomal degradation. In terms of vaccines, it has been reported that the lipidation of proteins plays a pivotal role in enhancing the vaccine candidates' immunogenicity. One of the studies by Zeng et al. (2011) investigated that the covalent attachment of Pam2‐Cys to the vaccine protein could generate a tremendous humoral immune response. In this study, they have modified hen egg‐white lysozyme (HEL) and bovine insulin by coupling them with three different moieties of soluble Pam2Cys (the first group comprises of Pam2Cys; in the second group HEL coupled with Freund, whereas the third group was coupled with Alum) and administered in mice to check the immune responses. The study data suggested that both lipidated proteins have shown much sturdier humoral immune response, i.e., antibody production, compared to proteins administered in coupled with Freund and Alum. The lipidated protein has also shown a similar immune response when administered in different mice strains; this concludes that the posttranslationally modified vaccine candidate has guaranteed T‐cell‐dependent immune response across the MHC (major histocompatibility complex) barricade.
Further, it was reported that the lipidated pneumococcal antigens (DacB and PnrA) have shown ameliorated humoral immune responses when administered in mice. Compared to non‐lipidated proteins, the lipidated modified protein was responsible for the induction of high antibody titers, hence accountable for suppressing Pneumococcus colonization (Voß et al., 2020).
Several studies were done to determine whether the enhanced immune response is due to the protein's lipidation or delivery vehicles, such as liposomes or immune stimulating complexes (Könnings et al., 2002). After the comparative studies, it was found that the candidate's enhanced immunogenicity was because of lipidation without the involvement of any adjuvant or delivery carriers. Hence, this concludes that lipidation modification is responsible for maintaining the proteins' stability without disordering its integrity, and it would be advantageous for the protein‐based vaccine designing and protect it from immediate proteasomal degradation.
3.8. Formylation
Formylation is the addition of a formyl group to the compound. The protein synthesis mechanism has great importance, as the process originates with a formylated methionine residue. This modification often occurs on histone proteins (lysine), involved in DNA binding and modulating gene expression (Leder & Bursztyn, 1966). This modification has been known to induce a tremendous protective immune response against intracellular pathogens. Compared to MHC Class 1a molecules, the Class 1b molecules remain active in the host to fight against the infection. Mir and Sharma (2013), in a review article, has summarized the role of H2‐M3 (MHC Class 1b molecule), which can recognize and represent the N‐formylated peptides for the activation of both innate (chemotactic property, ROS production, and phagocyte activation to secrete antimicrobial peptides) and adaptive (proliferation of T cell and the activation of CD8+ T‐cells) immune response against the infectious M. tuberculosis. MHC Class 1a recognizes N‐formylated methionine peptides by blocking both the C and N terminus, whereas MHC Class 1b (H2‐M3) recognizes only N termini of N‐formylated peptides by utilizing the frame‐shifted binding site. This H2‐M3 can also recognize and bind to the nonformylated peptides obtained from influenza viruses, but the formylated peptides have a 10,0000‐fold more possibility of binding effectively with the H2‐M3. Chun et al. (2001) have screened the genome of Mtb and had found that N‐formylated peptide sequences have an excellent binding affinity for M3, and they have tested the same by performing the peptide‐binding assay. With this study, they have come across that the mice immunized with M3‐restricted N‐formylated peptides have provoked cell‐mediated immunity, which led to the lysis of infected Mtb macrophages.
Further, it was reported that the bone marrow‐derived dendritic cells pulsed with TB2 (H2‐M3 binding peptide) when injected in mice have elicited enduring CD8+ based T cell‐mediated immune response against the Mtb pathogen (Doi et al., 2007). However, the vaccines that are already in the pipeline for Mtb, such as VMP1002, Ag85b, and others, are not restricted. This concludes that N‐formylated peptides represented by MHC class 1a molecules could be a groovy target and an innovative way to design vaccines against infectious diseases like tuberculosis.
3.9. Amidation
Amidation refers to amide formation by replacing or substituting the hydroxyl group. This kind of modification influences the immunogenicity of epitopes‐derived protein or peptide utilized to design vaccines. Peptide vaccines are the short antigenic fragments of amino acids that are responsible for the targeted immune responses. However, this type of vaccine is highly vulnerable to proteasomal degradation, making them less efficient (Li et al., 2014). Nowadays, the peptidomimetic approach is being used to overcome these kinds of complications. These are the synthetic pseudo or small modified peptide molecules (mimic peptide), which are engineered or modified at the N or C terminals to gain the high stability and immunogenicity of vaccine immunogens (Croft & Purcell, 2011). One of the studies done by Cotton et al. has shown that the amidation or modification at ψ[CH2‐NH] or ψ[CO‐NMe] (N‐methylated) in the amide bonds of epitopes (derived from snake venom; 24–36 peptides) at C‐terminal has the ability to bind with murine MHC Class II I‐Ed molecules, which ultimately is responsible for activating the T helper cells and leads to the generation of potential antibody immune responses (Cotton et al., 1998). The nonnatural pseudo peptides (MHC1 and MHCII ligands) can be generated with this approach, which directs to T cell responses' augmentation.
