Abstract
This study investigates the effect of the nanostructure of squalene in the form of microemulsion on COVID‐19 patients. In this blinded clinical trial, a comparison was made between the efficacy of squalene treatment and controls. A total of 30 COVID‐19 patients admitted to the emergency department, and the infection ward was equally allocated to case (n = 15) and control (n = 15) groups according to their age and underlying diseases. The baseline characteristics of subjects, including age, gender, time of treatment onset, underlying condition, white blood cells count, and lymphocyte count were similar (p < 0.05). Baseline laboratory tests and computed tomography (CT) scans were performed for the study groups. The treatment group received 5 mg of intravenous squalene twice a day and standard treatment for 6 days, while controls received only standard treatment. After 6 days of treatment, clinical and CT scan changes were evaluated and compared in intervention and control groups. The need for oxygen therapy (p = 0.020), 2 days of no fever (p = 0.025), cough alleviation (p = 0.010), and lung high‐resolution computed tomography improvement (p = 0.033) were significantly different between cases and controls within 7 days of admission. No adverse effects were observed in the treatment group. Our data suggest that squalene could be considered as a potential treatment for COVID‐19, and further studies are required to confirm the results.
Keywords: clinical study, COVID‐19, inflammation, microemulsion, pumpkin seed oil, squalene
1. INTRODUCTION
The new coronavirus (SARS‐CoV‐2) emerged in December 2019 and was declared a global pandemic by the World Health Organization (WHO). Due to its high infectivity and contagion rate, it has posed a severe global health threat. 1 , 2 After nearly a year, more than 69,000,000 confirmed cases and 1,500,000 deaths related to this virus had been reported worldwide (from December 2019 till December 2020). 3
The clinical course of COVID‐19 ranges from asymptomatic infection to life‐threatening pneumonia and ARDS, cytokine storm, multiorgan failure, and eventually death. 4 , 5 Despite the uncertainty surrounding the mechanism of lung injury, accumulating evidence suggests that proinflammatory cytokines and cells are excessively high in critically ill patients, which results in cytokine storm syndrome and hyper‐inflammation of the pathogenesis of COVID‐19. 6 , 7 In contrast, oxidative stress is also hypothesized to account for SARS‐CoV‐2 infection pathogenesis, which involves in a cytokine storm, coagulopathy, and cell hypoxia. 8
The urgent need for an efficacious treatment has initiated a global race to find a novel or repurposed antiviral medicine. For this purpose, several therapeutic agents have been evaluated; nonetheless, many clinical trials are still running, and so far, only Remdesivir has been approved by FDA for COVID‐19 treatment, and the need for therapeutic development persists. 9 , 10
Recently, there has been a rising demand for herbal and organic substances for treating and preventing medical conditions, and they have provided a valuable source of medical discoveries. Terpenoids, also known as Isoprenoids, are a broad group of natural products in many plants and demonstrate a wide range of biological activities. 11 They are classified into several subclasses based on their isoprene subunit number including hemiterpenes, sesquiterpenes, triterpenes, tetraterpenes, and polyterpenes. 12 Terpenoids are effective against cancer, inflammation, malaria, and various infectious diseases with multiple medical and therapeutic applications (e.g., Paclitaxel is an anticancer, and Artemisinin is an antimalaria drug). 11
Squalene (SQ) is a natural lipophilic biomolecule that belongs to the chemical class of triterpenes. With a formula of C30H50, it acts as a precursor of sterols and other bioactive terpenoids. 13 There is a small amount of this antioxidant component in the human body (skin, liver, and intestine). It can be extracted from shark liver oil or some plant oils. 14 Various studies exhibited certain bioactivities of this molecule, including antioxidant, antineoplastic, 15 , 16 and antiatherosclerotic properties, 17 , 18 and a chemopreventive effect. 19 SQ, as an effective ingredient in MF59, is a safe and potent influenza vaccine adjuvant. It can improve the immunogenicity of vaccines even in immunocompromised patients 20 , 21 , 22 without stimulating antibody response. 23
It is believed that SQ possesses anti‐inflammatory effects. 17 , 24 This natural substance prevents the over‐activation of neutrophils and monocytes and increases anti‐inflammatory enzymes. 24 In addition, the antioxidant feature of SQ highlighted in several studies could be linked to SQ molecular features, which makes it an efficient quencher of singlet oxygen and free radicals. 25 , 26 , 27
The antiviral, antifungal, and antimicrobial effects of terpenoids have been evaluated recently, and they are presumed to provide a promising novel antiviral medicine. 28 , 29 Terpenoids appear to act as an antiviral agent by activating membrane‐mediated mechanisms and inhibiting viral DNA synthesis. 30 In contrast, several studies have revealed the antiviral effect of some natural herbs against SARS‐CoV. 31 , 32 , 33 Glycyrrhizin, a triterpenoid compound, exhibits anti‐SARS‐CoV activity, which can completely block virus replication in high concentrations. 31 , 34 The antiviral effect of SQ in treating the hepatitis C virus has been shown in a patent. 35
Due to the lipophilic structure of SQ, oil‐in‐water microemulsions can offer a suitable form for SQ intravenous injection. The safety and biocompatibility of SQ microemulsions have been shown in various studies. 36 , 37 , 38 , 39
Developing novel therapeutics is crucial, but formulating new drugs and evaluating their safety and efficacy in a short time seems impractical due to the time‐consuming nature of this process. Therefore, it is reasonable to examine substances with confirmed antiviral potentials that are safe and well‐tolerated. Given that SQ possesses anti‐inflammatory, immunomodulatory, and antioxidant features with minimum side effects, it might affect SARS‐CoV‐2 infection as well. Accordingly, this clinical trial aims to obtain an effective SQ‐based formulation to examine the therapeutic effect of SQ among COVID‐19 patients. SQ was extracted from natural oil of high purity. To the best of our knowledge, this is the first study that investigates the SQ effect on SARS‐CoV‐2 infection.
