Abstract
Avidity is defined as the binding strength of immunoglobulin G (IgG) toward its target epitope. Avidity is directly related to affinity, as both processes are determined by the best fit of IgG to epitopes. We confirm and extend data on incomplete avidity maturation of IgG toward severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nucleoprotein (NP), spike protein‐1 (S1), and its receptor‐binding domain (RBD) in coronavirus disease 2019 (COVID‐19) patients. In SARS‐CoV‐2‐infected individuals, an initial rise in avidity maturation was ending abruptly, leading to IgG of persistently low or intermediate avidity. Incomplete avidity maturation might facilitate secondary SARS‐CoV‐2 infections and thus prevent the establishment of herd immunity. Incomplete avidity maturation after infection with SARS‐CoV‐2 (with only 11.8% of cases showing finally IgG of high avidity, that is, an avidity index > 0.6) was contrasted by regular and rapid establishment of high avidity in SARS‐CoV‐2 naïve individuals after two vaccination steps with the BioNTech messenger RNA (mRNA) Vaccine (78% of cases with high avidity). One vaccination step was not sufficient for induction of complete avidity maturation in vaccinated SARS‐CoV‐2 naïve individuals, as it induced high avidity only in 2.9% of cases within 3 weeks. However, one vaccination step was sufficient to induce high avidity in individuals with previous SARS‐CoV‐2 infection.
Keywords: avidity, protective immunity, receptor‐binding domain, recomLine SARS‐CoV‐2 IgG, SARS‐CoV‐2, vaccination
Highlights
After severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, immunoglobulin G (IgG) toward viral nucleoprotein (NP), receptor‐binding domain (RBD), and spike protein‐1 (S1) shows immature avidity.
The breakpoint of avidity maturation correlates with that of IgG production.
Patients with more severe clinical symptoms show increased avidity maturation.
Two vaccination steps lead to complete avidity maturation.
Therefore, vaccination seems to induce protective immunity toward SARS‐CoV‐2 and coronavirus disease 2019 (COVID‐19).
Preceding SARS‐CoV‐2 infections enhance avidity maturation after subsequent vaccination.

1. INTRODUCTION
The detection of viral genomes by real‐time polymerase chain reaction (RT‐PCR) is the primary approach to recognize infections with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Supplementary SARS‐CoV‐2‐specific antibody tests are necessary for an assessment of infection past viremia, for epidemiology, health system management, and the control of immunization programs. 1 , 2 , 3 , 4 , 5
Due to its complexity, the serological response toward SARS‐CoV‐2 does not allow to distinguish between acute and past infections by differential determination of SARS‐CoV‐2‐specific IgM and IgG. 6 Also, the humoral response toward SARS‐CoV‐2 seemed to decline after an initial rise. 7 , 8 , 9 , 10 , 11
Avidity of IgG is defined as the strength of IgG binding to its specific epitope. This strength can be easily determined by the degree of removal of bound IgG through treatment with chaotropic agents like urea. 6 , 12 Avidity maturation is strictly correlated with affinity maturation, as both characteristics depend on the best fit between IgG and epitope. 6 , 13 , 14 , 15 , 16
The avidity of neutralizing IgG is also crucial for protective immunity, 5 , 6 as will be discussed in detail under Introduction Section in Supporting Information Material. An initial analysis revealed that avidity maturation after SARS‐CoV‐2 infection remained at an untypical low level in most cases. 12 This is in line with the reports by other groups. 9 , 10 , 17 , 18 , 19 , 20
The mechanism(s) underlying incomplete avidity maturation after SARS‐CoV‐2 infections have not been clarified so far. Restricted availability of SARS‐CoV‐2 antigen for the immune system, either with respect to concentration or time, might hypothetically be the cause for incomplete avidity maturation after the first contact between SARS‐CoV‐2 antigens and the immune system. It has also been reported that SARS‐CoV‐2 infection/COVID‐19 can strongly impair the function of germinal centers in secondary lymphoid organs. 21 As germinal centers are the site of affinity/avidity maturation, 22 SARS‐CoV‐2 infections might as well contribute to incomplete avidity maturation through this mechanism.
The importance of high avidity IgG for the immunological defense toward SARS‐CoV‐2 is obvious, as the interaction between the SARS‐CoV‐2 RDB and the cellular angiotensin‐converting enzyme 2 (ACE2) receptor is driven by high affinity. 23 Therefore, protective immunity toward SARS‐CoV‐2 infection depends on neutralizing antibodies of high affinity/avidity. 5 , 23 It was therefore suggested that (a) natural SARS‐CoV‐2 infection would not lead to sustained protection toward SARS‐CoV‐2 and COVID‐19, and (b) that the goal of immunization should be the induction of neutralizing antibodies of high avidity. 5
Here we confirm and extend our previous findings on incomplete avidity maturation after natural SARS‐CoV‐2 infection by increasing (a) the number of patients studied and (b) the time of observation. We also show that two vaccination steps with the BioNTech vaccine lead to an IgG response that is different from the humoral response after SARS‐CoV‐2 infection both in quantitative and qualitative terms. Whereas avidity maturation after infection with SARS‐CoV‐2 remained at low or intermediate avidity levels, two vaccination steps allowed for complete avidity maturation of IgG directed toward RBD/S1 in most vaccinated subjects.
2. METHODS
2.1. Patients and sera
2.1.1. SARS‐CoV‐2‐positive sera
A. 93 sera from 70 adult outpatients (33 females, 37 males aged between 18 and 65 years) with clinical signs of COVID‐19 (such as fever, headache, loss of smell, sore throat, or pneumonia) and SARS‐CoV‐2 infection confirmed by RT‐PCR, and sera from 80 healthy individuals vaccinated with the COMIRNATY®/BNT162B2 vaccine from BioNTech/Pfizer (57 females, 23 males; 18–50 years: n = 62; 50–65 years: n = 11; 65–79 years: n = 1; >80 years: 6) were collected in the Munich area after a call for a voluntary donation of serum samples for serological analysis related to SARS‐CoV‐2. The samples were mainly drawn by the family doctors. The volunteers gave their written consent for testing. The logistic support of Mikrogen GmbH collected the sera and all necessary information.
The anonymized samples were transferred to Research and Development of Mikrogen GmbH for professional testing in the recomLine SARS‐CoV‐2 IgG line assay, including avidity testing. For the members of Research and Development and the senior author (G.B.), information was restricted to clinical symptoms and the time between the onset of clinical symptoms and extraction of the sera.
