Abstract
There have been few reports on the imaging characteristics of cryptococcus neoformans (C. neoformans) infection of the breast. Herein, we reported the imaging features of C. neoformans infection of the breast in a 41-year-old woman with immune thrombocytopenic purpura. Bilateral, diffuse, hyperechoic, and well-defined margin lesions were observed on breast ultrasounds. In addition, a global asymmetry in the left breast, and a focal asymmetry in the right breast were observed on mammograms. Breast fine needle aspiration and biopsy results revealed a C. neoformans infection. After 5 months of treatment with oral fluconazole and amphotericin B, the lesion on the right breast disappeared on repeated-breast ultrasounds.
Keywords: Immune thrombocytopenic purpura, cryptococcus neoformans, Breast infection, Case report
Introduction
Cryptococcus affects people of all ages worldwide [1]. Cryptococcus infection in humans generally occurs due to inhalation of a number of yeasts or spores of Cryptococcus neoformans (C. neoformans) existing in the environment [2]. Most cases (90%) with this infection evolve to hematogenous dissemination, with special damage for the central nervous system leading to cryptococcal meningitis [3].
Corticosteroids are the first-line therapy for patients with immune thrombocytopenic purpura (ITP). However, long-term treatment with corticoid is a risk factor for invasive C. neoformans diseases [4]. Clinical manifestations due to C. neoformans typically occur in the lung and central nervous system, and have been scarcely reported to occur in the breast [5]. The imaging characteristics of Cryptococcal infection in the breast have not been well documented in the current literature. Therefore, we reported a case of bilateral Cryptococcal infection of the breasts in a female patient who had been diagnosed with ITP for 3 years at Bach Mai Hospital, Vietnam.
Case presentation
A 41-year-old housewife was admitted to Bach Mai Hospital after 3 weeks of prolonged high fever (39-40°C), chilly feeling, headache, dry cough, and cutaneous lesion in the chest and back with painful masses and enlargement of both breasts. The patient's medical history was remarkable with using corticoid for ITP treatment. The patient had a primary ITP for about 3 years, and was treated with low-dose maintenance methylprednisolone (8 mg per day). The patient did not suffer other underlying diseases nor receive other immunosuppressive drugs. Besides, the human immunodeficiency virus (HIV) antibody was found to be negative.
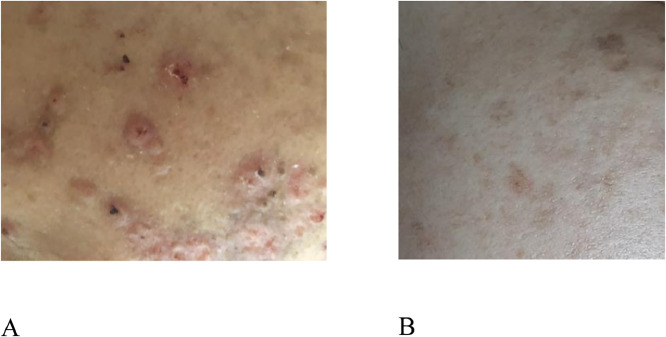
The patient's temperature was 40°C on admission. There were multiple tiny erythematous papules (5 mm in diameter) with center necrosis and brown crusts on her face, chest, abdomen, and back (Fig. 1A). One month after the first fever episode, the patient noted painful masses, and enlargement in both breasts. On physical examination, these masses were mobile, firm, and large. There was no change in the appearance of the breast skin. There was no nipple discharge nor axillary lymphadenopathy. The patient's dyspnea also became severe, requiring oxygen supplement.
Fig. 1.
Skin lesion on the patient's face: (A) Erythematous papules (5 mm in diameter) with center necrosis and brown crusts. (B) After 5 months of treatment, the skin lesion reduced.
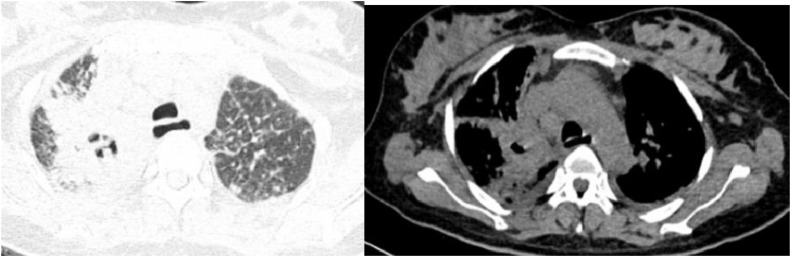
Lung computed tomography demonstrated a poor-margin consolidation with air-bronchogram and cavitation in the upper lobe of the right lung. There were also scattered and small nodules in the other parts of both lungs and bilateral pleural effusion (Fig. 2). Brain MRI showed no abnormality.