2. MATERIALS AND METHODS
2.1. Materials
FeCl3 • 6H2O (97%), FeCl2 • 4H2O (99%), potassium hydroxide, acetone, ethanol, hexane, toluene (99%), ammonium hydroxide (27%–30%) Tween 80 and Span 80 were purchased from Merck Company (Darmstadt Merck). Pumpkin seed oil was procured from Zarband Company. Standard squalene was purchased from Sigma Aldrich company for gas chromatography (GC) analysis.
3. METHODS
3.1. Squalene extraction
SQ was extracted from pumpkin seed oil described in our previous studies. 40 , 41 SQ extraction from pumpkin seed oil is performed in two steps: the adsorption of fatty acids (FAs) on the magnetic iron oxide nanoparticles and the solvent extraction method. Briefly, iron oxide (Fe3O4), as magnetic nanoparticles (MNPs) (5.2 mmol), were prepared using a coprecipitation reaction. First, FeCl3 and FeCl2 with a molar ratio of 2:1 were dissolved in 50 ml distilled water. Then, 4–5 ml ammonia was added to adjust pH to 11. This solution was placed in a balloon under atmospheric argon at 45°C stirred at a speed of 800 rpm. After 30 min, Fe3O4 nanoparticles appeared in the solution by changing the solution color to black. MNPs were washed with distilled water and separated from the solution by a magnet.
Afterward, the saponification reaction was induced for pumpkin seed oil. Five grams of the oil and 50 ml ethanolic potassium hydroxide were poured in a container and heated at 80°C under reflux conditions for 1 h. Then, the solution was heated in an oven for 4 h until all the ethanol solvent evaporated. Finally, a soap‐like dried sample was obtained, which was dissolved in 75 ml water before the addition of MNPs. This sample was placed in an oven at 130°C for the evaporation of water. Adsorption of FAs on the MNPs surface was done at this stage. Then, the dried sample was washed with acetone, and MNPs were separated with a magnet. In the last step, 20 ml hexane, 50 ml distilled water, and 20 ml ethanol were added to the remaining solvent and placed in a decanter to separate hexane containing squalene from other materials. Finally, hexane was removed under argon, and SQ was extracted.
3.2. Microemulsion preparation
The microemulsion containing SQ with a concentration of 10 mg/ml was prepared using the titration method. 42 A blend of hydrophilic and hydrophobic nonionic surfactants was selected for this aim. Tween 80 and Span 80 with the hydrophilic‐lipophilic balance (HLB) values of 15 and 4.3 were set, respectively. At first, surfactant components were mixed together with the mass ratio of 9:1 (Tween80:Span80), SQ was added with a mass ratio of 1:5 (SQ: surfactants) to the surfactant mixture, and the double‐distilled water was added slowly under moderate agitation (magnetic stirring). After equilibration, the samples were assessed visually by a clear and transparent liquid. 43 These mass ratios were selected based on the SQ ternary phase diagram. 44 At last, 10 mg SQ, 45 mg Tween 80, 5 mg Span 80, and 1 ml water was applied for microemulsion preparation. By using these contents, it is expected to prepare a microemulsion with appropriate particle size for IV usage.
3.3. Characterization
GC‐FID analysis specification (6890 Series GC system) was applied to measure the purity of extracted squalene. 41 A dynamic light scattering analyzer (DLS, CORDOUAN Tech.) was used to measure the hydrodynamic diameter of the microemulsion sample at 25°C in the deionized water. The sample morphology and size were determined using transmission electron microscopy (TEM) analysis (LEO). The zeta potential was computed by zeta meter (Zeta CAD) with SD = 11.49. GC‐mass (Agilent, 6890 Series GC) was applied to examine the purity of the extracted SQ. The refractive index (RI) and the electrical conductivity coefficient of the microemulsion sample were measured using Abbe's refractometer (Bellingham + Stanley Limited) and the conductivity meter (AZ 86503), respectively. The pH values of microemulsion samples were measured at 25°C by a pH meter (CibaCorningDiagnosticsLimited Sudbury, Suffolk CO106XD). The viscosity of the microemulsion formulation was determined using a viscometer (Bohlin software: Visco 88; Bohlin Instrument) at 25℃. To identify the content of the remaining Fe3O4 MNP or Fe2+/Fe3+ ion in the solution, Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP‐AES, 7604555, Spectro Acros) was applied.
Microemulsion samples were centrifuged (Sigma3–30k) at 10,000 rpm for 15 min at 25°C for the physical stability study. They were assumed as physically stable provided that no sign of drug precipitation, droplet conjugation, creaming, and phase separation was observed. The stability of samples over time was examined 3 and 6 months after preparation using droplet size determination. A significant increase in the average droplet size, polydispersity index, and unfavorable particle size distribution over time were considered as signs of instability over time. In addition, thermal stability studies of microemulsion at 4°C, 25°C, and 40°C were evaluated via clarity and phase separation observation.
3.3.1. SQ content in the prepared microemulsion was measured as follows
The microemulsion sample was centrifuged at 10,000 rpm for 15 min. A volume of 1 ml of the sedimented microemulsion sample was dissolved in 10 ml of hexane, which can dissolve SQ completely. The solution was analyzed by GC. The content of SQ in the microemulsion was determined based on the GC diagram and SQ calibration curve. By dividing the computed value by the initial SQ amount, the percentage of drug content was determined.
3.4. In vivo study
3.4.1. Study groups
The sample was selected from adults admitted to the emergency department and infectious ward of Shohada hospital. The patients with diagnostic signs and symptoms of COVID‐19 including acute loss of sense and smell, fever, dry cough, fatigue, lymphocytopenia, elevated c‐reactive protein (CRP), with/without contact with confirmed cases of COVID‐19, patients with diagnostic characteristics in high‐resolution computed tomography (HRCT) including ground‐glass opacities, vascular enlargement, bilateral abnormalities, lower lobe involvement, and posterior preference, 45 patients with positive real‐time polymerase chain reaction (PCR) test, eligible patients with respiratory symptoms (including dyspnea, chest pain or discomfort), oral temperature > 38°C and SpO2 < 93% were included in the study. Exclusion criteria were a history of myocardial ischemia, heart failure, and advanced COPD, mental retardation, direct admission to ICU, pregnancy, breastfeeding, a suspected or confirmed history of alcohol abuse or substance use disorder, and participation in other drug trials in the past month. After obtaining written informed consent, eligible patients were assigned to two groups of standard treatment (controls) and SQ plus standard treatment (cases) according to age, smoking habit, and past medical history, including DM, HTN, asthma, COPD, renal failure and liver failure.