B. Sera from 39 adult hospitalized patients with COVID‐19 and SARS‐CoV‐2 infection confirmed by positive RT‐PCR, together with information on the time of onset of clinical symptoms were kindly provided by Prof. P. Luppa, Technical University Munich.
2.2. Statements of ethics approval and of performance according to relevant guidelines and regulations are expressed after discussion
Sera were stored at – 20°C until they were tested in the immunoassays.
2.3. Immunoblot assay
A. Production of recomLine SARS‐CoV‐2 IgG nitrocellulose strips: Individual concentrations of purified recombinant antigens NP, RBD, S1 of SARS‐CoV‐2, as well as NP of 229E, NL63, OC43, HKU1 were applied directly onto nitrocellulose membranes in separate lanes. Production was standardized and the resultant strips were evaluated (see below for details), resulting in product # 7374 of Mikrogen GmbH. The assay has been CE‐marked.
B. Procedure of the line immunoassay: The reactivity of 1: 100 dilutions of serum antibodies against the recombinant antigens was detected with peroxidase‐labeled anti‐human IgG antibody and the use of precipitating tetramethylbenzidine. The first incubation of serum and test strips was for 1 h, followed by three washing steps with buffer. The incubation of the strips with peroxidase‐labeled anti‐human IgG antibody was for 45 min, followed by three washing steps. Treatment with tetramethylbenzidine was for 8 min.
The line immunoassays were carried out in a semi‐automatic processor Dynablot (Dynex Technologies GmbH) with manual serum pipetting according to the instruction manual provided by Mikrogen GmbH. An Epson J371A scanner (Epson) and recomScan software (Mikrogen GmbH) were used according to the instruction manuals.
C. Avidity determination: sera were incubated for 1 h with the recomLine SARS‐CoV‐2 IgG test strips in duplicate; then both replicates were incubated for 5 min with wash buffer, and one assay was incubated in the wash solution, while the parallel assay replicate was treated with 7 M urea for 3 min; after three additional washing steps both assay replicates were processed with anti‐human IgG antibody labeled with peroxidase and detected as outlined above to describe the line immunoassay procedure. The gray intensity area output by recomScan on the urea‐treated test strip was divided by the gray intensity of the parallel assay replicate to determine the avidity index arithmetically. Standard urea treatment for a sharp distinction between IgGs of low and high avidity was 7 M. Where indicated, other concentrations of urea were used during our analysis.
The precision and reproducibility of the immunoassay have been previously described. 12
The data analysis by G. Bauer was performed on the basis of raw data.
The Yates continuity corrected χ 2 test (two‐sided) was used for the statistical determination of significances (p < 0.01 = significant; p < 0.001 = highly significant).
3. RESULTS
3.1. Infection with SARS‐CoV‐2 leads to incomplete avidity maturation of IgG directed toward SARS‐CoV‐2 nucleoprotein (NP), spike protein‐1 (S1), and the receptor‐binding domain (RBD) of S1
The performance of the recomLine SARS‐CoV‐2 IgG immuno‐test with highly purified recombinant SARS‐CoV‐2 NP, S1, and RBD has been recently described. 12 Its analytical power was further evaluated (Figures S1 and S2). The long‐term kinetics of the IgG responses toward NP, S1, and RBD after symptomatic SARS‐CoV‐2 infections showed an initial rapid increase in the IgG concentration, followed by a continuous decline (Figure 1A−H). This is in line with the reports by several other groups. 8 , 9 , 10 , 11 As the IgG was efficiently removed by 7 M urea in the assays described in Figure 1A−G, most of these antibodies seemed to exhibit relatively low or intermediate avidity. The avidity indices showed a striking initial increase, but then remained at a plateau of low or intermediate values (Figure 1A−G). These findings confirm that the avidity maturation of the IgGs toward SARS‐CoV‐2 NP, S1, and RBD has not been completed. The curves obtained for IgG directed toward SARS‐CoV‐2 NP were gradually different from those obtained for IgG directed toward SARS‐CoV‐2 RBD or S1, whereas the values for IgG toward RBD and S1 were similar.
Figure 1.
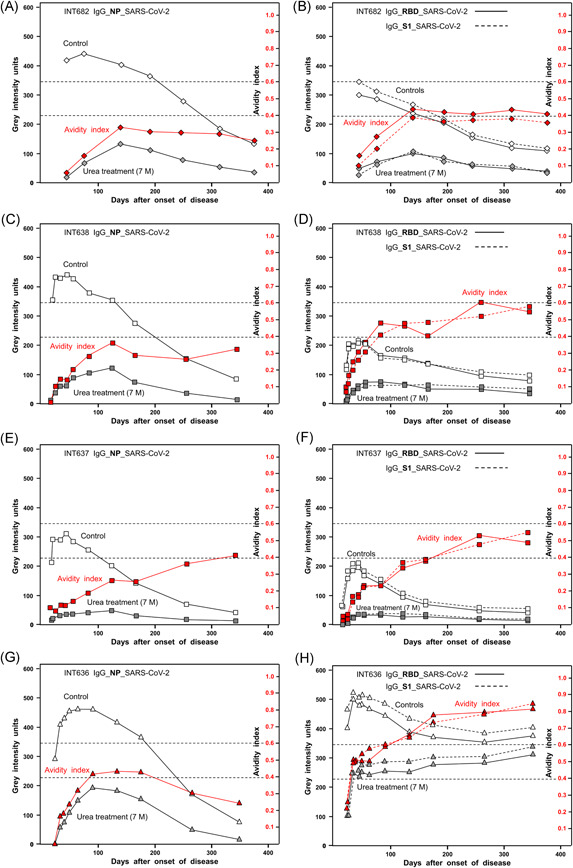
Kinetics of the serological responses to SARS‐CoV‐2 nucleoprotein (NP), receptor‐binding domain (RBD), and spike protein S1. Sera from four patients with COVID‐19 confirmed by positive RT‐PCR and clinical symptoms were tested for IgG toward SARS‐CoV‐2 NP (A, C, E, G) or RBD and S1 (B, D, F, H) using a line immunoassay with purified recombinant antigens, as described under Methods. The immunoassays were performed without (“Control”) and with urea treatment (7 M) for the determination of avidity. The determined gray intensity values (black) and the calculated avidity indices (red) are plotted toward the days after the onset of the disease. These findings demonstrate that, after a rapid initial increase, the concentrations of IgG directed toward NP, RBD, and S1 are declining. Incomplete avidity maturation seems to be characteristic for the IgG response toward SARS‐CoV‐2 NP, RBD, and S1, as the avidity indices remain at plateaus at low (avidity index < 0.4) or intermediate avidity (avidity index between 0.4 and 0.6). The avidities of IgG toward RBD and S1 under H are exceptional, showing an initial plateau of avidity after 50 days, followed by a secondary increase in avidity that is finalized at the second plateau of higher avidity. This pattern is indicative of secondary infection with SARS‐CoV‐2.COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; RT‐PCR, real‐time polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Avidity maturation of IgG directed toward SARS‐CoV‐2 RBD and S1 in the case described in Figure 1H was distinct from the other examples. After an initial rapid increase, the avidity index remained at a plateau for about 1 month, before it increased further to a second plateau. This pattern may be indicative of a second challenge with SARS‐CoV‐2 that triggered the continuation of avidity maturation. This secondary response seemed to be limited as well, as it also ended on a plateau.