Fig. 2.
Lung computed tomography: Poor-margin, large consolidation with air-bronchogram, and cavitation in the upper lobe of right lung. There were scattered and small nodules in the other parts of both lungs and bilateral pleural effusion.
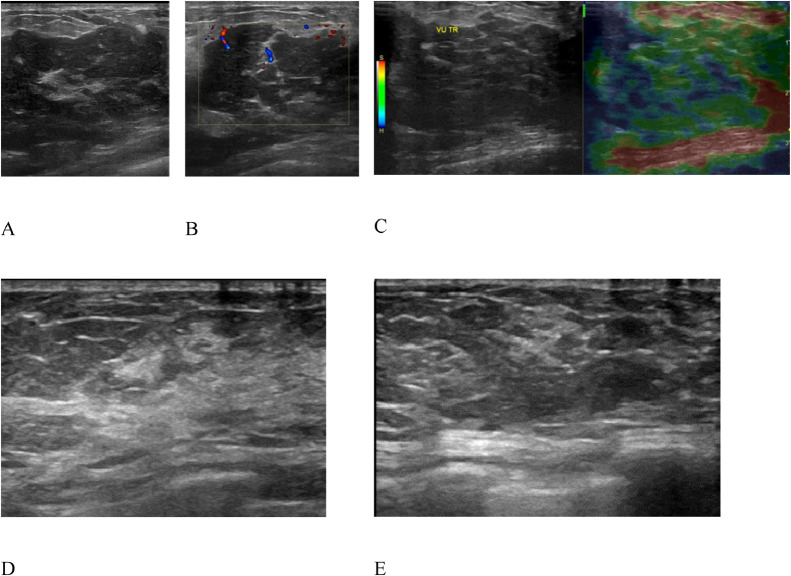
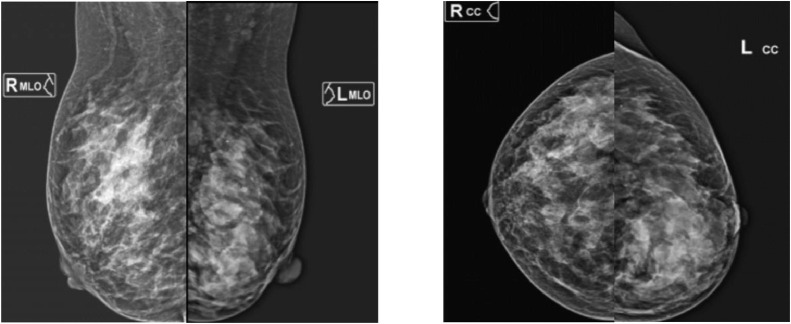
Breast ultrasound showed diffuse, hypoechoic, homogeneous, and well-defined margin lesions that dominant in the left breast. Slight edema and fat infiltration were observed in the surrounding area. The lesion was soft on elastography and had a vascular signal on Doppler mode, which was categorized as BI-RADS U4 (Fig. 3A-C). Mammography revealed a global asymmetry in the left breast. In the upper outer quadrant of the right breast, a focal asymmetry was denser than in other areas (Fig. 4). There was no mass, suspicious calcification, or architectural distortion in both breasts. Therefore, the lesions were classified as BI-RADS 3 on mammography.
Fig. 3.
Ultrasound examination of left breast: (A-C) Diffuse, hypoechoic, and well-defined margin lesion; dominant in the left side. After 5 months of treatment: no lesion on the right breast (D), a 25-mm hyperechoic, and ill-defined lesion on the left breast (E).
Fig. 4.
Mammography examination of bilateral breast: global asymmetry in the left breast, a focal asymmetry in the upper outer quadrant of the right breast.
Laboratory results revealed a white blood cell count of 16.2 g/L and a platelet count of 85 g/L. The culture of blood was positive with C. neoformans 4 times. Examination of cerebrospinal fluid (CSF) showed increased protein levels (1.12 g/L). Pandy's reaction and the culture of CSF were positive.
