Abstract
Background
The incidence of cardiovascular events in perioperative period of gastrointestinal tumor surgery cannot be ignored, and studies have shown that level of postoperative troponin is related to the postoperative risk of non-cardiac surgery. However, the relationship between pre-operative troponin levels and perioperative risk of gastrointestinal tumor surgery is unclear. Thus, we aimed to evaluate the value of high-sensitive cardiac troponin I (hs-cTnI) prior to gastrointestinal tumor surgery for perioperative risk assessment.
Methods
In this retrospective cohort study, 1259 patients who underwent gastrointestinal tumor surgery and had been tested for hs-cTnI on admission within 7 days prior to surgery were retrospectively recruited from January 2018 to June 2020. The primary combined endpoint including in-hospital all-cause mortality, acute myocardial infarction, cardiac arrest or ventricular fibrillation and acute decompensated heart failure. The secondary endpoint included total hospital stay and requirement of intensive care treatment.
Findings
Compared with patients with normal hs-cTnI, those with elevated hs-cTnI (> 0·028 ng/ml) were more likely to experience the combined endpoint (28·2% versus 2·7%, P < 0·001) and there was also an increasing rate of in mortality in elevated hs-cTnI group (2·4% versus 0·3%, P = 0·057). The length of total hospital stay was significantly longer in patients with elevated hs-cTnI (24·8 ± 16·3 versus 19·5 ± 7·9, P = 0·003) and the number of patients requiring intensive care treatment was also higher (22·6% versus 4·2%, P < 0·001). The area under the ROC curve assessing hs-cTnI in predicting in-hospital mortality was 0·787 [95% confidence interval (CI) 0·612–0·963, P = 0·015] and for combined endpoint was 0·822 [95% CI 0·766–0·879, P < 0·001]. Hs-cTnI > 0·028 ng/ml was associated with significantly higher cardiovascular event rate in patients with the revised cardiac index ≤ 1. The positive likelihood ratio of hs-cTnI (> 0·028 ng/ml) for predicting combined endpoint reaches 10.5 in patients with Lee index = 0. In multivariate logistic analyses, hs-cTnI was one of the best predictors for the combined endpoint [odds ratio (OR) 5·924 (95%CI: 2·869–12·233), P < 0·001].
Interpretation
Hs-cTnI provides powerful prognostic information for patients undergoing gastrointestinal tumor surgery, and therefore provides reliable prognostic information incremental to revised cardiac index.
Keywords: Gastrointestinal tumor surgery, Perioperative risk, High-sensitive troponin i
Research in context.
Evidence before this study
We searched Pubmed, Google scholar and China National Knowledge infrastructure for articles using the terms (“troponin” or “cardiac” or “cardiovascular” AND “surgery” or “operation”), with no date restrictions and not limited to English-language publications. We found one systematic review and several original studies showing that pre-operative troponin levels are associated with adverse events and mortality after noncardiac surgery. However, these studies mainly focused on patients undergoing vascular surgery or other operations, and the sample size of gastrointestinal tumor surgery is limited. As the incidence rates of gastrointestinal tumor increased markedly around the world, we therefore perform a study to evaluate the value of pre-operative troponin for risk stratification of patients undergoing gastrointestinal tumor surgery.
Added value of this study
We showed that high sensitive-cardiac troponin I (hs-cTnI) provide predictive information for the occurrence of serious cardiovascular events in receiving gastrointestinal tumor surgery patients, which is incremental to the revised cardiac risk index. Moreover, pre-operative levels of hs-cTnI were associated with the length of hospital stay and the necessity of intensive care treatment.
Implication of all the available evidence
Hs-cTnI plays an important role in perioperative risk stratification in patients with gastrointestinal tumors. This study provided additional prognostic information of surgery to many gastrointestinal surgeons in the world. Moreover, it can also help surgeons and oncologists determine the best course of action for gastrointestinal tumor patients, so as to promote the clinical development. Further multiple-center prospective studies are needed be carried out to verify this conclusion.
Alt-text: Unlabelled box
1. Introduction
The incidence rates of gastrointestinal tumor increased markedly around the world, which seriously threaten human health. Surgical resection is the most important treatment strategy [1,2]. Although great progress had been made in surgical technique, anesthetic management and postoperative care as well over the past decades, surgical interventions are still companied by relevant cardiovascular morbidity and mortality [3,4].
Therefore, preoperative risk assessment of patients is particularly important. Currently, several clinical risk indices are applied in practice. The most used one is the Lee's revised cardiac risk index [5]. However, the study used to develop the decision aids had relied on small numbers of gastrointestinal tumor patients (324 cases for abdominal surgery) and had not been validated with larger cohort of patients [5]. Therefore, it is urgently needed to improve the perioperative risk assessment of gastrointestinal tumor patients.
Cardiac troponin assays provide rapid and specific detection of myocardial injury and are essential for the diagnosis of acute myocardial infarction (AMI) [6,7]. Moreover, cardiac troponin is also a valuable prognosis marker for patients with acute coronary syndrome, non-cardiac medical inpatients, critically ill patients or COVID-19 patients [8], [9], [10], [11]. More importantly, level of troponin is heightened in patients with several cardiovascular disease such as stable coronary heart disease or heart failure, reflecting minor myocardial injury and affecting prognosis [12,13].
Several studies have established the association between pre-operative troponin levels and major adverse cardiac events (MACE) and mortality after non-cardiac surgery. However, these studies mainly focused on patients undergoing vascular surgery or other operations, and the number of patients with gastrointestinal tumors was relatively small (0–254 cases undergoing abdominal surgery) [4,[14], [15], [16], [17]]. Troponin elevation after non-cardiac surgery is a predictor of mortality after major abdominal colorectal surgery[18,19]. However, logically speaking, the study of preoperative troponin elevation on perioperative risk is more significant.
The objective of our study was to evaluate the value of cardiac troponin I (cTnI) measured by a new high-sensitive troponin I assay (hs-cTnI) for risk stratification of patients undergoing gastrointestinal tumor surgery.
2. Methods
2.1. Study design and participants
The study was conducted as a single center retrospective cohort study including patients with gastrointestinal tumor and scheduled for surgery. We collected the data of 1259 consecutive patients between 2018 and 01 and 2020–06 in the sixth affiliated hospital of Sun Yat-sen University, sited in Guang Zhou, China. The study methods were compliant with the STROBE checklist (supplement file - 1).
Inclusion criteria were major abdominal surgery in general anesthesia, an age ≥ 18 years and had been tested for hs-cTnI on admission whin 7 days prior to surgery. Exclusion criteria were emergent surgery, failure to perform surgery and there was clinical evidence of unstable coronary artery disease (cardiac chest pain with or without ischemic electrocardiograph changes) according to medical record at the time of pre-operative evaluation.
The study has been approved by the local ethical boards of the sixth affiliated hospital of Sun Yat-sen University.
2.2. Study definitions
The diagnosis of myocardial infarction was based on the universal definition of myocardial infarction [6]. History of coronary artery disease (CAD), defined as prior bypass surgery, coronary intervention, myocardial infarction or compliance with guideline definition [20,21].
The Lee index (revised cardiac index) was calculated as described previously. Briefly, one point was assigned to each of the following factors: high-risk type of surgery, ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease, insulin therapy for diabetes and preoperative serum creatinine > 2·0 mg/dl (176·8 μmol/L).