3.4.2. Study design
Laboratory tests including complete blood count, CRP, blood urea nitrogen, creatinine, lactate dehydrogenase (LDH), aspartate transaminase (AST), alanine aminotransferase (ALT), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), ferritin, D‐dimer, creatine phosphokinas (CPK), troponin, and total bilirubin were measured preintervention and then daily. Lung HRCT was performed for all participants at the baseline.
Standard treatment included oxygen therapy, 150 mg chloroquine (equivalent to 250 mg chloroquine phosphate), two tablets on the first day and one tablet every 12 h for a total of 10 days, or 400 mg hydroxychloroquine on the first day and then one tablet every 12 h for 10 days and Lopinavir/Ritonavir (200/50) every 12 h for 10–14 days and heparin 5000IU subcutaneous TDS (for body mass index [BMI] ≥ 40, 7500 IU SC TDS) or Enoxaparin 40 mg SC once a day (for BMI ≥ 40, 40 mg SC BID).
SQ treatment included intravenous injection of 5 mg SQ microemulsion twice a day for 6 days. The required dose for clinical study was selected based on the Fluad Influenza vaccine. Fluad Influenza vaccine contains 9.75 mg squalene in oily phase as an adjuvant. 46 In this study, 10 mg of SQ was chosen as the proposed dose for injection (5 mg SQ microemulsion twice a day). However, due to the severity of the COVID‐19 pandemic, this dose was repeated for 6 days.
Vital signs and clinical characteristics, including respiratory rate, SpO2%, oral temperature, cough alleviation, and oral tolerance, were constantly assessed and recorded during the hospital admission. The assessors of symptoms were blinded.
Lung HRCT was repeated 7 days after the last dose of SQ. The severity of lung involvement was defined according to score (0 for no involvement, 1 for GGO, consolidation or nodule in a maximum of 2 lobes in an area less than one‐third of each lobe, 2 for the involvement of 3 or 4 lobes with an area of less than one‐third of each lobe or in 1 or 2 lobes with an area more than one‐third of each lobe, 3 for the involvement of 5 lobes with an area of less than one‐third of each lobe or in 3 lobes with an area more than one‐third of each lobe and 4 for greater involvement). Variations in the lung involvement severity score were compared before and after treatment in the study groups.
All participants were monitored for adverse events including anaphylaxis, ALT or AST elevation more than 2.5‐fold beyond the normal upper limit, total bilirubin elevation more than 1.5‐fold beyond the normal upper limit, acute pancreatitis, and diarrhea.
It should be noted that squalene was previously administrated intravenously in the form of lipid emulsion. Toxicity or hypersensitive reaction was not reported. 38 In addition, in another study, vaccination with a subunit influenza vaccine with the MF59 adjuvant showed no antisqualene antibodies nor enhanced pre‐existing antisqualene antibody titers. It confirmed that antisqualene antibodies are not increased by immunization with vaccines with the MF59 adjuvant. 23
These data extended the safety profile of the SQ emulsion. Consequently, we applied SQ in the IV form for new application in COVID‐19 treatment.
3.4.3. Statistical analysis
Data were analyzed using SPSS 18 software. The data analyst was blinded to the study groups. Qualitative variables were reported as number and percentage, and quantitative variables were expressed as median ± SD. Categorical variables were analyzed using the χ 2 test. Clinical outcomes and improved lung HRCT score 7 days after treatment were compared between groups using an independent t test. A value of p < 0.05 was considered to be significant.
The study protocol was approved by the MUMS ethics committee (IR.MUMS.REC.1399.468) and IRCT (trial ID: 51922, date: 2020.11.04)
4. RESULTS
4.1. SQ extraction
The purity of SQ was detected using FID‐GC analysis. The result is shown in Figure 1. As the figure shows, the runtime for SQ is 17.918 min. The calibration curve has a residual standard deviation of RSD = 25.73 and the root mean square deviation of R 2 = 0.999. Results confirmed the successful extraction of SQ with high purity (>95%).
Figure 1.
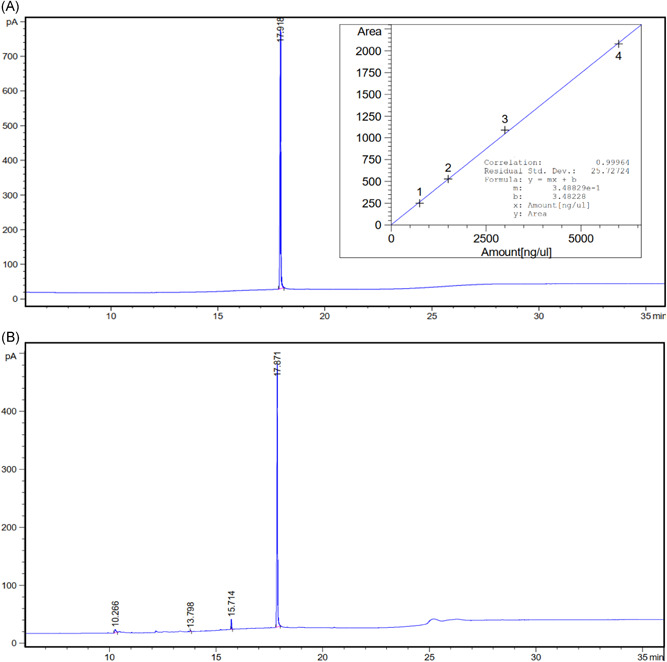
FID‐GC chromatogram diagram for (A) standard squalene at 6000 ppm and (B) extracted squalene at 3250 ppm (calibration curve is shown in the incident diagram)
4.2. Characterization results of microemulsion
TEM image and DLS diagram of microemulsion containing SQ are shown in Figure 2. As Figure 2A illustrates, the sample comprises spherical particles with an average particle size of about 30 nm. In addition, the DLS diagram shows an average particle size of 40.37 nm with a polydispersity index (PDI) of 0.247 (Figure 2B). This enlargement of particles in DLS could be attributed to the calculation of the hydrodynamic diameter by DLS analysis.