The vaccine for Alzheimer's AN1792, composed of full‐length Aβ1‐42, has come up with the threat of meningoencephalitis due to the autoimmune Th1 response in contrast to all forms of Aβ (Fox et al., 2005; Orgogozo et al., 2003). To overcome this risk and minimize the accumulation of Aβ, the authors have utilized the chemically synthesized AβpE3‐8 and AβpE11‐16 peptides that were having C‐terminal amidated and cysteine bridged with two‐glycine residues (pEFRHDSGGC and pEVHHQKGGC, respectively) (Vingtdeux et al., 2016). Targeting the N‐terminal truncated pyroglutamate‐3 Aβ (AβpE3), a potential Aβ product could be a possible way for the acceptability of immunotherapy in Aβ of amyloid plaques. The peptide vaccine formulated against AβpE3, composed of AβpE3‐8 and AβpE11‐16 peptides with CRM197 (Shinefield, 2010), a diphtheria toxin vaccine, used as a carrier protein and has proved its immunogenicity in clinical trials. Further, the mice were immunized with AβpE3:CRM197 vaccine to develop a unique anti‐AβpE3 antibody, which shows no cross‐reactivity with Aβ1‐42, N‐terminally truncated pyroglutamate‐11 Aβ (AβpE11), or AβE2 (noncyclized) (Graham, 2013). Antiserum analysis suggested that the presence of IgG1 isotype, anti‐AβpE3 antibodies were an indicator of an antiinflammatory Th2 response generated by the AβpE3:CRM197 vaccine. This study concluded that the C‐terminal amidated peptide AβpE3‐8 and AβpE11‐16 conjugated with CRM197 vaccine could be responsible for the generation of the potential immune response against AβpE3 and AβpE11, respectively. It has also been reported that few antigenic epitopes/peptides have more probabilities of rousing long‐lasting protection and proved to show more stability than whole protein vaccines (Purcell et al., 2003). RTS, S a well‐known vaccine registered against malaria and has received a positive response from the European Medicines Agency. This vaccine comprises CSP of sporozoites, T cell epitopes of CSP, and hepatitis B surface antigen. Later, after immunization in children some side effects were noted, such as fever, swelling at the site of vaccination, local pain, and convulsive seizures. The CSP protein has 37 reiterations of NPNA (Asn‐Pro‐Asn‐Ala), which was found to be a highly immunogenic epitope for subunit vaccine designing. By considering this protein, a peptidomimetic UK‐39 (20 amino acids and 5 NPNA reiterations) was designed, which is similar to native CSP NPNA repeats in terms of structure, function, and antigenicity (Abdulhaqq & Weiner, 2008). The amide bond was inserted between the glutamate amino acid residue and 4‐aminoproline, in addition to an N‐terminal phosphatidylethanolamine. The amide bond's purpose was to achieve stable hydrogen bonding to be linked up with the IRIV (immuno‐potentiating influenza virosomes), vaccine delivery system. When injected in mice, UK‐39 resulted in parasite inhibition by producing the sporozoite cross‐reactive IgGs antibody and, hence, found to be an effective therapeutic option (Okitsu et al., 2007).
4. CONCLUDING REMARKS AND FUTURE PERSPECTIVE
The identification of antigenic sequences is the primary step in designing next‐generation vaccines. The efficacy of vaccines can be enhanced by supplementation of adjuvant and by using the potential delivery systems. Nevertheless, even after these strategies, there is still some gap to achieve better quality and highly immunogenic vaccines. The mechanism or molecular pathways associated with the vaccine's immune response is still not clearly understood. We have come across the current knowledge present on posttranscriptional/translation modifications (chemically and naturally) in vaccine macromolecules, searching for potential interventions for obtaining the tremendous immune response and protective immunity. This review is specifically focused on the relationship between the PTMs and vaccine molecules in improving the rate of protective immune responses to combat both communicable and NCDs. A variety of PTMs occur naturally and are responsible for regulating the various type of cell functions.
In contrast, the unusual PTMs can lead to certain disorders in humans and responsible for microorganism's pathogenicity. As the classical vaccine approaches have proven ineffective, researchers exploited the PTM to develop efficacious next‐generation vaccines against a broad range of pathogens. PTM modifications in the vaccine molecules are not standard in active research, although it can become very generous and successful immunotherapy. This updated review article provides the vaccine candidates' collective information, which naturally possesses the PTM at their specific site or chemically synthesized to get a better immune response and protect the degradation. For example, glycosylation has dramatically enhanced the immunogenicity of vaccines compared to nonglycosylated and is in the pipeline of clinical trials. The newly generated or modified peptides have shown great immune responses in various infections and autoimmune diseases like influenza, malaria, arthritis, HIV infection, multiple sclerosis, cancer immunotherapy, and many more. Although this modification is responsible for enhancing immunity, the safety, efficacy, and quality standards of modified next‐generation vaccines should be assessed before their availability to the population. We firmly believe that this review will deliver fruitful information for vaccines' development as this is the current need for society. It will encourage the researchers working in this specific field and inspire basic and experimental molecular biologists.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
Rupal Ojha is thankful to UGC for providing a University fellowship. Vijay Kumar Prajapati is thankful to the Central University of Rajasthan for providing lab facilities.
