Abstract
Vaccination for SARS‐CoV‐2 is necessary to overcome coronavirus disease 2019 (COVID‐19). However, the time‐dependent vaccine‐induced immune response is not well understood. This study aimed to investigate the dynamics of SARS‐CoV‐2 antispike immunoglobulin G (IgG) response. Medical staff participants who received two sequential doses of the BNT162b2 vaccination on days 0 and 21 were recruited prospectively from the Musashino Red Cross Hospital between March and May 2021. The quantitative antispike receptor‐binding domain (RBD) IgG antibody responses were measured using the Abbott SARS‐CoV‐2 IgGII Quant assay (cut off ≥50 AU/ml). A total of 59 participants without past COVID‐19 history were continuously tracked with serum samples. The median age was 41 (22–75) years, and 14 participants were male (23.7%). The median antispike RBD IgG and seropositivity rates were 0 (0–31.1) AU/ml, 0.3 (0–39.5) AU/ml, 529.1 (48.3–8711.4) AU/ml, 18,836.9 (742.2–57,260.4) AU/ml, and 0%, 0%, 98.3%, and 100% on days 0, 3, 14, and 28 after the first vaccination, respectively. The antispike RBD IgG levels were significantly increased after day 14 from vaccination (p < 0.001) The BNT162b2 vaccination led almost all participants to obtain serum antispike RBD IgG 14 days after the first dose.
Keywords: COVID‐19, mRNA vaccine, quantitative antispike RBD IgG, SARS‐Cov‐2
Highlights
Highlights The quantitative SARS‐Cov‐2 anti‐spike receptor‐binding domain (RBD) IgG antibody responses were measured in the 59 medical staff participants who received two sequential doses (day 0, 21) of the BNT162b2 vaccination. The quantitative anti‐spike RBD IgG antibody responses were measured using the Abbott SARS‐CoV‐2 IgGⅡ Quant assay (cut off ≥50 AU/mL). The median anti‐spike RBD IgG and seropositivity rates were 0 (0–31.1) AU/mL, 0.3 (0–39.5) AU/mL, 529.1 (48.3–8711.4) AU/mL, 18836.9 (742.2–57260.4) AU/mL, and 0%, 0%, 98.3%, and 100% on days 0, 3, 14, and 28 after the first vaccination, respectively.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) was firstly reported in Wuhan, China, in December 2019, and the outbreak of COVID‐19 has become a big threat. In severe COVD‐19 cases, infection causes pneumonia, severe acute respiratory syndrome, kidney failure, and even death. 1 , 2 , 3 , 4 As of May 16, 2021, more than 160 million people had COVID‐19, and more than 3 million deaths had been reported globally. 5
Insufficient SARS‐CoV‐2 pandemic control required the rapid development of vaccines. Several vaccines, including adenoviral‐vectored, protein subunit, and whole‐cell inactivated virus vaccines, have now reported efficacy in phase III trials and have received emergency approval in many countries. 6 In recent years, modified methods of the synthetic messenger RNA (mRNA) paved the way to the efficient use of RNA vaccines. 7 , 8 A novel development in vaccine formulation generated two mRNA technology vaccines; BNT162b2 by BioNTech/Pfizer 9 and mRNA‐1273 by Moderna. 10 SARS‐CoV2 particles have four main structures: spike protein (S), nucleocapsid protein (N), envelope protein (E), and membrane protein (M). Of these, what is called a neutralizing antibody is essentially an immunoglobulin G (IgG) antibody against a spike protein. 11 SARS‐CoV2 has an infection pattern with invasion into cells expressing angiotensin‐converting enzyme type 2 (ACE2) via S protein. SARS‐CoV‐2 adheres to target cells by fusing ACE2 with the receptor‐binding domain (RBD) region located at the tip of the S protein. 12 The representative Pfizer vaccines were known as BNT162b1 and BNT162b2. The former is designed for the RBD region only, and the latter is designed for the entire sequence of the S protein. 13 Early studies on mRNA vaccines from BioNTech/Pfizer and Moderna showed high efficacy and safety. 9 , 10 Also, more promising reports on the effectiveness of the vaccines after completing the full vaccination schedule (prime and boost dose) have been published. 14 , 15 , 16 The vaccine's effectiveness in preventing death from COVID‐19 was 72% for days 14–20 after the first dose. 14
Although high efficiency was observed in clinical trials and initial real‐situation vaccinations, the first published studies with Pfizer–BioNTech mRNA vaccines have reported weaker immune responses and a higher number of nonresponders among older people after the single and second dose of the BNT162b2 vaccine. 17 , 18 Conversely, current vaccines cause an immune response to viral spike antigens, antispike antibodies, associated with neutralizing activity. 19 , 20 , 21 The antibodies against RBD of SARS‐CoV‐2 protein have been shown to inhibit interactions with ACE2, suggesting that RBD is an attractive target and marker for vaccination. However, the characteristics and the dynamics have not been well clarified.