Figure 2.
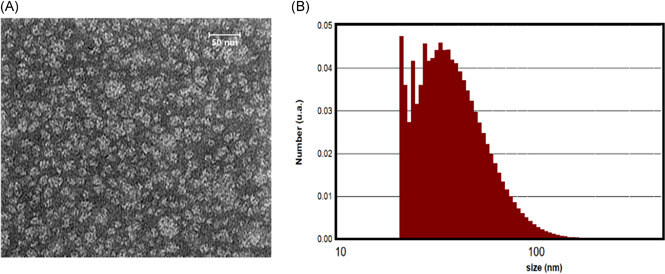
Size analysis of microemulsion particles (A) TEM image, and (B) DLS diagram
The zeta potential of the microemulsion sample was −32.01 mV, which is acceptable for a drug delivery system. The electrical conductivity coefficient of microemulsion was 467 μs/cm, which confirms the formation of an oil‐in‐water microemulsion. The refractive index of microemulsion was 1.391, which was near the aqueous phase. Therefore, it revealed the oil‐in‐water microemulsion with high transparency. The pH value was 6.52, which was close to pH value of the body environment and safe for oral usage. Following 3 and 6 months of preparation, no turbidity was observed in the sample, which confirms the high stability of the system. Furthermore, the sample was stable at 4℃, 25℃, and 40℃ without any sign of phase separation. Details of characterization at the preparation time and after 3 and 6 months are reported in Table 1.
Table 1.
Characterization results of the microemulsion sample
| After preparation | |||||||
|---|---|---|---|---|---|---|---|
| Average droplet size (nm) | Zeta potential (mV) | PDI | RI | Conductivity (μs/cm) | pH | Phase Separation after centrifuge | |
| 40.37 ± 0.2 | −32.01 ± 0.04 | 0.247 ± 0.011 | 1.391 ± 0.009 | 467 ± 3 | 6.52 ± 0.05 | Not observed | |
| After 3 months | After 6 months | ||||||
|---|---|---|---|---|---|---|---|
| Average droplet size (nm) | Zeta potential (mV) | PDI | Visual inspection | Average droplet size (nm) | Zeta potential (mV) | PDI | Visual inspection |
| 48.02 ± 0.1 | −27.03 ± 0.03 | 0.278 ± 0.009 | Transparent | 59.28 ± 0.3 | −24.35 ± 0.02 | 0.285 ± 0.012 | Transparent |
The rheogram of the SQ microemulsion is shown in Figure 3. The figure confirms that the sample behaves as a Newtonian fluid with the linearity of shear stress versus shear rate (R 2 > 0.999) and the viscosity is constant. The calculated viscosity of the formulation is 67.4 ± 1.1 mPa.s. This is in agreement with similar microemulsion samples. 47
Figure 3.
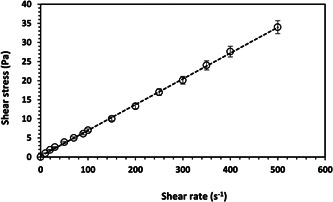
Rheogram of the SQ microemulsion formulation
SQ content analysis results confirmed that greater than 98% of the SQ was loaded in the microemulsion formulation.
ICP measurement was performed to confirm there is no residue of MNP following the SQ extraction from pumpkin seed oil. ICP determines the content of iron in the sample. Results showed that there are no iron ion/MNPs in the sample. Furthermore, the weight of the final SQ sample was checked every 15 min. Any sign of weight reduction shows the evaporation of solvent at the ambient temperature. After fixing the weight, it was assumed as the final SQ sample. This confirms the nonexistence of solvents in the final product.
4.3. In vivo results
A total of 30 patients (15 in each group) confirmed with COVID‐19 were studied. Baseline characteristics are shown in Table 2. The mean age of the control and experimental groups were 50.07 ± 17.9 and 51.07 ± 20.49 years, respectively, and the two groups were identical in this regard (p = 0.888). Out of 15 patients in the control group, 4 (26.7%) were female, and 11 (73.3%) were male while in the case group, 6 (40.0%) were female and 9 (60.0%) were male (p = 0.350). Time from symptoms onset to treatment varied between 2 and 6 days not significantly different between cases and controls (p = 0.470). In both groups, 7 (46.7%) patients had a history of underlying diseases, and the two groups were not significantly different in this regard (p = 0.642). Mean white blood cells count was 4.51 ± 2.31 in controls and 4.34 ± 2.01 in cases, and mean lymphocyte count was 1.73 ± 0.91 and 1.73 ± 0.95 in controls and cases, respectively. WBC count and lymphocyte count were statistically similar in cases and controls (p = 0.810 and 0.999, respectively).
Table 2.