Ojha, R. , Prajapati, V. K. (2021). Cognizance of post‐translational modifications in vaccines: A way to enhanced immunogenicity. J Cell Physiol, 236, 8020–8034. 10.1002/jcp.30483
REFERENCES
- Abdel‐Aal, A.‐B. M. , El‐Naggar, D. , Zaman, M. , Batzloff, M. , & Toth, I. (2012). Design of fully synthetic, self‐adjuvanting vaccine incorporating the tumor‐associated carbohydrate Tn antigen and lipoamino acid‐based Toll‐like Receptor 2 Ligand. Journal of Medicinal Chemistry, 55(15), 6968–6974. [DOI] [PubMed] [Google Scholar]
- Abdulhaqq, S. A. , & Weiner, D. B. (2008). DNA vaccines: Developing new strategies to enhance immune responses. Immunologic Research, 42(1‐3), 219–232. [DOI] [PubMed] [Google Scholar]
- Anassi, E. , & Ndefo, U. A. (2011). Sipuleucel‐T (provenge) injection: The first immunotherapy agent (vaccine) for hormone‐refractory prostate cancer. Pharmacy and Therapeutics, 36(4), 197–202. [PMC free article] [PubMed] [Google Scholar]
- Andersson, H. A. , & Barry, M. A. (2004). Maximizing antigen targeting to the proteasome for gene‐based vaccines. Molecular Therapy, 10(3), 432–446. [DOI] [PubMed] [Google Scholar]
- Breitling, J. , & Aebi, M. (2013). N‐linked protein glycosylation in the endoplasmic reticulum. Cold Spring Harbor Perspectives in Biology, 5(8), a013359–a013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhausen, I. , & Stanley, P. (2015). O‐GalNAc Glycans. In (Eds.) Varki, A. , Cummings, R. D. , Esko, J. D. , Stanley, P. , Hart, G. W. , Aebi, M. , Darvill, A. G. , Kinoshita, T. , Packer, N. H. , Prestegard, J. H. , Schnaar, R. L. & Seeberger, P. H. , Essentials of Glycobiology (pp. 113–123). Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Bungener, L. , Geeraedts, F. , Ter Veer, W. , Medema, J. , Wilschut, J. , & Huckriede, A. (2008). Alum boosts TH2‐type antibody responses to whole‐inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine, 26(19), 2350–2359. [DOI] [PubMed] [Google Scholar]
- Bystricky, S. , & Szu, S. C. J. B. C. (1994). O‐acetylation affects the binding properties of the carboxyl groups on the Vi bacterial polysaccharide. 51(1), 1‐7. [DOI] [PubMed]
- Campbell, D. J. , Kladis, A. , Duncan, A.‐M. , & Lawrence, A. C. (1995). [19] Strategies for measurement of angiotensin and bradykinin peptides and their metabolites in central nervous system and other tissues. Methods in Neurosciences (23, pp. 328–343). Elsevier; [Google Scholar]
- Campos, M. A. , Vargas, M. A. , Regueiro, V. , Llompart, C. M. , Albertí, S. , & Bengoechea, J. A. (2004). Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Journal of Infection and Immunity, 72(12), 7107–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, N. J. (2013). Multicomponent meningococcal serogroup B vaccine (4CMenB; Bexsero(®)): A review of its use in primary and booster vaccination. BioDrugs, 27(3), 263–274. [DOI] [PubMed] [Google Scholar]
- Chun, T. , Serbina, N. V. , Nolt, D. , Wang, B. , Chiu, N. M. , Flynn, J. L. , & Wang, C.‐R. J. T. J. O. E. M. (2001). Induction of M3‐restricted cytotoxic T lymphocyte responses by N‐formylated peptides derived from Mycobacterium tuberculosis. 193(10), 1213‐1220. [DOI] [PMC free article] [PubMed]
- Cimbro, R. , Peterson, F. C. , Liu, Q. , Guzzo, C. , Zhang, P. , Miao, H. , Van Ryk, D. , Ambroggio, X. , Hurt, D. E. , De Gioia, L. , Volkman, B. F. , Dolan, M. A. , & Lusso, P. (2016). Tyrosine‐sulfated V2 peptides inhibit HIV‐1 infection via coreceptor mimicry. EBioMedicine, 10, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, D. , Ashkenazi, S. , Green, M. S. , Gdalevich, M. , Robin, G. , Slepon, R. , Yavzori, M. , Orr, N. , Block, C. , Ashkenazi, I. , Shemer, J. , Taylor, D. N. , Hale, T. L. , Sadoff, J. C. , Pavliakova, D. , Schneerson, R. , & Robbins, J. B. (1997). Double‐blind vaccine‐controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet, 349(9046), 155–159. [DOI] [PubMed] [Google Scholar]
- Copyright 2015‐2017 by The Consortium of Glycobiology Editors, La Jolla, California. All rights reserved.