2.3. Exposure and clinical endpoints
The patients were divided into elevated hs-cTnI level group and normal hs-cTnI level group. The primary combined endpoint included all-cause mortality, acute myocardial infarction, cardiac arrest or ventricular fibrillation and acute decompensated heart failure during hospitalization. The secondary endpoints included total hospital stay and requirement of intensive care treatment. The definition of the "requirement of intensive care treatment" is that during the period from postoperative to discharge, patients was evaluated by anesthesiologists and surgeons and decided to be transferred to intensive care unit (ICU) for further support treatment or monitor for various reasons such as hemodynamic instability, low oxygen saturation requires ventilator assisted breathing, acute kidney injury needs continuous renal replacement therapy and so on.
2.4. Data collection
Clinical, laboratory, echocardiographic parameters at baseline, medication, surgery procedure, operation time and outcome were all collected from medical records. Patients were followed-up till discharge.
Blood samples were taken within 7 days prior to surgery. Samples were taken from an antecubital vein in tubes without additives and processed immediately. Serum was separated by centrifugation and frozen at −70 °C until analyses. All analyses were performed at the clinical lab of the sixth affiliated hospital of Sun Yat-sen university.
C-TnI was measured by a high-sensitive electrochemiluminescence-immunoassay on an automatic analyzer (ABBOTT, Architect i1000SR). The 99th percentile reference value of hs-cTnI was 0·028 ng/ml.
All echocardiographic examinations were performed during hospitalization before surgery. The images were obtained in accordance with the guidelines of the American Society of Echocardiography [22], and stored using a digitized ultrasound system (GE vivid E9).
2.5. Statistical analysis
We used PASS11 software to estimate the sample size in advance, with supposed beta = 0·2, α = 0·05, according to the results of previous literature [16]. The ratio of case in combined endpoints group versus control group was 1:26 (36/943), HR = 2·6 and the proportion of troponin increased in the control group was about 22·4%. We got the optimal sample size: there were 39 cases in the events group and 1014 cases in the non-events group.
Continuous variables are expressed as means ± standard deviation (SD). Categorical variables are expressed as frequencies (percentage). Continuous variables were compared using the independent t-test and categorical variables compared using the chi-squared test or Fisher's exact test where appropriate. The missing values on the database were excluded from the analysis.
To evaluate test performance of hs-cTnI and the Lee index as predictors for mortality, and combined endpoint, respectively, the area under the curve (AUC) of the receiver operating characteristics curve (ROC) has been calculated. The sensitivity, specificity, positive predictive value and negative predictive value of hs-cTnI in predicting combined endpoint were calculated and further analysis according to Lee index stratification. Odds ratios for all clinical variables were calculated by univariate logistic regression analyses. Multivariate logistic regression analysis was performed for the combined endpoint, candidate variables consisted of age, hs-cTnI, laparoscope, low density cholesterol (LDL), left ventricular ejection fraction (LVEF), left atrium (LA), interventricular septum (IVS), left ventricular posterior wall (LVPW), Lee score, neutrophil granulocyte ratio (NEUR), hypertension and atrium fibrillation. The statins, metformin and other drugs taken by patients are related to the history of coronary heart disease, diabetes, hypertension, and atrial fibrillation, but these indicators have been covered in the Lee index and the history of hypertension or atrial fibrillation. Therefore, considering the possibility of collinearity, we exclude the drugs information in the multivariate logistic regression analysis. Internal validation was performed by applying bootstrap resample method (1000 times) to assess model optimism. Calibration was based on the Hosmer and Lemeshow test and discrimination on the receiver operating characteristic curve.
Statistical analyses were done using IBM SPSS software (version 22). The area under ROC curve of the two groups was compared by MedCalc software (version 15). A two-sided alpha of less than 0·05 was considered statistically significant.
2.6. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
3. Results
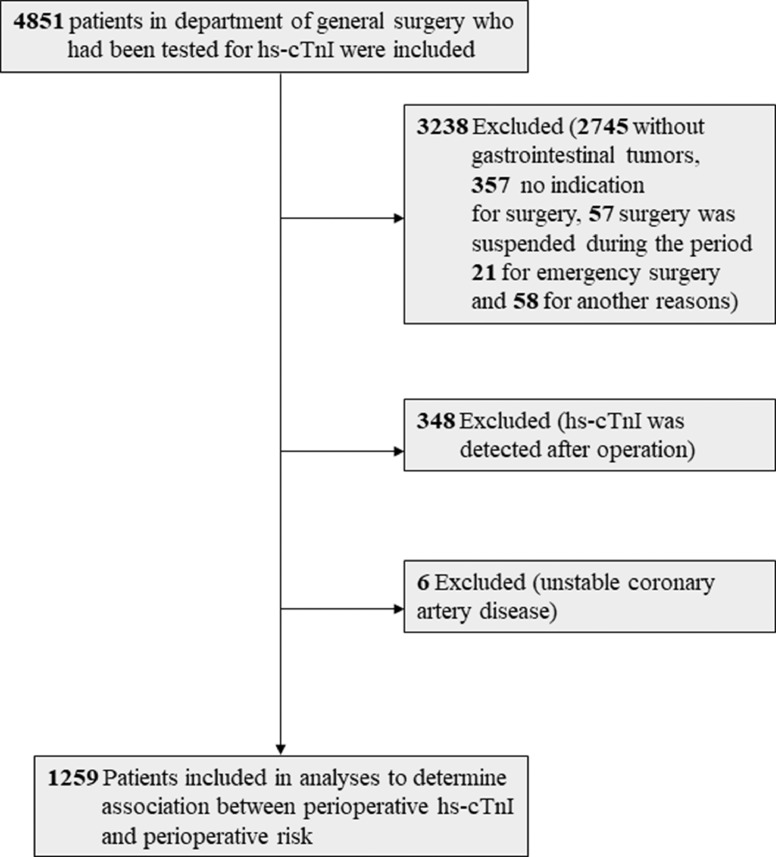
The final sample size consisted of 1259 patients (Fig. 1). Baseline characteristics of the patients are presented in Table 1. A total of 1259 patients were enrolled, 35·7% females, mean age 62·7 years. A history of CAD was present in 7·3% of the patients. Hypertension was the most common cardiovascular risk factor, followed by smoking and diabetes. 17% patients were under cardiovascular medical treatment (2·4% for aspirin, 2·6% for clopidogrel, 3·3% for β-blocker, 6·4% for ACE inhibitor/ARB, 9·7% for calcium channel blocker, 3·7% for statins and 3·5% for metformin). 12·5% of the patients received chemotherapy and 1·7% for radiation therapy. In Lee index, 88·4% patients scored 0, 9·7% scored 1, 1·9% scored 2 or above.
Fig. 1.
Flow diagram that shows the process of enrollment and exclusion. Hs-cTnI, high sensitive – cardiac troponin I.
Table 1.