The measurement of the dynamics of the immune response may provide a potential surrogate marker of protection. In the present study, we analyzed the dynamics of quantitative SARS‐CoV‐2 antispike RBD IgG response to BNT162b2 mRNA vaccination.
2. METHODS
2.1. Samples
The Pfizer–BioNTech BNT162b2 vaccine 9 was predominately provided to the medical staff at an acute hospital in Japan. The medical staff vaccination program started in March 2021 at the Musashino Red Cross Hospital. The protocol was a two‐step vaccination with a procedure in which the first vaccination is followed by a second vaccination with the same dose 21 days later as previously reported. 9 The medical staff was invited to participate in the study. Participants signed an informed consent form agreeing with sampling and usage of their clinical data. Blood samplings were performed before the first dose of the vaccine (baseline), at 3 days (time point 1), 14 days (time point 2), and 28 days after the first dose (time point 3). Participants who failed to take a blood test at least once were not included in this study. A total of 59 participants with every serum sample were tested for serum antibody assay.
2.2. Serum antibody assay
Serum samples were analyzed for the antibodies to SARS‐CoV‐2 S‐RBD IgG using quantitative SARS‐CoV‐2 IgG QN chemiluminescent microparticle immunoassay on an ARCHITECT i2000SR analyzer (Abbott Laboratories). The assay cutoff is ≥50 AU/ml, with linear quantification of detected results from 50 to 40,000 AU/ml reported by the manufacturer protocol (reference number 06S61; Abbott Laboratories). The specificity and sensitivity are 99.6% (95% confidence interval [CI], 99.20–99.80) and 100.0% (95% CI 95.72–100.00), respectively. A study was conducted to evaluate dilutions of the first World Health Organization (WHO) International Standard for anti‐SARS‐CoV‐2 immunoglobulin (human) (NIBSC Code 20‐136) with the SARS‐CoV‐2 IgG II Quant assay calibrated with Abbott internal reference calibrators. Serial dilutions were created using the prepared WHO standard and human recalcified plasma, negative for SARS‐CoV‐2. Samples were tested in replicates of seven using one lot each of SARS‐CoV‐2 IgG II Quant reagents, calibrators, and controls on one ARCHITECT i2000SR instrument. A linear regression analysis was performed regressing the mean observed concentration results (AU/ml) versus the expected WHO International Standard concentrations (binding antibody unit per milliliter [BAU/ml]) using samples with concentrations (BAU/ml = 0.142 × AU/ml). Furthermore, this assay can detect virus variants such as B.1.1.7, B.1.351, and P.1. 22 At the same time course, IgG against SARS‐CoV‐2 nucleocapsid (N) protein was also measured.
2.3. Statistical analysis
The χ 2 and Fisher's exact tests were used to compare categorical variables. The changes in continuous variables were analyzed using the one‐way analysis of variance (ANOVA) test. The threshold for statistical significance was set at p < 0.05. The GraphPad Prism software (GraphPad Software) was used for data analysis.
2.4. Ethical statement
This study was approved by the Ethical Committee of the Musashino Red Cross Hospital and was conducted according to the Declaration of Helsinki (Confirmation No.: 2103).
3. RESULTS
The median age of the 59 participants was 41 (22–75) years, and 14 of the patients were male (23.7%). There were 39 (66.1%) laboratory staff and 20 (33.9%) office workers. They all had no past history of COVID‐19. To ensure that no asymptomatic COVID‐19 participants were included, we measured RBD antibody titers on days 0 and 3 after the first vaccination and the serum IgG against SARS‐CoV‐2 N protein. If asymptomatic COVID‐19 participants were included, some of these assays would become positive. However, there were no positives for anti‐N IgG at all time points.
