Abstract
Aims
To explore the correlation between cardiac‐related comorbidities, cardiac biomarkers, acute myocardial injury, and severity level, outcomes in COVID‐19 patients.
Method
Pubmed, Web of Science, Embase, CNKI, VIP, Wanfang, Cochrane Library databases, medRxiv, and Sinomed were reviewed systemically. Various types of clinical research reporting cardiac‐related comorbidities, cardiac biomarkers including lactate dehydrogenase (LDH), troponin I (TnI), high sensitivity troponin I (hs‐TnI), creatine kinase (CK), creatine kinase–MB (CK‐MB), myoglobin (Myo), N‐terminal pro‐b‐type natriuretic peptide (NT‐proBNP) and acute cardiac injury grouped by severity of COVID‐19 were included. Outcome measures were events and total sample size for comorbidities, acute cardiac injury, and laboratory parameters of these biomarkers. The study was performed with Stata version 15.1.
Results
Seventy studies, with a total of 15,354 cases were identified. The results showed that COVID‐19's severity was related to cardiovascular disease. Similar odds ratios (ORs) were achieved in hypertension except for severe versus critical group (OR = 1.406; 95% CI, 0.942–2.097; p = .095). The relative risk (RR) of acute cardiac injury is 7.01 (95% CI, 5.64–8.71) in non‐survivor cases. When compared with the different severity of cardiac biomarkers, the pool OR of CK, CK‐MB, TnI, Myo and LDH were 2.683 (95% CI, 0.83–8.671; p = .106; I 2 = 0%), 2.263 (95% CI, 0.939–5.457; p = .069), 1.242 (95% CI, 0.628–2.457; p = .534), 1.756 (95% CI, 0.608–5.071; p = .298; I 2 = 42.3%), 1.387 (95% CI, 0.707–2.721; p = .341; I 2 = 0%) in the critical versus severe group, whose trends were not similar to other groups. The standard mean differences (SMD) of CK and TnI in the critical versus severe group were 0.09 (95% CI, −0.33 to 0.50; p = .685; I 2 = 65.2%), 0.478 (95% CI, −0.183 to 1.138; p = .156; I 2 = 76.7%), which means no difference was observed in the serum level of these indicators.
Conclusion
Most of the findings clearly indicate that hypertension, cardiovascular disease, acute cardiac injury, and related laboratory indicators are associated with the severity of COVID‐19. What is now needed are cross‐national prospectively designed observational or clinical trials that will help improve the certainty of the available evidence and treatment decisions for patients.
Keywords: acute myocardial injury, cardiac biomarkers, comorbidities, COVID‐19, meta‐analysis, SARS‐CoV‐2
We use a meta‐analysis to explore the correlation between cardiac‐related comorbidities, cardiac biomarkers, acute myocardial injury, and severity level, outcomes in patients with COVID‐19. Most of the findings clearly indicate that hypertension, cardiovascular disease, acute cardiac injury, and related laboratory indicators are associated with the severity of COVID‐19. What is now needed are cross‐national prospectively designed observational or clinical trials that will help improve the certainty of the available evidence and treatment decision for patients.

1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), a severe respiratory disease, has caused an unprecedented pandemic crisis. As of August 1, 2020, a total of 17,396,943 cases of COVID‐19 infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), 675,060 deaths have been confirmed by the WHO, and nearly 300 thousand new cases were reported in every 24 h all around the world. SARS‐CoV‐2 is approximately 80% gene sequence similarity to SARS‐CoV, 1 , 2 , 3 which infected host human cells by binding to the receptor proteins, known as angiotensin‐converting enzyme 2 (ACE2) receptor. And the disease transmission eventually contributed to a pneumonia epidemic outbreak in 2003. 4 , 5 ACE2 receptor is highly expressed in multiple organ systems 6 and plays an essential negative role in the ACE‐angiotensin II (Ang II)‐angiotensin II receptor type 1 (AT1R) pathway called the classical renin‐angiotensin‐aldosterone system (RAAS) axis, whose positive effect can increase sympathetic nervous system tension. 7
According to the current observation, it is the cardiovascular complications and myocardial injury that should be paid close attention to, while most of the attention has been paid to the pulmonary system. The relationship between coronavirus disease 2019 and clinical characters has been much more precise. Firstly cardiovascular diseases, including coronary heart disease and hypertension, can increase the risks for adverse outcomes such as the rate of critical situations and death. 8 Secondly, a notable proportion of patients experience cardiovascular symptoms, and myocardial injury indicators change at the initial presentation. Several recent studies have described the clinical characteristics and epidemiological findings of COVID‐19 that were tightly implicated in the cardiovascular system. 9 , 10 , 11 Nevertheless, due to the mounting clinical research, further and broader elucidation can be carried out. Here we summarize the current literature to explore the correlation between cardiac‐related comorbidities, cardiac biomarkers, acute myocardial injury, and severity level or outcomes in COVID‐19 patients.
2. METHODS
2.1. Search strategy
Our review followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) recommendations and criteria. 12 We conducted a literature retrieval by two independent reviewers without language restrictions (Cai Q and Zhang L) in Pubmed, Web of Science, Embase, CNKI, Wanfang, Cochrane Library databases, VIP, medRxiv, and Sinomed using “SARS‐CoV⁃2,” “COVID‐19,” and "2019⁃nCoV" from December 1, 2019, to June 27, 2020. All relevant research should report the outcome including cardiac‐related comorbidities and cardiac biomarkers, including lactate dehydrogenase (LDH), troponin I (TnI), high sensitivity troponin I (hs‐TnI), creatine kinase (CK), creatine kinase–MB (CK‐MB), myoglobin (Myo), N‐terminal pro‐b‐type natriuretic peptide (NT‐proBNP) or acute cardiac injury. Additionally, extra searches were performed in the title or abstract that reported “clinical characteristics” or “clinical data.” Studies that report cardiac biomarkers or acute cardiac injury but did not classify by patients' severity level or outcomes of COVID‐19 were excluded.
2.2. Selection criteria
Two independent reviewers (Zhengchuan Zhu and Miaoran Wang) scrutinized all titles and abstracts excluded irrelevant studies. Then they independently reviewed the full reports. Studies were eligible for inclusion if they met the following conditions: (1) study types: retrospective, prospective, cross‐sectional, observational, descriptive or case‐control studies which report cardiac‐related comorbidities, cardiac biomarkers (including CK, CK‐MB, TnI, Myo or NT‐proBNP, LDH) or patient suffered from acute cardiac injury with COVID‐19; (2) patient characteristics: patients should be diagnosed with COVID‐19 and grouped into moderate cases, severe cases or critical cases according to Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (trial version 7) from China or WHO interim guidance 13 ; (3) interventions: the study should include at least one cohort data for severe versus non‐severe, severe versus moderate, severe versus non‐severe, severe versus critical, or non‐survivor versus survivor cohorts; and (4) outcome: the mean (standard deviation SD) or median (interquartile range; IQR) for each laboratory parameters of these biomarkers should be involved as continuous outcomes. And the event and total sample size for comorbidities and acute cardiac injury should be involved as dichotomous outcomes.
The exclusion criteria were: (1) study types: case reports, reviews, editorial materials, conference abstracts, and summaries of discussions, (2) patient characteristics: patients in a specific condition such as those who were pregnant (3) recruiters were infected by other coronaviruses, including the severe acute respiratory syndrome (SARS) or the Middle East respiratory syndrome (MERS) human coronavirus or (4) study with insufficient data information in the event.
2.3. Data extraction
Independent reviewers (Qiaoyan Cai, Ling Zhang) extracted data into a predesigned sheet and the results were checked by each reviewer. In case of disagreements, a consensus was reached through discussion or another reviewer (Zhengchuan Zhu) examination. Extracted data included: the name of the author, date of publication for the articles, name of the journal, study design, sample size, the diagnostic criteria, the severity of the disease, cardiac‐related comorbidities, and cardiac biomarkers. Mean and standard deviation of indicator values are calculated from the median, interquartile range, and sample size, through Hong Kong Baptist University's website (http://www.math.hkbu.edu.hk/%7Etongt/papers/median2mean.html). The Newcastle‐Ottawa Scale (NOS) quality assessment scale was utilized by two investigators (Zhengchuan Zhu and Miaoran Wang) to assess the quality of studies included in the meta‐analysis. According to these criteria, each study can receive a maximum of nine points. Studies with NOS scores of ≥5 are considered to be high‐quality studies in this systematic review and meta‐analysis.
2.4. Statistical analysis
All patients were divided into four cohorts before conducting the study: severe versus moderate, severe versus critical, severe versus non‐severe, and survivor versus non‐survivor based on the diagnosis of COVID‐19 in every study. To reduce the influence from different detection methods, dichotomous outcomes (e.g., cardiac‐related comorbidities) were extracted as odds ratio (OR) with 95% confidence interval (CI), continuous outcomes were extracted as standard mean difference (SMD) with 95% CI. Summary relative risks (RRs) with 95% CI were carried out for the association between acute cardiac injury and the severity of COVID‐19. Heterogeneity among the included studies was assessed using the Cochran's Q test (p < .05) and the I2 statistic(considered I 2 values <25% to represent low heterogeneity, 25%–50% to moderate heterogeneity, and >50% to severe heterogeneity). The fixed effects models were applied when I 2 ≤ 50% or p ≥ .05, while the random effect models were used by the Mantel‐Haenszel method. To probe the sources of heterogeneity, we employed a leave‐one‐out analysis when I 2 > 50%. We generated funnel plots and Egger's test to examine the possibility of publication bias. Egger's test with p > .05 was considered as funnel symmetry and no publication bias. The statistical analysis was performed with Stata version 15.1 (Stata Corp LP) and GraphPad Prism 7.0 (GraphPad Software).
