Abstract
Housing sows in groups create the challenge of decreasing fighting amongst sows. One proposed method to do so is to feed a high tryptophan diet, but the effect on the fetus is unknown. To investigate this, 66 sows were fed one of three diets: Control (0.14% SID tryptophan), Medium (0.28% SID tryptophan), or High (0.42% SID tryptophan), from days 28 to 35 of gestation. Sows gestated in standard gestation stalls. Blood samples were taken on day 27 prior to and on day 35 after tryptophan supplementation. On days 1, 2, and 3, nursing bouts were observed so as to record disputes and displacements from teat competition. The piglets’ activity and fighting were recorded on days 3, 7, and 11 from 0700 to 1700 h. On day 12, four piglets per litter were blood sampled: two to be used in later behavior tests and two to act as controls for blood cortisol levels. On day 14, the two behavior test piglets from each litter were subjected to a 10-min Isolation Test and 5-min Human Approach Test. On day 15, the behavior test piglets were paired by sex and treatment (for example, a male Medium piglet paired with another male Medium piglet from a different crate) and each pair was subjected to a 10-min Social Challenge Test and immediately blood sampled. Piglet cortisol and serotonin did not differ among treatments (P > 0.10). There were no differences (P > 0.10) for number born (12.7 ± 0.4), born alive (11.7 ± 0.4), or mortality (1.1 ± 0.2). Behavior during nursing bouts was similar, with no treatment differences in number of disputes or displacements, and similar bout lengths among treatments (199.5 ± 4.6 s, P > 0.10). No differences were detected for any of the variables for Isolation or the Human Approach Tests (P > 0.10). During the Social Challenge Test, High piglets had more contacts approaching the head of the companion piglet than did either Medium or Control piglets (14.3 ± 1.1, 10.7 ± 1.1, and 9.69 ± 0.8, respectively, P < 0.02). Total number of aggressive interactions during the test tended to be greater for Medium piglets compared to High piglets (9.3 ± 1.5 vs 5.1 ± 0.9, P < 0.07). Time budget data of the litter indicate that piglets from all three treatments spent equal amounts of time active and inactive (P > 0.10). Aggression was low with 0.3 ± 0.04% of piglets displaying aggressive behavior. Feeding high concentrations of tryptophan for a short duration early in gestation does not have a negative impact on sows’ subsequent offspring.
Keywords: aggression, behavior, piglet, swine, stress, tryptophan
INTRODUCTION
Public pressure is changing animal agriculture practices including how we house sows. Currently, there is a move to change from stall housing of gestation sows to group housing. This creates another welfare problem due to sows fighting as they establish a social hierarchy. Methods to decrease this aggression and improve the welfare of sows are needed. One such method could be the feeding of tryptophan. Poletto et al. (2010) and Warner et al. (1998) found that feeding sows tryptophan was useful in decreasing aggression. However, the impact of elevated tryptophan on the developing fetus is unknown.
Arevola et al. (1991) reported that tryptophan concentrations increased in different fetal organs, including the brain, when pregnant rats were orally administered high doses of tryptophan, suggesting that high levels of tryptophan can cross the placental barrier. However, studies conducted in pregnant rats have shown that a tryptophan-enriched diet fed throughout pregnancy and lactation diminished serotonin and decreased activity of tryptophan hydroxylase in the cortex and in the brain stem of 5-d-old rat pups (Huether et al., 1992). A study by Dennis et al. (2013) demonstrated that chick hens hatched after being given excess embryonic serotonin exhibited significantly less aggressive behavior. However, no such studies have been conducted in pigs. Therefore, the objective of this study was to evaluate the behavior, physiology, and welfare of piglets born to sows fed high-tryptophan gestation diets from days 28 to 35 post-breeding, which is likely the time a producer would feed tryptophan and is about the time embryonic implantation occurs in swine.
MATERIALS AND METHODS
Application of Treatments
This study was conducted at the Swine Farm at Purdue University, West Lafayette, IN and approved by the Purdue University IACUC (# 1402001024). Sixty-six Yorkshire x Landrace multiparous sows, assigned to treatments by balancing for parity, were included in one of three treatments: Control (0.14% SID tryptophan, n = 19), Medium (0.28% SID tryptophan, MED, n = 20), or High (0.42% SID tryptophan, HIGH, n = 21). Diets were fed from gestational days 28 to 35 to mimic a protocol that might be used by producers to decrease aggression when mixing sows after breeding. Details of the diets are presented in Table 1. The concentration of tryptophan in the diets is based on the previous research (Poletto et al., 2010) which showed that a 0.42% digestible tryptophan diet raised blood tryptophan level, reduced time spent standing, and increased lying behavior in gilts. The high tryptophan diet also reduced agonistic interactions and aggressiveness.
Table 1.