Baseline characteristics.
| All patients | Non-Events | Events | P - value | |
|---|---|---|---|---|
| n (%) | 1259 | 1203 (95·6) | 56 (4·4) | |
| Gender (female) | 449 (35·7) | 431 (35·8) | 18 (32·1) | 0·213 |
| Age (years) | 62·7 ± 11·9 | 62·3 ± 11·8 | 72·6 ± 10·2 | < 0·001** |
| BMI (kg/m2) | 22·4 ± 3·3 | 22·4 ± 3·3 | 22·3 ± 3·5 | 0·732 |
| Smoke | 147 (11·7) | 124 (10·3) | 6 (10·7) | 0·953 |
| NYHA class II-IV | 16 (1·3) | 14 (1·2) | 6 (7·1) | < 0·001** |
| CAD | 92 (7·3) | 79 (6·6) | 13 (23·2) | < 0·001** |
| Hypertension | 285 (22·6) | 255 (21·2) | 30 (53·6) | < 0·001** |
| Atrial fibrillation | 14 (1·1) | 9 (0·7) | 5 (8·9) | < 0·001** |
| Diabetes mellitus | 132 (10·5) | 119 (9·9) | 13 (23·2) | 0·001* |
| Insulin dependent | 18 (1·4) | 16 (1·3) | 2 (3·6) | 0·189 |
| Aspirin | 30 (2·4) | 27 (2·2) | 3 (5·4) | 0·296 |
| Clopidogrel | 33 (2·6) | 29 (2·4) | 4 (7·1) | 0·082 |
| Anticoagulation | 23 (1·8) | 17 (1·4) | 6 (10·7) | < 0·001** |
| β-blocker | 42 (3·3) | 34 (2·8) | 8 (14·3) | < 0·001** |
| ACE inhibitor/ARB | 80 (6·4) | 68 (5·7) | 12 (21·4) | < 0·001** |
| ARNI | 2 (0·2) | 2 (0·2) | 0 (0) | 1 |
| CCB | 122 (9·7) | 115 (9·6) | 7 (12·5) | 0·467 |
| Statins | 47 (3·7) | 40 (3·3) | 7 (12·5) | 0·001* |
| Diuretic | 21 (1·7) | 18 (1·5) | 3 (5·4) | 0·063 |
| Metformin | 44 (3·5) | 38 (3·2) | 6 (10·7) | 0·008* |
| Chemotherapy | 158 (12·5) | 153 (12·7) | 5 (8·9) | 0·403 |
| Radiation therapy | 22 (1·7) | 22 (1·8) | 0 (0) | 0·618 |
| Lee index | ||||
| 0 | 1113 (88·4) | 1077 (89·5) | 36 (64·3) | < 0·001** |
| 1 | 122 (9·7) | 106 (8·8) | 16 (28·6) | |
| ≥ 2 | 24 (1·9) | 20 (1·7) | 4 (7·1) | |
| HR (beats per min) | 80·6 ± 12·8 | 80·6 ± 12·8 | 79·5 ± 13·0 | 0·544 |
| SBP (mmHg) | 126·1 ± 18·2 | 126·0 ± 18·3 | 128·3 ± 7·5 | 0·361 |
| DBP (mmHg) | 76·9 ± 10·6 | 77·0 ± 10·6 | 75 ± 11·4 | 0·165 |
Continuous variables are expressed as means ± SD. Categorical variables are expressed as frequencies (percentage). n, number; BMI, body mass index; NYHA, New York Heart Association; CAD, coronary artery disease; ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blocker; CCB, Calcium channel blocker; ARNI, angiotensin receptor-neprilysin inhibitors; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure. *P ≤ 0·05, **P < 0·001.
Patients who met events were elder, had more frequently a history of chronic disease (such as hypertension, CAD, diabetes and atrium fibrillation), a premedication (such as anticoagulation, β-blocker, Angiotensin-converting enzyme inhibitor/Angiotensin receptor blocker (ACEI/ARB), statins, or metformin), higher Lee index, neutrophil granulocyte ratio (NEUR), low density lipoprotein (LDL) and hs-cTnI and worse cardiac structure and function (Tables 1 and 2). The information of surgery was shown in Table 3. The results showed that there was no difference in operation site, operation time and anesthesia time between the two groups. Although the proportion of endoscopy in the event group is low, an explanation is that anesthesiologists usually think that patients with elevated hs-cTnI have high cardiovascular risk and do not tolerate pneumoperitoneum. The chemotherapy regimen, scheme and proportion of neoadjuvant chemotherapy were also provided (Supplementary file - 2). We can see that there was no significant difference in the chemotherapy regimens between the events group and the non-events group.
Table 2.
Laboratory index, electrocardiograph and echocardiography on admission.
| All patients | Non-Events | Events | P -value | |
|---|---|---|---|---|
| Hemoglobin, g/L (n = 1201 vs 56) | 119·0 ± 24·6 | 119·3 ± 24·7 | 114.9 ± 22.1 | 0·123 |
| WBC, × 109/L (n = 1200 vs 56) | 6·6 ± 3·1 | 6·5 ± 3·1 | 7·1 ± 2·3 | 0·151 |
| NEUR,% (n = 1201 vs 56) | 29·7 ± 30·8 | 28·8 ± 30·5 | 48·6 ± 30·9 | < 0·001** |
| PCT, ng/ml, (n = 153 vs 4) | 0·05 ± 0·59 | 0·008 ± 0·01 | 1·8 ± 3·7 | 0·391 |
| CRP, mg/L (n = 1125 vs 49) | 5·4 ± 17·53 | 5·2 ± 17·1 | 9·8 ± 25·2 | 0·212 |
| Creatine, μmol/L (n = 1193 vs 55) | 78·7 ± 44·3 | 77·5 ± 30·6 | 106·5 ± 154·4 | 0·169 |
| LDL, mmol/L (n = 1149 vs 56) | 3·2 ± 0·9 | 3·2 ± 0·9 | 2·8 ± 0·9 | 0·002* |
| AST, U/L (n = 1197 vs 55) | 21·8 ± 13·2 | 21·7 ± 12·8 | 23·3 ± 20·1 | 0·379 |
| ALT, U/L (n = 1197 vs 55) | 18·6 ± 17·3 | 18·6 ± 16·8 | 18·4 ± 25·4 | 0·907 |
| TBIL, g/L (n = 1174 vs 56) | 12·3 ± 7·8 | 12·4 ± 7·9 | 11·0 ± 4·1 | 0·185 |
| DBIL, g/L (n = 1173 vs 56) | 2·7 ± 4·0 | 2·7 ± 4·1 | 2·6 ± 2·3 | 0·901 |
| CKMB, U/L (n = 1102 vs 54) | 16·6 ± 23·2 | 16·6 ± 23·3 | 15·8 ± 21·5 | 0·799 |
| BNP, pg/ml (n = 72 vs 20) | 211·6 ± 469·6 | 169·6 ± 492·9 | 362·8 ± 342·5 | 0·104 |
| Myoglobin, ng/ml (n = 1203 vs 565) | 47·2 ± 87·8 | 44·5 ± 64·9 | 104·7 ± 284·3 | 0·12 |
| Hs-cTnI, ng/ml (n = 1203 vs 56) | 0·01 ± 0·11 | 0·008 ± 0·024 | 0·141 ± 0·498 | 0·049* |
| D-dimer, mg/L (n = 670 vs 38) | 0·9 ± 1·5 | 0·9 ± 1·5 | 1·3 ± 1·7 | 0·097 |
| CEA, ng/ml (n = 1184 vs 55) | 31·3 ± 289·5 | 29·3 ± 291·1 | 74·1 ± 249·9 | 0·262 |
| CA125, U/L (n = 1188 vs 55) | 23·7 ± 69·1 | 23·1 ± 70·0 | 35·6 ± 45·3 | 0·19 |
| CA199, U/L (n = 1177 vs 55) | 353·3 ± 5042·5 | 266·2 ± 3798·8 | 2216·3 ± 16,176·3 | 0·376 |
| LVEF,% (n = 1080 vs 53) | 66·6 ± 6·3 | 66·8 ± 6·2 | 63·6 ± 8·0 | 0·007* |
| LVDd, mm (n = 1080 vs 54) | 44·3 ± 5·5 | 44·2 ± 5·5 | 45·2 ± 6·0 | 0·224 |
| LA, mm (n = 1080 vs 54) | 30·5 ± 4·7 | 30·4 ± 4·7 | 32·4 ± 5·5 | 0·012* |
| IVS, mm (n = 1080 vs 54) | 9·5 ± 1·6 | 9·5 ± 1·6 | 10·1 ± 1·9 | 0·005* |
| LVPW, mm (n = 1080 vs 54) | 9·2 ± 1·4 | 9·2 ± 1·3 | 9·9 ± 1·7 | 0·003* |
Summary statistics are means ± SD or n (%), n, number; WBC, white blood cell; NEUR, neutrophil granulocyte ratio; PCT, Procalcitonin; CRP, C-reactive protein; LDL, low density lipoprotein; ALT, Alanine aminotransferase; AST, Aspartate transaminase; TBIL, total bilirubin; DBIL, direct bilirubin; BNP, brain natriuretic peptide; hs-cTnI, high sensitive-cardiac troponin I; CKMB, Creatine phosphokinase-Mb; CEA, carcino-embryonic antigen; CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 199; LVEF, left ventricular ejection fraction; LVDd, Left ventricular end-diastolic diameter; LA, left atrium; IVS, interventricular septum; LVPW, left ventricular posterior wall. *P ≤ 0·05, **P < 0·001.
