Abstract
Introduction
Our group has conducted extensive basic and preclinical studies of the use of human induced pluripotent cell (iPSC)-derived neural stem/progenitor cell (hiPSC-NS/PC) grafts in models of spinal cord injury (SCI). Evidence from animal experiments suggests this approach is safe and effective. We are preparing to initiate a first-in-human clinical study of hiPSC-NS/PC transplantation in subacute SCI.
Setting
NS/PCs were prepared at a Good Manufacturing Practice-grade cell processing facility at Osaka National Hospital using a clinical-grade integration-free hiPSC line established by the iPSC Stock Project organized by the Kyoto University Center for iPS Cell Research and Application. After performing all quality checks, the long-term safety and efficacy of cells were confirmed using immunodeficient mouse models.
Methods
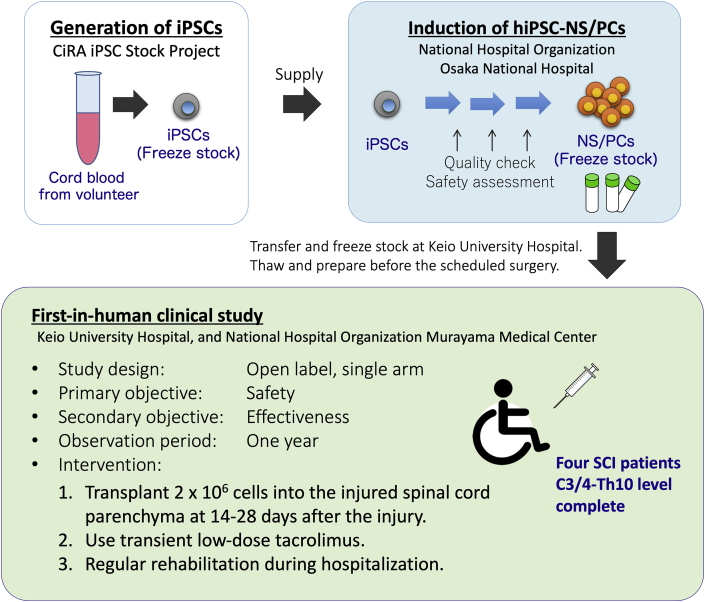
The forthcoming clinical study uses an open-label, single-arm design. The initial follow-up period is 1 year. The primary objective is to assess the safety of hiPSC-NS/PC transplantation in patients with subacute SCI. The secondary objective is to obtain preliminary evidence of its impact on neurological function and quality-of-life outcomes. Four patients with C3/4-Th10 level, complete subacute (within 24 days post-injury) SCI will be recruited. After obtaining consent, cryopreserved cells will be thawed and prepared following a multi-step process including treatment with a γ-secretase inhibitor to promote cell differentiation. A total of 2 × 106 cells will be transplanted into the injured spinal cord parenchyma 14–28 days post-injury. Patients will also receive transient immunosuppression. This study protocol has been reviewed and approved by the Certified Committee for Regenerative Medicine and the Japanese Ministry of Health, Labor and Welfare (University Hospital Medical Information Network Clinical Trials Registry [UMIN-CTR] number, UMIN000035074; Japan Registry of Clinical Trials [jRCT] number, jRCTa031190228).
Discussion/conclusion
We plan to start recruiting a patient as soon as the COVID-19 epidemic subsides. The primary focus of this clinical study is safety, and the number of transplanted cells may be too low to confirm efficacy. After confirming safety, a dose-escalation study is planned.
Keywords: Induced pluripotent stem cells, Neural stem/progenitor cells, Spinal cord injury, Regenerative medicine, Transplantation
Highlights
-
•
A first-in-human clinical study for spinal cord injury using iPSC-derived cells is about to begin.
-
•
The primary objective is to assess the safety of human iPSC-derived neural stem/progenitor cells.
-
•
Further clinical trials are expected to be conducted to statistically assess efficacy.
Abbreviations
- AIS
American Spinal Cord Injury Association Impairment Scale
- CiRA
Center for iPS Cell Research and Application
- CNS
central nervous system
- ESC
embryonic stem cell
- FCM
flow cytometry
- FDG-PET
fluorodeoxyglucose(18F) positron emission tomography
- GMP
good manufacturing practice
- GSI
γ-secretase inhibitor
- hiPSC
human induced pluripotent stem cell
- hiPSC-NS/PC
human induced pluripotent stem cell-derived neural stem/progenitor cell
- HLA
human leukocyte antigen
- ICC
immunocytochemistry
- IDMC
independent data monitoring committee
- iPSC
induced pluripotent stem cell
- ISNCSCI
International Standards for Neurological Classification of Spinal Cord Injury
- MPSS
methyl prednisolone sodium succinate
- MRI
magnetic resonance imaging
- NS/PC
neural stem/progenitor cell
- OMIN
Online Mendelian Inheritance in Man
- OPC
oligodendrocyte progenitor cell
- PBS
phosphate-buffered saline
- PET
positron emission tomography
- PMDA
Pharmaceuticals and Medical Devices Agency
- QC
quality control
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SCI
spinal cord injury
- SNV
single nucleotide variation
1. Introduction
Spinal cord injury (SCI) may lead to partial or complete paralysis of the sensory, motor, and/or autonomic nervous systems below the damaged region of the spinal cord parenchyma. Despite decades of intensive research and development, no breakthrough treatment has been developed, and functional recovery remains elusive, particularly in cases of complete SCI [1].There are more than 100,000 chronic SCI patients in Japan, with an incidence of about 5000 new cases per year (40.2 per 1 million population) [2]. An SCI database maintained by 30 Japanese hospitals indicates that 29.3% of all new cases were classified as complete injury (American Spinal Cord Injury Association Impairment Scale (AIS) grade A) on discharge between 2005 and 2017. Historically, high-energy impacts, such as may occur in traffic accidents (43.7%) and falls from heights (28.9%) [2], have been the main causes of SCI. More recently, a growing number of cases of SCI leading to incomplete paralysis in elderly patients are associated with lower-impact events, such as slips and falls in non-elevated settings [3].
The current standard treatment protocol for SCI recommends early surgical decompression, but the timing of surgery depends on factors such as the degree of compression and extent and severity of paralysis, and remains controversial. A 24 h infusion of high-dose methyl prednisolone sodium succinate (MPSS) is another option, but current recommendations limit the initiation of intravenous MPSS to within the first 8 h post-injury [4]. Upon stabilization of the patient's condition, long-term rehabilitation is typically begun immediately. All current treatment modalities focus on prevention of secondary complications and optimization of residual function. However, there is no effective treatment to repair the damaged spinal cord itself.
Spinal cord regeneration has been researched worldwide since at least the late 1970s, when survival of fetal nervous tissue grafts in the adult mammalian central nervous system (CNS) was first reported [5]. It has been speculated that the CNS can regenerate in human under appropriate environmental conditions. Since then, progress in the development of regenerative medicine approaches for SCI has been dramatic. Research into spinal regeneration may involve the use of chemical or biomolecular products (drugs) and/or the transplantation of cells or tissue. Drug therapy typically involves a clearly defined mechanism of action. Clinical studies involving the administration of drugs, including granulocyte colony-stimulating factor [6,7], a Rho inhibitor [8], riluzole [9], and hepatocyte growth factor [10], after SCI have been performed, but evidence of a clinical benefit is modest.