- Cotton, J. , Hervé, M. , Pouvelle, S. , Maillère, B. , & Ménez, A. (1998). Pseudopeptide ligands for MHC II‐restricted T cells. International Immunology, 10(2), 159–166. [DOI] [PubMed] [Google Scholar]
- Croft, N. P. , & Purcell, A. W. (2011). Peptidomimetics: Modifying peptides in the pursuit of better vaccines. Expert Review of Vaccines, 10(2), 211–226. [DOI] [PubMed] [Google Scholar]
- Cuccui, J. , Thomas, R. M. , Moule, M. G. , D'Elia, R. V. , Laws, T. R. , Mills, D. C. , Williamson, D. , Atkins, T. P. , Prior, J. L. , & Wren, B. W. (2013). Exploitation of bacterial N‐linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biology, 3(5), 130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow, J. J. , & Kesselheim, A. S. (2015). A new wave of vaccines for non‐communicable diseases: What are the regulatory challenges? Food and Drug Law Journal, 70(2), 243–258. [PubMed] [Google Scholar]
- de La Fuente, J. , Canales, M. , & Kocan, K. J. P. I. (2006). The importance of protein glycosylation in development of novel tick vaccine strategies. 28(12), 687‐688. [DOI] [PubMed]
- Dikid, T. , Jain, S. K. , Sharma, A. , Kumar, A. , & Narain, J. P. (2013). Emerging & re‐emerging infections in India: An overview. The Indian Journal of Medical Research, 138(1), 19–31. [PMC free article] [PubMed] [Google Scholar]
- Doi, T. , Yamada, H. , Yajima, T. , Wajjwalku, W. , Hara, T. , & Yoshikai, Y. (2007). H2‐M3‐restricted CD8+T cells induced by peptide‐pulsed dendritic cells confer protection against mycobacterium tuberculosis. Journal of Immunology, 178(6), 3806–3813. [DOI] [PubMed] [Google Scholar]
- Dudas, R. A. , & Karron, R. A. (1998). Respiratory syncytial virus vaccines. Clinical Microbiology Reviews, 11(3), 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faburay, B. , McGill, J. , & Jongejan, F. J. P. O. (2017). A glycosylated recombinant subunit candidate vaccine consisting of Ehrlichia ruminantium major antigenic protein1 induces specific humoral and Th1 type cell responses in sheep. 12(9), e0185495. [DOI] [PMC free article] [PubMed]
- Feldmann, H. , & Geisbert, T. W. (2011). Ebola haemorrhagic fever. Lancet, 377(9768), 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, N. C. , Black, R. S. , Gilman, S. , Rossor, M. N. , Griffith, S. G. , Jenkins, L. , & Koller, M. (2005). Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology, 64(9), 1563–1572. [DOI] [PubMed] [Google Scholar]
- Fu, C. , Zhao, H. , Wang, Y. , Cai, H. , Xiao, Y. , Zeng, Y. , & Chen, H. (2016). Tumor‐associated antigens: Tn antigen, sTn antigen, and T antigen. Hla, 88(6), 275–286. [DOI] [PubMed] [Google Scholar]
- Goddard‐Borger, E. D. , & Boddey, J. A. (2018). Implications of Plasmodium glycosylation on vaccine efficacy and design. Future Microbiology, 13(6), 609–612. [DOI] [PubMed] [Google Scholar]
- Gomes, C. P. , Fernandes, D. E. , Casimiro, F. , da Mata, G. F. , Passos, M. T. , Varela, P. , Mastroianni‐Kirsztajn, G. , & Pesquero, J. B. (2020). Cathepsin L in COVID‐19: From pharmacological evidences to genetics. Frontiers in Cellular and Infection Microbiology, 10, 589505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. S. (2013). Advances in antiviral vaccine development. Immunological Reviews, 255(1), 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H. C. , Sun, S. Q. , Jin, Y. , Yang, S. L. , Wei, Y. Q. , Sun, D. H. , Yin, S. H. , Ma, J. W. , Liu, Z. X. , Guo, J. H. , Luo, J. X. , Yin, H. , Liu, X. T. , & Liu, D. X. (2013). Foot‐and‐mouth disease virus‐like particles produced by a SUMO fusion protein system in Escherichia coli induce potent protective immune responses in guinea pigs, swine and cattle. Veterinary Research, 44(1), 48. 10.1186/1297-9716-44-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitri, K. , Kuttel, M. M. , De Benedetto, G. , Lockyer, K. , Gao, F. , Hansal, P. , Rudd, T. R. , Beamish, E. , Rijpkema, S. , Ravenscroft, N. , & Bolgiano, B. (2019). O‐acetylation of typhoid capsular polysaccharide confers polysaccharide rigidity and immunodominance by masking additional epitopes. Vaccine, 37(29), 3866–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek, Z. (2003). Emerging human infectious diseases: Anthroponoses, zoonoses, and sapronoses. Emerging Infectious Diseases, 9(3), 403–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huremović, D. (2019). Brief history of pandemics (pandemics throughout history). Psychiatry of Pandemics: A Mental Health Response to Infection Outbreak, 7–35. [Google Scholar]