Table 3.
Operation information.
| Non-Events | Events | P - value | |
|---|---|---|---|
| Main surgical sites, n (%) | 0·442 | ||
| Esophageal | 2 (0·2) | 0 (0) | |
| Gastric | 113 (9·4) | 7 (12·5) | |
| Intestinal | 22 (1·8) | 2 (3·6) | |
| Colorectum | 1063 (88·4) | 47 (83·9) | |
| Anal tube | 3 (0·2) | 0 (0) | |
| Laparoscope, n (%) | 1023 (85) | 41 (73·2) | 0·017* |
| Operation time (minutes), means ± SD | 222·9 ± 95·7 | 216·8 ± 81·6 | 0·668 |
| Anesthesia time (minutes), means ± SD | 273·6 ± 95·8 | 255·1 ± 91·0 | 0·156 |
n, number; *P ≤ 0·05.
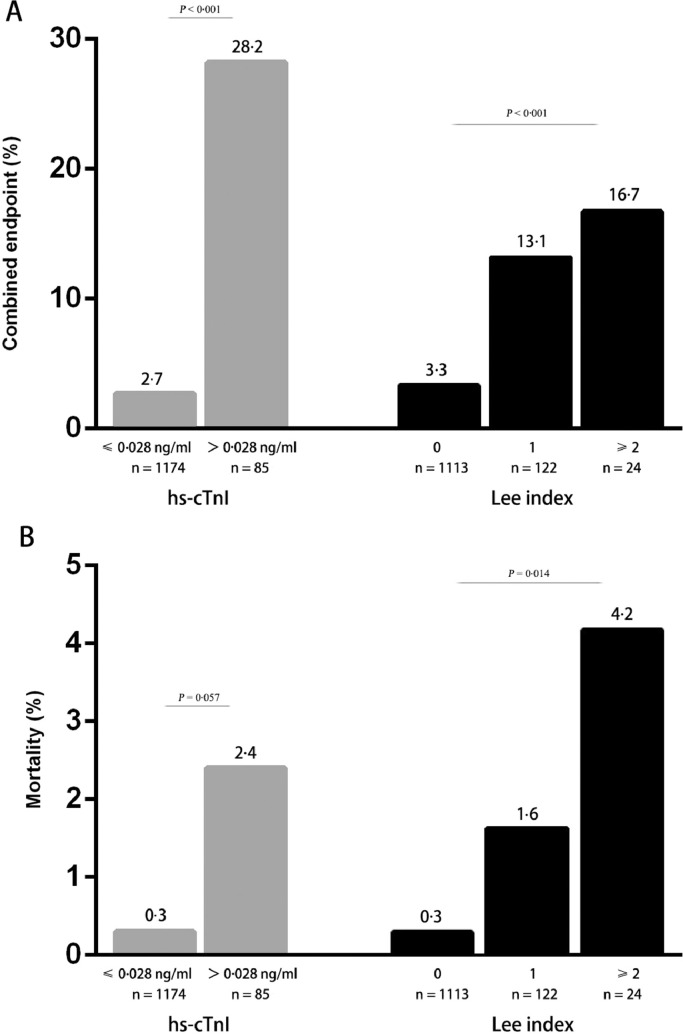
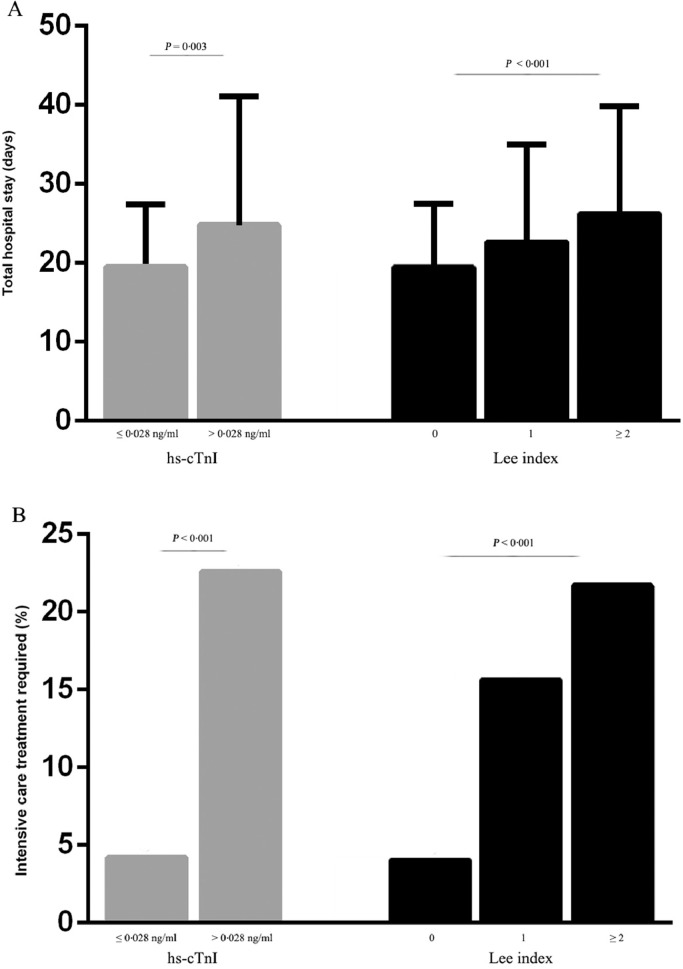
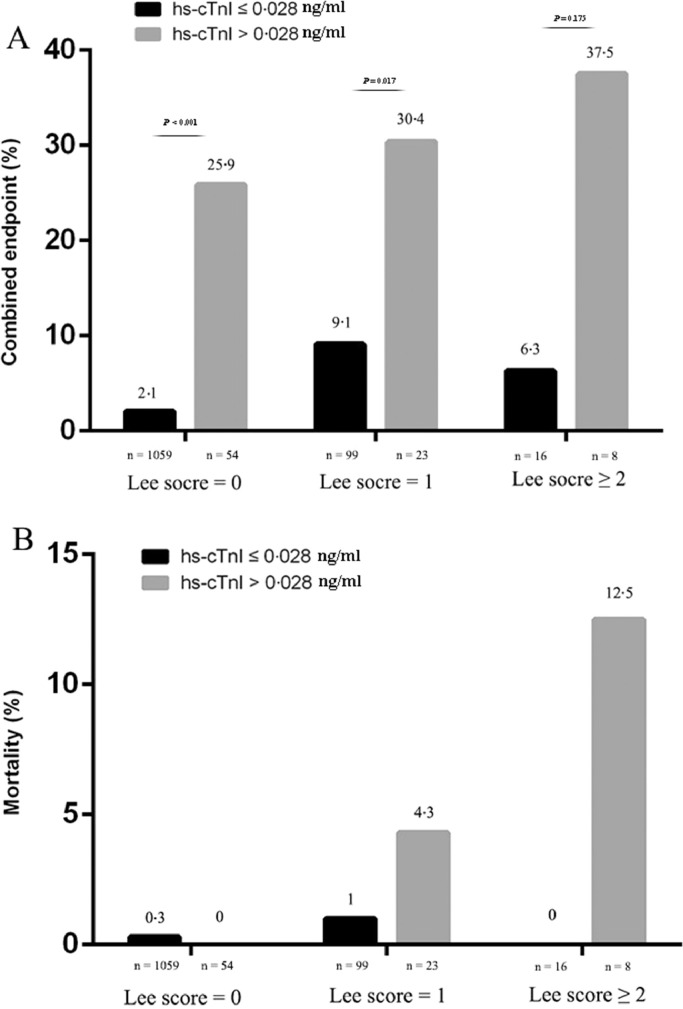
In the entire cohort, 85 (6·8%) patients had elevated hs-cTnI. As compared with patients with normal hs-cTnI level, the occurrence of the combined endpoint was significantly higher in patients with elevated hs-cTnI (specifically 24·7% patients with acute decompensated heart failure versus 2·3%, 3·5% patients with cardiac arrest or ventricular fibrillation versus 0·2%, 17·6 patients with acute myocardial infarction versus 0·5%). Comparable results could be observed for in-hospital mortality (2·4% versus 0·3%), although not statistically significant (Fig. 2). The perioperative risk also increased with the increase of Lee index. Furthermore, the length of total hospital stay was significantly longer in patients with elevated hs-cTnI (24·8 ± 16·3 versus 19·5 ± 7·9, P = 0·003). The number of patients requiring intensive care treatment was higher in patients with elevated hs-cTnI either (Fig. 3). Hs-cTnI > 0·028 ng/ml was associated with significantly higher cardiovascular event rate in patients with the revised cardiac index ≤ 1 (Fig. 4). According to Lee index stratification, the odds ratios (95% CI) of troponin elevation for predicting perioperative risk in patient with Lee index = 0, 1 and ≥ 2 were 16·5 (95%CI: 7·9–34·6), 4·4 (95%CI: 1·4–13·4), 9·0 (95%CI: 0·8–107·4), respectively.