3. RESULTS
3.1. Included studies
Detailed steps of the literature search process is shown in Figure 1. In total, 5259 potentially relevant publications were found from different databases in the initial search. After removing duplications, 3063 were carefully screened, and 2407 unrelated research were removed. And 389 of them were further excluded after screening titles and abstracts. Relevant articles were further selected by reading the full texts. Among these studies, 102 did not report the cardia injury biomarkers, 61 were not classified by the severity of COVID‐19 cases, 14 did not include clear diagnostic criteria, 14 investigated the pregnant and infant cases, 3 were not considered suitable because of their study design, and one suspected considerable overlapping of patients populations in the same hospital. Finally, 67 articles were included.
Figure 1.
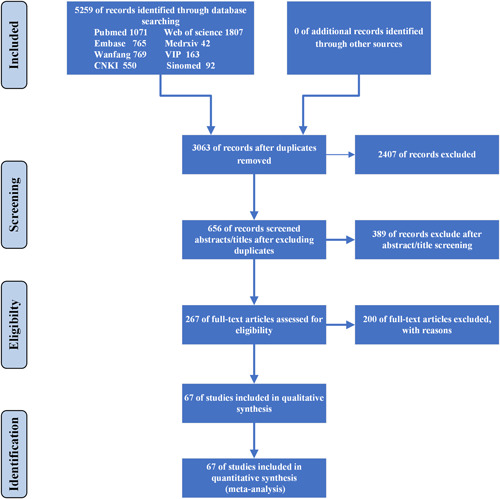
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart of the systematic literature review and article identification
3.2. Baseline characteristics and risk of bias
In total, 67 trails are summarized in the meta‐analysis, and their characteristics are presented in Table 1. A total of 14901 hospitalized patients from four countries were studied in 2020 and the average sample size was 222, which varied from 21 to 1449. The overall average age was greater than 40 years ranged from 1 year to 96 years old and slightly more than half of the sample population was male (51.4%) between 35.48% 22 to 80.9%. 66 The most general cardiac‐related comorbidities were coronary heart disease and hypertension. The quality assessment for the included studies is shown in Table 2, and all included studies were evaluated to be high quality with a NOS score ≥6. The P‐value of Egger's test in funnel plots was shown in Table 3.
Table 1.
The characteristics of the included studies in this meta‐analysis
| Study | Data | Study design | Region and Country | Diagnostic criteria | n | Age(mean ± SD)/median w (IQR25, 75) | Sex(male) | Group |
|---|---|---|---|---|---|---|---|---|
| 14 | 2020/01–2020/02 | Retrospective, observational study | Beijing, China | Trial 6th version protocol from China | 90 | 53.0 ± ″″16.9 | 43 | Mild cases (n = 2)/Moderate cases (n = 55)/severe cases (n = 22)/critical cases (n = 11) |
| 15 | 2020/01/01–2020/02/06 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 463 | 51 (43, 60) | 244 | Moderate cases (n = 282)/severe cases (n = 181) |
| 16 | 2020/01/20–2020/02/10 | Retrospective, observational study | Guangzhou, China | Trial 7th version protocol from China | 66 | Mild cases 38.5 (29, 60.3)/moderate cases 56 (38, 63)/severe cases 57 (55.5, 68.5)/critical cases 66 (60, 83) | 29 | Mild cases (n = 22)/moderate cases (n = 31)/severe cases (n = 8)/critical cases (n = 5) |
| 17 | 2020/01/17–2020/02/20 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 89 | 53.0 ± 16.9 | 41 | Mild cases (n = 18)/moderate cases (n = 40)/severe cases (n = 21)/critical cases (n = 10) |
| 18 | 2020/01/24–2020/02/23 | Retrospective, observational study | Chongqing, China | Trial 6th version protocol from China | 223 | 46.5 ± 16.1 | 105 | Moderate cases (n = 192)/severe cases (n = 31) |
| 19 | 2020/01/21–2020/03/15 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 30 | Death cases 63.6 (median)/survivor cases 65 (median) | 15 | Survivor cases (n = 12)/death cases (n = 18) |
| 20 | 2020/01/13–2020/02/12 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 274 | 62(44, 70) | 171 | Deaths (n = 113)/recovered patients (n = 161) |
| 21 | 2020/01/01–2020/02/21 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 225 | Deaths 69 (62, 74)/recover cases 40 (33, 57) | 124 | Deaths (n = 106)/recovered cases (n = 116) |
| 22 | 2020/01/01–2020/02/18 | Retrospective, observational study | Wuhan, China | WHO interim guidance | 273 | Mild cases 58.95 ± 10.80/severe cases 58.97 ± 14.38/critical cases 57.27 ± 17.25 | 97 | Mild cases (n = 198)/severe cases (n = 60)/critical cases (n = 15) |
| 23 | 2020/01/03–2020/02/24 | Retrospective, observational study | Wuhan, China | Trial 7th version protocol from China | 54 | 68 (59.8, 74.3) | 34 | Death cases (n = 26)/survivor cases (n = 28) |
| 24 | 2020/01/16–2020/02/25 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 95 | 49 (39.0, 58) | 53 | Death cases (n = 6)/survivor cases (n = 89) |
| 25 | 2020/01/05–2020/01/13 | Retrospective, observational study | Wuhan, China | Trial 4th version protocol from China | 34 | Severe cases 63 (58, 69)/critical cases 67 (66, 75) | 17 | Severe cases (n = 26)/critical cases (n = 8) |
| 26 | 2020/01/16–2020/02/20 | Retrospective, observational study | non‐Wuhan area of Hubei Province, China | Quantitative polymerase chain reaction assay | 91 | 46 (median) | 49 | Mild cases (n = 61)/severe cases (n = 30) |
| 27 | before 2020/01/31 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 191 | 56 (46, 67) | 119 | Survivors (n = 137)/non‐survivors (n = 54) |
| 28 | 2020/01/27–2020/02/27 | Retrospective, observational study | Henan, China | Trial 5th version protocol from China | 29 | 56 (31.5, 66) | 14 | Moderate cases (n = 8)/severe cases (n = 21) |
| 29 | 2020/01/08–2020/02/20 | Retrospective, observational study | Wuhan, China | WHO interim guidance | 323 | 61 (23, 91) | 166 | Non‐severe (n = 151)/severe (n = 146)/critical (n = 26) |
| 30 | 2020/01/01–2020/02/15 | Retrospective, observational study | Wuhan, Shanghai, and Anhui, China | Trial 5th version protocol from China | 476 | 53 (40, 64) | 271 | Moderate (n = 352)/severe (n = 54)/critical (n = 70) |
| 31 | 2020/01/01–2020/02/15 | Retrospective, observational study | Wuhan, China | Trial 7th version protocol from China | 25 | 51 (median) | 10 | Non‐severe (n = 16)/severe (n = 9)/; alive (n = 20)/dead (n = 5) |
| 32 | 2020/01/20–2020/02/5 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China and WHO interim guidance | 584 | 60 (48, 69) | 279 | Non‐severe (n = 279)/Severe (n = 269) |
| 33 | 2020/01/20–2020/04/01 | Retrospective, observational study | Wuhan, China | WHO interim guidance and trial 7th version protocol from China | 1449 | 57 (42, 66) | 733 | Alive (n = 1327)/died (n = 122) |
| 34 | 2020/01/21–2020/02/12 | Retrospective, observational study | Beijing, China | Trial 5th version protocol from China | 80 | 53 ± 20 | 38 | Severe (n = 27)/non‐severe (n = 53) |
| 35 | 2020/01/12–2020/02/20 | Retrospective, observational study | Ningbo, China | Trial 6th version protocol from China | 127 | 50.90 ± 15.26 | 54 | Non‐severe cases (n = 111)/severe cases (n = 16) |
| 36 | 2020/01/02–2020/02/10 | Retrospective, observational study | Wuhan, China | WHO interim guidance | 221 | 55 (39.0, 66.5) | 108 | Severe (n = 55)/non‐severe (n = 166) |
| 37 | 2020/01/22–2020/02/10 | Retrospective, observational study | Jiangsu, China | Trial 5th version protocol from China | 202 | 44.0 (33, 54) | 116 | Non‐severe patients (n = 179)/severe patients (n = 23) |
| 38 | 2019/12/11–2020/01/29 | Retrospective, observational study | mainland, China | WHO interim guidance | 1099 | 47.0 (35, 58) | 637 | Non‐severe (n = 926)/severe (n = 173) |
| 39 | 2020/01/24–2020/02/07 | Retrospective, observational study | Chongqing, China | Trial 6th version protocol from China | 114 | 46.05 ± 15.15 | 56 | Mild cases (n = 99)/severe cases (n = 4)/critical cases (n = 11) |
| 40 | 2020/01/20–2020/02/10 | Retrospective, observational study | Guangzhou, China | WHO interim guidance | 275 | 49 (34, 62) | 128 | Non‐severe Patients (n = 230)/severe patients (n = 45) |
| 41 | 2020/01/21–2020/02/25 | Retrospective, observational study | Chongqing, China | Trial 6th version protocol from China | 51 | 60.94 ± 14.87 | 27 | Severe (n = 35)/critical cases (n = 16) |
| 42 | 2020/01/24–2020/03/01 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 382 | 52.0 ± 14.1 | 190 | Moderate cases (n = 285)/severe cases (n = 63)/critical cases (n = 34) |
| 43 | 2020/01/01–2020/03/22 | Retrospective, observational study | Wuhan, China | Trial 7th version protocol from China | 102 | 51.6 ± 19.3 | 75 | Moderate cases (n = 63)/severe cases (n = 29)/critical cases (n = 10) |