Baseline characteristics of study groups
| Baseline characteristics | Controls (N = 15) | Cases (N = 15) | p value |
|---|---|---|---|
| Age (year) (Mean ± SD) | 50.07 ± 17.97 | 51.07 ± 20.49 | 0.888 |
| Gender, n (%) | |||
| Female | 4 (26.7%) | 6 (40.0%) | |
| Male | 11 (73.3%) | 9 (60.0%) | 0.350 |
| Time from symptom onset to treatment (days), n (%) | |||
| 2 | 0 (0.0%) | 1 (6.7%) | 0.470 |
| 3 | 1 (6.7%) | 4 (26.7%) | |
| 4 | 6 (40.4%) | 4 (26.7%) | |
| 5 | 7 (46.7%) | 5 (33.3%) | |
| 6 | 1 (6.7%) | 1 (6.7%) | |
| Underlying chronic diseases, n (%) | 7 (46.7%) | 7 (46.7%) | 0.642 |
| WBC (109/L) (mean ± SD) | 4.51 ± 2.31 | 4.34 ± 2.01 | 0.810 |
| Lymphocyte (109/L) (mean ± SD) | 1.73 ± 0.91 | 1.73 ± 0.95 | 0.999 |
After 7 days of standard or modified treatment in control and experimental groups, clinical outcomes were evaluated as listed in Table 3. As Table 3 shows, after 7 days of treatment, out of 15 patients in the control group, 1 (6.7%) needed no oxygen therapy, 9 (60%) received low flow, and 5 (33.3%) received high flow oxygen. In the experimental group, however, 8 (53.4%) patients did not need oxygen, 5 (33.3%) were on low flow and 2 (13.3%) were on high flow oxygen therapy. Hence, the need for oxygen therapy was significantly different between cases and controls after 7 days of treatment (p = 0.020). Conversion to severe or critical clinical status was 5 (33.3%) and 1 (16.7%), for control and case groups, respectively, which was statistically significant (p = 0.084). In 7 (46.7%) patients in the control group and 13 (86.7%) patients in the experimental group, no fever was registered at least for 2 days, which was significantly different between groups (p = 0.025). Cough alleviation was observed in 6 (40.0%) controls and 13 (86.7%) cases, and there was a significant difference between the two groups in this regard (p = 0.010).
Table 3.
Outcomes variables of study groups 7 days after treatment
| Outcomes | Controls (N = 15) | Cases (N = 15) | p value |
|---|---|---|---|
| Oxygen therapy, n (%) | |||
| Without | 1 (6.7%) | 8 (53.4%) | 0.020 |
| Low flow | 9 (60.0%) | 5 (33.3%) | |
| High flow | 5 (33.3%) | 2 (13.3%) | |
| Conversion to severe/critical* clinical status, n (%) | 5 (33.3%) | 1 (16.7%) | 0.084 |
| 2 days without fever, n (%) | 7 (46.7%) | 13 (86.7%) | 0.025 |
| Cough alleviation, n (%) | 6 (40.0%) | 13 (86.7%) | 0.010 |
| Improvement in chest CT scan, n (%) | 4 (26.7%) | 10 (66.7%) | 0.033 |
Critical status means RR > 30 per min, HR > 125 per min, SpO2 < 90% with oxygen therapy, SBP < 90 mmHg, capillary filling > 3 s, oliguria, loss of consciousness, LDH > 2 × upper limit of normal, progressive lymphopenia (especially less than 500), PT, PTT, INR > upper limit of normal, CRP > 2 × upper limit of normal (especially > 100), Ferritin > 500 µg/L, D‐dimer > 1000 ng/ml, CPK > 2 × upper limit of normal, elevated troponin.
Moreover, improvement in chest CT scan was reported in 4 (26.7%) controls and 10 (66.7%) cases, which varied significantly between the two groups (p = 0.033). Figures 4 and 5 show some CT scan images for control and case groups on the first and last days of treatment. No clinical and laboratory adverse effects were observed within 7 days of admission.
Figure 4.
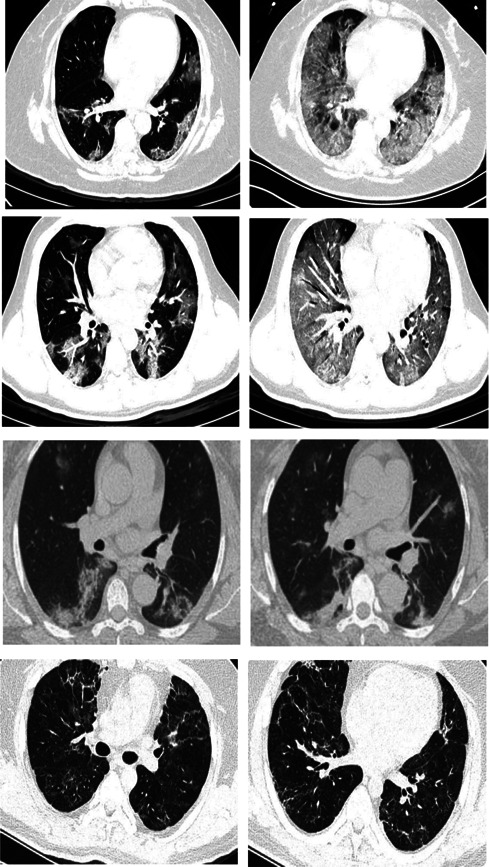
Chest computed tomography scan of the control group with standard treatment (left image: on the first day, right image: after 6 days of treatment)
Figure 5.
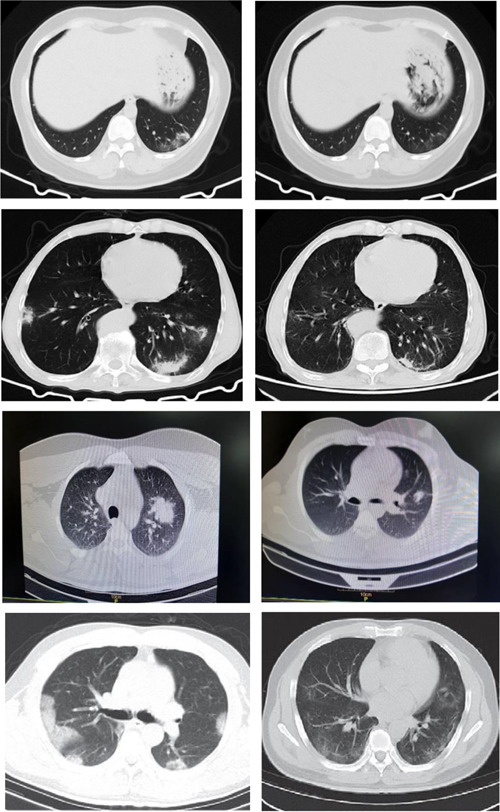
Chest computed tomography scan of the experimental group with standard treatment + SQ (left image: on the first day, right image: after 6 days of treatment)
5. DISCUSSION
This clinical trial investigated the efficacy of SQ microemulsion in the treatment of COVID‐19. Our findings provide evidence that this novel medicine can lead to clinical improvement of symptoms in ICU‐admitted patients, including fever, cough, chest CT scan, and oxygen therapy requirement. The therapeutic effect of SQ on SARS‐Cov‐2 has not been studied in the form of a clinical trial so far; however, the results of various studies have demonstrated the therapeutic potentials of this natural substance.