As shown in Table 1 and Figure 1, the median serum antispike RBD IgG was dependent on the days from vaccination. The median antispike RBD IgG were 0 (0–31.1), 0.3 (0–39.5), 529.1 (48.3–8711.4), and 18,836.9 (742.2–57,260.4) on days 0, 3, 14, and 28, respectively, after the first vaccination. The seropositive rates were 0%, 0%, 98.3%, and 100%, respectively.
Table 1.
The characteristics of study participants and antispike RBD IgG by gender and age
| All (n = 59) | |
|---|---|
| Male/female (n, %) | 14 (23.7%)/45 (76.3%) |
| Age (years) | 41 (22–75) |
| Occupation laboratory staff/office worker (n, %) | 39 (66.1%)/20 (33.9%) |
| Past history of COVID19 (n, %) | 0 (0%) |
| Past history of other diseases (n, %) | 19 (32.2%) |
| Gender | Day 0 (AU/ml) | Day 3 (AU/ml) | Day 14 (AU/ml) | Day 28 (AU/ml) |
|---|---|---|---|---|
| Male (n = 14) | 0.4 (0–6.9) | 0 (0–4.8) | 436.2 (78.2–1272.9) | 21,339.2 (4582.7–57,260.4) |
| Female (n = 45) | 0 (0–31.1) | 0.7 (0–39.5) | 536.6 (48.3–8711.4) | 18,439.4 (742.2–47,427.6) |
| p value | 0.563 | 0.249 | 0.52 | 0.407 |
| Age (years) | Day 0 (AU/ml) | Day 3 (AU/ml) | Day 14 (AU/ml) | Day 28 (AU/ml) |
|---|---|---|---|---|
| 21–30 (n = 15) | 0 (0–3.9) | 2.3 (0–39.5) | 424.7 (114.0–4428.9) | 19,199.0 (11,747.4–52,607.0) |
| 31–40 (n = 14) | 0 (0–6.9) | 0 (0–4.8) | 574.7 (78.2–2778.4) | 24,274.7 (742.2–57,260.4) |
| 41–50 (n = 14) | 0.5 (0–19.4) | 1.1 (0–18.7) | 543.2 (78.3–2576.3) | 19,215.5 (754.4–35,613.5) |
| 51–60 (n = 14) | 0.5 (0–31.1) | 0.4 (0–35.2) | 349.2 (76.0–8711.4) | 15,592.7 (8892.8–38,897.2) |
| 61–75 (n = 2) | 0 (0) | 0.65 (0–1.3) | 463.9 (48.3–879.5) | 16,214.1 (13,591.3–18,836.9) |
| p value | 0.547 | 0.498 | 0.549 | 0.523 |
| Age (years) | Day 0 (AU/ml) | Day 3 (AU/ml) | Day 14 (AU/ml) | Day 28 (AU/ml) |
|---|---|---|---|---|
| Male | ||||
| 21–30 (n = 3) | 0.7 (0–2.7) | 0 (0–0.1) | 424.7 (405.3–447.7) | 19,199.0 (16,199.7–52,607.0) |
| 31–40 (n = 7) | 0 (0–6.9) | 0 (0–4.8) | 563.2 (78.2–1272.9) | 23,479.4 (7952.4–57,260.4) |
| 41–50 (n = 3) | 2.7 (0–5.1 | 2.6 (0.3–4.4) | 233.5 (183.2–794.2) | 6489.4 (4582.7–33,389.8) |
| 51–60 (n = 1) | 1.8 | 0 | 124.5 | 25,463.7 |
| 61–75 (n = 0) | ND | ND | ND | ND |
| p value | 0.736 | 0.226 | 0.407 | 0.529 |
| Age (years) | Day 0 (AU/ml) | Day 3 (AU/ml) | Day 14 (AU/ml) | Day 28 (AU/ml) |
|---|---|---|---|---|
| Female | ||||
| 21–30 (n = 12) | 0 (0–3.9) | 2.8 (0–39.5) | 513.5 (114.0–4428.9) | 17,798.4 (1747.4–47,427.6) |
| 31–40 (n = 7) | 0 (0–4.1) | 0 (0–3.1) | 586.2 (282.6–2778.4) | 24,314.1 (742.2–37,351.1) |
| 41–50 (n = 11) | 0 (0–19.4) | 0.9 (0–18.7) | 549.9 (78.3–2576.3) | 22,163.8 (754.4–35,613.5) |
| 51–60 (n = 13) | 0.2 (0–31.1) | 0.6 (0–35.2) | 386.1 (76.0–8711.4) | 14,573.0 (8892.8–38,897.2) |
| 61–75 (n = 2) | 0 (0) | 0.7 (0–1.3) | 463.9 (48.3–879.5) | 16,214.1 (13,591.3–18,836.9) |
| p value | 0.681 | 0.498 | 0.648 | 0.681 |
Abbreviation: COVID19, coronavirus disease 2019.