| 44 | 2020/03/01–2020/03/30 | Retrospective, observational study | Esine, Brescia, Lombardy, Italy | Real‐time reverse transcriptase polymerase chain reaction | 144 | In‐hospital death 78.0 (64.2, 84.0)/discharged 62.1 (53.0, 72.8) | 96 | In‐hospital death (n = 70)/discharged (n = 74) |
| 45 | 2020/01/01–2020/02/15 | Retrospective cohort study | Wuhan, China | Not mention | 660 | 55.0 (34.0, 68.0) | 295 | Survivor (n = 578)/non‐survivor (n = 82) |
| 46 | 2020/01/01–2020/03/11 | Retrospective, observational study | Zhejiang, China | Trial 7th version protocol from China | 145 | 45.3 ± 13.6 | 79 | Non‐severely ill patients (n = 102)/severely ill patients (n = 43) |
| 47 | 2019/12/30–2020/02/16 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 73 | 58.36 ± 14.31 | 49 | Non‐survivors (n = 47)/survivors (n = 26) |
| 48 | 2020/01/17–2020/02/10 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 54 | 41 (31, 51) | 28 | Died (N = 68)/discharged (N = 82) |
| 49 | 2020/02/19–2020/04/15 | Retrospective, observational study | Daegu, Korea | ARDS Definition Task ForceRanieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. | 110 | 56.9 ± 17.0 | 48 | Severe patients (n = 23)/non‐severe patients (n = 87) |
| 50 | 2020/01/31–2020/03/05 | Retrospective, observational study | Wuhan, China | WHO interim guidance | 101 | 51.0 (37, 61) | 48 | Non‐survivor (n = 15)/survivor (n = 87)/ |
| 51 | 2020/01/10–2020/02/22 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 93 | 51.0 ± 17.5 | 41 | Survivors (n = 68)/non‐survivors (n = 25) |
| 52 | 2020/01/20–2020/02/10 | Retrospective, observational study | Guangzhou, China | Trial 6th version protocol from China | 278 | 48.1 ± 17.0 | 130 | Mild (n = 250)/moderate (n = 211)/severe (n = 28)/critical (n = 14) |
| 53 | 2020/01/10–2020/02/15 | Retrospective, observational study | Hubei, China | Trial 7th version protocol from China | 85 | 59.4 ± 15.3 | 45 | General cases (n = 46)/severe and critical cases (n = 39) |
| 54 | 2020/01/31–2020/02/05 | Retrospective, observational study | Wuhan, China | Trial 7th version protocol from China | 202 | 63 (51, 70) | 88 | non‐survival cases (n = 169)/survival cases (n = 33) |
| 55 | 2020/01/10–2020/03/13 | Retrospective, observational study | 8 provinces, China | Trial 6th version protocol from China | 703 | 46.1 ± 15.2 | 382 | Discharge cases (n = 659)/death cases (n = 33) |
| 56 | 2020/01/30–2020/02/11 | Retrospective cohort study | Huanggang, China | The WHO interim guidance the American Thoracic Society guidelines for community‐acquired pneumonia | 108 | 52 (37, 58) | 43 | Non‐severe (n = 83)/severe‐alive (n = 13)/severe‐dead (n = 12) |
| 57 | 2020/01–2020/02 | Retrospective cohort study | Beijing, China | Trial 6th version protocol from China | 80 | 51.2 ± 17.5 | 33 | Mild disease (n = 56)/severe (n = 24)/died (n = 3) |
| 58 | 2019/12/24–2020/01/28 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 109 | 52.5 ± 10.8 | 48 | Moderate cases (n = 65)/severe and critical cases (n = 44) |
| 59 | 2020/01/22–2020/02/18 | Retrospective, observational study | Anhui, China | Trial 6th version protocol from China | 79 | 45.1 ± 16.6 | 45 | General cases (n = 55)/severe and critical cases (n = 24) |
| 8 | 2020/01/24–2020/02/25 | Retrospective, observational study | Guangzhou, China | Trial 7th version protocol from China | 82 | 44.8 ± 15.6 | 48 | Moderate cases (n = 58)/severe and critical cases (n = 24) |
| 60 | 2020/01/20–2020/02/27 | Retrospective, observational study | Zhuzhou, China | Trial 5th version protocol from China | 80 | 47.8 ± 19 | 40 | Mild cases and moderate cases (n = 63)/severe cases and critical cases (n = 17) |
| 61 | 2020/01/20–2020/02/10 | Retrospective, observational study | Shanghai, China | Trial 6th version protocol from China | 292 | 49.9 ± 16.3 | 154 | Mild cases (n = 271)/severe cases (n = 21) |
| 62 | 2020/01/21–2020/02/11 | Retrospective, observational study | Beijing, China | Trial 6th version protocol from China | 74 | 52.7 ± 19.1 | 35 | Common cases (n = 56)/severe cases (n = 9)/critical severe cases (n = 9) |
| 63 | 2020/02–2020/03 | Retrospective, observational study | Tübingen, Germany | Real‐time reverse transcriptase polymerase chain reaction | 123 | 68 ± 15 | 77 | Non‐survivors (n = 16)/survivors (n = 107) |
| 64 | 2020/01/01–2020/02/23 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 671 | 63 (50, 72) | 322 | Death (n = 62)/survivors (n = 609) |
| 65 | 2020/02/21–2020/03/19 | Retrospective, observational study | Milan, Italy | WHO interim guidance | 233 | 61 (50, 72) | 161 | Survivors (n = 185)/non‐survivors (n = 48) |
| 66 | 2020/1/27 | Retrospective, observational study | Wuhan, China | Trial 6th version protocol from China | 21 | 56 (50.0–65.0) | 17 | Severe cases (n = 11)/moderate cases (n = 10) |
| 67 | 2020/01/01–2020/01/30 | Retrospective, observational study | Wuhan, China | RT‐PCR | 200 | Not mention | 99 | Alive cases (N = 166)/dead cases (N = 34) |
| 68 | 2020/02/01–2020/02/18 | Retrospective, observational study | Wuhan, China | American Thoracic Society guidelines for community acquired pneumonia | 47 | 64.91 (31–87) | 26 | Non‐severe patients (N = 23)/severe patients (N = 24) |
| 69 | 2020/01/10–2020/02/13 | Retrospective, observational study | Wuhan, China | Trial 5th version protocol from China | 204 | 49 (34–62) | 79 | Non‐severe (n = 135)/severe (n = 69) |
| 70 | 2020/01/30–2020/02/25 | Retrospective, observational study | Wuhan, China | Trial 4th version protocol from China | 403 | 56 (39–68) | 193 | Died (n = 100)/recovered (n = 303) |
| 71 | 2020/01/21–2020/03/02 | Retrospective cohort study | Chongqing, China | Trial 5th version protocol from China | 84 | 48 (42.3–62.5) | 48 | Severe (n = 20)/non‐severe (n = 64) |
| 72 | 2020/01/17–2020/02/20 | Retrospective, observational study | Changsha, China | The severe cases of COVID‐19 were defined according to the following criteria: respiratory rate ≥30/min or oxygen saturation ≤93% or PaO2/FiO2 ≤ 300 mmHg or mechanical ventilation or intensive care unit or death. | 242 | 45 (1–84) | 99 | Severe (n = 37)/non‐severe (n = 205) |
| 73 | 2020/01/25–2020/02/25 | Retrospective, observational study | Wuhan, China | WHO interim guideline | 344 | 64 (52–72) | 179 | Severely and critically ill patients survivors (n = 211)/non‐survivors (n = 133) |
| 74 | not mention | Retrospective, observational study | Anhui, China | Trial 5th version protocol from China | 167 | 42.31 (15.29) | 95 | Non‐severe (n = 137)/severe (n = 30) |
| 75 | 2020/01/20–2020/02/20 | Retrospective, observational study | Yancheng, Fuyang, Wuxi China | WHO interim guideline | 280 | 43.12 ± 19.02 | 151 | Mild‐and moderate type patients (n = 197)/severe‐and critically ill type patients (n = 83) |
| 76 | 2020/01/17–2020/02/07 | Retrospective, observational study | Changsha, China | Trial 5th version protocol from China | 161 | 45 (33.5, 57) | 80 | Non‐severe (N = 131)/severe (N = 30) |
| 77 | 2020/01/23–2020/02/08 | Retrospective, observational study | Chongqing, China | Trial 5th version protocol from China | 135 | 47 (36, 55) | 72 | Mild cases (n = 95)/severe cases (n = 40) |
| 78 | 2020/01/31–2020/02/05 | Retrospective, observational study | Wuhan, China | Trial 7th version protocol from China | 202 | 63 (51, 70) | 88 | Survivors (n = 169)/non‐survivors (n = 33) |
| 79 | 2020/03/10–2020/03/30 | Retrospective, observational study | England | RT‐PCR | 95 | 75 (59, 82) | 60 | Survivors (n = 75)/non‐survivors (n = 20) |
Note: The protocol from China: Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (the number of trial version) edited by the National Health Committee General Office; WHO interim guideline:Clinical management of severe acute respiratory infection when Novel coronavirus(nCoV) infection is suspected:2020 interim guidance.
Abbreviations: qPCR, quantitative polymerase chain reaction assay; RT‐PCR, real‐time reverse‐transcriptase polymerase chain reaction.
Table 2.