Additional examples of incomplete avidity maturation after SARS‐CoV‐2 infections are shown in Figure S3.
Figure 2 presents an overview of the kinetics of the IgG responses directed toward SARS‐CoV‐2 NP and RBD in 16 cases, followed for approximately 12 months. Practically all kinetics showed a rapid initial increase followed by continuous decline of the IgG response (A,B). However, the intensity of the response as well as the velocity of the decline showed remarkable variabilities. Except for one case, all sera remained in the range below an avidity index of 0.6 for IgG directed toward SARS‐CoV‐2 NP (C), whereas the majority of responses toward SARS‐CoV‐2 RBD reached plateaus of avidity indices below 0.6 (11/16), or higher in one‐third of the cases (5/16) (D). Even in those cases that reached higher avidity values, the process of avidity maturation seemed to be rather slow and discontinuous.
Figure 2.
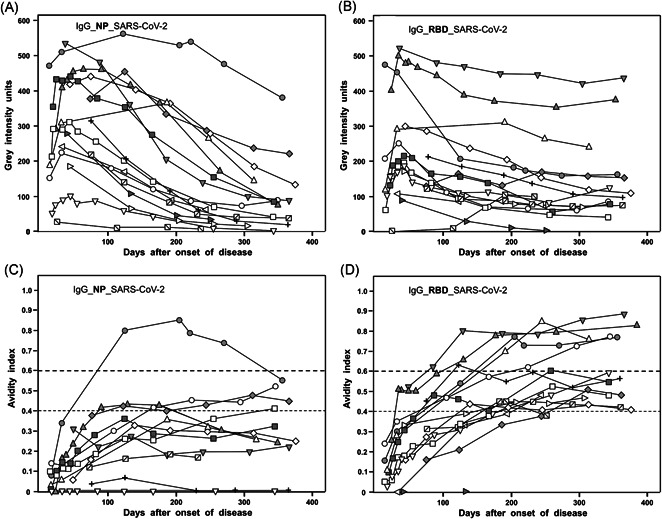
Summary of 16 long‐term kinetics of IgG responses toward NP and RBD after SARS‐CoV‐2 infection leading to COVID‐19. The responses of IgG directed toward NP (A) or RBD (B) in 16 cases of COVID‐19 cases were followed for about 12 months, and the corresponding avidity indices were calculated (IgG NP: C; IgG RBD: D). The results for the IgG levels described under A and B show a rapid increase in IgG toward NP or RBD, followed by a decline over time, characterized by a high degree of variability. Avidity indices (C, D) indicate that avidity maturation is not completed. In the case of IgG directed toward SARS‐CoV‐2 NP, only one serum reached high avidity (avidity index > 0.6). In the case of RBD, one 5/16 sera reached high avidity, whereas 11/16 sera remained at a plateau of intermediate avidity. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; NP, nucleoprotein; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Figure 3A summarizes the concentrations of IgG directed toward the three SARS‐CoV‐2 antigens (NP, RBD, and S1) at various time points after the onset of disease in 93 sera from 70 outpatients with COVID‐19. The gray intensities for the responses toward all the antigens showed broad variation over the time of observation, without indication of an underlying pattern. However, in all cases, antibody responses were developed toward all three antigens. Determination of avidity indices revealed relatively low values during the first 30 days, and a very moderate increase over time (Figure 3B). High avidity indices of more than 0.6 were the exception. They were only found in rare cases and later than 50 days after the onset of disease. In general, avidity indices of IgG toward RBD and S1 seemed to be higher than those for IgG toward NP, though they remained also at an untypical range of low avidity even long times after the onset of disease (see Figures S4−S9 for more details). These findings are in contrast to the findings for infection with other viruses, where low avidity of IgG is characteristic only for the early phase of infection, and then is regularly followed by the establishment of high avidity at later time points 6 (more details under Introduction section in Supporting Information Material). Avidity indices for IgG toward S1 and RBD of SARS‐CoV‐2 were nearly identical in most samples, in contrast to marked differences in avidity between IgG toward one of these two markers and NP (Figures S7−S9). A more refined analysis of avidity maturation, 12 using variable urea concentrations, illustrates the kinetics of incomplete avidity maturation, characterized by an initial onset of maturation, followed by an arrest at low avidity (Figure S10).
Figure 3.