Diet composition for sows on the Control diet, Medium diet (2× Tryp, 0.28% inclusion rate), and the High diet (3× Tryp, 0.42% inclusion rate)
| Ingredients, % | Gestation | 2× Tryp | 3× Tryp |
|---|---|---|---|
| Control | 2× | 3× | |
| Corn | 43.575 | 43.500 | 43.280 |
| Soybean meal, 48% | 11.47 | 11.47 | 11.47 |
| DDGS | 40.00 | 40.00 | 40.00 |
| Monocal. Phosphate | 0.51 | 0.51 | 0.51 |
| Limestone | 1.79 | 1.79 | 1.79 |
| Salt | 0.50 | 0.50 | 0.50 |
| Choice white grease | 1.00 | 1.00 | 1.00 |
| Lysine –HCl | – | – | – |
| dl-Methionine | – | – | – |
| l-Threonine | – | – | – |
| l-Tryptophan | – | 0.075 | 0.295 |
| Swine Vit. Premix | 0.25 | 0.25 | 0.25 |
| Swine TM Premix | 0.125 | 0.125 | 0.125 |
| Sow Vitamin Premix | 0.25 | 0.25 | 0.25 |
| Selenium premix | 0.05 | 0.05 | 0.05 |
| Phytase (600 PU/g) | 0.10 | 0.10 | 0.10 |
| Rabon Larvacide | 0.13 | 0.13 | 0.13 |
| Defusion Plus | 0.25 | 0.25 | 0.25 |
| ME, kcal/kg | 1492.1 | 1492.1 | 1492.1 |
| Crude Protein, % | 19.59 | 19.59 | 19.59 |
| Tot. Lysine, % | 0.83 | 0.83 | 0.83 |
| SID Lys, % | 0.60 | 0.60 | 0.60 |
| SID Thre, % | 0.56 | 0.56 | 0.56 |
| SID Tryp, % | 0.14 | 0.28 | 0.42 |
| SID Meth + Cys, % | 0.59 | 0.59 | 0.59 |
| Ca, % | 0.85 | 0.85 | 0.85 |
| P, % | 0.63 | 0.63 | 0.63 |
| Phytase avail. P, % | 0.45 | 0.45 | 0.45 |
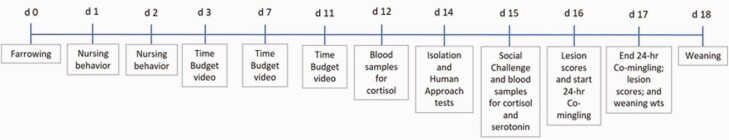
On day 27 after sows were bred, pregnancy was diagnosed by ultrasound. Treatment diets were fed to the sows from days 28 to 35. Throughout the study, sows were housed individually in 0.61 × 2.13 m (2′ × 7′) stalls and moved into farrowing crates on day 112 of gestation. Piglets were weaned at 18 ± 1.5 d. The study was conducted over four farrowing cycles with each treatment represented in every month (April, May, August, and September). A schematic of the timing and sequence of all procedures is presented in Figure 1.
Figure 1.
Time and sequence of data collection relative to farrowing and day of age.
Production Data and Nursing Behavior
The number of piglets born, weight of piglets at approximately 24 h of age and at weaning, number of live piglets, and preweaning mortality rate and reason were recorded. Litter behavior was recorded (Nuvico CB-HD2N-L IR Bullet Camera, Nuvico Inc., Englewood, NJ) from 0700 to 1700 h, when piglets were approximately 3, 7, and 11 d old. A 5-min scan sample, n = 360 scans/litter, was used to evaluate the time-budget for piglet behaviors and sow posture, using an ethogram adapted from that of Poletto et al. (2010) (Table 2). For piglet behaviors, litter was the experimental unit; the number of piglets performing each behavior was counted and then adjusted for litter size. To record disputes and displacements during nursing bouts, direct observations were conducted during a total of three nursing bouts for each sow over the first- and second-day post-farrowing, prior to any pigs being cross-fostered. Observations were completed in the morning before feeding. A nursing bout started when more than 50% of piglets were actively nursing and ended when more than 50% of piglets had stopped nursing. Data were corrected for litter size by dividing the number of disputes and displacements by the number of piglets in the litter, and an average of all three observed nursing bouts was calculated.
Table 2.
Ethogram for time-budget behaviors and postures observed in the piglets (adapted from Poletto et al., 2010)
| Variable | Description |
|---|---|
| Piglet behavior | |
| Active | Physically mobile: standing, walking, or running that does not involve aggressive interactions |
| Inactive | Physically immobile, without activity |
| Aggressive interaction | Engaging in agonistic interaction-pushing, biting, and/or head-knocking with another piglet |
| Nursing | Mouth on the teat |
| Sow posture | |
| Upright | Standing on all four legs or dog-sitting with rump on the floor and shoulders raised up with front legs extended |
| Lying Lateral | Lying on side |
| Lying Sternal | Lying on sternum |
Isolation Test
On day 12 of age, four piglets/litter, two males and two females, were selected to represent the average weight of the litters. Of these four, one male and one female piglet were used for the following behavior tests, and 3 mL i.v. blood samples were taken to measure cortisol and serotonin levels. The other male and female were not behavior tested to act as controls for cortisol levels before and after behavior testing. The ethogram for all behavioral tests is presented in Table 3.
Table 3.
Ethogram for the Isolation, Human Approach, Social Challenge, and Comingling behavior tests
| Variable | Description |
|---|---|
| Isolation Test | |
| Activity | Walking, running, or jumping |
| Activity start | Time the piglet starts walking after being placed in the pen |
| Investigation | Piglet’s nose oriented at the floor or walls with the appearance of sniffing or rooting |
| Resting | Lying or sitting |
| Escape attempt | Jumping or climbing against pen wall |
| Human Approach | |
| Contact | Any physical contact the piglet makes with the human, mostly sniffing |
| Social Challenge | |
| Activity start | Time the piglet starts walking after being placed in the pen |
| Social contact | Sniffing the penmate either head-on or along the body with piglet nose in contact with penmate |
| Nudge | Using its snout to push the penmate |
| Agonistic encounters | Biting, head tossing, mounting, and shoving the penmate |
| Duration in proximity | Being within one body length of the penmate |
| Comingling | |
| Active | Physically mobile: standing, walking, or running that does not involve aggressive interactions |
| Inactive | Physically immobile, without activity |
| Aggressive interaction | Engaging in agnostic interaction-pushing, biting, and/or head-knocking with another piglet |
| Nursing | Mouth on teat |
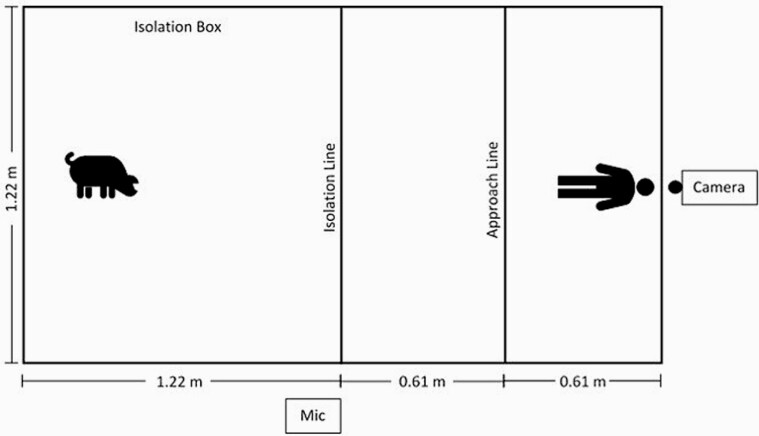
At day 14 of age, an Isolation Test was performed. The Isolation Test was conducted in a 1.2 × 1.2 m pen (Figure 2) in a room absent of other piglets and people. For each Isolation Test, the piglet was taken from its litter by an observer, who was not acting as the “human” during the following Human Approach Test, carried, and placed into the center of the pen. Video was recorded by placing a camera (Canon Vixia HFR 700 camcorder, Canon USA Inc., Melville, NY) directly outside of the pen behind the human at a height of 2.38 m. Vocalizations were recorded by placing the microphone (Song Meter SM3, Wildlife Acoustics, Maynard, MA) outside of the isolation testing pen near the Isolation Line and at a height just above that of the isolation pen’s wall. The microphone was set to the highest recording bandwidth (48 kHz).