Lately, the anti‐inflammatory and immunomodulatory effect of SQ has been examined in detail in different studies. Sánchez‐Quesada et al. demonstrated that SQ could affect inflammation by various means. 48 They reported that SQ enhances the switching of M1 macrophages, which mediate the proinflammatory response, to the M2 macrophages with anti‐inflammatory features. Also, SQ seems to increase anti‐inflammatory cytokines, such as interleukin (IL)‐10, IL‐4, and IL‐13, and raise the production of tissue inhibitor of metalloproteinases 2, a natural inhibitor of matrix metalloproteinase‐2 (MMP‐2). 48 MMP‐2 is a proteolytic enzyme that applies in the degradation of extracellular matrix proteins and pathologic tissue destruction. 49 , 50
Moreover, Sánchez‐Quesada et al. claimed that SQ is capable of decreasing nuclear factor κB (NF‐κB) expression. 48 NF‐κB is a crucial transcription factor that produces many inflammatory genes and functions in both innate and adaptive immune cells. 51 Terpenoids are considered to be natural NF‐κB pathway inhibitors with anti‐inflammatory and anticancer effects. 52
The NF‐κB pathway appears to be a key element in COVID‐19 natural progression and conversion to a severe phenotype. 53 The proteomic examination of SARS‐CoV‐2 infected lung tissue exhibited the upregulation of molecules associated with the NF‐κB pathways reflecting the significant role of NF‐κB in COVID pathogenesis. 54 Recent studies within vitro human lung models have shown that SARS‐CoV‐2 infection in the alveolar epithelium changes cell transcription toward an inflammatory program, and NF‐κB‐mediated inflammatory signaling is the most‐upregulated pathway. 55 Accordingly, targeting the NF‐κB pathway could reduce mortality in the severe form of COVID‐19. 53 , 54 , 55
The antioxidant defense is a critical element in determining the severity of viral infections. 56 Respiratory viruses are associated with reactive oxygen species production and redox imbalance linked to inflammation and subsequent tissue damage. 57 Studies show that antioxidant deficiency may lead to viral mutation and exacerbate viral virulence in RNA viruses. 58 The SQ antioxidant properties have received increasing attention because its molecular features make it a potent quencher of singlet oxygen and free radicals. 26 , 27 Sabeena et al. examined the effect of SQ on myocardial infarction in rats, concluding that the antioxidant and membrane‐stabilizing action of SQ might explain its cardioprotective impact. 59 Additionally, Das et al. reported that SQ is a nontoxic antioxidant that could decrease the production of reactive oxygen species and improve cellular glutathione homeostasis, fostering protection against cisplatin and carboplatin‐induced toxicity in mesenchymal stem cells. 60 In light of the latter points, the role of SQ as an anti‐inflammatory, immunomodulatory, and antioxidant agent highlights its potentials for the treatment of COVID‐19 patients.
SQ has a lipophilic structure and oil‐in‐water microemulsions of this substance have already been used in various studies. In humans and animal trials, intravenous administration of SQ emulsion is safe and well‐tolerated, with a slower clearance from the circulation compared to triglycerides and plant sterols. 38 , 61 SQ is a safe, effective, and preferred vaccine adjuvant, 37 , 62 as it improves antigen uptake by immune cells and evokes humoral and cell‐mediated immunity. 63
Immune‐modulating treatments in severe COVID‐19 cases and antioxidant applications are considered as potential treatment strategies. 8 , 64 The results of this clinical trial suggest that SQ microemulsion administration in severe cases of SARS‐CoV‐2 infected patients can improve several clinical outcomes.
6. CONCLUSIONS
In this clinical study, the efficacy of SQ microemulsion extracted from pumpkin seed oil was investigated to treatment COVID‐19. Some crucial parameters were measured at the first and final stages of the treatment. Results showed that SQ has a significant effect on improving symptoms of ICU‐admitted patients by decreasing fever and cough during the treatment period. In addition, comparing the chest CT scan on the first and final days of treatment exhibits significant improvement in those patients in the SQ treatment group. They did not require oxygen therapy. The antioxidant, anti‐inflammatory, and immunomodulatory effects of SQ may account for these observations. The results of this clinical trial suggest that SQ microemulsion administration as a novel medicine in SARS‐CoV‐2 infected patients can improve several clinical outcomes.
Due to the likely results of this clinical study, the efficacy of other forms of SQ microemulsion such as sublingual or inhalation for COVID‐19 treatment can be clinically investigated. In these new forms, the drug can be absorbed by the tongue or nasal mucosa. Finally, understanding the mechanism of action of SQ microemulsion can extend our knowledge in designing new drugs for this pandemic treatment. This can be the subject of future work.