Clinical studies of cell transplantation have examined the use of neural stem/progenitor cells (NS/PCs) [11,12], olfactory ensheathing glia cells [13], and mesenchymal stromal/stem cells [14], but it remains unclear which cell type(s) is best suited for clinical use. Proposed mechanisms of action include compensation or replacement of damaged cells, regeneration of neural circuits through promotion of axonal outgrowth, and protection of the undamaged parenchyma by graft-derived trophic factors. The predominant mechanism of action for any given therapy likely depends on a combination of factors, including the cell type and methods of cell processing and delivery. In the protocol described here, we will use NS/PCs, which are expected to function by proliferating, differentiating into neural cells, producing neurotrophic factors, and eliciting anti-inflammatory effects, leading to reductions in secondary damage proximal to the injury site [15].
The development of induced pluripotent stem cell (iPSC) [16] technology has provided a useful resource to generate differentiated somatic cells of interest, and several clinical studies regarding the potential applications of human iPSC (hiPSC)-derived cells have been initiated in recent years. The decision to use these cells in our study was partly based on the prohibitive processing times and cost considerations associated with the use of autologous hiPSC-derived cells. Moreover, the gene transduction technology used to generate clinical-grade hiPSCs in this study has advanced to a level supportive of clinical use [17], and animal studies have indicated its safety and effectiveness [18].
In this report, we describe the underlying rationale and detailed protocol for a first-in-human study of hiPSC-derived NS/PC (hiPSC-NS/PC) transplantation in patients with complete cervical/thoracic subacute SCI [19]. The study will be conducted as a “class I regenerative medicine” protocol, as permitted under Japan's Act on the Safety of Regenerative Medicine. The protocol has been reviewed and approved by the Certified Committee for Regenerative Medicine at Keio University (certification number R2016001; approval date: November 27, 2018) and the Japanese Ministry of Health, Labor and Welfare (approval date: March 13, 2019). This treatment protocol is not supervised by the national drug regulatory agency (Pharmaceuticals and Medical Devices Agency, PMDA) and is not designed to satisfy market approval requirements. Pending the results of this study, an Investigational New Drug application may be filed to enable further testing under PMDA supervision.
2. Setting: preparation of hipsc-ns/pcs for the clinical study
2.1. Induction of hiPSC-NS/PCs
NS/PCs were prepared at a Good Manufacturing Practice (GMP)-grade cell processing facility at Osaka National Hospital using the clinical-grade “human leukocyte antigen (HLA) super-donor”-derived, integration-free hiPSC line YZWJs513 established by the iPSC Stock Project organized by the Kyoto University Center for iPS Cell Research and Application (CiRA) [20,21]. hiPSCs were developed at the CiRA following the differentiation protocol previously reported for EB-NS/PCs [17]. In brief, neural differentiation was performed using the quick-aggregation procedure of the serum-free culture of embryoid body-like aggregates with quick reaggregation (SFEBq) protocol [22]. After neural induction for 14 days, NS/PCs were transferred to NS/PC expansion medium and expanded using the neurosphere culture technique [23]. The final product, hiPSC-NS/PCs at the fourth passage, was frozen and shipped to Keio University, and will be maintained until an eligible patient is enrolled.
2.2. Quality control (QC) of hiPSC-NS/PCs
For QC, hiPSC-NS/PCs will be examined in terms of their biological characteristics and contaminants. Expression levels of phenotype marker genes (SOX1, PAX6, PSA-NCAM, and ganglioside GD2) will be analyzed using quantitative reverse transcription polymerase chain reaction (qRT-PCR), flow cytometry (FCM), and/or immunocytochemistry (ICC) (Table 1). The terminal differentiation potential will be tested by analyzing expression of ELAVL3/4 (HuC/D), β–III–tubulin, or GFAP after incubation for 4 weeks in differentiation medium. Expression of OCT4 analyzed using qRT-PCR, FCM, and ICC will be used to detect residual undifferentiated cells or inadequately differentiated cells. We will also perform ICC after incubation for 1 week under pluripotent stem cell culture conditions (back culture) to detect any OCT4-positive colonies (Table 1; Fukusumi, in preparation). Expression levels of two other pluripotent stem cell markers (TRA-1-60 and SSEA3) will be analyzed by FCM. Chromosomal analysis will be performed using Giemsa staining and G-banding. Copy number variants, single nucleotide variations (SNVs), and indels in the genome of the final product will be examined using a microarray, whole genome sequencing, and whole exome sequencing. The ploidy of pluripotent cell derivatives is a potential risk factor. In the present protocol, we will remove all cells with clonal karyotypic abnormalities and/or <90% normal karyotype determined by Giemsa staining and G-banding (Table 1). Ongoing work by our group indicates that prolonged cell division induces chromosomal instabilities (Kanematsu, in preparation); therefore, we will limit the passage number (4 for NS/PCs) and number of days of culture (100 in total), and monitor the estimated number of cell divisions for the end product. Cells with defined numbers of de novo copy number variations, SNVs, and/or indels of cancer-related genes, as reported by Shibata [24] and listed in the COSMIC Cancer Gene Census [25,26] and Online Mendelian Inheritance in Man (OMIN) [27], will also be excluded from the end product. In addition, microbiological testing will be performed using standard techniques, including tests for sterility, endotoxins, mycoplasma, and virus infection (Table 1).
Table 1.
Quality standard values of the final product.
| Test | Material | Target | Method | Value |
|---|---|---|---|---|
| Specification | ||||
| Morphology | PCFP | Neurosphere | Microscopic observation | No significant mixture of adherent cells |
| Phenotypic marker expression | PCFP | SOX1 | qRT-PCR∗ | ΔCt ≤ 10 |
| FCM | ≥ 90% | |||
| ICC | ≥ 80% | |||
| PAX6 | qRT-PCR∗ | ΔCt ≤ 6 | ||
| PSA-NCAM | FCM | ≥ 90% | ||
| GD2 | FCM | ≥ 90% | ||
| Terminal neural differentiation | PCFP following 4 weeks of differentiation culture | ELAVL3/4 (HuC/D) | ICC | ≥ 50% |
| β–III–tubulin | ICC | Positive | ||
| GFAP | ICC | Positive | ||
| Impurities | ||||
| Phenotypic marker expression | PCFP | OCT4 | qRT-PCR∗∗ | ΔΔCt ≥ 7.643 |
| FCM | ≤ 0.01% | |||
| ICC | ≤ 0.0005% | |||
| TRA-1-60 | FCM | ≤ 0.01% | ||
| SSEA3 | FCM | ≤ 0.01% | ||
| Back culture | PCFP following 1 week of additional culture | OCT4-positive colony | ICC | none |
| Chromosomal analysis | ||||
| Karyotyping | PCFP following 1 week of additional culture | Rate of normal karyotype results | Giemsa staining (50 cells or more) | normal karyotype ≥90% |
| Identical abnormal karyotype | G-banding (20 cells or more) | Clonal abnormality (≥3 cells having the same chromosomal loss, or ≥ 2 cells having the same conformational abnormality or extra chromosomes)is not detected. | ||
| Rate of normal karyotype results | G-banding (20 cells or more) | ≥ 90% | ||
| Tetraploid | G-banding + Giemsa staining (Percentage among 100 metaphase cells or more) | ≤ 4% | ||
| Microbiological quality control | ||||
| Sterility test | CFP | Bacteria and fungi | JP | negative |
| Endotoxin test | Endotoxin | JP | ≤ 25 EU/mL | |
| Mycoplasma test | NAT + Culture | JP | Below the detection sensitivity | |
| Virus test | HBV, HCV, HIV, HTLV-1, and Parvovirus B19 | PCR | Below the detection sensitivity | |
| Genomic analysis | ||||
| CNV | PCFP | De novo CNV | Microarray | ≤ 5 amplification or deletion of 10 kb or more compared to the cells at the end of passage 3 NS/PCs |
| Highly frequent gene mutations causally implicated in hereditary tumor, and cancer (OMIM, CGC, and Shibata list) | WGS + WES (SNV, indel) | No CNV detected in areas where the risk of tumorigenesis is reported | ||
Abbreviations: PCFP, pre-cryopreserved final product; CFP, cryopreserved final product; qRT-PCR, quantitative reverse transcription polymerase chain reaction (comparative Ct); ∗, ΔCt value; ∗∗, ΔΔCt value; FCM, flow cytometry; ICC, immunocytochemistry; NAT, nucleic acid amplification technique; Culture, methods for mycoplasma expansion in broth culture and detection by colony formation on nutrient agar plates; JP, Japanese Pharmacopoeia; CNV, copy number variation; SNV, single nucleotide variation; indel, insertion/deletion; OMIM, Online Mendelian Inheritance in Man; CGC, The Cancer Gene Census; WGS, whole genome sequencing; WES, whole exome sequencing.