Figure 2.
Testing pen for the Isolation and Human Approach Test. For the Isolation Test, only the 1.2 × 1.2 m pen was used. For the Human Approach Test, the pen was opened up to create the 1.2 × 2.4 m pen. The latency for the piglet to explore and cross the Isolation line, cross the Approach Line, and Contact the Human was recorded. The same pen (1.2 × 2.4 m) was used for the Social Challenge Test but without a person present.
Continuous recording of behaviors was used to record the latencies to start moving around the pen, investigating the pen, and to the first escape attempt, as well as the number of resting bouts, total resting duration, and total number of escape attempts. Piglet activity started when the piglet started moving around the pen, i.e., walking or running. Investigatory behaviors consisted of the piglet orienting its nose towards the floor or walls of the pen in a manner that suggested sniffing or rooting. Escape attempts were classified as the piglet jumping against or attempting to climb up the wall. It was possible for Activity or Investigation to begin immediately; in which case the latency was recorded as 0.
Vocalizations were analyzed using Avisoft-SASLab Pro (Avisoft Bioacoustics, Glienicke, Germany) and each vocalization was analyzed for duration of call and interval between calls. A mean was developed across the entire call for the following parameters: peak frequency, bandwidth, entropy, and harmonic-to-noise ratio. Finally, the duration of all calls per animal was combined to analyze total duration. The definitions of vocal characteristics are presented in Table 4.
Table 4.
Definitions of vocalization characteristics recorded during the Social Challenge Test (adapted from Chapel et al. 2018)
| Variable | Description |
|---|---|
| Duration, s | Duration of each individual call |
| Peak frequency, Hz | Loudest frequency found within a call |
| Bandwidth, Hz | The difference between the minimum and maximum frequency |
| Entropy | The randomness within a call where zero is a pure-tone and one is completely random noise |
| Total duration, s | Total time of all calls together |
| Interval between calls, s | Time between calls |
| Harmonic to noise ratio | Ratio of the degree of harmonic sound to additional noise produced within a call |
Human Approach Test
To conduct a Human Approach Test, a human entered the pen (Figure 2) immediately after the Isolation Test, extended the pen’s length to 2.4 m by picking up the wall and moving it back 1.2 m, and stood motionless at this far end of the pen from the piglet for 2 min (Figure 2). The wall was moved easily with minimal noise or disturbance to the pig. An observer stood outside the area, out of view, to record the times when the piglet crossed the Isolation Line, Approach Line, and contacted the human, as well as the number and duration of contacts. No pigs contacted the human during the enlargement of the pen; if a pig walked with the human to cross the Isolation and/or Approach Lines, these times were recorded as 0.
At the end of 2 min, the human knelt motionless in the same location. At this time, the observer recorded the piglet’s response: neutral, which included no reaction or walk away, or fear, which included vocalization, freeze, startled jump, or run away. If the piglet moved away in reaction to the human’s movement, the test was stopped when the piglet once again made contact and this time was recorded as the latency to contact after human movement. If the piglet made no reaction to the human’s movement and remained in contact, then the latency was recorded as 0. The maximum length of the total Human Approach Test was 5 min. The pen had visually designated areas using lines on the floor (Figure 2) to allow the observer to record when the piglet crossed the “Isolation Line,” the “Approach Line,” and when it touched the observer. Total number of contacts with the human and duration of all contacts were recorded. Refer to Table 3 for an ethogram for all behavior tests.
Social Challenge Test
To conduct a Social Challenge Test, at 15 d of age the same piglets used for the Isolation Test were paired by sex and treatment (for example, a male Medium piglet paired with another male Medium piglet from a different crate) and each pair was placed together in the center of the 1.2 × 2.4 m pen (Figure 2) for 10 min. The test was recorded using the same camera and set up as the Isolation Test; vocalizations were not used, however, given the complexity of having two pigs vocalizing simultaneously. From the video, behavior of the piglet pair was continuously recorded for escape attempts, social interactions, and agonistic encounters (Table 3). The experimental unit was the pair of piglets. No tests required intervention due to piglet injury or excessive aggression. After the test, 3 mL i.v. blood samples were collected to measure cortisol and serotonin, and the piglets were returned to their dam.
Comingling
At 16 d of age, piglets in all litters were assigned a lesion score (0 = no lesions, 1 = old lesions, and 2 = fresh lesions with blood) for their ears and shoulders. Then the barrier between two farrowing crates of sows in like treatments was removed to allow piglets from both litters to comingle (n = 7, n = 8, and n = 9 paired litters for the Control, Medium, and High sows, respectively). The behavior of the piglets was recorded for 24 h (Nuvico CB-HD2N-L IR Bullet Camera, Nuvico Inc., Englewood, NJ) and a 5-min scan sample, n = 288 scans/litter, was used to record instances of activity, nursing, and aggression (Table 3). The experimental unit was the litter, and the number of piglets involved in each behavioral category was recorded. Categories of behavior were mutually exclusive, such that a piglet counted as engaging in aggression was not then counted as active. Twenty-four hours later, the barrier was replaced, litters were returned to their respective sow, and weights and lesions scores were recorded for each piglet.
Sow Bloodwork
On days 27 and d of gestation, 5 mL i.v. blood samples were collected from sows using EDTA-treated tubes. Whole blood was stored at −80 °C until analyzed to evaluate the change in peripheral blood 5-HT and TRP concentrations before and after supplementation using an HPLC method adapted from Poletto et al (2010).