Fig. 2.
The frequency of the combined endpoint (A) and in-hospital mortality (B) in association with hs-cTnI (gray bars), and the revised cardiac index ‘Lee index’ (dark bars). Compared with patients with normal hs-cTnI on admission, patients with elevated hs-cTnI are more likely to suffer combined endpoint (P < 0·001), There is also an increasing trend in mortality of elevated hs-cTnI group (P = 0·057). The occurrence of the combined endpoint was also related to the Lee index (P < 0·001), Comparable results could be observed for in-hospital mortality (P = 0·014).
Fig. 3.
The frequency of the total hospital stays (A) and intensive care treatment required (B) in association with hs-cTnI (gray bars), and the revised cardiac index ‘Lee index’ (dark bars). Error bars represent standard deviation (SD). The length of hospital stay (means ± SD) was significantly longer in those patients with a higher Lee index (19·4 ± 8·1 versus 22·5 ± 12·5 versus 26·2 ± 13·6 days; P < 0·001) and in patients with elevated hs-cTnI (19·5 ± 7·9 versus 24·8 ± 16·3 days; P = 0·003). The number of patients requiring intensive care treatment was higher in patients with elevated hs-cTnI and was related to the Lee index.
Fig. 4.
The frequency of the combined endpoint (A) and in-hospital mortality (B) of hs-cTnI levels according to the stratification of Lee index. Hs-cTnI > 0·028 ng/ml was significantly associated with a higher event rate in each risk category according to the Lee index especially for Lee index < 2 (Lee index = 0, 2·1% versus 25·9%, P < 0·001, OR 16·498 (7·865–34·605), P < 0·001; Lee index = 1, 9·1% versus 31·4%, P < 0·017, OR = 4·375 (95%CI:1·425–13·433), P = 0·01).