In preparation for our first-in-human study, we have completed all the processes and tests described above and confirmed the safety both in vitro (Fig. 1) and in vivo (Fig. 2). After obtaining the approval of the Certified Committee for Regenerative Medicine based on these results with cells of non-clinical grade, we manufactured clinical-grade (under GMP conditions) hiPSC-NS/PCs using the same cord blood cell-derived iPSC line (YZWJs513) and confirmed their safety in vitro. As a further test of long-term safety, the end product was transplanted into immunodeficient mice using the method described in our previous report [17], and safety was confirmed after 3 months in the spinal cord and 6 months in the brain (Fig. 3).
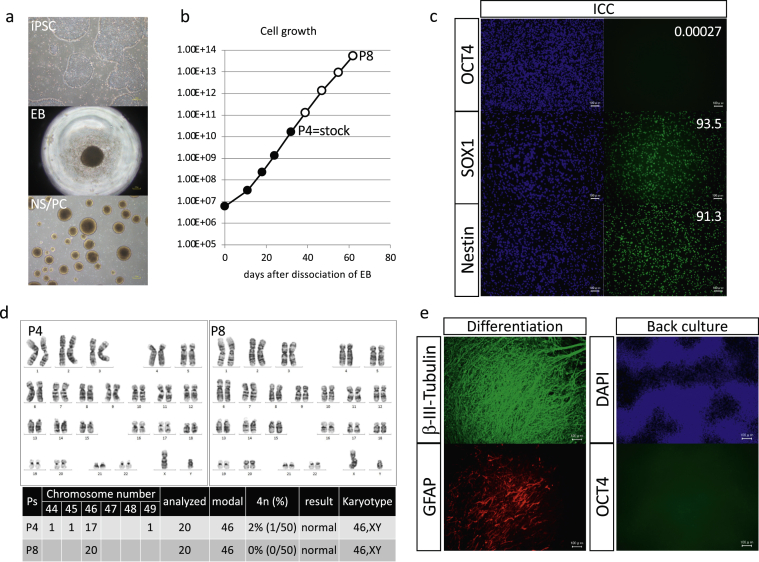
Fig. 1.
In vitro QC steps used to manufacture NS/PCs from YZWJs513 iPSCs. (a) Images of iPSCs, embryoid bodies (EBs, intermediate product), and NS/PCs (scale bar = 200 μm). (b) During culture, the passage number, days of culture, and number of cell divisions were monitored and all within the threshold. (c) ICC of the final product showed no expression of OCT4 (marker of pluripotency). More than 90% of cells expressed SOX1 and Nestin (markers of NS/PCs; scale bar = 100 μm). (d) Chromosomal analysis of the final product (P4) and the longer culture product (P8) yielded normal results. (e) ICC after 4 weeks of differentiation culture showed the potential of NS/PCs to differentiate into β–III–tubulin-positive neurons and GFAP-positive astrocytes. Proliferation of OCT4-positive cells was not observed after culture under pluripotent stem cell culture conditions (scale bar = 100 μm).
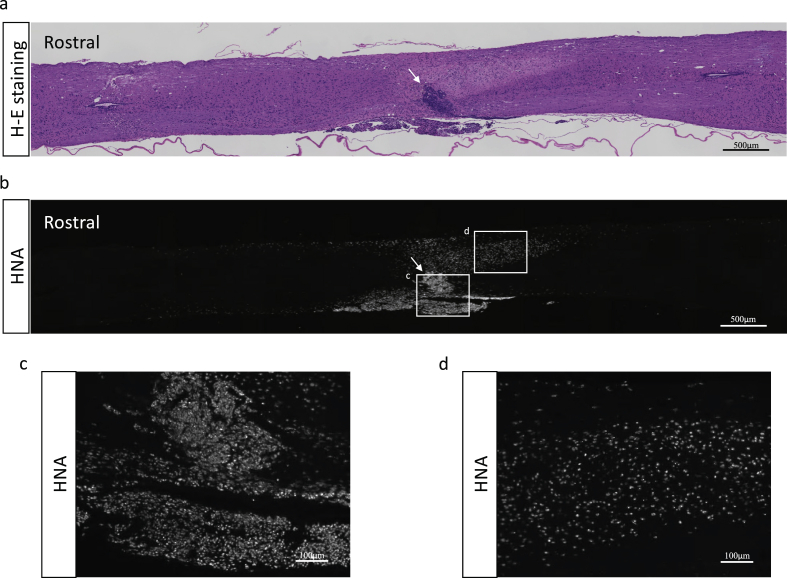
Fig. 2.
Representative histological images of sagittal spinal cord sections at 15 weeks after transplantation of YZWJs513 hiPSC-NS/PCs into the lesion epicenter of NOD/scid mice. (a) Tumor formation was not observed in a hematoxylin & eosin (H-E)-stained section (b–d) Immunohistochemical staining of human nucleotide antigen (HNA) showed engraftment of transplanted NS/PCs. Arrows indicate the lesion epicenter.
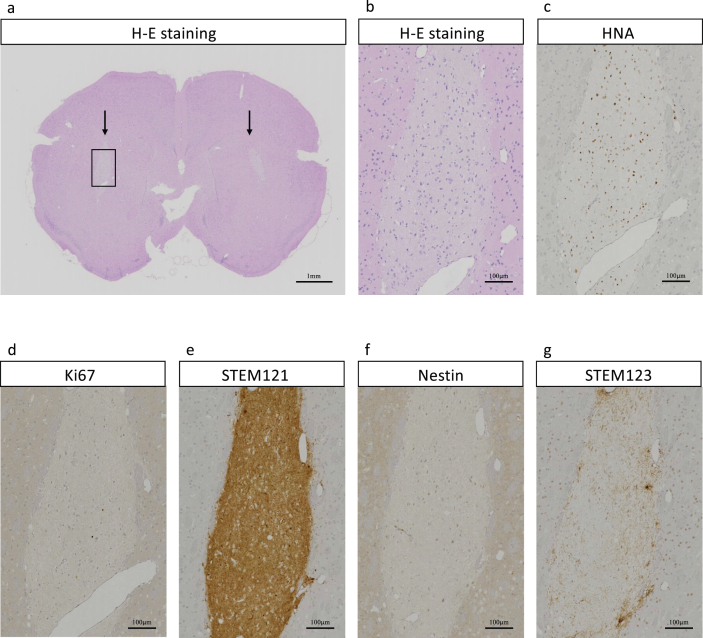
Fig. 3.