NEWCASTLE‐OTTAWA quality assessment scale for cross‐sectional studies
| Selection | Comparability | Exposure | Total score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate definition of cases | Representativeness of the cases | Selection of controls | Definition of controls | Control for important factor | Ascertainment of exposure | Same method of ascertainment for cases and controls | Nonresponse rate | ||
| 13 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 14 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 15 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 16 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 17 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 18 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| 19 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 20 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 21 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 22 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 23 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 24 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 25 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 26 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 27 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 28 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 29 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 30 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 31 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 32 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 33 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 34 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 35 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 36 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 37 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 38 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 39 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 40 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 41 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 42 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 43 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 44 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 45 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 46 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 47 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 48 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 49 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 50 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 51 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 52 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 53 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 54 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 55 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 56 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 57 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 58 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 7 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 59 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 60 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 61 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 62 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 63 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 64 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 65 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 66 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 67 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 68 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 69 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 70 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 71 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 72 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 73 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 74 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 75 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 76 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 77 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| 78 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
Table 3.
p value of Egger's test in funnel plots
| Items | p value of Egger's test | ||
|---|---|---|---|
| Dichotomous outcomes | Continuous outcomes | ||
| Severe versus moderate | Hypertension | .97 | – |
| Cardiovascular disease | .137 | – | |
| Acute cardiac damage | – | – | |
| CK | .405 | .571 | |
| CK‐MB | .197 | .421 | |
| LDH | .057 | .37 | |
| Myo | .12 | .709 | |
| TnI | .541 | .661 | |
| NT‐BNP | – | – | |
| Severe versus non‐severe | Hypertension | .708 | – |
| Cardiovascular disease | .109 | – | |
| CK | .091 | .068 | |
| CK‐MB | .059 | .767 | |
| TnI | .604 | .455 | |
| LDH | .255 | .322 | |
| Myo | – | .573 | |
| NT‐BNP | – | .156 | |
| Severe versus critical | Hypertension | .037 | – |
| Cardiovascular disease | .079 | – | |
| CK | – | .85 | |
| CK‐MB | .086 | .923 | |
| LDH | .602 | .354 | |
| TnI | .092 | .074 | |
| Myo | – | .627 | |
| Non‐survior versus survivor | Hypertension | .986 | – |
| Cardiovascular disease | .166 | – | |
| TnI | .445 | .162 | |
| CK | .419 | .052 | |
| CK‐MB | .343 | .693 | |
| NT‐BNP | – | .654 | |
| Acute cardiac injury | .46 | – | |
| LDH | .103 | .254 | |
| Myo | .793 | .088 | |
| Severe versus moderate | Hypertension | .97 | – |
| Cardiovascular disease | .137 | – | |
| Acute cardiac damage | – | – | |
| CK | .405 | .571 | |
| CK‐MB | .197 | .421 | |
| LDH | .057 | .37 | |
| Myo | .12 | .709 | |
| TnI | .541 | .661 | |
| NT‐BNP | – | – | |
| Severe versus non‐severe | Hypertension | .708 | – |
| Cardiovascular disease | .109 | – | |
| CK | .091 | .068 | |
| CK‐MB | .059 | .767 | |
| TnI | .604 | .455 | |
| LDH | .255 | .322 | |
| Myo | – | .573 | |
| NT‐BNP | – | .156 | |
3.3. Efficacy analysis
3.3.1. Cardiac‐related comorbidities
The data on cardiac‐related comorbidities among patients were extracted from 4 groups, then pooled respectively for meta‐analysis. Figure 2 displays an overview of the number of COVID‐19 cases suffered from hypertension in different severities. The results show that there were marked differences for hypertension between severe and moderate cases (OR = 1.53; 95% CI, 1.17–1.99, p = .002; I 2 = 9.1%), severe and non‐severe cases (OR = 1.78; 95% CI, 1.52–2.09; p = 0; I 2 = 57.8%), non‐survivor and survivor cases (OR = 2.07; 95% CI, 1.79–2.40; p = 0; I 2 = 12.9%), while no differences between critical and severe cases (OR = 1.41; 95% CI, 0.94–2.10, p = .00; I 2 = 0%).
Figure 2.
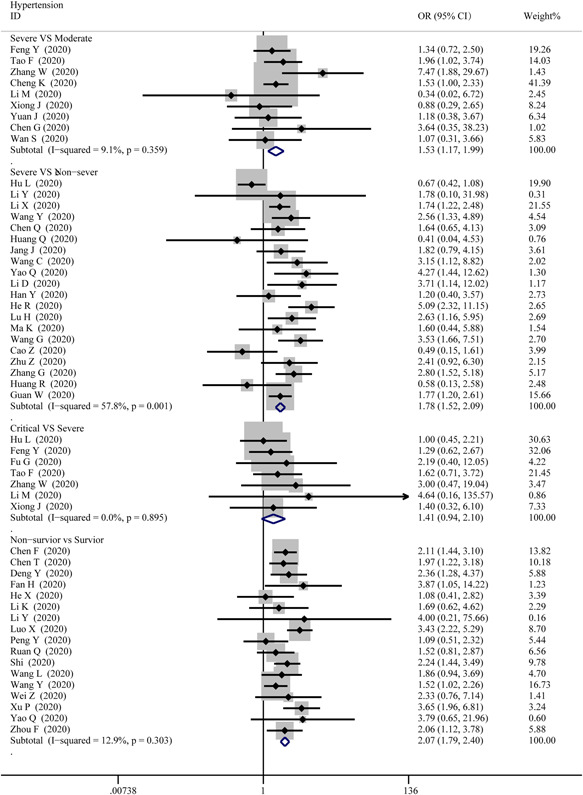
Forest plot comparisons of the number of COVID‐19 cases suffered from hypertension in severe versus moderate, severe versus critical, severe versus non‐severe, and survivor versus non‐survivor group
As presented in Figure 3, cardiovascular disease was significantly associated with the severity level of COVID‐19 in every group. The combined OR were 2.50 (95% CI, 1.64–3.82; p = 0; I 2 = 25%), 3.60 (95% CI, 2.61–4.97; p = 0; I 2 = 25.0%), 2.18 (95% CI, 1.26–3.76; p = .005; I 2 = 0%), 2.75 (95% CI, 2.25–3.36; p = 0; I 2 = 53.6%), respectively. There were no publishing biases based on the results of Egger's test in funnel plots, except for hypertension in the critical versus severe group (p = .037).
Figure 3.
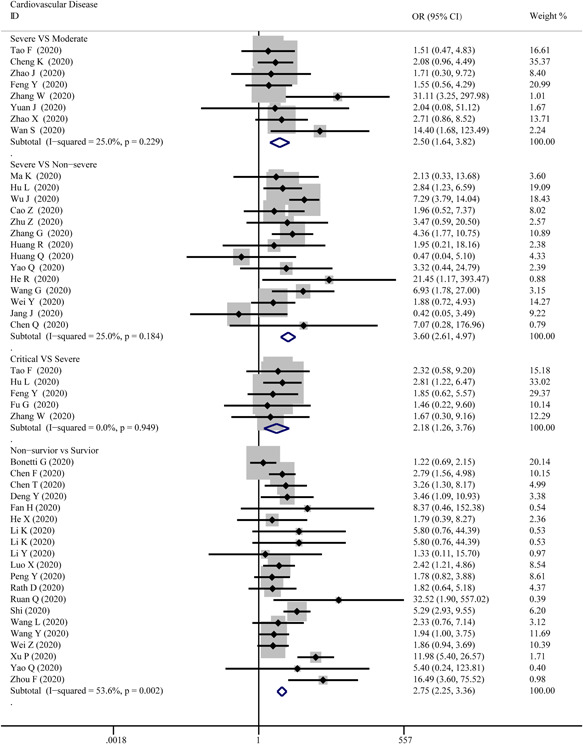
Forest plot comparisons of the number of COVID‐19 cases suffered from cardiovascular disease in severe versus moderate, severe versus critical, severe versus non‐severe, and survivor versus non‐survivor group
3.3.2. Laboratory indicators
-
1.
CK
The abnormal cases and increasing serum levels were all pooled for meta‐analysis. As can be seen from Figure 4, the combined OR were 2.66 (95% CI, 1.554–4.545; p = 0; I 2 = 0%) in the severe versus moderate group, 2.80 (95% CI, 1.86–4.23; p = 0; I 2 = 51.6%) in the severe versus non‐severe group after removing “Guan W 2020,” 2.683 (95% CI, 0.83–8.671; p = .106; I 2 = 0%) in critical versus severe group which means the number of the COVID‐19 patients with increased CK level was no different, and 2.42 (95% CI, 1.81–3.23; p = 0; I 2 = 0%) in the survivor versus non‐survivor group. In Figure 5, the SMD was showed similar trends that: 0.65 (95% CI, 0.27–1.04; p = .001; I 2 = 87.1%) in severe versus moderate group, 0.53 (95% CI, 0.37–0.68; p = 0; I 2 = 60.4%) in severe versus non‐severe group, and 1.19 (95% CI, 0.79–1.59; p = 0; I 2 = 91.7%) in survivor versus the non‐survivor group that higher serum levels of CK were observed in more severe cases except for critical versus severe group in which SMD is 0.09 (95% CI, −0.33 to 0.50; p = .685; I 2 = 65.2%). Sensitivity analysis by removing “Ling Y 2020,” “Li Q 2020” showed that overall estimates did not depend on a single publication in severe versus moderate (SMD = 0.42; 95% CI, 0.28–0.56; p = 0; I 2 = 32.7%) and survivor versus non‐survivor group (SMD = 1.04; 95% CI, 0.78–1.29; p = 0; I 2 = 71.1%) respectively. The funnel plot is presented in Figure 6. There were no publishing biases based on the results of Egger's test in the funnel plot.