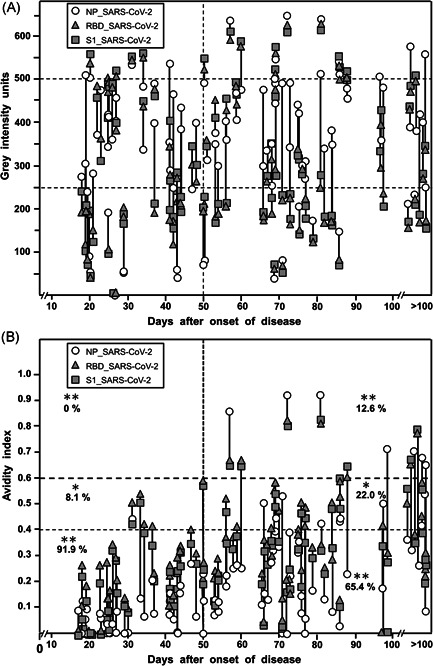
IgG responses in 93 serum samples from patients with confirmed SARS‐CoV‐2 infection and COVID‐19. Ninety‐three sera from 70 patients with clinical signs of COVID‐19, quite predominantly showing ambulatory mild disease, and with positive SARS‐CoV‐2 RT‐PCR test were tested with the recomLine SARS‐CoV‐2 IgG test. The gray intensities (A), which reflect the concentrations of the respective antibodies and the avidity indices (B) are shown in correlation to the time after the onset of disease for each serum. (A) Reflectometric gray intensity values. The result shows a broad distribution of gray scales, which was similar at all time points. The percentages of sera showing values in the ranges 0–250, 250–500, and more than 500 gray intensity were not significantly different when sera were taken before or after 50 days after the onset of the disease were compared, with the exception of IgG toward NP at less than 250 units. Importantly, all sera that gave a positive result showed positivity toward all three antigens tested. In 93.5% of the sera with positive IgG toward S1, IgG toward RBD showed a value that was less than 20 gray intensity units different from that obtained for IgG S1, whereas, in 6.5% of the sera, IgG values toward S1 and RBD were different more than 20 gray intensity units (p < 0.001). In contrast, only 15% of sera showed IgG toward NP at a value that differed less than 20 gray intensity units from that of the respective IgG S1 value, and 84.9% showed a higher difference (p < 0.001). (B) Avidity indices. Whereas the gray intensity values (indicative of the respective concentrations of IgG) were broadly similar between sera taken within 50 days or after 50 days after the onset of the disease; the avidity of the IgG was increasing between these groups. However, no complete avidity maturation was reached. A total of 91.9% of the sera taken before Day 50 showed an avidity index below 0.4, whereas only 65.4% of the sera taken after 50 days showed similar low avidity (p < 0.001). As an indication of partial avidity maturation, 8.1% of sera taken before 50 days showed an avidity index between 0.4 and 0.6, in contrast to 22.0% of sera in the group where sera were taken after 50 days (p < 0.01). Avidity indices above 0.6 were only found at 12.6% in the group where sera had been taken later than 50 days after the onset of the disease (p < 0.001). In summary, an overall low degree of avidity maturation was seen, with a minority of samples of high avidity at late time points. These findings confirm incomplete avidity maturation after SARS‐CoV‐2 infection. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; RT‐PCR, real‐time polymerase chain reaction; NP, nucleoprotein; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.2. Avidity maturation of IgG toward SARS‐CoV‐2 antigens increases with the severity of clinical symptoms of COVID‐19 patients
The comparison of the antibody concentrations and avidity indices of sera from COVID‐19 patients without and with the requirement for hospitalization showed that there was a trend for higher gray intensities and a marked increase in avidity in the hospitalized patients (Figure S11). These data show that at least in a low percentage of patients, one of the outcomes of severe illness due to COVID‐19 is the final establishment of anti‐SARS‐CoV‐2 IgG of higher avidity.
3.3. Two vaccination steps cause rapid induction of high avidity IgG toward SARS‐CoV‐2 spike protein
Vaccination with the BioNTech/Pfizer vaccine caused efficient induction of IgG directed toward SARS‐CoV‐2 RBD (Figure 4A). Despite a marked variability with regard to the onset of IgG generation after the first vaccination, an additional strong increase in the IgG concentration was seen after the second vaccination in all sera. This strong induction of IgG after vaccination was in contrast to the less pronounced IgG response after infection, which was also characterized by a subsequent decline.
Figure 4.
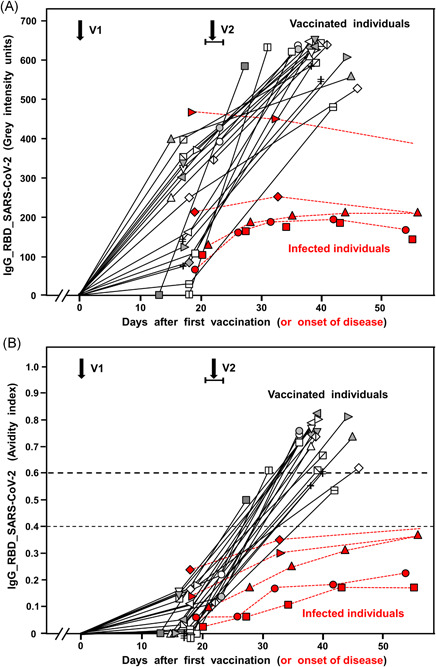
IgG responses after vaccination toward SARS‐CoV‐2. The increase in gray intensity units (representing IgG concentration) (A) and in avidity (B) of IgG directed toward SARS‐CoV‐2 RBD after vaccination with the BioNTech/Pfizer vaccine are summarized for 21 cases (black curves). For comparison, five representative cases of natural SARS‐CoV‐2 infections (labeled in red) are included. V1 and V2 indicate the time of the first and second vaccination. V1 is defined as Day zero. V2 was varying between Days 21–23 after the first vaccination. The results show high variability of the IgG response after the first vaccination, followed by an additional stronger response after the second vaccination. Avidity was low after the first vaccination but reached intermediate avidity (2/20 cases) and high avidity (18/20 cases) after the second vaccination (p < 0.001). In comparison with natural infection, vaccination induces a strong and continuous IgG response (p < 0.001) with uninhibited avidity maturation, in contrast to incomplete avidity maturation after natural infection, characterized by plateau values in the low avidity range (p < 0.001). IgG, immunoglobulin G; NP, nucleoprotein; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
The first vaccination step essentially induced IgG of low avidity, whereas the second vaccination step caused rapid and nearly uniform maturation to high avidity (Figure 4B). At 19 days after the second vaccination, in most of the sera, high avidity IgG directed toward SARS‐CoV‐2 RBD (AI > 0.6) had been reached. This impressive effect is contrasted by the delayed and incomplete avidity maturation after natural infection, which resulted in a plateau within the lower avidity range. Figure S20 summarizes and further strengthens these findings by presenting the respective median values.
Figure 5 summarizes some special cases of IgG responses after vaccination. Cases a–d had been infected with SARS‐CoV‐2 up to 300 days before vaccination, as confirmed by positive RT‐PCR and antibody titers directed toward SARS‐CoV‐2 NP, RBD, and S1. These titers had been declining over time, thereby maintaining low avidity (see Figures S14−S17 for detailed kinetics). Except for case “a,” antibody titers toward RBD were at a level of 100 gray intensity units and less, shortly before vaccination. The first vaccination step was then sufficient to induce an immediate rise to high concentrations of RBD‐specific IgG (Figure 5A) and to the highest possible level of avidity (Figure 5B). This finding shows that a preceding SARS‐CoV‐2 infection, even after a strong decline of the IgG concentration directed toward SARS‐CoV‐2, provides strong and immediate support for highly efficient avidity maturation and strong IgG production already after one vaccination step. These findings are extended in Figure S19.