- Imhof, A. , Yang, X. J. , Ogryzko, V. V. , Nakatani, Y. , Wolffe, A. P. , & Ge, H. (1997). Acetylation of general transcription factors by histone acetyltransferases. Current Biology, 7(9), 689–692. [DOI] [PubMed] [Google Scholar]
- Ioannou, X. P. , Griebel, P. , Hecker, R. , Babiuk, L. A. , & van Drunen Littel‐van den Hurk, S. (2002). The immunogenicity and protective efficacy of bovine herpesvirus 1 glycoprotein D plus Emulsigen are increased by formulation with CpG oligodeoxynucleotides. Journal of Virology, 76(18), 9002–9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, S. R. , Cedeno, C. D. , Sharma, A. , Zhang, W. , Mohler, J. L. , Odunsi, K. , Wilson, E. M. , & Karpf, A. R. (2013). DNA methylation and nucleosome occupancy regulate the cancer germline antigen gene MAGEA11. Epigenetics, 8(8), 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis, R. (2016). Posttranslational modifications and the immunogenicity of biotherapeutics. Journal of Immunology Research, 2016, 5358272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner, E. (1909). The three original publications on vaccination against smallpox: Bartleby. com.
- Jin, B. , Li, Y. , & Robertson, K. D. (2011). DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer, 2(6), 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury, G. A. , Baliban, R. C. , & Floudas, C. A. (2011). Proteome‐wide post‐translational modification statistics: Frequency analysis and curation of the swiss‐prot database. Scientific Reports, 1(1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. I. , Park, S. , Bae, J.‐Y. , Lee, S. , Kim, J. , Kim, G. , & Shin, J. S. J. P. B. (2020). Glycosylation generates an efficacious and immunogenic vaccine against H7N9 influenza virus. 18(12), e3001024. [DOI] [PMC free article] [PubMed]
- Klausen, U. , Holmberg, S. , Holmström, M. O. , Jørgensen, N. G. D. , Grauslund, J. H. , Svane, I. M. , & Andersen, M. H. (2018). Novel strategies for peptide‐based vaccines in hematological malignancies. Frontiers in immunology, 9, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könnings, S. , Copland, M. J. , Davies, N. M. , & Rades, T. (2002). A method for the incorporation of ovalbumin into immune stimulating complexes prepared by the hydration method. International Journal of Phamaceutics, 241(2), 385–389. [DOI] [PubMed] [Google Scholar]
- Kontsekova, E. , Zilka, N. , Kovacech, B. , Novak, P. , & Novak, M. (2014a). First‐in‐man tau vaccine targeting structural determinants essential for pathological tau‐tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimer's Research & Therapy, 6(4), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontsekova, E. , Zilka, N. , Kovacech, B. , Skrabana, R. , & Novak, M. (2014b). Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer's disease. Alzheimer's Research & Therapy, 6(4), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler, H. , Scheel, B. , Gnad‐Vogt, U. , Miller, K. , Schultze‐Seemann, W. , Vom Dorp, F. , Parmiani, G. , Hampel, C. , Wedel, S. , Trojan, L. , Jocham, D. , Maurer, T. , Rippin, G. , Fotin‐Mleczek, M. , Mülbe, F. , Probst, J. , Hoerr, I. , Kallen, K. J. , Lander, T. , & Stenzl, A. (2015). Self‐adjuvanted mRNA vaccination in advanced prostate cancer patients: A first‐in‐man phase I/IIa study. Journal for Immunotherapy of Cancer, 3, 26. von der. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder, P. , & Bursztyn, H. J. P. O. T. N. A. O. S. O. T. U. S. O. A. (1966). Initiation of protein synthesis, I. Effect of formylation of methionyl‐tRNA on codon recognition, 56(5), 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.‐D. , Yan, Y.‐P. , Liang, S.‐M. , & Wang, T.‐F. (2009). Production of FMDV virus‐like particles by a SUMO fusion protein approach in Escherichia coli. Journal of Biomedical Science, 16(1), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , von Schaewen, M. , Wang, X. , Tao, W. , Zhang, Y. , Li, L. , Heller, B. , Hrebikova, G. , Deng, Q. , Ploss, A. , Zhong, J. , & Huang, Z. (2016). Altered glycosylation patterns increase immunogenicity of a subunit hepatitis C virus vaccine, inducing neutralizing antibodies which confer protection in mice. Journal of Virology, 90(23), 10486–10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Joshi, M. D. , Singhania, S. , Ramsey, K. H. , & Murthy, A. K. (2014). Peptide vaccine: Progress and challenges. Vaccines, 2(3), 515–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaticki, S. , Yang, A. , John, A. , Scott, N. E. , Lingford, J. P. , O'neill, M. T. , Erickson, S. M. , McKenzie, N. C. , Jennison, C. , Whitehead, L. W. , Douglas, D. N. , Kneteman, N. M. , Goddard‐Borger, E. D. , & Boddey, J. A. (2017). Protein O‐fucosylation in Plasmodium falciparum ensures efficient infection of mosquito and vertebrate hosts. Nature Communications, 8(1), 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca, S. , & Mihaescu, T. (2013). History of BCG vaccine. Maedica, 8(1), 53–58. [PMC free article] [PubMed] [Google Scholar]