Figure 1.
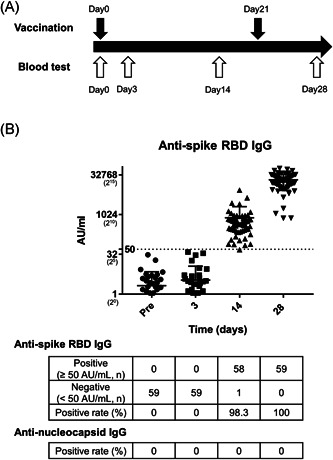
Dynamics of SARS‐CoV‐2 antispike RBD IgG response after vaccination. (A) Schema of the schedule for vaccination and blood test. (B) Antispike RBD IgG titer (AU/ml) and seropositive rate of antispike RBD IgG and antinucleocapsid IgG in a time‐dependent manner. RBD, receptor‐binding domain
The RBD IgG levels were significantly increased after day 14 from vaccination (p < 0.0001, one‐way ANOVA test). In our cohort, there were no significant differences when compared with gender or age. However, a trend was observed for younger participants; the median RBD IgG titer of under 50 years old (n = 43) tended to be higher on day 14 (median, 549.7/349.3, p = 0.137) and day 28 (median, 22,163/15,592, p = 0.264) as compared with over 50 years old (n = 16) (Table 1).
4. DISCUSSION
This study provides evidence regarding the dynamics of quantitative SARS‐CoV‐2 antispike RBD IgG response to BNT162b2 mRNA vaccination. BNT162b2 mRNA vaccination led the antibodies of almost all participants to seropositive after the first vaccination.
In this study with observation for 28 days, the median RBD IgG levels on day 28 were the highest (18,836.9 (742.2–57,260.4), p < 0.001). Conversely, the IgG levels on day 3 were not significantly changed from day 0 (p = 0.82). This result supported the previous report that estimated the effectiveness in preventing death from COVID‐19 for days 14–20 after the first vaccination. 14 In terms of characteristics of participants, there were no significant differences when compared with gender or age with IgG procedure. Muller et al. 17 reported that background differences between the antibody responses were raised after the first and second BNT162b2 vaccinations, at particular lower frequencies of neutralizing antibodies in the elderly group (>80 years of age). As a premise, physical binding such as RBD and neutralization assays are different and cannot be compared. If we had enough same‐generation participants, referential data regarding RBD might have been given. Our cohort did not include such elderly groups, and most of the participants (56/59) were younger (<60 years of age). Although there was no significant difference between those under and over 50 years old, the antibody titer of under 50 years old tended to be numerically higher on days 14 and 28. Such an analysis including more older people is also expected.
This study has certain novel points. First, we were tracking the serum IgG data of the same person over time. Furthermore, our tracking data included the day 3 point. Previously, we investigated the plasma anti‐SARS‐CoV‐2 antibody for the patients infected with COVID‐19. 23 We could exclude the fact that every participant was either infected or was in the process of producing antibodies by measuring RBD antibody titers on days 0 and 3 after the first vaccination and the serum IgG against SARS‐CoV‐2 N protein.