Representative histological images of axial brain section of NOG mouse at 6 months after the transplantation of 1 × 106 cells of YZWJs513 hiPSC-NS/PCs for clinical use to each striatum. (a) Arrows indicate the transplantation sites, and the cell engraftment site is observed as light-colored area by H-E staining. (b) Enlarged image from a (c–g) Immunostained images of the same area as b: human nuclear antigen (c); Ki67 (d); STEM121 (human cytoplasm antigen) (e); human specific Nestin (f); and STEM123 (human specific GFAP) (g). The cells in the transplanted cites were uniform regardless of the engraftment site such as the brain surface, the striatum, and the white matter, and almost no anti-Nestin antibody staining-positive cells were observed, and the differentiation tendency was STEM123 staining-positive. In addition, the anti-Ki67 antibody staining positive cell rate was lower than 5%, and it was considered that the proliferative ability and neoplasticity were negligible.
2.3. Efficacy of hiPSC-NS/PC transplantation in a mouse model
We previously reported the efficacy of transplantation of hiPSC-NS/PCs into mouse and primate models of SCI [18,28] using a iPSC line (201B7) that was generated with retroviral vectors.
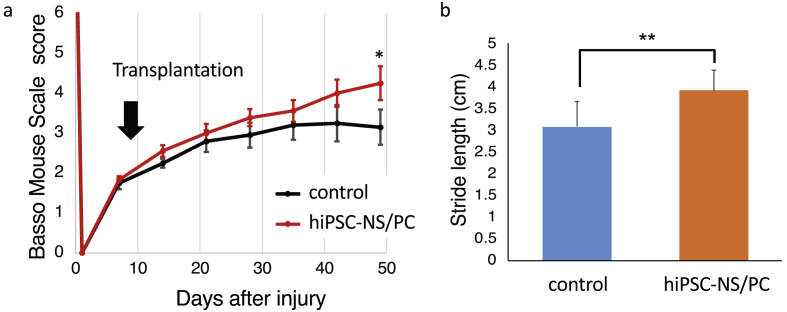
Accordingly, for the preclinical study, we have additionally studied the use of NS/PCs induced from the human umbilical cord-derived iPSC YZWJs513 strain (established under the same conditions and from the same donor as the clinical-grade line) and confirmed their efficacy following transplantation into the spinal cord of immunodeficient NOD-scid mice (n = 8) at 9 days after contusion injury at the ninth thoracic vertebra level. Compared with control animals (n = 10), which received an injection of phosphate-buffered saline (PBS), the transplanted mice showed significant recovery of motor function by 49 days post-injury (Fig. 4).
Fig. 4.
Transplantation of YZWJs513 hiPSC-NS/PCs promotes motor functional recovery after SCI in immunodeficient mice. (a) The motor function of each mouse was evaluated weekly after injury using the Basso Mouse Scale by two investigators who were blinded to the treatment of experimental mice. Sensitivity analyses with mixed-effects models for repeated measures revealed a significant difference at day 49. Data represent the means ± SEM (n = 10 for the control group and n = 8 for the transplantation group. ∗p < 0.05) (b) Treadmill gait analyses using the DigiGait System was performed at the sixth week after transplantation. Stride length was significantly longer in the transplantation group than in the control group. Data represent the means ± SEM (n = 10 for the control group and n = 8 for the transplantation group. ∗∗p < 0.01).
3. Methods: The protocol of the clinical study
3.1. Objectives
The primary objective of this clinical trial is to examine the outcome of hiPSC-NS/PCs transplantation in subacute SCI patients at the 12 month follow-up. Post-transplant mortality and the incidence of adverse events, including hyperproliferation of transplanted cells, will be evaluated. The efficacy of treatment on motor function, sensory function, spasticity, and quality of life will also be evaluated. Changes in motor function will be assessed following the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) in comparison with a historical control. An overview from cell preparation to the clinical study is shown in Fig. 5.
Fig. 5.
An overview from cell preparation to the clinical study. hiPSCs prepared at the CiRA were induced into NS/PCs at National Hospital Organization Osaka Medical Hospital and cryopreserved at Keio University Hospital. A first-in-human clinical study for subacute complete SCI using hiPSC-NS/PCs will be performed at Keio University Hospital in cooperation with National Hospital Organization Murayama Medical Hospital.
3.2. Trial design
The study design is open-label and single-arm. The decision not to use a sham-operated control was made for ethical reasons because the study is not designed to yield conclusive evidence of safety or efficacy. Patients will be recruited at Keio University Hospital (Tokyo, Japan). After transplantation of hiPSC-NS/PCs and confirmation of stabilization of the patient's condition, patients will be transferred to Murayama Medical Center (Tokyo, Japan), a partner hospital specializing in treatment of SCI patients.
3.3. Eligibility criteria
Detailed inclusion and exclusion criteria are provided in Table 2.
-
-Main inclusion criteria
- ∙Up to 24 days post-SCI.
- ∙Injury level between the third/fourth cervical vertebrae and the tenth thoracic vertebra.
- ∙AIS classification A.
- ∙Able to transplant hiPSC-NS/PCs at 14–28 days after SCI.
- ∙Age 18 years or older.
-
-Main exclusion criteria
- ∙Multiple injury, transection injury, or rupture of the dura mater apparent on magnetic resonance imaging (MRI).
- ∙History of previous SCI or diseases related to the spinal cord or subarachnoid space.
- ∙Severe respiratory failure or damage of other organs that would make it difficult to adhere to the rehabilitation program or assess neurological function.
Table 2.
Inclusion and exclusion criteria of the clinical study.