- MacRae, T. H. (1997). Tubulin post‐translational modifications‐‐enzymes and their mechanisms of action. European Journal of Biochemistry, 244(2), 265–278. [DOI] [PubMed] [Google Scholar]
- McGettigan, J. P. (2010). Experimental rabies vaccines for humans. Expert Review of Vaccines, 9(10), 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettu, R. , Chen, C.‐Y. , & Wu, C.‐Y. (2020). Synthetic carbohydrate‐based vaccines: Challenges and opportunities. Journal of Biomedical Science, 27(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor, P. D. (2015). Live attenuated vaccines: Historical successes and current challenges. Virology, 479‐480, 379–392. [DOI] [PubMed] [Google Scholar]
- Mir, S. A. , & Sharma, S. (2013). Role of MHC class Ib molecule, H2‐M3 in host immunity against tuberculosis. Vaccine, 31(37), 3818–3825. [DOI] [PubMed] [Google Scholar]
- Munir Ahmad, S. , Martinenaite, E. , Hansen, M. , Junker, N. , Borch, T. H. , Met, Ö. , Donia, M. , Svane, I. M. , & Andersen, M. H. (2016). PD‐L1 peptide co‐stimulation increases immunogenicity of a dendritic cell‐based cancer vaccine. Oncoimmunology, 5(8), e1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance, K. D. , & Meier, J. L. (2021). Modifications in an emergency: The role of N1‐methylpseudouridine in COVID‐19 vaccines. ACS Central Science, 7, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento, I. P. , & Leite, L. C. C. (2012). Recombinant vaccines and the development of new vaccine strategies. Brazilian Journal of Medical and Biological Research, 45(12), 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussberger, J. , Matsueda, G. R. , Re, R. , & Haber, E. (1983). Selectivity of angiotensin II antisera. Journal of Immunological Methods, 56(1), 85–96. [DOI] [PubMed] [Google Scholar]
- Nuttall, P. , Trimnell, A. , Kazimirova, M. , & Labuda, M. J. P. I. (2006). Exposed and concealed antigens as vaccine targets for controlling ticks and tick‐borne diseases. Parasite Immunology, 28(4), 155–163. [DOI] [PubMed] [Google Scholar]
- Ojha, R. , Gupta, N. , Naik, B. , Singh, S. , Verma, V. K. , Prusty, D. , & Prajapati, V. K. (2020). High throughput and comprehensive approach to develop multiepitope vaccine against minacious COVID‐19. European Journal of Pharmaceutical Sciences, 151, 105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha, R. , Pandey, R. K. , & Prajapati, V. K. (2020). Vaccinomics strategy to concoct a promising subunit vaccine for visceral leishmaniasis targeting sandfly and leishmania antigens. International Journal of Biological Macromolecules, 156, 548–557. [DOI] [PubMed] [Google Scholar]
- Okitsu, S. L. , Silvie, O. , Westerfeld, N. , Curcic, M. , Kammer, A. R. , Mueller, M. S. , Sauerwein, R. W. , Robinson, J. A. , Genton, B. , Mazier, D. , Zurbriggen, R. , & Pluschke, G. (2007). A virosomal malaria peptide vaccine elicits a long‐lasting sporozoite‐inhibitory antibody response in a phase 1a clinical trial. PLoS One, 2(12), e1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgogozo, J. M. , Gilman, S. , Dartigues, J. F. , Laurent, B. , Puel, M. , Kirby, L. C. , Jouanny, P. , Dubois, B. , Eisner, L. , Flitman, S. , Michel, B. F. , Boada, M. , Frank, A. , & Hock, C. (2003). Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology, 61(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Ozdilek, A. , Paschall, A. V. , Dookwah, M. , Tiemeyer, M. , & Avci, F. Y. (2020). Host protein glycosylation in nucleic acid vaccines as a potential hurdle in vaccine design for nonviral pathogens. Proceedings of the National Academy of Sciences of the United States of America, 117(3), 1280–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi, N. , Hogan, M. J. , Pelc, R. S. , Muramatsu, H. , Andersen, H. , DeMaso, C. R. , & Parks, R. J. N. (2017). Zika virus protection by a single low‐dose nucleoside‐modified mRNA vaccination. 543(7644), 248‐251. [DOI] [PMC free article] [PubMed]