Samples were acidified using 4 M perchloric acid and freshly prepared 3% ascorbic acid. Supernatants were collected after centrifugation and injected in duplicate onto an Alliance e2695 HPLC system (Waters Corporation, Milford, MA) and C18 column with 3 μm pore size and 3 mm width by 150 mm length (ThermoFisher Scientific, Waltham, MA). Samples were run for 3 min in a fluorescence detector at 1 mL/min. A standard curve was generated using commercially available 5-HT and TRP standards (Sigma-Aldrich, St. Louis, MO). The intra- and inter-assay CVs for TRP were 1.1% and 3.4%, respectively, and for 5-HT, 1.1% and 5.8%.
Piglet Bloodwork
Blood collected from piglets on days 12 and 15 of age was allowed to coagulate at room temperature for less than 2 h, at which time serum was separated and stored at −80 °C until analyzed. Serum from days 12 and 15 was analyzed for cortisol concentrations using radioimmunoassay (RIA) Corti-Cote kits (0722110, MP Biomedicals LLC, Orangeburg, NY), and kit instructions were followed with samples ran in duplicate. Intra- and interassay CVs were 8.2% and 13.5%, respectively. Cortisol concentrations from dat 12 samples were considered the baseline levels and subtracted from day 15 levels to determine the change in cortisol after behavior tests were finished. Serum from day 15 was analyzed for serotonin concentrations using RIA kits (IB88189, IBL-America, Minneapolis, MN), kit instructions were followed, and samples were run in duplicate. Intra- and interassay CVs were 6.1% and 8.9%, respectively.
Statistics
All data were analyzed in SAS 9.4 (Cary, NC). Analysis of variance using fixed and random effects (treatment, sex, and day as fixed effects when appropriate and rep served as a random effect) was used to analyze most data. Interactions were explored and included in the model when appropriate to account for their effects. Repeated measures analysis was included for multiple measures over time, and a Kramer–Tukey adjustment was made for multiple comparisons. Data that were not normal were transformed, and if normality could not be achieved data were analyzed using the Wilcoxon–Mann–Whitney test. These data included piglet mortality, scours, and all measures of aggression; for the Isolation Test, latency to investigate, resting duration, and number of resting bouts; and for the Social Challenge Test, time in proximity, number of escape attempts, and escape attempt latency. Sow or litter was considered the statistical unit for all the production and litter data. Piglet was considered the experimental unit for the Isolation and Human Approach Tests; piglet pair was considered the experimental unit for the Social Challenge Test; and litter pair for comingling data. Significance was set at P < 0.05 and trends set at P < 0.10. Data are presented as means ± SE.
RESULTS
To verify that diets were formulated as prescribed, a pooled sample (batches and replicates) of each dietary treatment was taken for later tryptophan analysis. Diet samples were analyzed by an independent laboratory (Experiment Station Chemical Laboratories, Univ. MO, Columbia MO) and found to contain 0.16% (Control), 0.29% (Medium), and 0.37% (High) tryptophan, respectively. These concentrations were considered to be close enough to the targeted concentrations of 0.14%, 0.28%, and 0.42% tryptophan, respectively. Blood collected from sows prior to starting on the diet on day 27 of gestation, and after tryptophan supplementation ended on day 35 of gestation indicate a change in tryptophan of 1.23 ± 0.47, 7.59 ± 0.74, and 10.36 ± 1.44 μg/mL for Control, Medium, and High sows, respectively. Medium and High sows had a significant increase in blood tryptophan from days 0 to 7 of being fed diets compared to Control sows (P < 0.001). The change in tryptophan for Control sows was not significant. During the same time period, the change in serotonin was −0.001 ± 0.04, 0.038 ± 0.03, and 0.019 ± 0.02 for Control, Medium, and High sows, respectively (P < 0.63).
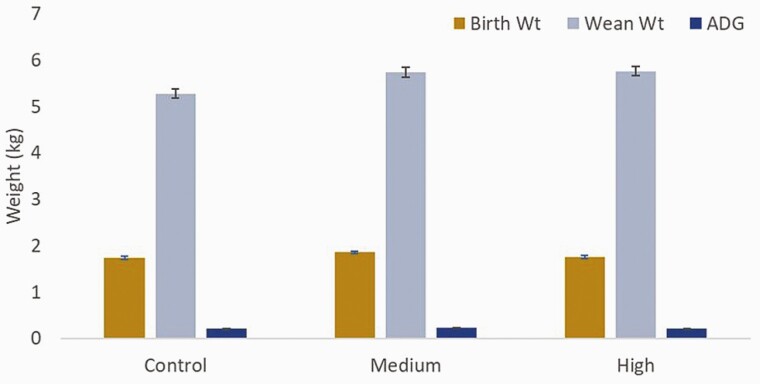
Sows delivered approximately 12 piglets per litter with one stillborn (Table 5) and did not differ by treatment (P > 0.10). Mortality was also similar among treatments with approximately one piglet per litter dying prior to weaning. Birth weight, weaning weight, and average daily gain were similar among treatments (Figure 3).
Table 5.
Mean production data by treatment
| Variable | Control n = 18 litters |
Medium n = 21 litters |
High n = 21 litters |
|---|---|---|---|
| Born, # | 13.6 ± 0.7 | 11.5 ± 0.6 | 13.2 ± 0.7 |
| Born Alive, # | 12.3 ± 0.7 | 10.7 ± 0.6 | 12.2 ± 0.6 |
| Mortality1, # | 1.5 ± 0.4 | 0.8 ± 0.3 | 1.0 ± 0.2 |
| Scours, # | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.1 |
1Mortality prior to weaning.
Figure 3.
Birth weight (Birth Wt), Weaning weight (Wean Wt), and average daily gain (ADG). Control n = 18 litters; Medium n = 21 litters; High n = 21 litters.
Treatments did not differ in the number of nursing disputes or displacements when piglets were observed during three nursing bouts (P > 0.10, Table 6). On average, there were 0.32 ± 0.05 disputes and 0.09 ± 0.02 displacements per pig. Nursing bout duration was similar among treatments lasting approximately 3 min.
Table 6.