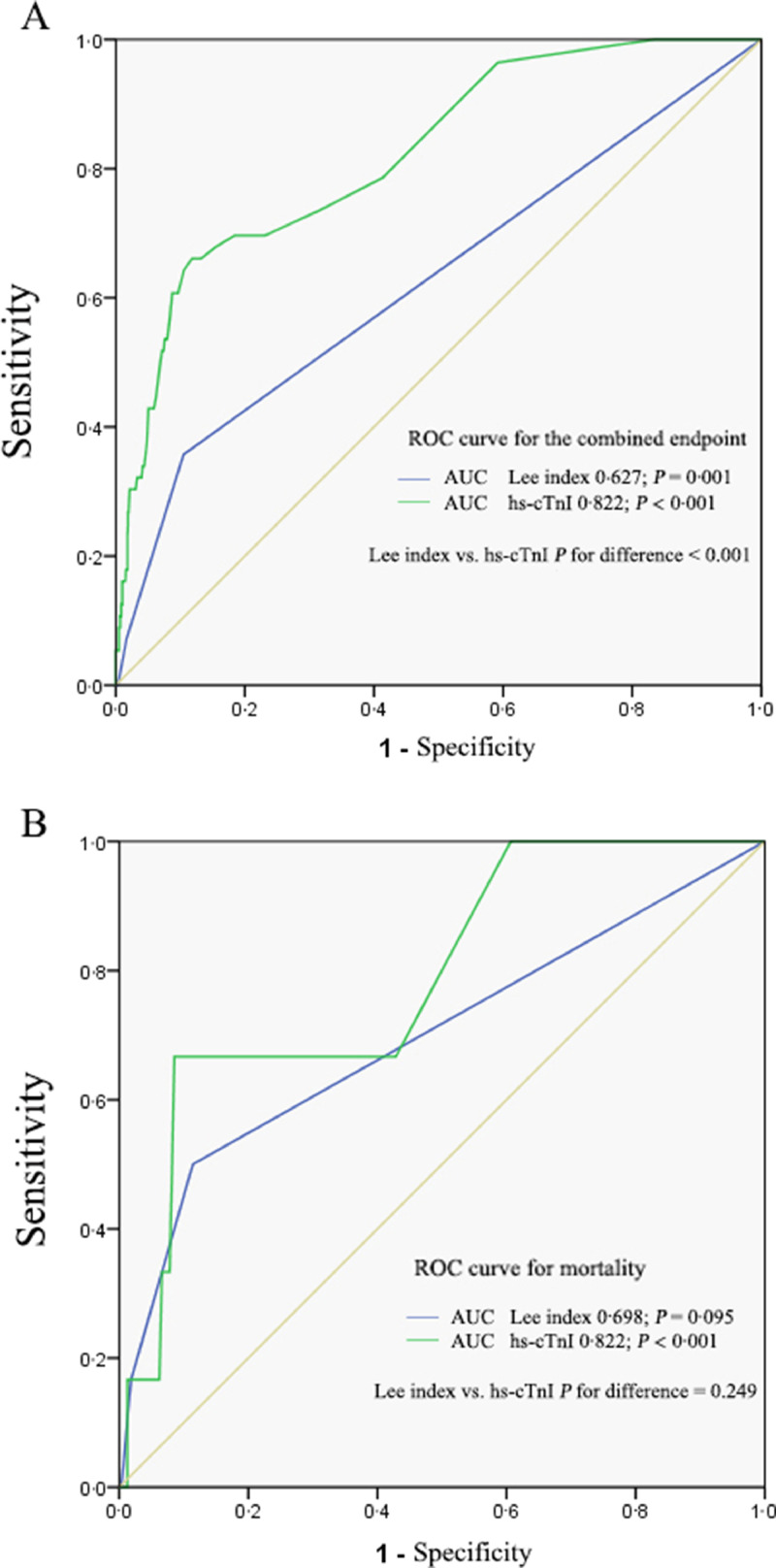
The ROC curves of hs-cTnI and the Lee index are shown in Fig. 5. The area under the ROC curve assessing hs-cTnI in predicting in-hospital mortality was 0·787 [95% confidence interval (CI) 0·612–0·963, P = 0·015] and combined endpoint was 0·822 [95% confidence interval (CI) 0·766–0·879, P < 0·001], which was larger than Lee index, 0·698 [95% confidence interval (CI) 0·447–0·949, P = 0·095] for mortality and 0·627 [95% confidence interval (CI) 0·543–0·711, P = 0·001] for combined endpoint. Comparing the AUC of Lee index and hs-cTnI, we found no difference exists in terms of predicting mortality. However, for the combined endpoint, the AUC for hs-cTnI was significantly larger (P < 0·001). The sensitivity, specificity, positive predictive value and negative predictive value of hs-cTnI in predicting combined endpoint were 42·9%, 94·4%, 28·2% and 97·3%, respectively. Although the positive likelihood ratio (PLR) of hs-cTnI for perioperative risk assessment was only 7·7 in the whole study population, in patients with Lee index = 0, the PLR of hs-cTnI (> 0·028 ng/ml) reaches 10·5 (as shown in supplement file - 3).
Fig. 5.
ROC curves of hs-cTnI, and the revised cardiac index ‘Lee index’ for the combined endpoint (A) and in-hospital mortality (B). The area under the ROC curve assessing hs-cTnI in predicting in-hospital mortality and combined point were larger than Lee index (0·787 versus 0·698, P < 0·001 and 0·822 versus 0·627, P = 0·249).
Univariate logistic regression analysis revealed that the age, chronic disease history (hypertension, atrium fibrillation, diabetes), elevated hs-cTnI, Lee score above 2, as well as laparoscope and some echocardiography data (LVEF, LA, IVS, LVPW) were significantly associated with an increased risk for the combined endpoint (Table 4). In a multivariate logistic regression analysis, elevated hs-cTnI, age, NEUR, hypertension and atrium fibrillation were five best predictors for the combined endpoint (Table 5). Internal validation was performed by applying bootstrap resample method to assess model optimism, The results were consistent and showed that the increase of hs-cTnI was still the strongest prognostic factor. The final model validated showed an AUC of 0·85 95%CI 0·794–0·905 and is well calibrated (Hosmer–Lemeshow test, P = 0·526).
Table 4.
Univariate logistic regression.
| OR | 95% CI | P - value | |
|---|---|---|---|
| Age | 1·093 | 1·063 - 1·125 | < 0·001 |
| Hypertension | 4·29 | 2·492 - 7·384 | < 0·001 |
| Atrial fibrillation | 13·007 | 4·208 - 4·206 | < 0·001 |
| Diabetes mellitus | 2·751 | 1·440 - 5·268 | 0·002 |
| Anticoagulation | 8·374 | 3·165 - 22·145 | < 0·001 |
| β-blocker | 5·73 | 2·518 - 10·043 | < 0·001 |
| ACE inhibitor/ARB | 4·552 | 2·298 - 9·018 | < 0·001 |
| Statins | 4·154 | 1·771 - 9·741 | 0·001 |
| Metfomin | 3·679 | 1·486 - 9·106 | 0·005 |
| Laparoscope | 0·481 | 0·261 - 0·887 | 0·019 |
| Lee score≥2 | 4·55 | 1·501 - 13·791 | 0·007 |
| NEUR | 1·022 | 1·012 - 1·032 | < 0·001 |
| LDL | 0·571 | 0·404 - 0·807 | 0·002 |
| Hs - cTnI>0·028 ng/ml | 14 | 7·8 - 25·3 | < 0·001 |
| LVEF | 0·939 | 0·906 - 0·973 | < 0·001 |
| LA | 1·082 | 1·027 - 1·140 | 0·003 |
| IVS | 1·255 | 1·071 - 1·471 | 0·005 |
| LVPW | 1·426 | 1·193 - 1·705 | < 0·001 |
Univariate logistic regression analyses of various variables as a predictor for the combined endpoint of mortality, acute myocardial infarction, cardiac arrest or ventricular fibrillation, and acute decompensated heart failure. ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blocker; NEUR, neutrophil granulocyte ratio; LDL, low density lipoprotein; hs-cTnI, high sensitive-cardiac troponin I; LVEF, left ventricular ejection fraction; LA, left atrium; IVS, interventricular septum; LVPW, left ventricular posterior wall. Depicted are all variables with a P - value < 0·1.
Table 5.
Multivariate logistic regression analyses.
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1·059 | 1·026–1·094 | < 0·001 |
| Hs-cTnI>0·028 ng/ml | 5·924 | 2·869–12·233 | < 0·001 |
| NEUR | 1·015 | 1·004–1·027 | 0·006 |
| Hypertension | 2·503 | 1·298–4·826 | 0·006 |
| Atrial fibrillation | 5·168 | 1·254–21·297 | 0·023 |
Abbreviations: OR, Odds ratio; hs-cTnI, high sensitive – cardiac troponin I; NEUR, neutrophil granulocyte ratio.