The present study had several limitations. First, although the study showed the dynamics of the SARS‐CoV‐2 antispike RBD IgG, this result was found in only Japanese medical staff populations. They may not be adaptable to racial differences and other populations. Second, the observation period and number of patients may be insufficient for solid comparisons. Our study results were at the same timing or comparison of samples derived from the same patients in a time‐dependent manner. Further long‐term observational studies enrolling large numbers of participants are required, including side effects and prevention effects for COVID‐19. Third, this study had no control group. After we understood that asymptomatic COVID‐19 patients cannot be ruled out by interview alone, we tried to exclude that each participant was infected. If asymptomatic COVID‐19 participants were included, RBD IgG on days 0 and 3 and the anti‐N IgG. would become positive. As a result of this study, there were no positives in those assays. We finally considered no asymptomatic COVID 19 participants included.
Given that the degree of qualitative antispike RBD results is a surrogate marker of protection against infection, other endpoints of interest such as hospitalization and death remain unclear.
In conclusion, BNT162b2 mRNA vaccination led to almost 100% seropositive SARS‐CoV‐2 antispike RBD IgG after 14 days. The antispike RBD IgG levels were significantly increased from days 14 to 28. SARS‐CoV‐2 antispike RBD IgG response may contribute to understanding the immune response and the appropriate prevention of COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study concept and design: Shun Kaneko, Masayuki Kurosaki, and Namiki Izumi. Acquisition of data: Shun Kaneko, Toru Sugiyama, Yuka Takahashi, Yoshimi Yamaguchi, and Masayuki Nagasawa. Analysis and interpretation of data: Shun Kaneko, Masayuki Kurosaki, and Namiki Izumi. Drafting of the manuscript: Shun Kaneko, Masayuki Kurosaki, and Namiki Izumi. Statistical analysis: Shun Kaneko and Masayuki Kurosaki. Study supervision: Namiki Izumi. Final approval: All of the authors. Agreement to be accountable for all aspects of the work: All of the authors.
ACKNOWLEDGMENTS
The authors appreciate the cooperation of the COVID‐19 countermeasure team, support staff, and their families at Musashino Red Cross Hospital.
Kaneko S, Kurosaki M, Sugiyama T, et al. The dynamics of quantitative SARS‐CoV‐2 antispike IgG response to BNT162b2 vaccination. J Med Virol. 2021;93:6813‐6817. 10.1002/jmv.27231
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Virol. 2020;127:104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Coronavirus disease (COVID‐19). Situation report. Accessed, May 17th, 2021.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 6. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine‐induced protection against COVID‐19 in humans. Nat Rev Immunol. 2021:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuller DH, Berglund P. Amplifying RNA vaccine development. N Engl J Med. 2020;382(25):2469‐2471. [DOI] [PubMed] [Google Scholar]
- 8. Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol. 2020;65:14‐20. [DOI] [PubMed] [Google Scholar]
- 9. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poland GA, Ovsyannikova IG, Crooke SN, Kennedy RB. SARS‐CoV‐2 vaccine development: current status. Mayo Clin Proc. 2020;95(10):2172‐2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verbeke R, Lentacker I, De Smedt SC, Dewitte H. The dawn of mRNA vaccines: the COVID‐19 case. J Control Release. 2021;333:511‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amit S, Regev‐Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS‐CoV‐2 infection and COVID‐19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller L, Andrée M, Moskorz W, et al. Age‐dependent immune response to the Biontech/Pfizer BNT162b2 COVID‐19 vaccination. Clin Infect Dis. 2021. Published online April 27, 2021. 10.1093/cid/ciab38 [DOI] [Google Scholar]
- 18. Abu Jabal K, Ben‐Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID‐19 vaccine: real‐world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370(6521):1227‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. An evaluation of COVID‐19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11(1):3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodgers MA, Batra R, Snell LB, et al. Detection of SARS‐CoV‐2 variants by Abbott molecular, antigen, and serological tests. medRxiv. 2021. 10.1101/2021.04.24.21256045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaneko S, Nukui Y, Arashiro T, et al. Clinical validation of an immunochromatographic SARS‐Cov‐2 IgM/IgG antibody assay with Japanese cohort. J Med Virol. 2021;93(1):569‐572. [DOI] [PubMed] [Google Scholar]