Figure 5.
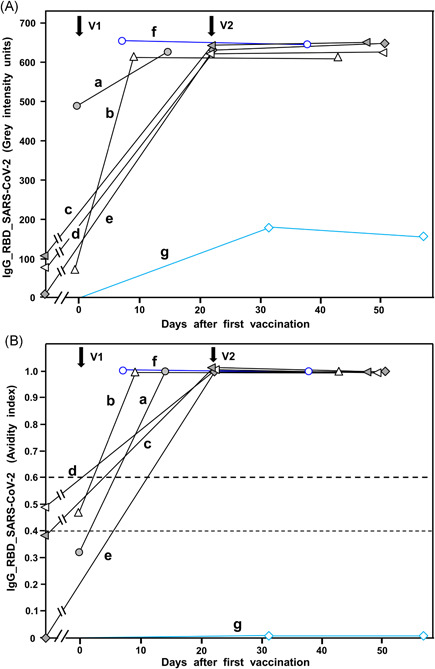
Analysis of special cases of vaccination. The figure shows the increase in gray intensity units (A) or avidity index (B) of special cases of vaccinated individuals. Cases a−e represent individuals with natural coronavirus infections several months before vaccination. Before vaccination, in four out of the five cases, the antibody titers derived from primary infection had declined to very low levels. One vaccination step was sufficient to induce a strong IgG response with extremely high avidity in all five cases. Case f represents a case without known preceding SARS‐CoV‐2 infection. However, the high response with high avidity already after the first vaccination step and the detection of IgG directed toward NP (not shown in the figure) indicates that this case is analogous to cases a‐e, that is, it seems to represent a vaccinated individual with (clinical inapparent) SARS‐CoV‐2 infection. Finally, Case g represents the only case seen so far in our study, which was characterized by a complete failure in avidity maturation after vaccination, paralleled by a low IgG response. V1 and V2 indicate the time point of the first and second vaccination. IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
There were no clinical signs of SARS‐CoV‐2 infection before vaccination in Case f. However, a very weak residual IgG directed toward SARS‐CoV‐2 NP and the immediate induction of high avidity IgG after first vaccination allowed the assumption that this individual might have been infected before vaccination.
Case g represents the exemption within all cases studied by us so far. The concentration of IgG induced by two vaccination steps and determined after the second vaccination was of unusually low concentration and was completely removed by urea treatment. This example seems to represent a rare case of completely failing avidity maturation. This rare case is of specific individual importance, as it is questionable whether the vaccinated subject has established protective immunity.
A summary of our data shows that vaccination with the BioNTech/Pfizer vaccine caused rapid induction of IgG directed toward SARS‐CoV‐2 RBD (Figure 6A,B). Within less than 23 days after the first vaccination, 46.1% of sera showed more than 200 gray intensity units. Intensities ranged between 0 and 650 units (median: 168 gray intensity units). Thus 13–30 days after the second vaccination, 97.6% of sera were in the range of more than 400 gray intensity units (median: 613). With one exception, avidity was low after the first vaccination (Figure 6C). After the second vaccination (Figure 6D), 78% of the sera reached high avidity, whereas only 17.1% remained at intermediate avidity. Avidity maturation seemed to have completely failed in one serum. This failure has been confirmed in a follow‐up test. Another serum showed an avidity index at the border between low and intermediate avidity. A follow‐up study performed after the first submission of our manuscript confirmed these findings for a substantially increased number of subjects (Figure S20).
Figure 6.
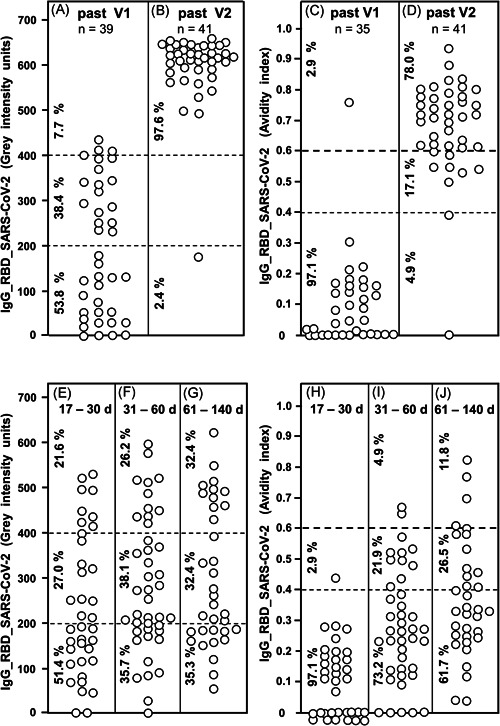
IgG responses after vaccination and natural infection with SARS‐CoV‐2. Thirty‐nine sera taken between 9 and 22 days after first vaccination with the BioNTech/Pfizer vaccine (A) and 41 sera taken between 6 and 25 days after second vaccination with the BioNTech/Pfizer vaccine (B) were tested for IgG directed toward SARS‐CoV‐2 RBD. The determined gray intensity units are shown under A and B, whereas the avidity indices, determined by parallel treatment with 7 M urea after incubation of serum with test antigens, are shown under C (sera obtained after the first vaccination) and D (sera obtained after the second vaccination). For comparison, 37 sera were taken from COVID‐19 outpatients between 17 and 30 days after the onset of the disease (E), 42 sera from COVID‐19 outpatients taken between 31 and 60 days after the onset of the disease (F) and 34 sera from COVID‐19 outpatients taken between 61 and 140 days after the onset of the disease were tested for IgG directed toward RBD (gray intensity units). The avidity indices of these sera were determined and are shown (H−J). The figure shows a variable induction of IgG after the first vaccination (A), which extends to uniformly high values after the second vaccination (B) (p < 0.001). This is paralleled by low avidity indices after the first vaccination (with one exception) and high avidity in 78.0% of the samples taken after the second vaccination (p < 0.001). Though natural infection with SARS‐CoV‐2 can also induce IgG concentrations of higher levels, a broad range of intensities is maintained over long times without statistically significant changes (E−G). Though a certain degree of avidity maturation in the low and intermediate range was seen with time for sera from patients with natural SARS‐CoV‐2 infection, only a minority of sera (11.8%) reached high avidity values (>0.6) (H−J) at 61−140 days after natural infection, whereas 78% of sera taken from vaccinated individuals had reached avidity indices above 0.6 between 6 and 25 days after the second vaccination (p < 0.001). The median values calculated from the data presented in Figure 6 are summarized in Figure S20. The confirmation of the data presented in Figure 6A−D through the increase in the number of vaccinated subjects are presented in Figure S18. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
The response toward SARS‐CoV‐2 S1 corresponded very well to the response toward RBD, whereas there was no detectable response toward SARS‐CoV‐2 NP, as expected (Figures S12, S13).