| Eligibility inclusion criteria | |
| 1) | Within 24 days after spinal cord injury with the injury level between the third/fourth cervical vertebrae and the tenth thoracic vertebra, with paralysis of AIS grade A, at the time of consent. |
| 2) | Able to transplant hiPSC-NS/PCs at 14–28 days after SCI. |
| 3) | Patient able to be hospitalized at Keio University Hospital in the acute period after cell transplantation surgery, followed by Murayama Medical Hospital at least until the sixth month after transplantation. Patient also able to visit Keio University Hospital for up to 52 weeks after cell transplantation. |
| 4) | Patient 18 years or older at the time of consent. |
| 5) |
Written informed consent voluntarily provided by the patient. When the patient is unable to provide a signature, written proof of the patient's agreement by witness of an adult relative. When the patient is younger than 20 years, written proof of the agreement of an adult relative, in addition to the patient's consent. |
| Eligibility exclusion criteria | |
| 1) | The state of the injured spinal cord. |
| Patients with multiple injuries, transection injury, or injury with a dural tear on preoperative MRI. | |
| 2) | Past history, comorbidity |
| History of spinal cord injury, or history or coexistence of abnormalities in the spinal cord or intrathecal space (a spinal cord tumor, meningitis, spinal subarachnoid hemorrhage, etc.). | |
| Difficult or impossible to capture MRI images (e.g., patients with a pacemaker in the heart). | |
| History or coexistence of intoxication due to alcohol or other drugs. | |
| 3) | Comorbidity |
| Major respiratory complications that require tracheal intubation, tracheostomy, or ventilation. | |
| Trauma or organ injuries that interfere with safety/efficacy evaluation. | |
| Other severe or uncontrolled medical complications including heart failure, diabetes mellitus, hypertension, interstitial pneumonia, renal failure, autoimmune disease, cancer, and mental illness. | |
| Active infection that becomes a contraindication for surgery. | |
| Dementia or high risk of dementia. | |
| 4) | Laboratory data |
| White blood cells ≤3.5 × 103/mm3, neutrophils ≥90%, platelets ≤1.5 × 105/mm3, hemoglobin ≤10.0 g/dL, PT-INR ≥ 1.2 (excluding patients taking anticoagulants), APTT ≥41 s (excluding patients taking anticoagulants), creatinine ≥1.1 mg/dL in males and 0.8 mg/dL in females, hepatic transferase (AST or ALT) ≥ 70 IU/L, and total bilirubin ≥1.0 mg/dL. | |
| 5) | History of allergy |
| Allergy to FK506. | |
| Allergy to ARTCEREB irrigation and perfusion solution for cerebrospinal surgery. | |
| 6) | Combination therapy |
| Cyclosporine, bosentan, and potassium-sparing diuretics at the time of consent. | |
| Use of another investigational new drug within 1 month from the time of consent. | |
| Use of steroids after spinal cord injury. | |
| 7) | Pregnancy |
| Women who are pregnant, lactating, or may be pregnant or are planning to become pregnant during the participation period of the clinical study. | |
| Men whose partners will be or plan to become pregnant during the participation period of the clinical study. | |
| 8) | Other patients who are deemed inappropriate by researchers. |
Abbreviations:AIS, American Spinal Cord Injury Association Impairment Scale; hiPSC-NS/PC, human induced pluripotent stem cell-derived neural stem/progenitor cell; SCI, spinal cord injury; MRI, magnetic resonance imaging.
3.4. Interventions: treatment description
3.4.1. Cell transplantation
hiPSC-NS/PCs will be transplanted between the 14th and 28th day post-injury.
Cells will be thawed 4 days prior to the scheduled transplant surgery. After recovery culture, the cells cultured as neurospheres will be treated with DAPT (a γ-secretase inhibitor (GSI)) for 24 h. On the day of transplantation, after washout by PBS, neurospheres having a diameter larger than 200 μm will be removed using a filter. Among the flasks prepared, one will be used specifically for cell count. Briefly, after dissociating the neusosphere using TrypLE™ select (Thermo Fisher), the cells will be diluted stepwise by DMEM/F12 and the number of alive cells will be counted using an ATP assay kit (CellTiter Glo® 3D Cell Viability Assay, Promega) by Enspire® Multimode Plate Reader (PerkinElmer). For transplantation, 2 × 106 cells from the other flasks will be suspended in 20 μL artificial cerebrospinal fluid. The cells will be delivered to the operation room and will be kept at 4 °C until transplantation.
At the operation room, under general anesthesia, dorsal spinal cord of the patient will be exposed at the scheduled time. Cells will be transplanted into the center of the site of injury, as confirmed by preoperative MRI imaging and intraoperative ultrasound imaging. The cells will be manually delivered under a surgical microscope using a Neuros syringe (Hamilton Company, USA) and a 30 gauge needle by a spine surgeon highly experienced in spinal cord surgery. This syringe has a blind stop, which allows the operator to ensure cells are precisely administered to the desired depth. After slow intraspinal injection of the cells, the needle will be removed after being held in place for about 3 min to prevent leakage of cells.
After the operation, the patient will remain in bed for 3–4 days to prevent a spinal fluid fistula. Following this initial monitoring period, they will be allowed to continue rehabilitating while not in bed.
3.4.2. Immunosuppressants
Low-dose tacrolimus at blood concentration of 5–10 ng/mL will be administered from 1 day before the transplantation and discontinued at the expected completion of remyelination post-transplantation. The time required for remyelination of the adult human spinal cord is unknown, but remyelination promoted by transplanted allogenic human embryonic stem cell (ESC)-derived NS/PCs was confirmed at 3 months post-transplantation in common marmosets [29]. Considering that the pregnancy period is about twice as long in humans as in common marmosets, we have decided to keep tacrolimus at 5–10 ng/mL by the 6th month post-transplantation, taper afterwards by 10–20% by every 1–2 weeks, and discontinue by the 9th month. This is longer than in previous studies [12,30]; however, it is possible that the NS/PCs used here may require more time to remyelinate because the cell source is different.
We will also collect immunological data, such as the HLA type and reaction of host lymphocytes to the transplant, in a similar way to that in a recent report [31]. We will analyze these data together with information about the HLA type of the source hiPSCs and clinical course of the recipient to further optimize the use of immunosuppressants in future studies.
3.4.3. Rehabilitation therapy
The degree of recovery after SCI may differ dependent on the quality and degree of rehabilitation therapy. Therefore, we will use a standardized rehabilitation program within the range allowed for SCI patients under Japan's National Health Insurance, which is up to 180 min per day until 60 days after the transplant operation and 120 min per day thereafter, which is as much as possible during hospitalization. The use of special equipment such as gait-assist robots as part of rehabilitation is unacceptable considering that therapeutic effects of these equipment and the transplanted cells could be mixed-up. Using this standard, we will attempt to achieve a uniform rehabilitation program for all patients, using data from patients treated in facilities specializing in SCI therapy as a historical control. Rehabilitation will be performed at Keio University Hospital in the first month post-transplant and at Murayama Medical Center thereafter. After patients have undergone at least 6 months of rehabilitation in hospital, they will be released, upon request.
3.5. Enrollment of patients
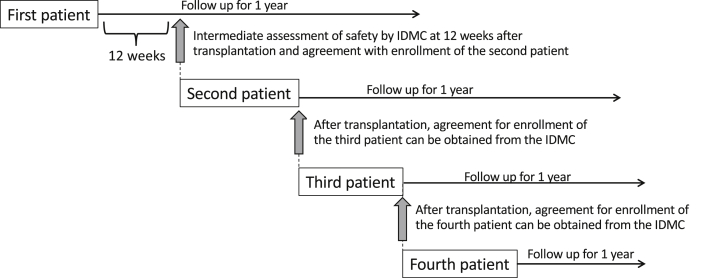
Patients will be enrolled sequentially, and each patient will be followed up for 1 year after transplantation. In the preclinical study with mice, abnormal growth of the transplant was estimated from the eighth week after transplantation by deterioration of motor function and could be confirmed histologically by the 12th week [32]. Moreover, abnormal cell growth can be detected by positron emission tomography (PET) imaging by day 28 at the earliest [33]. Based on these results from animal studies, the time interval between the first and second transplantation is set to a minimum of 12 weeks. After 12 weeks’ follow-up of the first patient, an independent data monitoring committee (IDMC) will meet to review the data and determine whether it is appropriate to continue the trial, before enrollment of the second patient. The IDMC will reconvene before enrollment of the third and fourth patients (Fig. 6).
Fig. 6.
Schema of patient enrollment. The safety of the first patient at 12 weeks after transplantation must be confirmed by the IDMC before the second patient is enrolled. After enrollment of the second patient, the IDMC must agree with recruitment of the third and fourth patients, although there is no fixed waiting period after the second and subsequent trans plantations.