-
2.
CK‐MB
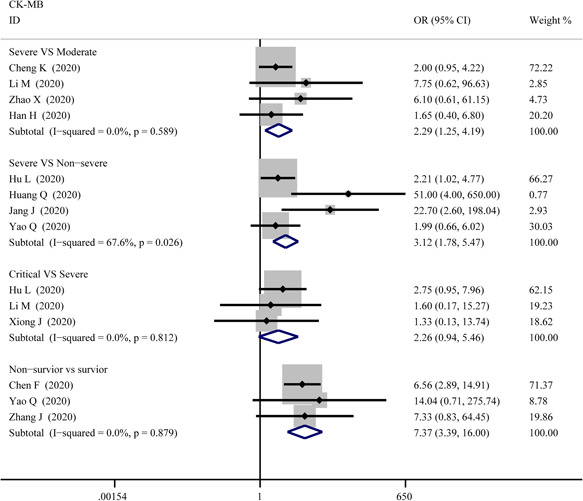
Figure 7 compares the results obtained from the meta‐analysis of CK‐MB. The combined OR were 2.29 (95% CI, 1.25–4.19; p = .007; I 2 = 0%) in severe versus moderate group, 3.12 (95% CI, 1.78–5.47; p = 0; I 2 = 67.6%) in severe versus non‐severe group, and 7.37 (95% CI, 3.39–16.00; p = 0; I 2 = 0%) in survivor versus non‐survivor group which indicated a larger amount cases with higher CK‐MB were in more severe cases whereas no difference in critical versus severe group (OR = 2.26; 95% CI, 0.94–5.46; p = .069; I 2 = 0%). A significantly increased in serum level of CK‐MB was also observed in more severe COVID‐19 cases. The SMD of CK‐MB is the following in Figure 5: 0.53 (95% CI, 0.33–0.72; p = 0; I 2 = 51.8%) in severe versus moderate group, 0.60 (95% CI, 0.29–0.91; p = 0; I 2 = 85%) in severe versus non‐severe group, 0.37 (95% CI, 0.044–0.70; p = .026; I 2 = 55.4%) in critical versus severe group, and 1.49 (95% CI, 0.61–2.37; p = .00; I 2 = 96.9%) in survivor versus non‐survivor group. Sensitivity analysis by removing “Wang G 2020,” “Gao W 2020,” “Shi S 2020” showed that overall estimates did not depend on a single publication in severe versus non‐severe (SMD = 0.70; 95% CI, 0.44–0.96; p = 0; I 2 = 74.8%), critical versus severe (SMD = 0.22; 95% CI, 0.01–0.42; p = .039; I 2 = 0%) and survivor versus non‐survivor group (SMD = 1.10; 95% CI, 0.80–1.41; p = 0; I 2 = 64.1%), respectively. Based on the results of Egger's test, we found no evidence of publication bias in for CK‐MB in all groups (Figure 6).
-
3.
TnI
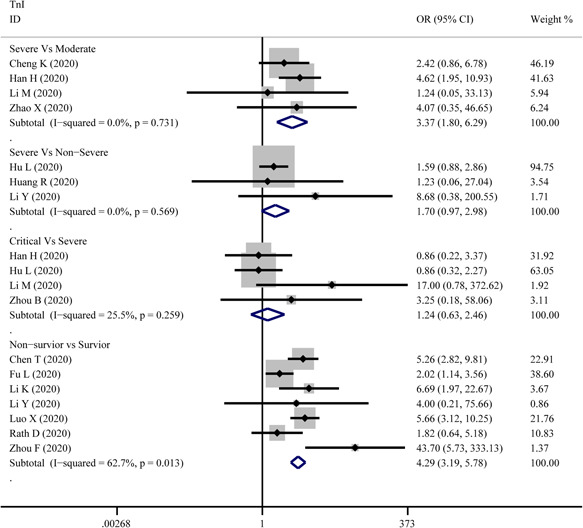
The combined OR of TnI were following in Figure 8 that 3.37 (95% CI, 1.80–6.292; p = 0; I 2 = 0%) in the severe versus moderate group, 1.70(95% CI, 0.97–2.98,p = .066; I 2 = 0%) in severe versus non‐severe group, 1.24 (95% CI, 0.63–2.46, p = .534; I 2 = 25.5%) in critical versus severe group, and 4.29 (95% CI, 3.19–5.78; p = 0; I 2 = 62.7%) in non‐survivor versus survivor group. The result of SMD obtained from critical versus severe group was 0.48 (95% CI, −0.183 to 1.14; p = .156; I 2 = 76.7%) displayed in Figure 5. The results of SMD showed that: 0.839 (95% CI, 0.308–1.371; p = .002; I 2 = 70.5%) in severe versus moderate group, 0.96 (95% CI, 0.784–1.13; p = 0; I 2 = 0.6%) in severe versus non‐severe group, and 1.13 (95% CI, 0.928–1.327; p = 0; I 2 = 76.9%) in survivor versus non‐survivor group after the sensitivity analysis by removing “Ma K 2020,” “Liu C 2020,” “Li Q 2020,” receptively, which means that higher serum levels of TnI were observed in more severe cases. According to the funnel plot in Figures 6 and 9 the results of Egger's test, there were no publish biases were observed.
-
4.
Myo
Figure 10 illustrates the patients of abnormal Myo in different severity The combined OR were 4.39 (95% CI, 2.44–7.91; p = 0; I 2 = 0%) in the severe versus moderate group, 4.36 (95% CI, 2.60–7.299; p = 0; I 2 = 0%) in the survivor versus non‐survivor group, which indicated larger amount cases with higher Myo were in more severe cases, while no difference was found in critical versus severe group (OR = 1.756; 95% CI, 0.608–5.071; p = .29; I 2 = 42.3%). As shown in Figure 5, the SMD of Myo are the following: 0.84 (95% CI, 0.36–1.32; p = .001; I 2 = 87.4%) in severe versus moderate group, 0.67 (95% CI, 0.25–1.086; p = .00; I 2 = 57.9%) in critical versus severe group, 2.09 (95% CI, 0.63–3.556; p = .005; I 2 = 95.8%) in severe versus non‐severe group, and 1.68 (95% CI, 1.23–2.13; p = 0; I 2 = 92.7%) in survivor versus non‐survivor group. Sensitivity analysis by removing “Li M 2020,” “Ling Y 2020,” and “Li Q 2020” showed that overall estimates did not depend on a single publication in severe versus moderate (SMD = 0.63; 95% CI, 0.35–0.91; p = 0; I 2 = 55.6%), severe versus non‐severe (SMD = 0.88; 95% CI, 0.59–1.17; p = 0; I 2 = 0%), and survivor versus non‐survivor group (SMD = 1.53; 95% CI, 1.18–1.88; p = 0; I 2 = 82.4%), respectively. The funnel plot is shown in Figures 6 and 9, respectively. Based on the results of Egger's test, we found no evidence of publication bias for Myo in all groups.
-
5.
NT‐proBNP
The combined OR was 4.285 (95% CI, 2.596–7.072; p = 0; I 2 = 0%) in the survivor versus non‐survivor group presented in Figure 11, which indicated a larger amount of cases with higher NT‐proBNP were in more severe cases. The serum level of NT‐proBNP significantly increased was also observed in more severe COVID‐19 cases. The SMD of NT‐proBNP are the following: 1.33 (95% CI, 0.48–2.17; p = .002; I 2 = 89.3%) in severe versus moderate group, 1.47 (95% CI, 0.71–2.22; p = 0; I 2 = 86%) in severe versus non‐severe group, and 1.50 (95% CI, 1.07–1.92; p = 0; I 2 = 87.2%) in survivor versus non‐survivor group. Sensitivity analysis by removing Shi S study showed that overall estimates did not depend on a single publication in survivor versus non‐survivor group (SMD = 1.35; 95% CI, 1.18–1.52; p = 0; I 2 = 22.3%). The results of the funnel plot are summarized in Figures 6 and 9, respectively. Based on the results of Egger's test, we found no evidence of publication bias in NT‐proBNP in all groups.
-
6.
LDH
Figure 4.
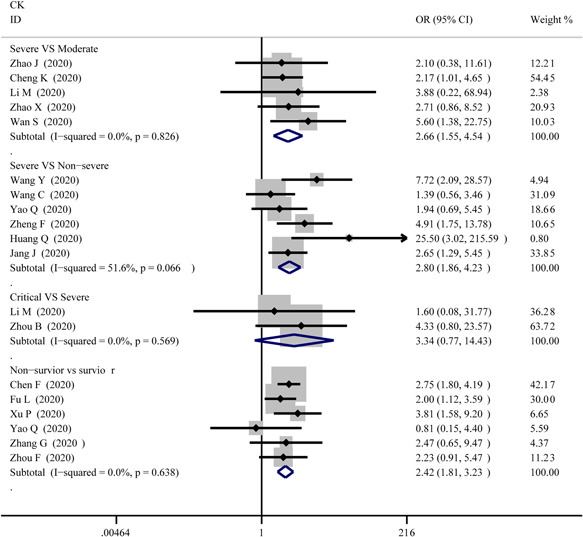
Forest plot comparisons of increasing creatine kinase (CK) in patients with different severity of COVID‐19
Figure 5.