AUTHOR CONTRIBUTIONS
The conception and design of this study have been performed by Dr. Ebrahimi, Dr. Karimi, and Dr. Farhadian. Analysis of data in SQ extraction has been done by Ms. Hataminia. Data at in vivo study has been completed by Mr. Amiri and Ms. Saffar Soflaei. Drafting the manuscript has been done by Ms. Saffar soflaei. Revising the manuscript critically for important intellectual content was done by Dr. Ebrahimi, Dr. Karimi, and Dr. Farhadian. Approval of the final version of the manuscript to be published was done by all authors.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Ebrahimi M, Farhadian N, Amiri AR, Hataminia F, Soflaei SS, Karimi M. Evaluating the efficacy of extracted squalene from seed oil in the form of microemulsion for the treatment of COVID‐19: A clinical study. J Med Virol. 2021;94:119‐130. 10.1002/jmv.27273
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mahase E. Covid‐19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. [DOI] [PubMed] [Google Scholar]
- 2. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus. StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 3. World Health Organization . WHO Coronavirus Disease (COVID‐19) Dashboard 2020, December 12. https://covid19.who.int/
- 4. Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm”. Relevant to COVID‐19? JAMA Internal Med. 2020;180(9):1152‐1154. [DOI] [PubMed] [Google Scholar]
- 5. Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4(4):66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255‐2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID‐19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cecchini R, Cecchini AL. SARS‐CoV‐2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubin D, Chan‐Tack K, Farley J, Sherwat A. FDA approval of Remdesivir—a step in the right direction. N Engl J Med. 2020;383(27):2598‐2600. [DOI] [PubMed] [Google Scholar]
- 10. Saeed H, Osama H, Madney YM, et al. COVID‐19; current situation and recommended interventions. Int J Clin Pract. 2020;75:e13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang G, Tang W, Bidigare RR. Terpenoids as therapeutic drugs and pharmaceutical agents. In: Zhang L, Demain AL, eds. Natural Products: Drug Discovery and Therapeutic Medicine. Humana Press; 2005:197‐227. [Google Scholar]
- 12. Ruchika NJ, Pandey A. Synthetic metabolism and its significance in agriculture. In: Singh SP, Pandey A, Du G, Kumar S, eds. Current Developments in Biotechnology and Bioengineering. Elsevier; 2019:365‐391. Chapter 15. [Google Scholar]
- 13. Micera M, Botto A, Geddo F, et al. Squalene: more than a step toward sterols. Antioxidants. Vol 9. Basel; 2020:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popa O, Băbeanu NE, Popa I, Niță S, Dinu‐Pârvu CE. Methods for obtaining and determination of squalene from natural sources. BioMed Res Int. 2015;2015:367202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warleta F, Campos M, Allouche Y, et al. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA‐MB‐231 human breast cancer cells. Food Chem Toxicol. 2010;48(4):1092‐1100. [DOI] [PubMed] [Google Scholar]
- 16. Shimizu N, Ito J, Kato S, Eitsuka T, Miyazawa T, Nakagawa K. Significance of squalene in rice bran oil and perspectives on squalene oxidation. J Nutr Sci Vitaminol. 2019;65(Suppl S62‐s6). [DOI] [PubMed] [Google Scholar]
- 17. Lou‐Bonafonte JM, Martínez‐Beamonte R, Sanclemente T, et al. Current insights into the biological action of squalene. Mol Nutr Food Res. 2018:e1800136. [DOI] [PubMed] [Google Scholar]
- 18. Guillén N, Acín S, Navarro MA, et al. Squalene in a sex‐dependent manner modulates atherosclerotic lesion which correlates with hepatic fat content in apoE‐knockout male mice. Atherosclerosis. 2008;197(1):72‐83. [DOI] [PubMed] [Google Scholar]
- 19. Smith TJ. Squalene: potential chemopreventive agent. Expert Opin Investig Drugs. 2000;9(8):1841‐1848. [DOI] [PubMed] [Google Scholar]
- 20. Ko EJ, Kang SM. Immunology and efficacy of MF59‐adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041‐3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Black S. Safety and effectiveness of MF‐59 adjuvanted influenza vaccines in children and adults. Vaccine. 2015;33(Suppl 2):B3‐B5. [DOI] [PubMed] [Google Scholar]
- 22. O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6(5):699‐710. [DOI] [PubMed] [Google Scholar]
- 23. Del Giudice G, Fragapane E, Bugarini R, et al. Vaccines with the MF59 adjuvant do not stimulate antibody responses against squalene. Clin Vaccine Immunol. 2006;13(9):1010‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cárdeno A, Aparicio‐Soto M, Montserrat‐de la Paz S. Squalene targets pro‐ and anti‐inflammatory mediators and pathways to modulate over‐activation of neutrophils, monocytes and macrophages. J Functional Foods. 2015;14:779‐790. [Google Scholar]
- 25. Bhilwade HN, Tatewaki N, Nishida H, Konishi T. Squalene as novel food factor. Curr Pharm Biotechnol. 2010;11(8):875‐880. [DOI] [PubMed] [Google Scholar]
- 26. Kim SK, Karadeniz F. Biological importance and applications of squalene and squalane. Adv Food Nutr Res. 2012;65:223‐233. [DOI] [PubMed] [Google Scholar]
- 27. Kohno Y, Egawa Y, Itoh S, Nagaoka S, Takahashi M, Mukai K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n‐butanol. Biochim Biophys Acta. 1995;1256(1):52‐56. [DOI] [PubMed] [Google Scholar]
- 28. Brahmkshatriya PP, Brahmkshatriya PS, Ramawat KG, Mérillon J‐M. Terpenes: chemistry, biological role, and therapeutic applications. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer; 2013:2665‐2691. [Google Scholar]
- 29. Pu JY, He L, Wu SY, Zhang P, Huang X. Anti‐virus research of triterpenoids in licorice. Bing Du Xue Bao. 2013;29(6):673‐679. [PubMed] [Google Scholar]
- 30. Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95(3):412‐427. [DOI] [PubMed] [Google Scholar]
- 31. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet. 2003;361(9374):2045‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li SY, Chen C, Zhang HQ, et al. Identification of natural compounds with antiviral activities against SARS‐associated coronavirus. Antiviral Res. 2005;67(1):18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu CY, Jan JT, Ma SH, et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci USA. 2004;101(27):10012‐10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoever G, Baltina L, Michaelis M, et al. Antiviral activity of glycyrrhizic acid derivatives against SARS‐coronavirus. J Med Chem. 2005;48(4):1256‐1259. [DOI] [PubMed] [Google Scholar]
- 35.Elsherbini SH. Squalene is an antiviral compound for treating hepatitis C virus carriers. Google Patents; 1999.