3.6. Outcome measures and follow-up
Safety evaluation is the primary objective of this study (Table 3). All adverse events, as listed in Table 4, will be recorded throughout the study period. When an adverse event is observed, the date, content, severity (grade 1: mild; grade 2: not mild but not serious; and grade 3: serious, meaning there is a possibility that it could result in permanent malfunction, such as death or impediment of daily living, depending on the condition of the patient) will be recorded. Treatment taken, outcome (disappearance, alleviation, invariance, deterioration, death, sequelae, etc.), post-treatment evaluation date, and the assessed causal relationship with the protocol treatment (transplanted hiPSC-NS/PCs, transplantation operation, immunosuppression, and rehabilitation) graded at five levels (definite, probable, possible, not likely, and not related) will be evaluated for each adverse event.
Table 3.
Primary and secondary end points.
| Primary end point: safety | All adverse events will be recorded throughout the study period. All serious and unexpected adverse events will be reported to the IDMC. |
| Secondary end points: efficacy | Change of ISNCSCI total motor scores at the 52 nd week from the baseline. |
| Sequential transition of ISNCSCI motor scores. | |
| Sequential transition of AIS classification. | |
| Sequential transition of ISNCSCI sensory scores. | |
| Sequential transition of modified Frankel classification. | |
| Sequential transition of the zone of partial preservation. | |
| Sequential transition of PainDETECT scores (Japanese version). | |
| Sequential transition of the Neuropathic Pain Symptom Inventory (Japanese version). | |
| Sequential transition of the modified Ashworth scale. | |
| Sequential transition of Spinal Cord Independence Measure version III (Japanese version). | |
| Another end point: efficacy | Change of ISNCSCI motor scores at the 24th week from the baseline. |
Abbreviations: IDMC, independent data monitoring committee; ISNCSCI, International Standards for Neurological Classification of Spinal Cord Injury; AIS, American Spinal Cord Injury Association Impairment Scale.
Table 4.
List of assumed adverse events that may happen during the clinical trial.
| Due to | Possible adverse events |
|---|---|
| Spinal cord injury | Motor/sensory paralysis of upper and lower limbs, orthostatic hypotension, hypotension, dizziness, autonomic dysreflexia, thermoregulatory disorder, bladder and bowel dysfunction, paralytic ileus, neuropathic pain, spasticity, edema, joint contracture, pneumonia, urinary tract infection, pressure ulcers, deep vein thrombosis, pulmonary infarction/embolism, ectopic ossification, urinary calculus, hypercalcium crystal formation, liver disorder, sexual dysfunction, spinal cavities, and spinal atrophy. |
| Surgery | Bleeding, infections (bacterial meningitis, wound infection, pneumonia, urinary tract infection, etc.), spinal fluid leakage, skin and nerve damage due to fixation of the posture during surgery, complications accompanying general anesthesia (blood pressure fluctuation, hoarseness, drug allergy, etc.), arrhythmia, fever, malaise, wound pain, and hematologic test abnormality (glutamic-oxaloacetic transaminase, glutamate pyruvate transaminase, C-reactive protein, white blood cells, etc.). |
| Cell transplantation | Rejection reaction to transplanted cells, tumorigenesis derived from transplanted cells, and deterioration of neurological symptoms (motor/sensory function, neurological pain, etc.) |
| Immunosuppressants | Infectious diseases, drug allergic reaction, gastrointestinal disorder (diarrhea, constipation, stomach ache, loss of appetite, gastrointestinal bleeding, etc.), renal failure, abnormal serum magnesium value, hyperkalemia, abnormal heart function, hyperglycemia, tremor, liver failure, and cytopenia. |
| Others | Trauma caused by unexpected falls during rehabilitation, subcutaneous hemorrhage after blood collection, tape rash, fatigue, symptoms caused by comorbidities (hay fever, high blood pressure, headache, etc.), and insomnia. |
All serious and unexpected adverse events will be reported to the director of the hospital and will be judged by the IDMC as soon as practicable. Additionally, serious adverse events with a causal relationship with regenerative medicine will be reported to the Certified Committee for Regenerative Medicine and the Ministry of Health, Labor and Welfare within 15 days. This time limit will be 7 days in cases of fatal or life-threatening events. As for the adverse events that are not serious, they will be reported to the director of the hospital every 60 days. Additionally, among these, events with a causal relationship with regenerative medicine will be reported to the Certified Committee for Regenerative Medicine and the Ministry of Health, Labor and Welfare every 60 days.
Secondary outcome measures on efficacy will be compared with the baseline, as measured on day 14 post-injury, and will include motor function measured by ISNCSCI motor scores and the modified Frankel classification [34]; sensory function measured by ISNCSCI sensory scores as well as changes in activities of daily living measured by Spinal Cord Independence Measure version III [35]; and spasticity measured by the modified Ashworth scale [36] (Table 3). In an exploratory evaluation, data from a database of Japanese SCI patients generated by the Spinal Injuries Center (Fukuoka, Japan) will be used as a historical control.
The schedule of the study is summarized in Table 5.
Table 5.
Schedule of study activities.
| Admission/screening | 14 days after injury (if applicable) | 1 day before transplant | 14–28 days after injury | Post-transplant (days) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 |
21 |
35 |
56 |
84 |
112 |
140 |
168 |
365 |
|||||
| ±2 | ±7 | ±7 | ±7 | ±7 | ±7 | ±7 | ±7 | ±14 | |||||
| Sign ICD | × | ||||||||||||
| Health information | ×a | ||||||||||||
| Inclusion/exclusion | × | × | × | ||||||||||
| Enrollment | × | ||||||||||||
| Screening laboratories | × | ||||||||||||
| HLA/MLR test | × | ||||||||||||
| Cellular transplantation | × | ||||||||||||
| Immunosuppression b | × | × | × | × | × | × | × | × | × | × | |||
| Report AEs and SAEs | × | × | × | × | × | × | × | × | × | × | × | ||
| Neurological examinationsc,d | × | × | × | × | × | × | × | × | × | × | × | ||
| AIS d | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Rehabilitation therapy e | × | × | × | × | × | × | × | × | × | × | × | × | × |
| General status/vital signs e | × | × | × | × | × | × | × | × | × | × | × | × | × |
| MRI | ×a | × | ×g | ×g | ×g | ||||||||
| FDG-PET | ×g | ||||||||||||
| X-rays | ×a | ||||||||||||
| Blood exams | ×a | × | × | × | × | × | × | × | × | × | × | ||
| Urinary exams | ×a | ||||||||||||
| Ultrasonography of leg vein f | ×a | × | × | × | × | × | × | × | × | ||||
| Discharge information h | × | ||||||||||||
Abbreviations: ICD, informed consent document; HLA, human leukocyte antigen; MLR, mixed lymphocyte reaction; AE, adverse event; SAE, severe adverse event; AIS, American Spinal Cord Injury Association Impairment Scale; MRI, magnetic resonance imaging; FDG-PET, fluorodeoxyglucose(18F) positron emission tomography.
Items that can use the data taken before admission.
Immunosuppressants will be used every day from day −1 to day 280 after transplantation.
Including the International Standards for Neurological Classification of Spinal Cord Injury, modified Frankel classification, zone of partial preservation, Pain DETECT, Neuropathic Pain Symptom Inventory, Modified Ashworth scale, and Spinal Cord Independence Measure version III.
Neurological examinations should be performed and AIS should be checked once per month after day 56.
Rehabilitation therapy and monitoring of general status/vital signs will be performed every day during hospitalization.
Ultrasonography of a leg vein will be performed once per month after transplantation.