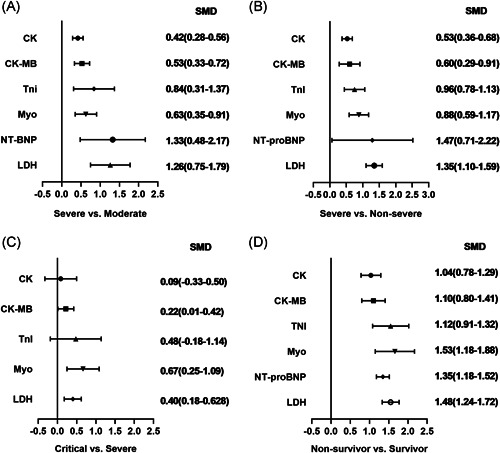
Forest plot comparisons of cardiac biomarkers: (A) severe versus moderate group, (B) severe versus critical group, (C) severe versus non‐severe group, (D) survivor versus non‐survivor group. SMD, standard mean difference
Figure 6.
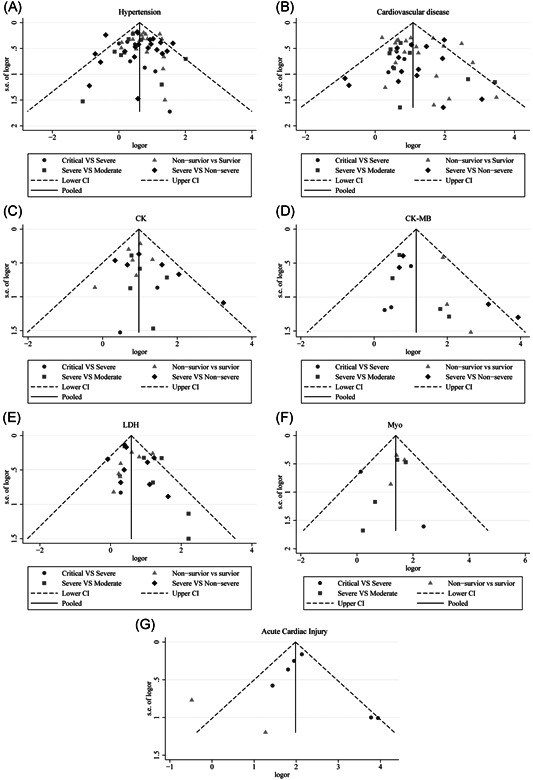
Funnel plot for the assessment of publication bias in dichotomous outcomes: (A) hypertension in different severity and outcome of COVID‐19, (B) cardiovascular disease in different severity and outcome of COVID‐19, (C) CK in different severity and outcome of COVID‐19, (D) CK‐MB in different severity and outcome of COVID‐19, (E) LDH in different severity and outcome of COVID‐19, (F) Myo in different severity and outcome of COVID‐19, (G) TnI in different severity and outcome of COVID‐19
Figure 7.
Forest plot comparisons of increasing creatine kinase–MB (CK‐MB) in patients with different severity of COVID‐19
Figure 8.
Forest plot comparisons of increasing troponin I (TnI) in patients with different severity of COVID‐19
Figure 9.
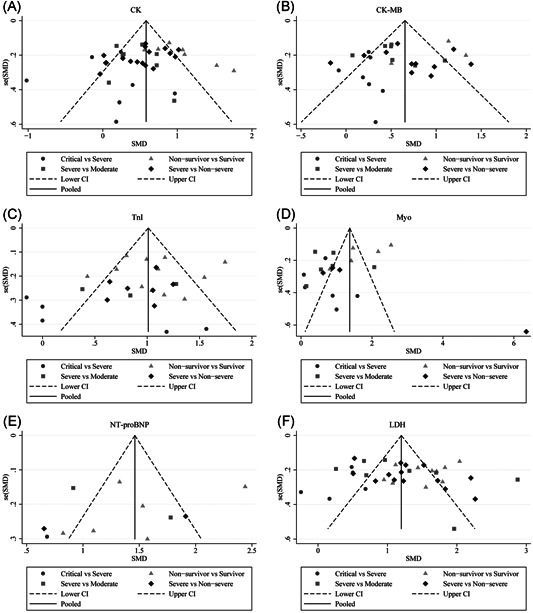
Funnel plot for the assessment of publication bias in continuous outcomes: (A) CK in different severity and outcome of COVID‐19, (B) CK‐MB in different severity and outcome of COVID‐19, (C) TnI in different severity and outcome of COVID‐19, (D) Myo in different severity and outcome of COVID‐19, (E) NT‐proBNP in different severity and outcome of COVID‐19, (F) LDH in different severity and outcome of COVID‐19
Figure 10.
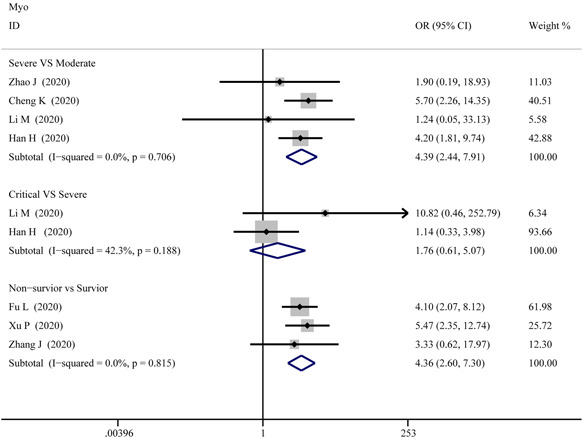
Forest plot comparisons of increasing myoglobin (Myo) in patients with different severity of COVID‐19
Figure 11.
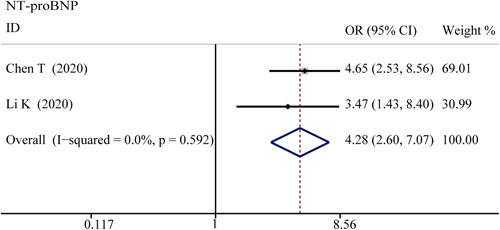
Forest plot comparisons of increasing N‐terminal pro‐b‐type natriuretic peptide (NT‐proBNP) in paitents with different severity of COVID‐19
As displayed in Figure 12, the combined OR were 2.01 (95% CI, 1.57–2.57; p = .025; I 2 = 61.1%) in the severe versus moderate group, 1.62 (95% CI, 1.36–1.93; p = 0; I2 = 39.5%) in the severe versus non‐severe group, and 1.98 (95% CI, 1.52–2.58; p = 0; I 2 = 11.4%) in survivor versus non‐survivor group, whereas, 1.39 (95% CI, 0.71–2.72; p = .34; I 2 = 0%) in the critical versus severe group which means there was no difference among the severity of COVID‐19 cases. The SMD of LDH was showed that higher serum levels of LDH were observed in severe cases (Figure 5). The results are following: 1.26 (95% CI, 0.75–1.78.; p = 0; I 2 = 92.3%) in the severe versus moderate group, 1.28 (95% CI, 1.01–1.56; p = 0; I 2 = 82.6%) in the severe versus non‐severe group, 0.40 (95% CI, 0.18–0.62; p = 0; I 2 = 27.8%) in the critical versus severe group, and 1.48 (95% CI, 1.24–1.72; p = 0; I 2 = 0.76%) in the survivor versus non‐survivor group. Sensitivity analysis by removing “Wu J 2020” showed that overall estimates did not depend on a single publication in severe versus non‐severe (SMD = 1.35; 95% CI, 1.10–1.59; p = 0; I 2 = 74.2%). There were no publishing biases based on the results of Egger's test in funnel plots (Figures 6 and 9).
Figure 12.
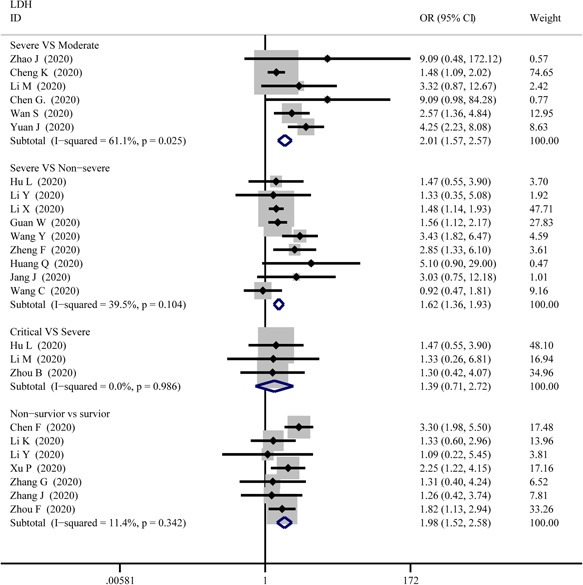
Forest plot comparisons of increasing lactate dehydrogenase (LDH) in patients with different severity of COVID‐19
Figure 13.
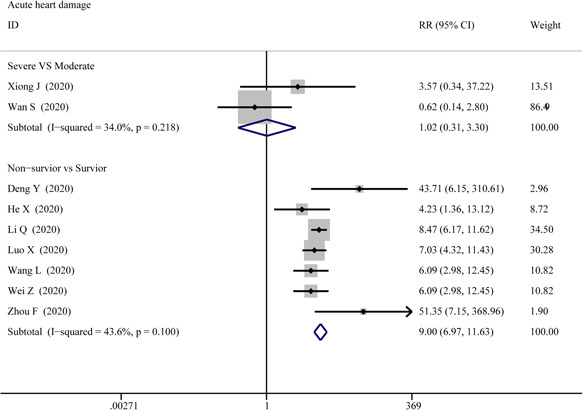
Forest plot comparisons of acute cardiac injury in patients with different severity of COVID‐19
3.3.3. Acute cardiac injury
The data on acute cardiac injury among patients were extracted from 2 groups: the severe versus moderate group and the survivor versus non‐survivor group, then pooled respectively for meta‐analysis. The results are set out in Figure 12 that there were marked differences for acute cardiac injury between survivor versus non‐survivor cases (RR = 7.01; 95% CI, 5.64–8.71; p = 0; I 2 = 68.3%), while no differences between severe versus moderate cases (RR = 1.017; 95% CI, 1.017–0.313; p = .978; I 2 = 34%). Sensitivity analysis by removing “Chen T 2020” showed that overall estimates did not depend on a single publication in survivor versus non‐survivor group (RR = 9.005; 95% CI, 6.974–11.626; p = 0; I 2 = 43.6%). Based on the results of Egger's test, we found no evidence of publication bias for acute cardiac injury in all groups.