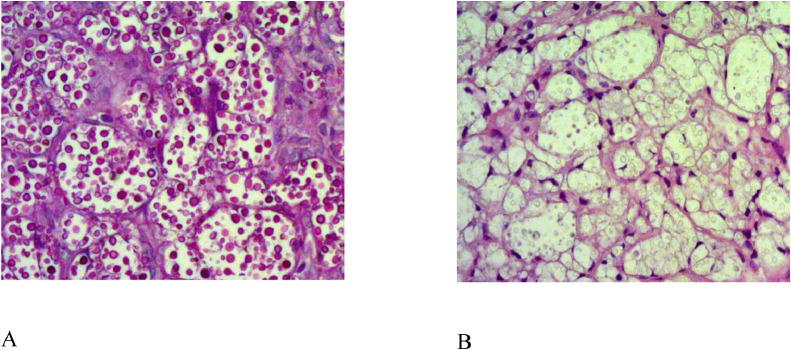
Specimens in the upper-outer quadrant of the left breast were obtained using a 14G-core needle biopsy. In addition, a fine needle aspiration was performed in the lesion of the right breast. Pathology result demonstrated C. neoformans infection of the left breast (Fig. 5). Gram stain of the right aspiration showed yeast cells. Culture at 37°C identified as C. neoformans.
Fig. 5.
Histologic tissue section of the left breast biopsy shown: encapsulated yeast form consistent with Cryptococcus (A) Hematoxylin-Eosin staining, (B) Periodic Acid Schiff staining.
The patient was treated with oral fluconazole 900 mg daily and intravenous amphotericin B 50 mg daily for 2 weeks and maintained with fluconazole alone. After 5 months of treatment, the cutaneous presentation reduced (Fig. 1B). On follow-up ultrasound findings, the right breast parenchyma became normal, whereas on the left breast, the lesion diminished as a hyperechoic, ill-defined small mass (Fig. 3D-E).
Discussion
C. neoformans is a ubiquitous environmental fungus that can cause human disease. A case-control study investigating the risk factors of invasive C. neoformans infection confirms autoimmune disease is an independent risk factor for the occurrence of cryptococcemia [6]. Immunodeficiency is one of the common features reported in most cases of cryptococcal breast infection or abscess [5,7,8].
Before the Acquired Immunodeficiency Syndrome (AIDS) epidemic, the number of C. neoformans infection among patients with AIDS, and immunocompromised patients without AIDS was equal [9]. Nowadays, the incidence of Cryptococcosis infection in patients with HIV decreases due to antiviral treatment. However, patients without HIV, especially those with underlying medical conditions or predisposing factors, are still at a high risk of C. neoformans infections [10]. In our case report, the patient had a negative HIV test but had been on long-term ITP treatment with corticosteroids, which is a risk factor for C. neoformans infection.
Cryptococcal infection and abscess formation can affect almost all parts of the body; however, lesion in the human breast is uncommonly seen. The common initial sites of Cryptococcal infection include the lung and the brain, which primarily suggests pathogenesis through the respiratory tract. However, various infection sites reported on the skin, lung, parotid, muscle, breast, or mediastinal abscess in the literature favors the hypothesis of hematogenous or lymphatic dissemination [5,11,12]. In the present reported patient, the culture of blood, CSF, and breast aspiration were positive, which proved hematogenous spreading and might explain why the breast infection was bilateral, and diffuse.
Several case reports have shown that cryptococcus mastitis presenting as an isolated focus of infection that may mimic a neoplasm in imaging modalities. A study on 9 patients described a patient with a 4-cm well-circumscribed mass of the right breast on a mammogram [7]. Another case report described a woman infected with C. neoformans whose mammography and ultrasound examinations revealed a 3-cm oval lesion in the left side with hyperechoic content, suggesting a breast abscess [4].
Interestingly, our patient had diffuse lesions on both the left, and the right breasts. Theoretically, the breast can be diffusely affected by a variety of disorders such as physiology changes, inflammation or infection, as well as benign or malignancy tumors [13]. Although diffuse lesions related to malignancy are rare, they should be considered the most important differential diagnosis in our case.
Bilateral inflammatory breast carcinoma is the first differential diagnosis for its rapid onset of enlargement and diffuse lesions on imaging with a “peau d'orange” texture in the breast skin [13]. However, our patient had no change in breast skin appearance. Besides, bilateral breast lesions related to secondary malignancy should be considered [14]. While hematogenous metastasis manifests as a single mass or multiple masses, lymphangitic metastasis usually shows diffuse infiltration. Therefore, the patients’ clinical history, the detailed information concerning unilateral and/or bilateral diffuse enlargement or asymmetry, and biopsy are helpful. In our case, C. neoformans was demonstrated in all specimens.