Items that can be assessed within 1 month from the scheduled time-point.
Patients can be discharged after day 168. If desired, patients can opt to remain in hospital until the end of the initial observation period.
3.7. Statistical analysis
All patients who receive the study transplant will be included in safety analysis. To assess efficacy, we define the following two analysis populations:
-
(1)
Full Analysis Set (FAS): An analysis population based on the Intention-to-Treat principle. All patients who receive the study transplant and have at least one follow-up data point will be included in this population.
-
(2)
Per Protocol Set (PPS): All patients who adhere to the trial protocol will be included in this population. Details of the population will be finalized before the database lock. The primary analysis population in the efficacy evaluation is the FAS.
For safety analysis, the number and proportion of patients who experience one or more adverse event will be summarized. The number and proportion of patients will be also summarized for individual adverse events and their grade. The change from the baseline at each time-point over 12 months in each laboratory test will be summarized. For efficacy evaluation, the change from baseline at each time point over 12 month in each endpoint will be summarized. The summary will be generated for completers and for all patients in which last observation carried forward will be used for dropouts. The changes will be visually compared with those from a database of Japanese SCI patients generated by the General Spinal Injuries Center (Fukuoka, Japan).
4. Discussion
iPSCs are starting to be used in several clinical studies in Japan [37,38], and their safety is becoming clear. Preclinical studies suggest that hiPSC-NS/PCs help to reconstruct neural circuits in the injured spinal cord and improve functional recovery. This first-in-human clinical trial will be conducted to investigate the safety and efficacy of hiPSC-NS/PCs for the treatment of human subacute SCI.
In this trial, safety is the top priority, and QC is essential for this. The key point to understand QC of the cells used in this trial is that the nature of hiPSCs and hiPSC-NS/PCs differs between clones. Upon QC, only qualified high-quality iPSC-NS/PCs will be used in clinical trials. In the preclinical study, we excluded low-quality clones at the QC stage and picked a candidate clone for this clinical trial from the viewpoint of safety and effectiveness.
Considering safety, the risk of tumorigenicity is a key concern in the transplantation of cells derived from pluripotent sources. In the preclinical study with an animal model, several NS/PC clones exhibited abnormal overgrowth in vivo [32]. Although we did not observe cellular invasion into the surrounding spinal canal or metastasis to other organs, expansion of transplanted cells into the spinal column caused paralysis of the lower limbs. Moreover, the transplanted cells had some properties characteristic of neuroepithelial tumor cells in the adult CNS [17]. Consequently, monitoring and regulation of the proliferation and differentiation of NS/PCs are critical.
Our previous studies of proliferative tumor-like tissues obtained following transplantation of hiPSC-NS/PCs into animal models indicated that such tissues are composed of proliferating cells with an immature neural phenotype and differ from teratomas. Thus, cellular hyperproliferation (tumorigenicity) appears to be attributable to genetic and/or epigenetic transformation of NS/PCs, not to contamination of undifferentiated iPSCs [17]. NS/PCs derived from specific iPSC clones tended to form a tumor-like mass (and therefore such clones were designated “unsafe”) [32,39]. In our previous preclinical studies [28,39], we initially used hiPSC lines (201B7 and 253G1 clones) that had been generated with retroviral vectors [16,40]. According to the results, we considered the possibility that a subset of cells displaying genomic mutations or excessive proliferation represented NS/PCs that had lost their multipotency (i.e., the potency to give rise to neurons and glia), but which nonetheless retained either residual or induced activation of the OCT4 transgene [32].
In contrast to these previous studies, in our forthcoming clinical study, we use integration-free iPSCs; however, we believe that the persistence of such differentiation-resistant cells is still a risk factor for tumorigenesis. Genomic instability due to incomplete reprogramming at the time of iPSC generation and subsequent differentiation culture may also significantly contribute to tumorigenicity or hyperproliferation, even in integration-free iPSC clones [17,39]. Importantly, genomic instability increases with the number of cell divisions during serial cell culture [39].
To exclude such differentiation-resistant cells among cells that will be used in the present clinical trial, we established an in vitro QC strategy to detect OCT4-positive cells using multiple techniques including qRT-PCR, FCM, ICC, and back culture with high sensitivity. We will also perform chromosomal and genomic analyses to lower the risk of tumorigenicity and hyperproliferation. This QC process is used to establish the standard characteristics of the end product (Table 1).
In the present protocol, it will be difficult to track cell engraftment post-transplantation. To minimize patient risk and regulatory testing requirements, transplanted cells will not be labeled using transgenes; cellular products must comply with quality standards for both cell- and gene-based products and thus cannot be marked by transgenes. For similar reasons, we have opted to use integration-free iPSCs and not employ a “suicide gene” strategy to eliminate engrafted cells. The primary signals used to detect excessive proliferation of transplanted cells during the follow-up period will be deterioration of neurological symptoms and the results of MRI or PET. In a study involving transplantation of hiPSC-NS/PCs with an abnormal growth capacity into mouse brain, we detected hyperproliferating cells by MRI or PET as early as 28 days [33]. In addition, previous studies indicate that “unsafe” cells have a particularly high proliferation rate in the first 3 months after transplantation [17,32]. Therefore, we plan to perform MRI on days 21, 84, 168, and 365 after surgery, and fluorodeoxyglucose (18F) PET around day 168 after surgery. If hyperproliferation or malignant transformation of NS/PCs is detected, we will immediately terminate immunosuppression; surgical resection of the graft will be considered if we determine there is a high risk of further deterioration of neurological symptoms due to cell proliferation.
Regarding effectiveness, putative mechanisms underlying the observed recovery of motor function are thought to be combinatorial and may include 1) remodeling of the injury environment by trophic factors secreted by transplanted cells and/or their progeny (functional improvement of residual cells, promotion of regeneration of the spinal cord, suppression of inflammation, etc.), 2) repair of neural circuits by transplanted cells (replacement of lost or damaged nerve cells), and 3) remyelination by oligodendrocytes differentiated from transplanted cells [18,28,41]. In this study, we will use a GSI to improve the safety of hiPSC-NS/PCs by inducing cells into a more mature state. A GSI acts as an inhibitor of Notch signaling, which has been shown to limit the proliferation of transplanted hiPSC-NS/PCs without inhibiting the improvement of motor function in vivo by animal studies [42]. hiPSC-NS/PCs (253G1 strain) prepared using the same technique exhibited an engraftment rate of 12% at the third month after transplantation into the injured spinal cord of NOD-scid mice (n = 10), and tended to acquire a neuronal phenotype (5% of cells were Nestin-positive (immature neural marker), 51% of cells were ELAVL-positive (neuronal marker), 11% of cells were GFAP-positive (astrocyte marker), 4% of cells were APC-positive (oligodendrocyte marker), and 4% of cells were Ki67-positive (proliferating marker)). We observed life-long engraftment of transplanted cells in immunodeficient mice and anticipate long-term cellular engraftment in humans as well. However, the cellular differentiation rate in humans may differ from that observed in animal models, as suggested by the different differentiation rates of hiPSC-NS/PCs transplanted into NOD-scid mice and common marmosets [18,28]. However, biopsy of human spinal cord tissue is precluded for ethical reasons, making it difficult to investigate the engraftment, survival, and differentiation patterns of transplanted cells in this study.