The high percentage of sera that had reached high avidity after the second vaccination was contrasted by the low percentage of sera with high avidity induced by natural infection within a similar time range (Figure 4E−J). Though a broad range in the concentration of IgG directed toward SARS‐CoV‐2 RBD was reached after natural infection, the avidity of this IgG was remaining in the range of low and intermediate avidity values. This was even true for an extended time window between 61 and 140 days after the onset of the disease (Figure 4E−J). These data show that two rounds of vaccination can induce high titers of high avidity IgG directed toward RBD/S1, whereas IgG induced after natural infection shows incomplete avidity maturation. This conclusion is further substantiated and visualized by a comparison of the median values obtained for IgG concentration and avidity in infected versus vaccinated persons (Figure S20).
4. DISCUSSION
4.1. Infection with SARS‐CoV‐2 leads to immature avidity maturation of IgG directed toward NP, RBD, and S1
Our data confirm that specific IgG responses after SARS‐CoV‐2 infections are characterized by an initial rapid increase in concentration, followed by a continuous decline. This finding, obtained for IgG toward NP, RBD, and spike protein S1, is in line with the reports by other groups. 7 , 8 , 9 , 10 , 11 Importantly, after SARS‐CoV‐2 infection, a limited rapid initial increase in avidity was followed by a continuous plateau of low or intermediate avidity. High avidity was only reached in a few cases. To our knowledge, such atypical kinetics of incomplete avidity maturation have not been reported so far for the immunological response toward other viruses 6 (see Introduction section in Supporting Information Material for more details). Even within 1 year after the onset of clinical symptoms, the vast majority of COVID‐19 outpatients in our study did not develop high avidity IgG toward SARS‐CoV‐2 NP, and 68% of the patients showed no high avidity IgG toward SARS‐CoV‐2 RBD. These findings of incomplete avidity maturation after natural infection with SARS‐CoV‐2 are in line with the findings by several other groups. 9 , 10 , 17 , 18 , 19 , 20 Also, the study by Luo et al. 24 is indirectly confirming this view, as Luo et al. used 3 M urea. This resulted in an overestimation of avidity in comparison with the use of the discriminatory concentration of 7 M urea. This argument is also valid for the discussion of avidity maturation after SARS‐CoV‐1 infection in a study that applied 4 M urea. 25 Incomplete avidity maturation has also been shown for a substantial number of cases of seasonal coronavirus infections. 6 , 26
4.2. Two rounds of vaccination with the BioNTech vaccine induce high avidity IgG directed toward RBD
The findings related to the IgG responses after natural SARS‐CoV‐2 infection are strongly contrasted by the responses seen after vaccination, as presented in Figures 4, 5, 6. Two vaccination steps with the BioNTech/Pfizer vaccine caused rapid induction of high avidity IgG toward SARS‐CoV‐2 RBD and S1. This indicates that RBD and S1 are excellent antigens for the induction of high concentrations of IgG with high avidity. This implies perfect triggering of the immune response, as well as constant and sufficiently long support of affinity maturation in the germinal centers through vaccine‐induced viral antigens.
As suggested by Khatri et al., 23 high avidity IgG induced by vaccination seems to be essential for interference with the high‐affinity interaction between ACE2 and viral spike protein. This concept is in line with the suggestion that protective immunity toward SARS‐CoV‐2 infection and COVID‐19 should require high avidity neutralizing antibodies. 5 These considerations are also in perfect agreement with the highly protective effect of the BioNTech/Pfizer vaccine in Phase III studies that preceded its approval.
Our findings therefore define S1 and RBD as excellent antigens for the performance of avidity testing, based on the application of the chaotropic agent urea. The observed span between avidity indices from 0 to 1, depending on the time of assessment, demonstrates that the antigens do not lose their binding capacity through treatment with 7 M urea.
As natural infection with SARS‐CoV‐2 induces a response that is characterized by incomplete avidity maturation, it may be speculated that SARS‐CoV‐2 infection either is suboptimal with respect to the concentrations of antigens reached or with respect to the time of availability of the antigens for the immune system. Alternatively or in addition, SARS‐CoV‐2 infection might have a direct negative impact on avidity/affinity maturation. A look at other viral systems shows that negative effects on avidity maturation have been observed (i) as the consequence of interference with immunological mechanisms, as well as (ii) due to suboptimal availability of antigen. Nair et al. 27 have reported on HIV‐dependent interference with avidity maturation, resulting in a low avidity IgG response after measles virus infection or vaccination of HIV‐positive patients. On the other side, it has been shown that lowering the antigenic load through treatment of HIV or tuberculosis infections was leading to the prevalence of low avidity IgG directed toward the respective agents. 28 , 29
The impairment of germinal centers in secondary lymphoid organs by SARS‐CoV‐2, as reported by Kaneko et al., 21 would allow a straightforward explanation for incomplete avidity maturation after SARS‐CoV‐2 infection. However, as the important findings by Kaneko et al. 21 have been established in lymph nodes derived from patients who had died from COVID‐19, it is uncertain whether this mechanism also applies to patients with milder disease. Furthermore, the relative increase in avidity in patients, who required hospitalization compared with outpatients, as shown in Figure S11, and also reported by other groups, 7 , 20 , 30 cannot be explained by the findings presented by Kaneko et al. 21 It rather could be explained by a higher viral load and longer time of interaction with SARS‐CoV‐2 in patients with more severe disease and a lower viral load in patients with milder disease.