- 36. Deng J, Cai W, Jin F. A novel oil‐in‐water emulsion as a potential adjuvant for influenza vaccine: development, characterization, stability and in vivo evaluation. Int J Pharm. 2014;468(1‐2):187‐195. [DOI] [PubMed] [Google Scholar]
- 37. Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions. AAPS PharmSciTech. 2012;13(2):498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Relas H, Gylling H, Miettinen TA. Fate of intravenously administered squalene and plant sterols in human subjects. J Lipid Res. 2001;42(6):988‐994. [PubMed] [Google Scholar]
- 39. Shah RR, Dodd S, Schaefer M, et al. The development of self‐emulsifying oil‐in‐water emulsion adjuvant and an evaluation of the impact of droplet size on performance. J Pharm Sci. 2015;104(4):1352‐1361. [DOI] [PubMed] [Google Scholar]
- 40. Hataminia F, Farhadian N. A novel experimental method for adsorption of fatty acids from pumpkin seed oil in the presence of iron oxide nanoparticles: Experimental and SA–LOOCV–GRBF mathematical modeling. Colloids Surf A. 2017;528:30‐40. [Google Scholar]
- 41. Hataminia F, Farhadian N, Karimi M, Ebrahimi M. A novel method for squalene extraction from pumpkin seed oil using magnetic nanoparticles and exploring the inhibition effect of extracted squalene on angiogenesis property. J Taiwan Inst Chem Eng. 2018;91:1‐9. [Google Scholar]
- 42. Golmohammadzadeh S, Farhadian N, Biriae A, Dehghani F, Khameneh B. Preparation, characterization and in vitro evaluation of microemulsion of raloxifene hydrochloride. Drug Dev Ind Pharm. 2017;43:1619‐1625. [DOI] [PubMed] [Google Scholar]
- 43. Dehghani F, Farhadian N, Golmohammadzadeh Sh, Biriae A, Ebrahimi M, Karimi M. Preparation, characterization and in‐vivo evaluation of microemulsions containing tamoxifen citrate anti‐cancer drug. Eur J Pharm Sci. 2017;96:479‐489. [DOI] [PubMed] [Google Scholar]
- 44. Shah RR, Dodd S, Schaefer M, et al. The development of self‐emulsifying oil‐in‐water emulsion adjuvant and an evaluation of the impact of droplet size on performance, pharmaceutics, drug delivery and pharmaceutical technology. Comparative Study. 2015;104:1352‐1361. [DOI] [PubMed] [Google Scholar]
- 45. Adams HJ, Kwee TC, Yakar D, Hope MD, Kwee RM, Chest CT. Imaging signature of coronavirus disease 2019 infection: in pursuit of the scientific evidence. Chest. 2020;158(5):1885‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. FLUAD® QUADRIVALENT—Seqirus Inc. US Package Insert ; 15 March 2021.
- 47. Ghorbanzadeh M, Farhadian N, Golmohammadzadeh Sh, Karimi M, Ebrahimi M. Formulation, clinical and histopathological assessment of microemulsion based hydrogel for UV protection of skin. Colloids Surfaces B: Biointerfaces. 2019;179:393‐404. [DOI] [PubMed] [Google Scholar]
- 48. Sánchez‐Quesada C, López‐Biedma A, Toledo E, Gaforio JJ. Squalene stimulates a key innate immune cell to foster wound healing and tissue repair. Evid Based Complement Alternat Med. 2018;2018:9473094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parks WC, Wilson CL, López‐Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617‐629. [DOI] [PubMed] [Google Scholar]
- 50. Sarker H, Hardy E, Haimour A, Maksymowych WP, Botto LD, Fernandez‐Patron C. Identification of fibrinogen as a natural inhibitor of MMP‐2. Sci Rep. 2019;9(1):4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu T, Zhang L, Joo D, Sun S‐C. NF‐κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF‐kappaB signaling with anti‐inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65(19):2979‐2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The role and therapeutic potential of NF‐kappa‐B pathway in severe COVID‐19 patients. Inflammopharmacology. 2020;29:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leng L, Cao R, Ma J, et al. Pathological features of COVID‐19‐associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduct Target Ther. 2020;5(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang J, Hume AJ, Abo KM, et al. SARS‐CoV‐2 infection of pluripotent stem cell‐derived human lung alveolar type 2 cells elicits a rapid epithelial‐intrinsic inflammatory response. Cell Stem Cell. 2020;27(6):962‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keles ES. Mild SARS‐CoV‐2 infections in children might be based on evolutionary biology and linked with host reactive oxidative stress and antioxidant capabilities. New Microbes New Infect. 2020;36:100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV. Redox biology of respiratory viral infections. Viruses. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12(9):417‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sabeena Farvin KH, Anandan R, Kumar SH, Shiny KS, Sankar TV, Thankappan TK. Effect of squalene on tissue defense system in isoproterenol‐induced myocardial infarction in rats. Pharmacol Res. 2004;50(3):231‐236. [DOI] [PubMed] [Google Scholar]
- 60. Das B, Antoon R, Tsuchida R, et al. Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin‐induced cytotoxicity in vivo without protecting tumor growth. Neoplasia. 2008;10(10):1105‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tegenge MA, Von Tungeln LS, Mitkus RJ, et al. Pharmacokinetics and biodistribution of squalene‐containing emulsion adjuvant following intramuscular injection of H5N1 influenza vaccine in mice. Regul Toxicol Pharmacol. 2016;81:113‐119. [DOI] [PubMed] [Google Scholar]
- 62. Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14(9):3286‐3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kantipakala R, Bonam SR, Vemireddy S, Miryala S, Halmuthur MS. Squalane‐based emulsion vaccine delivery system: composition with murabutide activate Th1 response. Pharm Dev Technol. 2019;24(3):269‐275. [DOI] [PubMed] [Google Scholar]
- 64. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID‐19: immunology and treatment options. Clin Immunol. 2020;215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