Furthermore, bilaterality, and other organ infection suggested a disseminated disorder in our patient. Disseminated cryptococcosis is defined by (1) a positive culture from at least 2 different sites, or (2) a positive blood culture [15]. Cryptococcal infection in other organs was reported; however, cryptococcal mastitis is extremely rare. The diagnosis of cryptococcal mastitis required isolation of fungus in the culture, histologic evidence of fungus, or positive latex agglutination testing for serum or CSF cryptococcal antigen [7]. All positive criteria were found in our patient.
Cryptococcus infection may become more dangerous if not diagnosed and treated early. The antifungal pharmacologic treatment is limited to amphotericin B, fluconazole, and 5-flucytosine [16]. Systemic antifungal therapy has been shown to be effective in previous reported cases and has been highly recommended for reducing the risk of recurrent infection or spreading to other sites [4,7]. This case was successfully treated using the first dose of 50 mg of amphotericin B and 900 mg of fluconazole for 5 months, followed by maintained therapy with fluconazole only. If the lesion is a focal mass, surgical excision combined with antifungal therapy can also be considered [7].
Conclusion
Diffuse hypoechoic patchy on ultrasound and focal or global asymmetric density on mammography are the special radiological characteristics in our case with C. neoformans infection of the breasts. Diagnostic imaging techniques can be used in this case as a method to diagnose and evaluate the disease progression and therapeutic response.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Footnotes
Competing Interests: The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Nidhi A., Meena A., Sreekumar A., Daga M.K. Corticosteroid-induced cryptococcal meningitis in patient without HIV. BMJ Case Rep. 2017 doi: 10.1136/bcr-2016-216496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May R.C., Stone N.R.H., Wiesner D.L., Bicanic T., Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14(2):106–117. doi: 10.1038/nrmicro.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maziarz E.K., Perfect J.R. Cryptococcosis. Infect Dis Clin North Am. 2016;30(1):179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanbury R.M., Graham E.M. Systemic corticosteroid therapy–side effects and their management. Br J Ophthalmol. 1998;82(6):704–708. doi: 10.1136/bjo.82.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schouten W.E.M., Damen M., Davids P.H.P., Ketel R.J.V., Prins J.M. Cryptococcal breast abscess. Scand J Infect Dis. 2002;34(4):309–310. doi: 10.1080/00365540110080197. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y.-Y., Shiau S., Fang CT. Risk factors for invasive cryptococcus neoformans diseases: a case-control study. PLoS One. 2015;10(3):e0119090. doi: 10.1371/journal.pone.0119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman M., Pottage J.C. Cryptococcal infection of the breast. Clin Infect Dis. 1995;21(5):1166–1169. doi: 10.1093/clinids/21.5.1166. [DOI] [PubMed] [Google Scholar]
- 8.Haddow L.J., Sahid F., Moosa M.-Y.S. Cryptococcal breast abscess in an HIV-positive patient: Arguments for reviewing the definition of immune reconstitution inflammatory syndrome. J Infect. 2008;57(1):82–84. doi: 10.1016/j.jinf.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Speed B., Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21(1):28–34. doi: 10.1093/clinids/21.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Kiertiburanakul S., Wirojtananugoon S., Pracharktam R., Sungkanuparph S. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis. 2006;10(1):72–78. doi: 10.1016/j.ijid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T., Fukuda K. Cryptococcus: a rare cause of parotid abscess in liver cirrhosis. Case Reports Hepatol. 2020;2020:2020. doi: 10.1155/2020/8849448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashida M.Z., Seque C.A., Pasin V.P., Enokihara M.M.S.E.S, Porro A.M. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92(5 Suppl 1):69–72. doi: 10.1590/abd1806-4841.20176343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An Y.Y., Kim S.H., Cha E.S., Kim H.S., Kang B.J., Park C.S. Diffuse infiltrative lesion of the breast: clinical and radiologic features. Korean J Radiol. 2011;12(1):113–121. doi: 10.3348/kjr.2011.12.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vizcaíno I., Torregrosa A., Higueras V., Morote V., Cremades A., Torres V. Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. Eur Radiol. 2001;11(9):1659–1665. doi: 10.1007/s003300000807. [DOI] [PubMed] [Google Scholar]
- 15.Yehia B.R., Eberlein M., Sisson S.D., Hager D.N. Disseminated cryptococcosis with meningitis, peritonitis, and cryptococcemia in a HIV-negative patient with cirrhosis: a case report. Cases Journal. 2009;2(1):170. doi: 10.1186/1757-1626-2-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spadari C.C., de C., Wirth F., Lopes L.B., Ishida K. New approaches for cryptococcosis treatment. Microorganisms. 2020;8(4) doi: 10.3390/microorganisms8040613. [DOI] [PMC free article] [PubMed] [Google Scholar]