Nursing bout duration and proportion (number of disputes or displacements divided by the number of piglets in the litter) of disputes and displacements during nursing
| Variable | Control n = 16 litters |
Medium n = 20 litters |
High n = 21 litters |
|---|---|---|---|
| Disputes | 0.32 ± 0.04 | 0.26 ± 0.04 | 0.37 ± 0.06 |
| Displacements | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.11 ± 0.02 |
| Nursing bout length, s | 192.9 ± 10.4 | 193.8 ± 7.4 | 210.0 ± 6.3 |
Sows spent most of their time lying; the majority of this time was spent lying laterally (Table 7), with Control sows lying laterally more than High sows (P < 0.05), but not Medium sows. Piglets from all three treatments were equally active and inactive (Table 7). During the observation times, approximately 50% of piglets were observed inactive and resting and 15% were actively exploring their pen, dam, or littermates. Aggression was low with only 0.3 ± 0.04% of piglets engaged in aggressive interactions during the observation times.
Table 7.
Time budget of sow posture (expressed as the average proportion of time sows spent in a specific posture) and the performance of piglet behaviors (expressed as the average proportion of piglets performing a specific behavior) during light hours (0700 to 1700 h) over days 3, 7, and 11
| Variable | Control n = 12 litters |
Medium n = 15 litters |
High n = 16 litters |
|---|---|---|---|
| Sow posture | |||
| Lying sternal | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.01 |
| Lying lateral | 0.67 ± 0.02a | 0.64 ± 0.02a,b | 0.56 ± 0.02b |
| Upright | 0.18 ± 0.02 | 0.20 ± 0.02 | 0.23 ± 0.02 |
| Piglet behavior1 | |||
| Active | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.01 |
| Inactive | 0.52 ± 0.01 | 0.52 ± 0.01 | 0.51 ± 0.01 |
| Nursing | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| Aggression | 0.003 ± 0.0006 | 0.003 ± 0.0005 | 0.003 ± 0.0005 |
a,
bMeans within a row with different superscripts differ (P < 0.05).
1During the time budget observations, approximately 15% of piglets were out of the observer’s sight, and thus the proportions do not add to 1.
No differences were detected for any of the variables for Isolation Test (P > 0.10, Table 8). Piglets tried to escape the enclosure approximately 10 times during the test.They rested only about 15 s. No differences were observed for duration, peak frequency, bandwidth, or entropy of each vocalization (P > 0.10, Table 9), with total duration during the Isolation Test showing a tendency for Medium piglets to have shorter durations than Control and High piglets (P > 0.07). Interval between calls was affected by sex (P < 0.03), with females (0.30 ± 0.02 s) having a shorter interval than males (0.35 ± 0.02 s). There was a treatment by sex effect for harmonic-to-noise ratio (P < 0.01) indicting greater ratios in Medium male piglets (26.65 ± 0.77) and lower in High male piglets (23.5 ± 0.71) with Control piglets intermediary.
Table 8.
Mean (± SE) of behavioral variables of piglets when subjected to a 10-min Isolation Test
| Variable | Control n = 27 piglets |
Medium n = 32 piglets |
High n = 36 piglets |
|---|---|---|---|
| Activity latency, s | 20.63 ± 4.66 | 16.72 ± 2.51 | 15.42 ± 2.54 |
| Latency to investigate pen, s | 14.17 ± 4.20 | 9.06 ± 2.22 | 9.16 ± 2.24 |
| Latency to escape attempt, s | 307.04 ± 13.47 | 310.15 ± 36.43 | 313.83 ± 34.12 |
| Escape attempts, # | 12.79 ± 2.82 | 8.39 ± 1.51 | 11.19 ± 1.62 |
| Resting duration, s | 11.92 ± 5.35 | 21.81 ± 9.64 | 19.35 ± 6.87 |
| Resting bouts, # | 0.50 ± 0.18 | 0.45 ± 0.16 | 0.80 ± 0.26 |
Table 9.
Mean (± SE) vocal characteristics of piglets during the 10-min Isolation Test
| Variable | Control n = 27 piglets |
Medium n = 32 piglets |
High n = 36 piglets |
|---|---|---|---|
| Duration, s | 0.38 ± 0.03 | 0.37 ± 0.02 | 0.35 ± 0.02 |
| Peak frequency, Hz | 424.32 ± 104.67 | 304.67 ± 68.09 | 374.00 ± 83.74 |
| Bandwidth, Hz | 1,167.39 ± 217.97 | 1,108.81 ± 197.08 | 1,175.62 ± 202.72 |
| Entropy | 0.38 ± 0.02 | 0.36 ± 0.02 | 0.34 ± 0.02 |
| Total duration, s | 204.11 ± 18.52c | 157.79 ± 18.65d | 192.27 ± 14.75c |
| Interval between calls, s | 0.27 ± 0.04 | 0.31± 0.04 | 0.27 ± 0.03 |
| Harmonic to noise ratio | 23.69 ± 0.97 | 24.55 ± 0.89 | 23.74 ± 0.85 |
c,
dMeans within a row with different superscripts tended to differ (P < 0.07).
When the human entered the enclosure, the piglets responded similarly with no differences detected among treatments (P > 0.10, Table 10). During the Human Approach Test, piglets contacted the human within approximately 40 s, and stayed in contact with the human for only 10 s (Table 10).
Table 10.
Mean (± SE) of behavioral variables of piglets when subjected to a 5-min Human Approach Test
| Variable | Control n = 27 piglets |
Medium n = 32 piglets |
High n = 36 piglets |
|---|---|---|---|
| Latency to cross Isolation Line, s | 33.81 ± 8.60 | 31.71 ± 7.19 | 27.86 ± 5.50 |
| Latency to cross Approach Line, s | 37.00 ± 6.62 | 42.43 ± 7.92 | 33.80 ± 6.07 |
| Latency to physically contact human, s | 45.56 ± 6.46 | 41.89 ± 6.01 | 38.18 ± 4.57 |
| Contacts, # | 4.96 ± 0.48 | 4.75 ± 0.52 | 5.03 ± 0.44 |
| Duration in contact, s | 9.69 ± 1.09 | 10.33 ± 1.28 | 12.50 ± 1.38 |
| Latency to contact after human movement, s | 28.27 ± 6.31 | 35.68 ± 8.52 | 23.26 ± 3.27 |
| Duration of contact after human movement, s | 12.85 ± 3.50 | 11.91 ± 3.16 | 11.00 ± 2.47 |
During the Social Challenge Test, High piglets had more contacts approaching the head of the companion piglet than did either Medium or Control piglets (P < 0.007, Table 11). Total number of aggressive interactions during the test tended to be greater for Medium piglets than for High piglets (P < 0.06), with Control piglets being intermediate and not different than Medium piglets (P > 0.10).