4. Discussion
The principal finding of this study is that hs-cTnI provide predictive information for the occurrence of serious cardiovascular events in receiving gastrointestinal tumor surgery patients, which is incremental to the revised cardiac risk index. Hs-cTnI, age neutrophil granulocyte ratio (NEUR), history of hypertension and atrium fibrillation were significant risk predictors. Moreover, we found that pre-operative levels of hs-cTnI were associated with the length of hospital stay and the necessity of intensive care treatment. In our study, the positive likelihood ratio of elevated hs-cTnI (> 0·028 ng/ml) for perioperative risk assessment reaches 10·5 in patients with Lee index = 0, which suggested the clinical significance of elevated hs-cTnI in these patients. A major strength of our study is that the analysis cohort was based on a large number of patients with gastrointestinal tumors, especially colorectal cancer.
Gorgun et al. had shown an association of postoperative plasma troponin levels with mortality in patients undergoing major abdominal colorectal surgery [18]. However, since the purpose is to predict the risk of perioperative death, the preoperative indicators seem to be more valuable than the postoperative troponin. If we can predict the perioperative risk through some indicators before surgery, clinicians have enough time to adjust the treatment strategy and make emergency plans for the possible risks.
Recent studies have demonstrated a prognostic value of pre-operative troponin levels for cardiovascular events in patients undergoing non-cardiac surgery [[14], [15], [16],[23], [24], [25]]. In all these studies, troponin was a significant risk predictor. The clinical status of tumor patients is more complex than that of general patients, such as appendicitis, vascular surgery and other related operations. Abnormal coagulation function, immune imbalance, nutritional status and other conditions will increase the risk of perioperative period [26,27]. In addition, the special psychological state and strong desire for survival of tumor patients will affect the risk of perioperative period [28,29]. Therefore, it is very important for surgeons and anesthesiologist to evaluate the perioperative risk of tumor patients more carefully before operation. Unfortunately, evidence regarding the value of risk assessment about troponin to the gastrointestinal tumor surgery is limited.
The incidence of major cardiac complications with major non-emergent non-cardiac surgery has been reported as being significantly associated to the revised Lee index [5]. Both Weber's and ours’ studies confirmed this result [16]. In the present study, we applied a cut-off value of 0·028 ng/ml which is a number for the 99th percentile of healthy population and is recommended for the diagnosis of myocardial infarction according to the universal definition of myocardial infarction. We found that hs-cTnI > 0·028 ng/ml was associated with significantly higher cardiovascular event rate in the whole cohort, particularly in patients with lower the revised cardiac index, which indicating hs-cTnI can provides reliable prognostic information incremental to revised cardiac index. However, hs-cTnI lost its predictive value in mortality, possibly due to insufficient sample size in the present study.
The doubt about the clinical significance of our study is mainly due to the low positive predictive value. However, the positive predictive value may strongly related to the prevalence of the disease [31]. The lower the prevalence, the lower the positive predictive value. Since the incidence of perioperative cardiovascular events is low, the positive predictive value will inevitably be low, which has been reported in previous studies [16,30]. Considering the likelihood ratio is not affected by the prevalence rate, which seems to be more objective and clinically significant than predictive value. Compared with previous results, our results are still better than those reported by previous studies in term of positive likelihood ratio (7·7 versus 2·7, 3·6) [16,30], although the results in all studies are not satisfactory. The possible reason is that perioperative risk assessment is too complex to be accurately assessed by a single index [32]. we further analyzed the role of troponin in perioperative risk assessment according to Lee index stratification, just as the role of d-dimer in pulmonary embolism which must be combined with the risk stratification of pulmonary embolism [33]. We finally found that the positive likelihood ratio of hs-cTnI (> 0·028 ng/ml) reaches 10·5 for patients with Lee index = 0. It is generally believed that 10 is the dividing line between good and bad detection method. Therefore, we think our research has important clinical significance, especially for those with Lee index = 0.
The possible explanations for the correlation between pre-operative high-sensitive troponin I elevation and perioperative cardiovascular risk are as follows: 1. the increasing of troponin indicates the existence of myocardial injury, which may be associated with unstable plaque exists in the coronary artery of this kind of patients, plaque are more likely to rupture, leading to the occurrence of perioperative cardiovascular adverse events under stress; 2. The mild increase of troponin indicates that the patients may have the risk of abnormal coagulation status, and are more likely to induce disseminated intravascular coagulation (DIC) and other serious clinical syndromes under stress; 3. Some gastrointestinal tumor patients with elevated troponin are suffering from malnutrition and others may have more comorbidity such as heart failure, anemia and renal dysfunction, which induce complex clinical status and poor prognosis.
Age and hypertension are the risk factors of cardiovascular disease [34] and atrial fibrillation has been strongly associated with heart failure and stroke [35,36]. Many studies have found that these 3 factors are related to the perioperative risk of non-cardiac surgery [37], [38], [39], our study further confirmed the previous results. The neutrophil ratio (NEUR) is a readily available marker of systemic inflammation driven by elevated concentrations of circulating cytokines which have been shown to modulate myocardial injury [40], Framingham based risk prediction of cardiovascular mortality also proved this value [41]. These may explain the strong correlation between preoperative elevated NEUR and perioperative risk in our study, consistent with previous study [42].
The findings of this study should be considered with some limitations. Firstly, as a retrospective study, several other parameters that may help predict serious cardiovascular events and all-cause death for patients undergoing gastrointestinal tumor surgery were missing. Secondly, in clinical practice, not every patient with gastrointestinal tumor who intends to undergo surgery will routinely detect hs-cTnI, and the surgery were canceled in some high-risk patients with elevated hs-cTnI. Therefore, the possibility of selection bias may be inevitable, we enrolled all patients who met the inclusion criteria to reduce potential bias. Thirdly, due to the small number of patients with Lee index ≥ 2, our study has some limitations for this kind of population. Fourthly, we did not perform external validity of the results. Our further prospective study will be carried out to verify this conclusion. Finally, this research was conducted in single center and lack of external validation, data from larger populations and multiple centers are warranted to further confirm the results.
In conclusion, hs-cTnI provides powerful prognostic information for patients undergoing gastrointestinal tumor surgery, and therefore provides reliable prognostic information incremental to revised cardiac index.
Contributors
Yitao Zhang, Jiaojie Xue, Ling Zhou, Zhichong Chen, Daici Chen and Weijie Zeng were responsible for the study concept and design and data anyalysis. Yitao Zhang, Jinhong Si and Weijie Zeng accessed and were responsible for the raw data associated with the study.
Jinhong Si and Shiyao Cheng, Kanglin Cheng and Mao Ouyang were responsible for the methods and data collection.
Kanglin Cheng, Shuqi Yu run the statistical analysis, data curation and interpreted the data.
Yitao Zhang, Zhichong Chen, Ling Zhou, Jiaojie Xue, Daici Chen and Weijie Zeng drafted the paper.
The corresponding author had the final responsibility to submit for publication.
All authors agree to be accountable for all aspects of the work and gave final approval for the version to be published.
Data sharing statement
The dataset supporting this article is available upon demand to the corresponding author and to the promoter (the sixth affiliated hospital of Sun Yat-sun University).
Funding
This work was supported by National Nature Science Foundation of China, Grant No. 81400301; The Fundamental Research Funds for the Central Universities and the National Natural Science Foundation of China, Grant No. 19ykpy10; the National Natural Science Foundation of China, Grant No. 31970703; and the Natural Science Foundation of Guangdong Province, Grant No. 2021A1515010544.