- Park, J. , Cho, J. , & Song, E. J. (2020). Ubiquitin–proteasome system (UPS) as a target for anticancer treatment. Archives of Pharmacal Research, 43(11), 1144–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin, S. A. (2005). Vaccines: past, present and future. Nat Med, 11(4 Suppl), S5–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polevoda, B. , & Sherman, F. (2002). The diversity of acetylated proteins. Genome Biology, 3(5), 0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, A. W. , McCluskey, J. , & Rossjohn, J. (2007). More than one reason to rethink the use of peptides in vaccine design. Nature reviews. Drug discovery, 6(5), 404–414. [DOI] [PubMed] [Google Scholar]
- Purcell, A. W. , Zeng, W. , Mifsud, N. A. , Ely, L. K. , Macdonald, W. A. , & Jackson, D. C. (2003). Dissecting the role of peptides in the immune response: theory, practice and the application to vaccine design. Journal of Peptide Science, 9(5), 255–281. [DOI] [PubMed] [Google Scholar]
- Ran, X. , Ao, Z. , Trajtman, A. , Xu, W. , Kobinger, G. , Keynan, Y. , & Yao, X. (2017). HIV‐1 envelope glycoprotein stimulates viral transcription and increases the infectivity of the progeny virus through the manipulation of cellular machinery. Scientific Reports, 7(1), 9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli, R. (2000). Reverse vaccinology. Current Opinion in Microbiology, 3(5), 445–450. [DOI] [PubMed] [Google Scholar]
- Rappuoli, R. , Pizza, M. , Masignani, V. , & Vadivelu, K. (2018). Meningococcal B vaccine (4CMenB): The journey from research to real world experience. Expert Review of Vaccines, 17(12), 1111–1121. [DOI] [PubMed] [Google Scholar]
- Richter, M. , Mewes, A. , Fritsch, M. , Krügel, U. , Hoffmann, R. , & Singer, D. (2014). Doubly phosphorylated peptide vaccines to protect transgenic P301S mice against Alzheimer's Disease like Tau aggregation. Vaccines, 2(3), 601–623. 10.3390/vaccines2030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, F. , Zhang, J. , & Whitton, J. L. (1997). DNA immunization: Ubiquitination of a viral protein enhances cytotoxic T‐lymphocyte induction and antiviral protection but abrogates antibody induction. Journal of Virology, 71(11), 8497–8503. 10.1128/jvi.71.11.8497-8503.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, S. Y. , Denu, J. M. , & Allis, C. D. (2001). Histone acetyltransferases. Annual Review of Biochemistry, 70, 81–120. [DOI] [PubMed] [Google Scholar]
- Scully, I. L. , Pavliak, V. , Timofeyeva, Y. , Liu, Y. , Singer, C. , & Anderson, A. S. (2018). O‐Acetylation is essential for functional antibody generation against Staphylococcus aureus capsular polysaccharide. Human Vaccines & Immunotherapeutics, 14(1), 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serruto, D. , Bottomley, M. J. , Ram, S. , Giuliani, M. M. , & Rappuoli, R. (2012). The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine, 30(Suppl 2), (02) B87–B97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She, Y.‐M. , Li, X. , & Cyr, T. D. (2019). Remarkable structural diversity of N‐glycan sulfation on influenza vaccines. Analytical Chemistry, 91(8), 5083–5090. [DOI] [PubMed] [Google Scholar]
- Shental‐Bechor, D. , & Levy, Y. (2008). Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proceedings of the National Academy of Sciences, 105(24), 8256–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinefield, H. R. (2010). Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use. Vaccine, 28(27), 4335–4339. [DOI] [PubMed] [Google Scholar]
- Siebenkäs, C. , Chiappinelli, K. B. , Guzzetta, A. A. , Sharma, A. , Jeschke, J. , Vatapalli, R. , Baylin, S. B. , & Ahuja, N. (2017). Inhibiting DNA methylation activates cancer testis antigens and expression of the antigen processing and presentation machinery in colon and ovarian cancer cells. PLoS One, 12(6), e0179501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepensky, D. , Tzehoval, E. , Vadai, E. , & Eisenbach, L. (2006). O‐glycosylated versus non‐glycosylated MUC1‐derived peptides as potential targets for cytotoxic immunotherapy of carcinoma. Clinical and Experimental Immunology, 143(1), 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, M. D. , Ritterhoff, T. , Klevit, R. E. , & Brzovic, P. S. (2016). E2 enzymes: more than just middle men. Cell Research, 26(4), 423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, M. M. , & Bird, A. (2008). DNA methylation landscapes: Provocative insights from epigenomics. Nature Reviews Genetics, 9(6), 465–476. [DOI] [PubMed] [Google Scholar]