Table 11.
Mean (± SE) of behavioral variables of paired piglets when subjected to a 10-min Social Challenge Test
| Variable | Control n = 13 paired piglets |
Medium n = 16 paired piglets |
High n = 19 paired piglets |
|---|---|---|---|
| Activity latency, s | 12.62 ± 2.70 | 26.47 ± 8.89 | 17.95 ± 3.61 |
| Social interaction latency, s |
27.46 ± 3.92 | 42.67 ± 11.46 | 36.05 ± 4.85 |
| Contacts approaching the head, # | 9.69 ± 0.84a | 10.67 ± 1.09a | 14.26 ± 1.11b |
| Contacts not approaching the head, # | 21.85 ± 2.37 | 16.27 ± 2.05 | 18.42 ± 1.53 |
| Nudges, # | 2.46 ± 0.58 | 3.67 ± 0.87 | 4.37 ± 0.67 |
| Latency to aggressive interactions, s | 186.42 ± 26.42 | 194.73 ± 31.34 | 271.17 ± 42.72 |
| Aggressive interactions, # | 7.08 ± 1.53c,d | 9.27 ± 1.45d | 5.05 ± 0.92c |
| Total duration of aggressive interactions, s |
42.62 ± 10.30 | 60.47 ± 13.78 | 32.68 ± 8.09 |
| Time spent in proximity, s | 571.23 ± 8.20 | 570.13 ± 5.60 | 569.21 ± 6.13 |
| Escape attempts, # | 0.08 ± 0.08 | 0.73 ± 0.36 | 0.53 ± 0.25 |
a,
b Means within a row with different superscripts differ (P < 0.02).
c,
d Means within a row with different superscripts tend to differ (P < 0.07).
There was no difference (P > 0.10) in the change of serum cortisol from before the behavioral test to after tests were completed (days 12 to 15) or serotonin levels at day 15. Serum cortisol increased similarly by 11.50 ± 2.86, 10.99 ± 2.33, and 17.75 ± 2.66 ng/mL for the Control, Medium, and High piglets, respectively. Serum serotonin levels did not differ (P > 0.10) at 1.44 ± 0.05, 1.49 ± 0.05, and 1.47 ± 0.06 μg/mL for the Control, Medium, and High piglets, respectively.
When the litters were allowed to comingle between two sows, we found no treatment differences in their behavior (P > 0.10). Throughout the duration of the comingling period, 18 ± 2% of piglets were active, 58 ± 2% inactive, 12 ± 1% nursing, and 2 ± 0.3% engaged in aggressive behavior. There was no difference in lesion severity after comingling. Lesions to the ears were scored 1.53 ± 0.08 (n = 14 litters), 1.29 ± 0.10 (n = 16 litters), and 1.4 ± 0.07 (n = 18 litters) for the Control, Medium, and High piglets, respectively (P > 0.10). Lesions to the shoulders were scored 0.84 ± 0.11, 0.64 ± 0.09, and 0.66 ± 0.11 for the Control, Medium, and High piglets, respectively (P > 0.10).
DISCUSSION
Several states in the United States have already banned individual gestation stalls for pregnant sows and other states are considering the regulation of sow gestational housing. This will require housing sows in groups. Pigs have a natural behavior to maintain social hierarchies in groups, and the order of hierarchy is established by fighting which typically settles in 72 h after they are mixed. Various approaches have been studied to reduce aggression in sows and improve welfare.
One method is to increase tryptophan in the diet (Poletto et al., 2014; Peden et al., 2018). Tryptophan is an essential amino acid which serves as a precursor for serotonin (5-hydroxytryptamine, 5-HT). Serotonin, in turn, regulates behavioral and physiological processes, such as elevation of mood, appetite, immunity, stress hormone secretion, and reduction of aggressive behavior (Bacqué-Cazenave et al., 2020). Li et al. (2006) found decreased fighting in tryptophan-fed, growing pigs after regrouping. Similarly, Poletto et al. (2010) have shown that aggression in young pigs when mixed could be reduced by short-term, high tryptophan dietary supplementation. In sows, they found that supplementing tryptophan decreased aggression and increased activity (Poletto et al., 2014). Warner et al. (1998) also reported similar results of reduced number of aggressive acts and a lower number of mounts in lairage after transportation. However, Li et al. (2011) found little impact of feeding tryptophan on sow aggression after mixing. It is unclear if these discrepancies are due to genetics, environment, or the duration/dose in which tryptophan is fed. Whether feeding higher amounts of tryptophan to pregnant sows will impact welfare and behavior of subsequent piglets has not been studied.
The impetus for this project developed around the idea of how prenatal stress, sometimes called fetal programming, alters development of the brain, which is thought to be due to the altering of feedback mechanisms and brain structures (Welberg and Seckl, 2001). If elevated concentrations of tryptophan enter the brain of developing piglets, then it is possible that the serotonergic system would be similarly altered. This could then have implications for mood, fear, aggression, and how animals respond to stress.
Serotonin is required for maturing post-synaptic areas that serotonin neurons project to as well as controlling the direction and degree of the growth of neurons (Whitaker-Azmitia et al., 1987). Serotonin, applied only 1 time to a fertile egg prior to being placed into an incubator (day 0), caused subsequent hens to be more fearful and less aggressive up to 18 wk of age (Dennis et al., 2013). The fetuses in our study would have been further developed, as they begin to attach to the uterus about day 14 and to implant about day 20, with neural development starting by day 25, and thus even more susceptible to change. Arevola et al. (1991) reported that tryptophan concentrations increased in different fetal organs, including the brain, when pregnant rats were orally administered high doses of tryptophan. Additional studies conducted in pregnant rats have shown that a tryptophan-enriched diet fed throughout pregnancy and lactation diminished serotonin and decreased activity of tryptophan hydroxylase in the cortex and in the brain stem of 5-d old rat pups (Huether et al., 1992). These data suggest that high levels of tryptophan can cross the placental barrier to further affect serotonin levels and action in offspring. However, no such studies have been conducted in pigs and it is unknown if increased dietary tryptophan in the sow diet will alter the serotonergic system in her offspring.