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
Acknowledgments
Thanks to Huijing He and Yuanxin Zhong for their technical support.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101128.
Contributor Information
Yitao Zhang, Email: zhangyt73@mail.sysu.edu.cn.
Zhichong Chen, Email: chenzhch3@mail.sysu.edu.cn.
Daici Chen, Email: chendc3@mail.sysu.edu.cn.
Weijie Zeng, Email: zengweijie@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Fitzmaurice C., Allen C., Barber R.M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Cheng X., Tong J., Hu Q., Chen S., Yin Y., Liu Z. Meta-analysis of the effects of preoperative renin-angiotensin system inhibitor therapy on major adverse cardiac events in patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2015;47(6):958–966. doi: 10.1093/ejcts/ezu330. [DOI] [PubMed] [Google Scholar]
- 4.Shen J.T., Xu M., Wu Y. Association of pre-operative troponin levels with major adverse cardiac events and mortality after noncardiac surgery: a systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35(11):815–824. doi: 10.1097/EJA.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 5.Lee T.H., Marcantonio E.R., Mangione C.M. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 7.Apple F.S., Sandoval Y., Jaffe A.S., Ordonez-Llanos J. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63(1):73–81. doi: 10.1373/clinchem.2016.255109. [DOI] [PubMed] [Google Scholar]
- 8.Morrow D.A., Cannon C.P., Jesse R.L. National academy of clinical biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53(4):552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 9.Vasile V.C., Chai H.S., Abdeldayem D., Afessa B., Jaffe A.S. Elevated cardiac troponin T levels in critically ill patients with sepsis. Am J Med. 2013;126(12):1114–1121. doi: 10.1016/j.amjmed.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A.N., Blonde K., Hackam D., Iansavichene A., Mrkobrada M. Prognostic significance of elevated troponin in non-cardiac hospitalized patients: a systematic review and meta-analysis. Ann Med. 2014;46(8):653–663. doi: 10.3109/07853890.2014.959558. [DOI] [PubMed] [Google Scholar]
- 11.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latini R., Masson S., Anand I.S. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116(11):1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 13.Omland T., De Lemos J.A., Sabatine M.S. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarveswaran J., Ikponmwosa A., Asthana S., Spark J.I. Should cardiac troponins be used as a risk stratification tool for patients with chronic critical limb ischaemia? Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2007;33(6):703–707. doi: 10.1016/j.ejvs.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Nagele P., Brown F., Gage B.F. High-sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long-term mortality after noncardiac surgery. Am Heart J. 2013;166(2):325–332.e1. doi: 10.1016/j.ahj.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber M., Luchner A., Seeberger M. Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J. 2013;34(11):853–862. doi: 10.1093/eurheartj/ehs445. [DOI] [PubMed] [Google Scholar]
- 17.Maile M.D., Jewell E.S., Engoren M.C. Timing of preoperative troponin elevations and postoperative mortality after noncardiac surgery. Anesth Analg. 2016;123(1):135–140. doi: 10.1213/ANE.0000000000001309. [DOI] [PubMed] [Google Scholar]
- 18.Gorgun E., Lan B.Y., Aydinli H.H. Troponin elevation after colorectal surgery: significance and management. Ann Surg. 2016;264(4):605–611. doi: 10.1097/SLA.0000000000001854. [DOI] [PubMed] [Google Scholar]
- 19.Devereaux P.J., Chan M.T., Alonso-Coello P. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 20.Neumann F.J., Sousa-Uva M., Ahlsson A. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 21.Montalescot G., Sechtem U., Achenbach S. ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 22.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Ma J., Xin Q., Wang X., Gao M., Wang Y., Liu J. Prediction of perioperative cardiac events through preoperative NT-pro-BNP and cTnI after emergent non-cardiac surgery in elderly patients. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thielmann M., Pasa S., Wendt D. Prognostic significance of cardiac troponin I on admission for surgical treatment of acute pulmonary embolism: a single-centre experience over more than 10 years. Eur J Cardiothorac Surg. 2012;42(6):951–957. doi: 10.1093/ejcts/ezs122. [DOI] [PubMed] [Google Scholar]
- 25.Gibson S.C., Marsh A., Berry C. Should pre-operative troponin be a standard requirement in patients undergoing major lower extremity amputation? Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2006;31(6):637–641. doi: 10.1016/j.ejvs.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Falanga A., Marchetti M., Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 27.ME I.J., Sanz-Pamplona R., Hermitte F., de Miranda N. Colorectal cancer: a paradigmatic model for cancer immunology and immunotherapy. Mol Aspects Med. 2019;69:123–129. doi: 10.1016/j.mam.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Breitbart W., Pessin H., Rosenfeld B. Individual meaning-centered psychotherapy for the treatment of psychological and existential distress: a randomized controlled trial in patients with advanced cancer. Cancer. 2018;124(15):3231–3239. doi: 10.1002/cncr.31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breitbart W., Rosenfeld B., Pessin H., Applebaum A., Kulikowski J., Lichtenthal W.G. Meaning-centered group psychotherapy: an effective intervention for improving psychological well-being in patients with advanced cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(7):749–754. doi: 10.1200/JCO.2014.57.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puelacher C., Lurati Buse G., Seeberger D. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221–1232. doi: 10.1161/CIRCULATIONAHA.117.030114. [DOI] [PubMed] [Google Scholar]
- 31.Simon R. Sensitivity, specificity, PPV, and NPV for predictive biomarkers. J Natl Cancer Inst. 2015;107(8) doi: 10.1093/jnci/djv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisher L.A., Fleischmann K.E., Auerbach A.D. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;130(24):2215–2245. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinides S.V., Meyer G., Becattini C. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC) Eur Respir J. 2019;54(3) doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 34.Gersh B.J., Sliwa K., Mayosi B.M., Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31(6):642–648. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 35.Ling L.H., Kistler P.M., Kalman J.M., Schilling R.J., Hunter R.J. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13(3):131–147. doi: 10.1038/nrcardio.2015.191. [DOI] [PubMed] [Google Scholar]
- 36.Kamel H., Healey J.S. Cardioembolic Stroke. Circ Res. 2017;120(3):514–526. doi: 10.1161/CIRCRESAHA.116.308407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen P.W., Gislason G.H., Jørgensen M.E. Influence of age on perioperative major adverse cardiovascular events and mortality risks in elective non-cardiac surgery. Eur J Intern Med. 2016;35:55–59. doi: 10.1016/j.ejim.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Smilowitz N.R., Gupta N., Guo Y., Beckman J.A., Bangalore S., Berger J.S. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart. 2018;104(14):1180–1186. doi: 10.1136/heartjnl-2017-312391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Diepen S., Bakal J.A., McAlister F.A., Ezekowitz J.A. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124(3):289–296. doi: 10.1161/CIRCULATIONAHA.110.011130. [DOI] [PubMed] [Google Scholar]
- 40.Kantola T., Klintrup K., Väyrynen J.P. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah N., Parikh V., Patel N. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: insights from the national health and nutrition examination survey-III. Int J Cardiol. 2014;171(3):390–397. doi: 10.1016/j.ijcard.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Ackland G.L., Abbott T.E.F., Cain D. Preoperative systemic inflammation and perioperative myocardial injury: prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2019;122(2):180–187. doi: 10.1016/j.bja.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.