In subjects with preceding SARS‐CoV‐2 infection, one vaccination step was sufficient to induce high concentrations of high avidity IgG toward RBD, even if the infection had occurred many months ago. This finding indicates that a preceding SARS‐CoV‐2 infection fully substitutes for one vaccination step in the avidity maturation process. Therefore, in previously infected individuals, only one vaccination step is sufficient to reach maximal avidity maturation, whereas two steps are required in the case of uninfected individuals. These response patterns of IgG avidity maturation are in excellent agreement with analogous findings on the levels of specific memory B cells after vaccination and infection. 31
4.3. Avidity and protective immunity
Moura et al. 20 also reported on higher IgG avidity in patients with more severe diseases. They showed that all of the patients who died presented avidity indices of IgG toward SARS‐CoV‐2 RBD lower than 0.3. This allows the assumption that a higher viral load associated with the more severe disease might increase avidity maturation, but that the failure to reach higher avidity increases the risk of a more severe outcome of the disease. The latter finding strengthens the role of high avidity for protective immunity. 5
The SARS‐CoV‐2 infection has been shown to lead to the generation of neutralizing antibodies. 32 , 33 It was demonstrated that the parallel determination of the IgG response toward RBD and spike protein of SARS‐CoV‐2 correlated very well with the presence of neutralizing antibodies. 29 Only one out of 5882 individuals positive for both IgG toward RBD and spike protein did not show neutralizing antibodies. This is in line with findings by other groups. 9 , 10 , 11 However, classical neutralization tests are performed by preincubation of virus and serum, before the mixture is applied to test cells. Therefore, neutralization tests determine the concentration of IgG with specific binding to epitopes that are actually involved in the attachment of the virus to the cellular receptor. However, as the test systems for neutralization do not involve direct competition between antibodies and cellular receptors for viral spike proteins, they do not allow to determine the role of IgG avidity, which might be critical for virus neutralization in vivo. 23 This deficiency can be compensated by direct avidity determination of IgG directed toward SARS‐CoV‐2 RBD. The importance of neutralization, as well as avidity for antibody functionality, has also been explicitly pointed out by Gaspar and De Gaspari. 34
Protective immunity can be predicted to depend on a sufficiently high concentration of neutralizing antibodies, ensuring optimal load of the virus with antibody. In addition, a sufficiently high affinity/avidity of these antibodies is most likely required, to cope with the competition of ACE2 for viral spike protein. The determination of avidity of IgG directed toward SARS‐CoV‐2 RBD in the small percentage of patients who developed COVID‐19 despite vaccination might allow to clarify the predictive value of avidity determination for the determination of individual protective immunity.
4.4. Induction of low avidity IgG: Potential advantage for coronaviruses?
As seasonal coronavirus infections are also frequently characterized by subsequent incomplete avidity maturation, 12 , 26 it may be speculated that maintaining low avidity of the IgG response, either through the suboptimal supply of the immune system with viral antigens or through impairment of avidity maturation, is part of the biology of coronaviruses in general. This might ensure the observed repeated waves of reinfection over time. 35 , 36 , 37 From this perspective, the establishment of high avidity IgG directed toward SARS‐CoV‐2 RBD through vaccination seems to be a rational approach to break the dynamics of SARS‐CoV‐2 infections and reinfections. The determination of avidity of IgG directed toward RBD might thereby play an essential role in the determination of individual protective immunity.
CONFLICT OF INTERESTS
E. Soutschek and M. Motz are owners of Mikrogen GmbH. E. Soutschek is the present CEO of Mikrogen GmbH. F. Struck, E. Stachik, K. Wochinz‐Richter, S. Schulz, and P. Schreiner are employees of Mikrogen GmbH. The determination of avidity of antibodies, using immunoblots or other techniques that allow the parallel measurement of humoral immune reactions toward several antigens in one assay, has been patented by Mikrogen GmbH (WO 00/54055; PCT/EP00/01883). In addition, a new patent application for a method to determine the antibodies toward SARS‐CoV‐2 has been filed by Mikrogen GmbH and is pending (EP 2019/2550). Mikrogen GmbH develops and produces test systems for serological analysis of infectious diseases. G. Bauer is a member of the Medical Faculty of the University of Freiburg. He is the inventor of WO 00/54055; PCT/EP00/01883 and one of the coinventors of EP 2019/2550.
ETHICS STATEMENT
The testing of serological parameters of SARS‐CoV‐2 infection in sera from hospitalized patients has been approved by the ethics committee of the Technical University of Munich (TUM) (# 147/20 S‐KH). Outpatients and vaccinated individuals who voluntarily took part in the serological study agreed individually and gave individual written consent for the study. The use of sera obtained from outpatients and from vaccinated individuals, for in vitro testing for antibodies toward coronaviruses was performed according to the legal specifications communicated to us by the Ethics commission of the Bavarian Medical Board [Ethik‐Kommission der Bayerischen Landesärztekammer](R008‐067 mat/ch), based on §24 MPG (Medizinproduktegesetz) and European norm http://eur-lex.europa.eu/legal-content/DE/TXT/?uri=OJ:L:2017:117:TOC. All research related to our study was performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants. The use of sera given by human research participants has been performed in accordance with the Declaration of Helsinki.
AUTHORS CONTRIBUTIONS
Development and evaluation of the test system, organizing and supervising testing in‐house, documentation and discussion of data, commenting and correcting the manuscript: Friedhelm Struck, Patrick Schreiner, Eva Staschik, Karin Wochinz‐Richter, Sarah Schulz, Erwin Soutschek, and Manfred Motz. Analysis of raw data, generation of the graphs, conceptualization, writing the manuscript: Georg Bauer.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank Dr. P. Luppa (Technical University Munich) and many voluntary donors for providing sera for this study. The authors thank Dr. P. Aichele (University of Freiburg) for the helpful discussion. The technical assistance during the preparation of the final graphs by Jürgen Brandel (Freiburg) is acknowledged. Open Access funding enabled and organized by Projekt DEAL.
Struck F, Schreiner P, Staschik E, et al. Vaccination versus infection with SARS‐CoV‐2: Establishment of a high avidity IgG response versus incomplete avidity maturation. J Med Virol. 2021;93:6765‐6777. 10.1002/jmv.27270
DATA AVAILABILITY STATEMENT
All raw data used for this study are presented within the study as “Gray intensity units”. Additional data are presented under Supplementary Materials.