Overall, this research found that feeding high concentrations of tryptophan to pregnant sows from gestational days 28 to 35 has very little effect on them or their subsequent offspring. Feeding tryptophan had little effect on the behavior of the sows, although lying lateral was decreased for the sows fed the high tryptophan diet, which may indicate that they were more restless or possibly warmer. Poletto et al., (2014) also found that sows fed tryptophan were more active and spent more time standing. This contrasts with Li et al. (2006), however, who found increased lying behavior in tryptophan fed pigs, although they did not specify the lying posture. In the current study, productivity of the sows proved to be equal, with sows producing similar sized litters and number of weaned piglets, and thus lying posture did not affect piglet crushing rates. In addition to similar sow behavior, the time budget data also indicate similar amounts of activity for piglets in all three treatments, suggesting that tryptophan did not have profound effects on these animals.
The serotonergic system is implicated in the expression of aggression. For instance, pigs defined as aggressive, based on the results of a Resident-Intruder Test, had fewer cells in the amygdala that expressed mRNA for the serotonin receptor 1A (D’Eath et al., 2005). We examined if the treatments affected measures of aggression by assessing the pigs’ behavior during nursing, a Social Challenge Test, and comingling. Examining aggression during nursing bouts found that piglets from all three treatments had equal amounts of displacements and disputes. It is well known that piglets engage in aggressive behavior to gain access and control to a specific teat in the first days of life, and Scheel et al. (1977) showed that this is associated with dominance/subordinate behavior later in life. The observation that aggression was not altered relative to the amount of tryptophan the dam received during gestation indicates that the increased tryptophan did not alter the serotonergic system’s influence on aggression at this age and for this duration of supplementation.
The Social Challenge Test in this study did find that High piglets approached the head of another piglet more often, with Medium piglets in between. This measure was taken as a sign of boldness, because the approach was not followed by aggression, and thus may indicate less fear in these piglets. Cortisol is often used as a measure of stress when pigs are exposed to novel or challenging situations (Lay and Wilson, 2004). We found no differences among treatments in cortisol concentrations after the pigs were taken out of the social challenge, another indicator that the treatments did not affect the ability of the pigs to cope. Similarly, when we challenged the pigs with comingling of two separate litters, we found no difference in behavior. Activity, nursing, and aggression were equal among treatments and no differences in lesion scores were detected. Across all treatments, only 2% of piglets were observed in aggressive interactions, indicating that fights were low overall. It is possible that comingling is a significant stress and that any possible effect of serotonergic programming was over-ridden as Li et al. (2006) suggest that when tryptophan fed pigs are not being challenged with a stressor, or only mildly so, they are less excitable but when in a stressful situation, tryptophan is not useful in altering their response. However, given that measures during relatively mild stressors were not altered, this is unlikely. Thus, most measures of fear and aggression taken in this study were not different, indicating that any effect of maternal tryptophan is slight.
The Isolation Test and Human Approach Test were conducted to assess fear and anxiety-like behavior. Behavior during Isolation Tests and Novel Object Tests (similar to our Human Approach Test) have also been associated with differences in the serotonergic system. For instance, Ursinus et al. (2013) found that pigs subjected to such tests had negative correlations for serotonin turnover, but positive correlations for serotonin, in pigs that exhibited standing alert behavior during the test. In contrast, they found positive correlations for turnover and negative correlations for serotonin in the hippocampus for pigs exhibiting exploration. When rats are exposed to an anxiety inducing state, such as an Open Field Test, they spend more time in the center of the open field test and serotonin function in the amygdala, striatum, and ralphe nuclei is decreased (Wang et al., 2019). In sheep defined as calm or nervous based on behavior exhibited in an Open Field Test, Ding et al. (2020) found that four single nucleotide polymorphisms in genes for tryptophan 5-hydroxylase and two for the serotonin receptor differentiated the two populations.
We, however, did not find treatment differences for fear measurements in the Isolation Test. There were no differences in any behaviors, with pigs having similar latencies to activity, investigation, escape attempts, etc. Medium piglets did tend to have a shorter duration of vocalizations, and Medium male pigs had a greater harmonic-to-noise ratio. Harmonic-to-noise ratio measures tonality or harshness of a call, which can be an indicator of individual stress. Increases in harmonic-to-noise ratios can be found with increasing arousal in piglets (Puppe et al., 2005; Linhart et al., 2015) and therefore may be indicative of higher stress levels in Medium male piglets during the Isolation Test. However, Medium pigs tended to also have shorter durations of vocalizations, so the meaning of these findings are unclear.
In summary, feeding high concentrations of tryptophan for a short duration early in gestation does not have a negative impact on sows’ subsequent offspring, based on the measures used in this experiment. A great deal of research has examined the mechanisms and implications of prenatal stress (fetal programming), a process by which the brain is modified in response to peripheral factors during fetal development. This study tested the hypothesis that a similar phenomenon may occur in fetuses receiving elevated concentrations of tryptophan. This is an important question if producers begin feeding gestating sows tryptophan in order to decrease aggression when mixing, given that mixing occurs after placental implantation. Is is possible, however, that if tryptophan did alter the serotonergic system of piglets, it may occur when the brain is more fully developed at a later stage of gestation than the one explored here. Therefore, if tryptophan were to be fed to sows at high levels during later stages of gestation, future research would be warranted.
FUNDING
This work was partially funded by the National Pork Board (grant no. 15-066). The authors thank the Purdue University Farm staff and the employees of the USDA-ARS Livestock Behavior Research Unit for their support in animal care and data collection. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The findings and conclusions in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy.
Conflict of interest statement. None declared.