4. DISCUSSION
SARS‐CoV‐2, the third newly severe epidemic coronaviruses that have led to significant outbreaks and caused a big challenge after the SARS‐CoV occurred in 2002 and the MERS‐CoV that was identified in 2012. 3 No pre‐existing immunity and definitive treatments can protect people, especially older adults and vulnerable members of the community, with prevalent comorbidities. 80 , 81 , 82 Cardiac‐related comorbidities and myocardial damage have been identified in a non‐negligible number of COVID‐19 patients, and the interpretation of these features based on the COVID‐19's severity is essential for further interventions and therapeutics. This comprehensive meta‐analysis and systematic review mainly summarize the cardiac‐related complications, outcomes, and laboratory findings of 15,354 COVID‐19 cases from 70 studies. All subjects were classified into the severe versus moderate group, severe versus critical group, severe versus non‐severe group, and survivor versus non‐survivor group according to the selected articles, respectively. We found that a higher severity of COVID‐19 was associated with higher rates of hypertension, cardiovascular diseases, and acute cardiac injury in the four groups. Similarly, this clean pattern was also found in the number of COVID‐19 patients with increased serum levels of CK, CK‐MB, Myo, LDH, TnI, and NT‐proBNP, whereas no difference was witnessed in the severe versus critical group. Another important finding was compared with milder infection patients, those with a severe infection showed a significant increase in CK, CK‐MB, Myo, LDH, and TnI.
Various mechanisms can be suggested to explain our results in the meta‐analysis that patients suffering from hypertension or underlying cardiovascular diseases have a high risk of developing severe manifestations of COVID‐19. The mismatch of supply and demand of myocardial oxygen is a common phenomenon of severe viral diseases accompanied by insufficient systemic oxygenation during pneumonia in elderly patients with cardiovascular diseases and other chronic diseases. 82 Another possible explanation for this might be that hypertension, cardiovascular diseases, and their treatments upregulate ACE2, especially with the use of RAAS inhibitors. 83 As a membrane‐bound aminopeptidate receptor that expresses on epithelial cells, ACE2 converts the vasoconstrictor AngII which is hydrolyzed by ACE1 into the vasodilator angiotensin 1–7 (Ang1–7). 84 ACE2 is higher expressed in the heart after receiving ARB or ACEI, 85 interacts with SARS‐CoV‐2 spike protein, which leads to endothelial dysfunction and myocardial damage directly. 86 Meanwhile, SARS‐CoV‐2 binding to ACE2 led to a reduction of the external ACE2 catalytic effect and replaced by internalization. 87 Therefore, the possible downregulation of ACE2 and the subsequent increase of the pro‐inflammatory AngII together with the decrease of the cardioprotective Ang1–7 in patients with COVID‐19 may ultimately compromise heart function. 88
The elevated serum levels of biomarkers of myocardial injury including CK, CK‐MB, LDH, TnI, and NT‐proBNP were strongly associated with severe forms of COVID‐19 from our research. Myocardial injury from SARS‐CoV‐2 infection involves many factors, and the mechanisms have not been fully elucidated yet. As mentioned previously, as a consequence of ACE2 expression in cardiac tissue, SARS‐CoV‐2 can directly cause heart damage. There have been studies showing that the heart is susceptible to SARS‐Cov‐2 infections. 89 A further mechanism for explanations involves a cytokine storm, which is described as an excessive immune response towards SARS‐CoV‐2 infection. 90 , 91 Cytokines are essential to infection control, but when the immune system is deregulated including the imbalanced response of type 1 and type 2 T helper cells, a cytokine storm will be observed, resulting in an excessively elevated level of cytokines, such as C‐reactive protein interleukin 8 (IL‐8), IL‐10, procalcitonin, IL‐1b, and tumor necrosis factor‐α particularly IL‐6 coupled with tissue damage and multiple organ dysfunctions. 92
There are several limitations that should be mentioned in our study. First, significant publication bias was observed in several results. A possible explanation is that some of the studies were published at the preprint server, which means some of the included studies are non‐peer‐reviewed scientific manuscripts. Secondly, as a novel infection disease, most of the including studies were retrospective case series, and no randomized controlled trial was included. The lack of RCT or prospective studies causes the consequence that it is hard to adjust for potentially confounding factors, and that might be a source of high heterogeneity. Third, different clinical laboratories used different ranges of normal values for laboratory indicators; therefore, we used SMD to value the relationship between increased serum level and severity of COVID‐19, which can achieve a definite ratio instead of numerical laboratory results. Finally, another limitation is that the majority of included studies were from China, only five of them are from other countries experiencing the COVID‐19 outbreak, which might lead to bias from races and geographic scope.
5. CONCLUSION
This meta‐analysis comprehensively investigates the differences in cardiac‐related comorbidities, outcomes, and laboratory indicators between patients with COVID‐19 who have different disease severity. The findings clearly indicate that hypertension, cardiovascular disease, acute cardiac injury, and related laboratory indicators are associated with the severity of COVID‐19. What is now needed are cross‐national prospectively designed observational or clinical trials that will help improve the certainty of the available evidence and treatment decisions for patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Zhengchuan Zhu and Miaoran Wang designed the study and wrote the manuscript. Qiaoyan Cai and Ling Zhang extracted the data and evaluated the RCTs quality. Jianfeng Chu, Jun Peng, and Keji Chen contributed to interpreting the results, draft reviewing, and finalizing the paper. All authors revised the report and approved the final version before submission.
ACKNOWLEDGEMENTS
This study is supported by funds from the Science and Technology Major Project of Fujian Province (No. 2019YZ014004), the Fujian Provincial Health and Family Planning Commission (2018‐CX‐42), Natural Science Foundations of Fujian Province (2018J01884) and National Natural Science Foundation of China of China (81774135 and 81232884).
Zhu Z, Wang M, Lin W, et al. Cardiac biomarkers, cardiac injury, and comorbidities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. Immun Inflamm Dis. 2021;9:1071‐1100. 10.1002/iid3.471
Zhengchuan Zhu and Miaoran Wang are shared first authors and contributed equally to this study.
Contributor Information
Jianfeng Chu, Email: jianfengchu999@yeah.net.
Jun Peng, Email: pjunlab@hotmail.com.
Keji Chen, Email: kjchenvip@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Xu JB, Zhao SZ, Teng TS, et al. Systematic comparison of two animal‐to‐human transmitted human coronaviruses: SARS‐CoV‐2 and SARS‐CoV. Viruses‐Basel. 2020;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belete TM. Review on up‐to‐date status of candidate vaccines for COVID‐19 disease. Infect Drug Resist. 2021;14:151‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bortolotti D, Gentili V, Rizzo S, Rotola A, Rizzo R. SARS‐CoV‐2 spike 1 protein controls natural killer cell activation via the HLA‐E/NKG2A pathway. Cells. 2020;9:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li M, Schifanella L, Larsen PA. Alu retrotransposons and COVID‐19 susceptibility and morbidity. Hum Genomics. 2021;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE‐angiotensin II‐AT(1) receptor axis vs. ACE2-angiotensin‐(1‐7)‐Mas receptor axis. Hypertension Res. 2009;32:533‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li B, Yang J, Zhao FM, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109:531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aikawa T, Takagi H, Ishikawa K, Kuno T. Myocardial injury characterized by elevated cardiac troponin and in‐hospital mortality of COVID‐19: an insight from a meta‐analysis. J Med Virol. 2020;93:51‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 11. Polverino F, Kheradmand F. COVID‐19, COPD, and AECOPD: immunological, epidemiological, and clinical aspects. Front Med. 2020;7:627278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777‐784. [DOI] [PubMed] [Google Scholar]
- 13. World Health O. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (2019‐nCoV) Infection is Suspected: Interim Guidance, 28 January 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 14. Gao W, Yang X, Zheng X, Su X. Clinical characteristic of 90 patients with COVID‐19 hospitalized in a Grade‐A hospital in Beijing. Academic J Chin Pla Med School. 2020:1‐4. [Google Scholar]
- 15. Cheng K, Wei M, Shen H, Wu C, Chen D. Clinical characteristics of 463 patients with common and severe type coronavirus disease 2019. Shanghai Med J. 2020:1‐15. [Google Scholar]
- 16. Li L, Yang L, Gui S, et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID‐19 in Wuhan, China. Theranostics. 2020;10:6113‐6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiong J, Jiang W, Zhou Q, Hu X. Clinical characteristics, treatment, and prognosis in 89 cases of COVID‐2019. Med J Wuhan Univ. 2020:1‐5. [Google Scholar]
- 18. Yuan J, Sun Y, Zuo Y, Chen T, Cao Q. Clinical characteristics of 223 novel coronavirus pneumonia cases in Chongqing. J Southwest Univ (Natural Science Edition). 2020:1‐7. [Google Scholar]
- 19. Zhang J, Ding D. Analysis of myocardial enzymes novel coronavirus pneumonia patients with severe disease. J Jiangsu Univ (Med Ed). 2020:1‐3. [Google Scholar]
- 20. Chen S, Wu J, Li Z, Xu D, Zhu Z. Clinical features of 109 cases of novel coronavirus pneumonia. Chin J Infect Dis. 2020;38:145‐149. [Google Scholar]
- 21. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: a retrospective study. Chin Med J. 2020;133:1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID‐19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. [DOI] [PubMed] [Google Scholar]
- 24. Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou B, She J, Wang Y, Ma X. The Clinical Characteristics of Myocardial injury 1 in Severe and Very Severe Patients with 2019 Novel Coronavirus Disease. J Infect. 2020;81:147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao XY, Xu XX, Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non‐Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
28.