- Swearingen, K. E. , Lindner, S. E. , Shi, L. , Shears, M. J. , Harupa, A. , Hopp, C. S. , & Kappe, S. H. J. P. P. (2016). Interrogating the Plasmodium sporozoite surface: identification of surface‐exposed proteins and demonstration of glycosylation on CSP and TRAP by mass spectrometry‐based proteomics. 12(4), e1005606. [DOI] [PMC free article] [PubMed]
- Szu, S. C. , Li, X. , Stone, A. L. , & Robbins, J. B. J. I. , immunity . (1991). Relation between structure and immunologic properties of the Vi capsular polysaccharide. 59(12), 4555‐4561. [DOI] [PMC free article] [PubMed]
- Szu, S. C. J. E. R. O. V. (2013). Development of Vi conjugate–a new generation of typhoid vaccine. 12(11), 1273‐1286. [DOI] [PubMed]
- Tarhini, A. A. , Leng, S. , Moschos, S. J. , Yin, Y. , Sander, C. , Lin, Y. , & Kirkwood, J. M. (2012). Safety and immunogenicity of vaccination with MART‐1 (26‐35, 27L), gp100 (209‐217, 210M), and tyrosinase (368‐376, 370D) in adjuvant with PF‐3512676 and GM‐CSF in metastatic melanoma. Journal of Immunotherapy, 35(4), 359–366. 10.1097/CJI.0b013e31825481fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux, V. , Zhao, H. , Chandakkar, P. , Acker, C. M. , Davies, P. , & Marambaud, P. (2016). A modification‐specific peptide‐based immunization approach using CRM197 carrier protein: Development of a selective vaccine against pyroglutamate Aβ peptides. Molecular Medicine, 22, 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voß, F. , van Beek, L. F. , Schwudke, D. , Ederveen, T. , van Opzeeland, F. J. , Thalheim, D. , Werner, S. , de Jonge, M. I. , & Hammerschmidt, S. (2020). Lipidation of pneumococcal antigens leads to improved immunogenicity and protection. Vaccines, 8(2).310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa, A. , Aljabbari, A. , Lokras, A. , Foged, C. , & Thakur, A. (2020). Opportunities and challenges in the delivery of mRNA‐based vaccines. Pharmaceutics, 12(2), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, Y. , Bowden, T. A. , Wilson, I. A. , & Crispin, M. (2019). Exploitation of glycosylation in enveloped virus pathobiology. Biochimica et Biophysica Acta (BBA)—General Subjects, 1863(10), 1480–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J. R. (1963). Margin of safety. Doubleday; [Google Scholar]
- Yadav, D. K. , Yadav, N. , & Khurana, S. M. P. (2014). Vaccines: Present status and applications. In Animal Biotechnology (pp. 491–508). Elsevier; [Google Scholar]
- Yi, Y. , Lagniton, P. N. P. , Ye, S. , Li, E. , & Xu, R.‐H. (2020). COVID‐19: What has been learned and to be learned about the novel coronavirus disease. International Journal of Biological Sciences, 16(10), 1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling, A. L. , Ficarro, S. B. , White, F. M. , Shabanowitz, J. , Hunt, D. F. , & Engelhard, V. H. (2000). Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. Journal of Experimetnal Medicine, 192(12), 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling, A. L. , Polefrone, J. M. , Evans, A. M. , Mikesh, L. M. , Shabanowitz, J. , Lewis, S. T. , Engelhard, V. H. , & Hunt, D. F. (2006). Identification of class I MHC‐associated phosphopeptides as targets for cancer immunotherapy. Proceedings of the National Academy of Sciences of the United States of America, 103(40), 14889–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, W. , Eriksson, E. M. , Lew, A. , & Jackson, D. C. (2011). Lipidation of intact proteins produces highly immunogenic vaccine candidates. Molecular Immunology, 48(4), 490–496. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Obata, C. , Hisaeda, H. , Ishii, K. , Murata, S. , Chiba, T. , Tanaka, K. , Li, Y. , Furue, M. , Chou, B. , Imai, T. , Duan, X. , & Himeno, K. (2005). A novel DNA vaccine based on ubiquitin–proteasome pathway targeting ‘self’‐antigens expressed in melanoma/melanocyte. Gene Therapy, 12(13), 1049–1057. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Cao, F. , Sun, Z. , Yu, W. , Zhao, L. , & Wang, T. (2013). Sulfation of Agrocybe chaxingu polysaccharides can enhance the immune response in broiler chicks. Journal of Applied Poultry Research, 22(4), 778–791. [Google Scholar]