REFERENCES
- 1. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Disease. 2020;71:2027‐2034. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jin Y, Wang M, Zuo Z, et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease. Int J Infect Dis. 2020;94:49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiao S‐Y, Wu Y, Liu H. Evolving status of the 2019 novel coronavirus infection: Proposal of conventional serologic assays for disease diagnosis and infection monitoring. J Med Virol. 2020;92:464‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wölfel R, Corman V, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 5. Bauer G. The potential significance of high avidity IgG for protective immunity towards SARS CoV‐2. Int J Infect Dis. 2021;106:61‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauer G. The variability of the serological response to SARS‐corona virus‐2: Potential resolution of ambiguity through determination of avidity (functional affinity). J Med Virol. 2021;93(2020):311‐322. 10.1002/jmv.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long Q‐X, Tang X‐J, Shi Q‐L, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26:845‐848. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 8. Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS‐CoV‐2 infection. Nat Microbiol. 2020;5:1598‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strömer A, Rose R, Grobe O, et al. Kinetics of nucleo‐ and spike protein‐specific immunoglobulin G and of virus‐neutralizing antibodies after SARS‐CoV‐2 infection. Microorganisms. 2020;8(2020):1572‐1583. 10.3390/microorganisms8101572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strömer A, Grobe O, Rose R, Fickenscher H, Lorentz T, Krumbholz A. Diagnostic accuracy of six commercial SARS‐CoV‐2 IgG/total antibody assays and identification of SARS‐CoV‐2 neutralizing antibodies in convalescent sera. medRxiv. 2020. 10.1101/2020.06.15.20131672 [DOI] [Google Scholar]
- 11. Beaudoin‐Bussières G, Laumaea A, Anand SP, et al. Decline of humoral responses against SARS‐CoV‐2 Spike in convalescent individuals. mBio. 2020;11:e02590‐20. 10.1128/mBio.02590-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauer G, Struck F, Schreiner P, Staschik E, Soutschek E, Motz M. The challenge of avidity determination in SARS‐CoV‐2 serology. J Med Virol. 2021;93:3092‐3104. 10.1002/jmv.26863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisen HN, Siskind GW. Variations in the affinities of antibodies during the immune response. Biochemistry. 1964;3:996‐1008. [DOI] [PubMed] [Google Scholar]
- 14. Foote J, Milstein C. Kinetic maturation of an immune response. Nature. 1991;352:530‐531. [DOI] [PubMed] [Google Scholar]
- 15. Hedman K, Lappalainen M, Söderlund M, Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1997;4:123‐129. [Google Scholar]
- 16. Hazel SL. Clinical utility of avidity assays. Expert Opin Med Diagn. 2007;1:511‐519. [DOI] [PubMed] [Google Scholar]
- 17. Benner S, Patel EU, Laeyendecker O, et al. SARS‐CoV‐2 antibody avidity responses in covid‐19 patients and convalescent plasma donors. J Inf Dis. 2020;222:1974‐1984. 10.1093/infdis/jiaa581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein SL, Pekosz A, Park H‐S, et al. Sex, age, and hospitalization drive antibody responses in a COVID‐19 convalescent plasma donor population. J Clin Invest. 2020;130:6141‐6150. 10.1172/JCI142004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu T, Hsiung J, Zhao S, et al. High‐accuracy multiplexed SARS‐CoV‐2 antibody assay with avidity and saliva capability on a nano‐plasmonic platform. Nat Biomed Eng. 2020;4:1188‐1196. 10.1101/2020.06.16.155580 [DOI] [PubMed] [Google Scholar]
- 20. Moura A, da Costa HH, Correa V, et al. Serological assessment of COVID‐19 patients in Brazil: Levels, avidity, and subclasses of IgG against RBD. Res Square. 10.21203/rs.3.re-131195/v1 [DOI] [Google Scholar]
- 21. Kaneko N, Kuo H‐H, Boucau J, et al. The Massachusetts Consortium on Pathogen Readiness Specimen Working Group Loss of Bcl‐6‐expressing T follicular helper cells and germinal centers in COVID‐19. Cell. 2020;183:143‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429‐457. [DOI] [PubMed] [Google Scholar]
- 23. Khatri I, Staal FJT, Van Dongen JJM. Blocking of the high‐affinity interaction‐synapse between SARS‐CoV‐2 spike and human ACE2 proteins likely requires multiple high‐affinity antibodies: an immune perspective. Front Immunol. 2020;11:570018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo YR, Chakraborty I, Yun C, Wu AHB, Lynch KL. Kinetics of SARS‐CoV‐2 antibody avidity maturation and association with disease severity. Clin Infect Dis. 2020:ciaa1389. 10.1093/cid/ciaa1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan PKS, Lim P‐L, Liu EYM, Cheung JLK, Leung DTM, Sung JJY. Antibody avidity maturation during severe acute respiratory syndrome–associated coronavirus infection. J Inf Dis. 2005;192:166‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Struck F, Schreiner P, Staschik E, et al. Incomplete IgG avidity maturation after seasonal coronavirus infections. J Med Virol. Under review. [DOI] [PubMed]
- 27. Nair N, Moss WJ, Scott S, et al. HIV‐1 infection in Zambian children impairs the development and avidity maturation of measles virus–specific immunoglobulin G after vaccination and infection. J Infect Dis. 2009;200:1031‐1038. 10.1086/605648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Re MC, Schiavone P, Bon I, et al. Incomplete IgG response to HIV‐1 proteins and low avidity levels in recently converted HIV patients treated with early antiretroviral therapy. Int J Infect Dis. 2010;14:(2010) e1008‐e1012. [DOI] [PubMed] [Google Scholar]
- 29. Arias‐Bouda LMP, Kuijper S, Van Der Werf A, Nguyen LN, Jansen HM, Kolk AHJ. Changes in avidity and level of immunoglobulin g antibodies to mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Society. 2003;10:702‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ripperger TJ, Uhrlaub JL, Watanabe M, et al. Orthogonal SARS‐CoV‐2 serological assays enable surveillance of low‐prevalence communities and reveal durable humoral. Immunity. 2020;53:925‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2‐naïve and recovered individuals after mRNA vaccination. Sci Immunol. 2021;6:eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370:1227‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after SARS‐CoV‐2 infection. J Infect Dis 2021;223:197‐205. 10.1101/2020.08.06.20169367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gaspar EB, De Gaspari E. Avidity assay to test functionality of anti‐SARS‐CoV‐2 antibodies. Vaccine. 2021;39:1473‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Callow KA, Parry HF, Sergeant M, Tyrrell DAJ. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short‐lasting. Nat Med. 2020;26:1691‐1693. 10.1101/2020.05.11.20086439 [DOI] [PubMed] [Google Scholar]
- 37. Galanti M, Shaman J. Direct observation of repeated infections with endemic coronaviruses. J Infect Dis. 2021;223:409‐415. 10.1093/infdis/jiaa392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All raw data used for this study are presented within the study as “Gray intensity units”. Additional data are presented under Supplementary Materials.