Zhao J, Gao HY, Feng ZY, Wu QJ. A Retrospective Analysis of the Clinical and Epidemiological Characteristics of COVID‐19

 Patients in Henan Provincial People's Hospital, Zhengzhou, China. Front Med. 2020;7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patients in Henan Provincial People's Hospital, Zhengzhou, China. Front Med. 2020;7:286. [DOI] [PMC free article] [PubMed] [Google Scholar] - 29. Hu L, Chen S, Fu Y, et al. Risk Factors Associated with clinical outcomes in 323 COVID‐19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71:2089‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Q, Cao Y, Chen L. Hematological features of persons with COVID‐19. Leukemia. 2020;34(8):2163‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li M, Lv M, Li C, Tong Y. Analysis on the cardiac features of patients with different clinical types of novel coronavirus disease 2019. Guangdong Med J. 2020:1‐4. [Google Scholar]
- 34. Cao Z, Li T, Liang L, et al. Clinical characteristics of Coronavirus Disease 2019 patients in Beijing, China. PLOS One. 2020;15:e0234764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Z, Cai T, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi‐center study. PLOS Neglected Trop Dis. 2020;14:e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang A, Qiu Q, Kong X, et al. Clinical and epidemiological characteristics of COVID‐19 patients in Chongqing China. Front Public Health. 2020;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Liao B, Guo Y, et al. Clinical characteristics of patients infected with the novel 2019 coronavirus (SARS‐Cov‐2) in Guangzhou, China. Open Forum Infect Dis. 2020;7:ofaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fu G, Deng J, Xiang J, Liu Y. Clinical characteristics and prognosis in 51 severe cases of COVID‐2019. J Chongqing Med Univ. 2020:1‐8. [Google Scholar]
- 42. Tao F, Yang F, Ruan X, Xia D, Li J. Clinical characteristics of 382 hospitalized patients with COVID⁃19. J Pract Med. 2020:1‐5. [Google Scholar]
- 43. Wu W, Huang H, Zhang M, Yu Q, Ding X. Clinical features of COVID‐19 patients: a 102‐case study. J Pract Med. 2020:1‐5. [Google Scholar]
- 44. Bonetti G, Manelli F, Patroni A, et al. Laboratory predictors of death from coronavirus disease 2019 (COVID‐19) in the area of Valcamonica, Italy. Clin Chem Lab Med. 2020;58:1100‐1105. [DOI] [PubMed] [Google Scholar]
- 45. Chen F, Sun W, Sun S, Li Z, Wang Z, Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID‐19 in Wuhan, China. Clin Transl Med. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fan H, Zhang L, Huang B, et al. Cardiac injuries in patients with coronavirus disease 2019: Not to be ignored. Int J Infect Dis. 2020;96:294‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang Q, Deng X, Li Y, et al. Clinical characteristics and drug therapies in patients with the common‐type coronavirus disease 2019 in Hunan, China. Int J Clin Pharm. 2020;42:837‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jang JG, Hur J, Choi EY, Hong KS, Lee W, Ahn JH. Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35:e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li D, Long Y, Huang P, Guo W, Wu S. Clinical characteristics of 80 patients with COVID‐19 in Zhuzhou City. Chin J Infect Control. 2020:227‐233. [Google Scholar]
- 51. Li K, Chen D, Chen S, et al. Predictors of fatality including radiographic findings in adults with COVID‐19. Respir Res. 2020;21:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu C, Deng X, Pan Y, et al. Clinical characteristics and CT imaging features of patients with different clinical types of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:548‐553. [DOI] [PubMed] [Google Scholar]
- 53. Wang CZ, Hu SL, Wang L, Li M, Li HT. Early risk factors of the exacerbation of Coronavirus disease 2019 pneumonia. J Med Virol. 2020;92:2593‐2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang L, He WB, Yu XM, Liu HF, Zhou WJ, Jiang H. Prognostic value of myocardial injury in patients with COVID‐19]. Zhonghua Yan Ke Za Zhi. 2020;56:E009. [DOI] [PubMed] [Google Scholar]
- 55. Xu PP, Tian RH, Luo S, et al. Risk factors for adverse clinical outcomes with COVID‐19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10:6372‐6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yao Q, Wang P, Wang X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Polish Arch Int Med. 2020;130:390‐399. [DOI] [PubMed] [Google Scholar]
- 57. Zhang C, Qin L, Li K, et al. A Novel Scoring System for Prediction of Disease Severity in COVID‐19. Front Cell Infect Microbiol. 2020;10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID‐19) in Taizhou, Zhejiang, China. Infection. 2020. [DOI] [PMC free article] [PubMed]
- 59. Fang X, Mei Q, Yang T, Zhang L, Yang Y. Immune imbalance mechanism and intervention strategy in patients with coronavirus disease 2019 (COVID‐19). Chin Pharmacol Bull. 2020:453‐459. [Google Scholar]
- 60. Li C, Li M, Gan L, Zhang Y. Heart and liver damages in coronavirus disease - 2019. Guangdong Med J. 2020:1‐4. [Google Scholar]
- 61. Ling Y, Lin X, Qian Z, Huang D, Zhang D, Li T. Clinical analysis of risk factors for severe patients with corona virus diseases 2019. Chin J Infect Dis. 2020;38:193‐198. [Google Scholar]
- 62. Zhang W, Hou W, Li A. Clinical characteristics of 74 hospitalized patients with COVID‐19. J Capital Med Univ. 2020:161‐167. [Google Scholar]
- 63. Rath D, Petersen‐Uribe A, Avdiu A, et al. Impaired cardiac function is associated with mortality in patients with acute COVID‐19 infection. Clin Res Cardiol. 2020;109:1491‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shi S, Qin M, Cai Y, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giacomelli A, Ridolfo AL, Milazzo L, et al. 30‐day mortality in patients hospitalized with COVID‐19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol Res. 2020;158:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fu L, Fei J, Xiang H‐X, et al. Influence factors of death risk among COVID‐19 patients in Wuhan, China: a hospital‐based case‐cohort study. medRxiv. 2020; 2020.2003.2013.20035329.
- 68. Han Y, Zhang H, Mu S, et al. Lactate dehydrogenase, a risk factor of severe COVID‐19 patients. medRxiv. 2020; 2020.2003.2024.20040162.
- 69. He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID‐19 pneumonia. J Clin Virol. 2020;127:104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID‐19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020; 2020.2003.2019.20033175.
- 71. Ma K‐L, Liu Z‐H, Cao C‐F, et al. COVID‐19 myocarditis and severity factors: an adult cohort study. medRxiv. 2020; 2020.2003.2019.20034124.
- 72. Wang G, Wu C, Zhang Q, Wu FEpidemiological and Clinical Features of Corona Virus Disease 2019 (COVID‐19) in Changsha, China (3/1/2020). medRxiv. 2020. https://ssrn.com/abstract=3548770
- 73. Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID‐19. Am J Respir Crit Care Med. 2020;201:1430‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wei YY, Wang RR, Zhang DW, et al. Risk factors for severe COVID‐19: Evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020;81:e89‐e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID‐19). J Intern Med. 2020;288:128‐138. [DOI] [PubMed] [Google Scholar]
- 76. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID‐19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404‐3410. [DOI] [PubMed] [Google Scholar]
- 77. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wei ZY, Qian HY. Myocardial injury in patients with COVID‐19 pneumonia. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E006. [DOI] [PubMed] [Google Scholar]
- 79. Tomlins J, Hamilton F, Gunning S, Sheehy C, Moran E, Macgowan A. letter to Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID‐19), the first UK cohort. J Infect. 2020;81:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ganji R, Reddy PH. Impact of COVID‐19 on mitochondrial‐based immunity in aging and age‐related diseases. Front Aging Neurosci. 2020;12:614‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu J, Zheng X, Tong QX, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J Med Virol. 2020;92:491‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Prompetchara E, Kettoy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9. [DOI] [PubMed] [Google Scholar]
- 83. Babapoor‐Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID‐19: possible mechanisms. Life Sci. 2020;253:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gembardt F, Sterner‐Kock A, Imboden H, et al. Organ‐specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lan J, Ge J, Yu J, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581:215‐220‐+. [DOI] [PubMed] [Google Scholar]
- 87. South AM, Diz DI, Chappell MC. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084‐H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9:e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ni W, Yang X, Yang D, et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Crit Care. 2020;24:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID‐19: an overview of the involvement of the chemokine/chemokine‐receptor system. Cytokine Growth Factor Rev. 2020;53:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kasozi, KI, Nidebala G, Alqarni M, et al. Bee Venom—a potential complementary medicine candidate for SARS‐CoV‐2 infections. Front Public Health. 2020;8:594458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