LITERATURE CITED
- Arevalo, R., Afonso D., Castro R., and Rodriguez M.. . 1991. Fetal brain serotonin synthesis and catabolism is under control by mother intake of tryptophan. Life Sci. 49:53–66. doi: 10.1016/0024-3205(91)90579-z [DOI] [PubMed] [Google Scholar]
- Bacqué-Cazenave, J., Bharatiya R., Barrière G., Delbecque J.-P., Bouguiyoud N., Giovanni G. D., Cattaert D., and Deurwaerdère P. D.. . 2020. Serotonin in animal cognition and behavior. International Int. J. Mol. Sci. 21:1649. doi: 10.3390/ijms21051649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel, N. M., Lucas J. R., Radcliffe J. S., Stewart K. R., and Lay D. C. Jr. 2018. Comparison of vocalization patterns in piglets which were crushed to those which underwent human restraint. Animals 8(8):138. doi: 10.3390/ani8080138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eath, R. B., Ormandy E., Lawrence A. B., Sumner B. E. H., and Meddle S. L.. . 2005. Resident-intruder trait aggression is associated with differences in lysine vasopressinand serotonin receptor 1A (5-HT1A) mRNA expression in the brain of pre-pubertal female domestic pigs (Sus scrofa). J. Neuroendocrinol. 17:679–686. doi: 10.1111/j.1365-2826.2005.01359.x [DOI] [PubMed] [Google Scholar]
- Dennis, R. L., Fahey A. G., and Cheng H. W.. . 2013. Alterations to embryonic serotonin change aggression and fearfulness. Aggress Behav. 39:91–98. doi: 10.3390/ani9110938 [DOI] [PubMed] [Google Scholar]
- Ding, L., Maloney S. K., Wang M., Rodger J., Chen L., and Blache D.. . 2020. Association between temperament related traits and single nucleotide polymorphisms in the serotonin and oxytocin systems in Merino sheep. Genes. Brain. Behav. 20:e12714. doi: 10.1111/gbb.12714 [DOI] [PubMed] [Google Scholar]
- Huether, G., Thömke F., and Adler L.. . 1992. Administration of tryptophan-enriched diets to pregnant rats retards the development of the serotonergic system in their offspring. Brain Res. Dev. Brain Res. 68:175–181. doi: 10.1016/0165-3806(92)90059-6 [DOI] [PubMed] [Google Scholar]
- Lay, D. C.Jr, and Wilson M. E.. . 2004. Considerations when using physiological data in assessing animal well-being. Anim. and Vet. Adv. 3(9):626–629. [Google Scholar]
- Li, Y. Z., Baidoo S. K., Johnston L. J., and Anderson J. E.. . 2011. Effects of tryptophan supplementation on aggression among group-housed gestating sows. J. Anim. Sci. 89:1899–1907. doi: 10.2527/jas.2010-3125 [DOI] [PubMed] [Google Scholar]
- Li, Y. Z., Kerr B. J., Kidd M. T., and Gonyou H. W.. . 2006. Use of supplementary tryptophan to modify the behavior of pigs1. J. Anim. Sci. 84:212–220. doi: 10.2527/2006.841212x [DOI] [PubMed] [Google Scholar]
- Linhart, P., Ratcliffe V. F., Reby D., and Špinka M.. . 2015. Expression of emotional arousal in two different piglet call types. PLoS One 10:e0135414. doi: 10.1371/journal.pone.0135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden, R. S., Turner S. P., Boyle L. A., and Camerlink I.. . 2018. The translation of animal welfare research into practice: The case of mixing aggression between pigs. Appl. Anim. Behav. Sci. 204:1–9. doi: 10.1016/j.applanim.2018.03.003 [DOI] [Google Scholar]
- Poletto, R., Kretzer F. C., and Hötzel M. J.. . 2014. Minimizing aggression during mixing of gestating sows with supplementation of a tryptophan-enriched diet. Physiol. Behav. 132:36–43. doi: 10.1016/j.physbeh.2014.04.043 [DOI] [PubMed] [Google Scholar]
- Poletto, R., Meisel R. L., Richert B. T., Cheng H.-W., and Marchant-Forde J. N.. . 2010. Aggression in replacement grower and finisher gilts fed a short-term high-tryptophan diet and the effect of long-term human–animal interaction. Appl. Anim. Behav. Sci. 122:98–110. doi: 10.1016/j.applanim.2009.11.015 [DOI] [Google Scholar]
- Puppe, B., Schӧn P., Tuchscherer A., and Manteuffel G.. . 2005. Castration-induced vocalization in domestic piglets, Sus scrofa: Complex and specific alterations of the vocal quality. Appl. Anim, Behav. Sci. 95:67–78. doi: 10.1016/j.applanim.2005.05.001 [DOI] [Google Scholar]
- Scheel, D. E., Graves H. B., and Sherritt G. W.. . 1977. Nursing order, social dominance and growth in swine. J. Anim. Sci. 45:219–229. doi: 10.2527/jas1977.452219x [DOI] [Google Scholar]
- Ursinus, W. W., Bolhuis J. E., Zonderland J. J., Rodenburg T. B., Souza A. S. D., Koopmanschap R. E., Kemp B., Korte-Bouws G. A., Korte S. M., and Reenen C. G. V.. . 2013. Relations between peripheral and brain serotonin measures and behavioral responses in a novelty test in pigs. Physiol. Behav. 118:88–96. doi: 10.1016/j.physbeh.2013.05.018 [DOI] [PubMed] [Google Scholar]
- Wang, L., Han D., Yin P., Teng K., Xu J., and Ma Y.. . 2019. Decreased tryptophan hydroxylase 2 mRNA and protein expression, decreased brain serotonin concentrations, and anxiety-like behavioral changes in a rat model of simulated transport stress. Stress 22:707–717. doi: 10.1080/10253890.2019.1625328 [DOI] [PubMed] [Google Scholar]
- Warner, R. D., Eldridge G. A., Hofmeyr C. D., and Barnett J. L.. . 1998. The effect of dietary tryptophan on pig behavior and meat quality-preliminary results. Anim. Prod. Aust. 22: 325. [Google Scholar]
- Welberg, L. A. M., and Seckl J. R.. . 2001. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13:113–128. doi: 10.1111/j.1365-2826.2001.00601.x [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia, P. M., Lauder J. M., Shemmer A., and Azmitia E. C.. . 1987. Postnatal changes in serotonin receptors following prenatal alterations in serotonin levels: further evidence for functional fetal serotonin receptors. Brain Res. 430:285–289. doi: 10.1016/0165-3806(87)90161-1 [DOI] [PubMed] [Google Scholar]