Immune rejection may interfere with the engraftment of transplanted cells even in the CNS, which is protected by the blood–brain barrier, just as it does in other organ systems. Previous studies of xenogeneic transplants reported that engrafted cells do not survive without immunosuppression [43], although a fraction of transplanted hiPSC-NS/PCs survive without immunosuppression in cases of allogeneic transplantation [44]. Moreover, hiPSC-NS/PCs only present antigens at low levels and exert immunosuppressive effects [41]. In a study of allogenic hiPSC-NS/PC transplantation into the spinal cord of minipigs, histological evaluation at 3.5 months after cessation of transient immunosuppression (duration: 4 weeks) revealed remyelination by transplanted cells, suggesting that temporary use of immunosuppressants may be sufficient to elicit long-term immune tolerance following transplantation of allogenic NS/PCs into the spinal cord [45]. Therefore, temporal use of immunosuppression is frequently employed in clinical studies of NS/PC transplantation into SCI patients, and no evidence of rejection or appearance of anti-donor HLA has been reported [12,30]. In the present study, we have opted to use low-dose tacrolimus, as was used in the SCiStar study [30]. We do not check patients' anti-HLA antibodies in the present study, but it may help determine the appropriate dose and administration period of immunosuppressants in other studies in the future.
Considering the phase of this first-in-human study, we have conservatively opted to transplant 2 × 106 cells per patient. In our preclinical studies, we engrafted comparatively larger numbers of cells by volume (5 × 105 cells/mouse; body weight 17–20 g; 1 × 106 cells/marmoset; body weight 250–500 g). Many first-in-human trials started using a similar number of cells in cases of direct injection into the spinal cord, including Geron's trial for the subacute phase of SCI using human ESC-derived oligodendrocyte progenitor cells (OPCs) (2 million cells), which has been taken over by Lineage Cell Therapeutics via Asterias Biotherapeutics [30,46], and UCSD's trial for chronic SCI using human fetus-derived NS/PCs (1.2 million cells) [12]. We believe it is reasonable to start from a small number of cells for first-in-human trials considering the safety issues. On the other hand, the SCiStar study by Asterias reported efficacy using 10 million cells, and UCSD's trial reported efficacy in two of four patients. Although 2 million cells in our trial might show some effectiveness, dose escalation may induce better functional recovery. Therefore, after confirming the safety issue, a dose-escalation study is planned, similar to Asterias' trial using human ESC-derived OPCs [30].
Considering that various clinical studies have begun, and safety and efficacy have been reported [[7], [8], [9],[12], [13], [14],30,47], it is also necessary to consider the proper choice of the treatment method for patients with different medical conditions. The appropriate treatment option may differ according to the patients’ condition such as their age (children, adults, or the elderly), time from injury (acute or chronic), cause of injury (trauma, spinal cord tumors, involvement of spinal or disc diseases, etc.), and complications (other trauma, heart or renal failure, etc.). In the future, when different treatments become available, it will be necessary to evaluate which combination of treatments is appropriate for each patient, taking into account the risks and benefits of each treatment.
5. Current status/conclusion
At the time of writing (June 2, 2021), we are preparing to recruit a patient. We completed the cell preparation and all QC by the end of August 2020, but the COVID-19 epidemic delayed the start of patient recruitment. We plan to start recruiting a patient as soon as the epidemic subsides (University Hospital Medical Information Network Clinical Trials Registry [UMIN-CTR] number, UMIN000035074; Japan Registry of Clinical Trials [jRCT] number, jRCTa031190228.)
Funding support
This study is supported by the Research Center Network for Realization of Regenerative Medicine (Centers for Clinical Application Research on Specific Disease/Organ), Grant No. 22 (18bm0204001h0006), and the Research Project for Practical Applications of Regenerative Medicine from the Japan Agency for Medical Research and Development (AMED), Grant No. JP18bk0104017, JP19bk0104017, JP20bk0104017, and JP21bk0104120.
Declaration of competing interest
R.Y. is an employee of Sumitomo Dainippon Pharma Co., Ltd. M.N. has a consultancy role with K-Pharma, Inc. and has received research funding from RMiC. H.O. is a compensated scientific consultant for San Bio Co., Ltd., K-Pharma, Inc., RMiC, and intellim Holdings Corporation and has received research funding from SanBio Co., Ltd., K-Pharma, Inc., and RMiC. S.Y. declared consulting, research funding from iPS Academia Japan. TA has a consultancy role with Kringle Pharma, Inc. The other authors declare no potential conflicts of interest.
Acknowledgments
We thank the CiRA (Kyoto, Japan) for kindly providing iPSCs. We are also very grateful to the members of the spinal cord research team at the Department of Orthopaedic Surgery, as well as the members of the Institute for Clinical Research National Hospital Organization Osaka National Hospital and the Foundation for Biomedical Research and Innovation at Kobe for their valuable assistance with the in vitro and in vivo experiments.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Keiko Sugai, Email: ksugai@keio.jp.
Miho Sumida, Email: sumida.miho.yc@mail.hosp.go.jp.
Tomoko Shofuda, Email: shofuda.tomoko.td@mail.hosp.go.jp.
Ryo Yamaguchi, Email: ryo-yamaguchi@ds-pharma.co.jp.
Takashi Tamura, Email: tamura@fbri.org.
Tsuneo Kohzuki, Email: kohzuki_2650@keio.jp.
Takayuki Abe, Email: abetk@yokohama-cu.ac.jp.
Reo Shibata, Email: leos0519@icloud.com.
Yasuhiro Kamata, Email: yasuhiro.kamata99@gmail.com.
Shuhei Ito, Email: shuheiazb@gmail.com.
Toshiki Okubo, Email: t.okubo@z8.keio.jp.
Osahiko Tsuji, Email: osahiko.z8@keio.jp.
Satoshi Nori, Email: satoshi_nori@2003.jukuin.keio.ac.jp.
Narihito Nagoshi, Email: nagoshi@2002.jukuin.keio.ac.jp.
Shinya Yamanaka, Email: yamanaka@cira.kyoto-u.ac.jp.
Shin Kawamata, Email: kawamata@fbri.org.
Yonehiro Kanemura, Email: kanemura.yonehiro.hk@mail.hosp.go.jp.
Masaya Nakamura, Email: masa@keio.jp, masa@keio.jp.
Hideyuki Okano, Email: hidokano@keio.jp.
References
- 1.Fawcett J.W., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2006:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 2.Shingu H., Ohama M., Ikata T., Katoh S., Akatsu T. A nationwide epidemiological survey of spinal cord injuries in Japan from January 1990 to December 1992. Paraplegia. 1995;33(4):183–188. doi: 10.1038/sc.1995.42. [DOI] [PubMed] [Google Scholar]
- 3.Sakai H., Ueta T., Shiba K. Epidemiological survey of spinal cord injury in Fukuoka prefecture. Bone Joint Nerve. 2011;1(3):475–480. [Google Scholar]
- 4.Fehlings M.G., Tetreault L.A., Wilson J.R., Kwon B.K., Burns A.S., Martin A.R. A clinical practice guideline for the management of acute spinal cord injury: introduction, rationale, and scope. Global Spine J. 2017;7(3 Suppl):84S–94S. doi: 10.1177/2192568217703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorklund A., Stenevi U. Intracerebral neural implants: neuronal replacement and reconstruction of damaged circuitries. Annu Rev Neurosci. 1984;7:279–308. doi: 10.1146/annurev.ne.07.030184.001431. [DOI] [PubMed] [Google Scholar]
- 6.Koda M., Hanaoka H., Sato T., Fujii Y., Hanawa M., Takahashi S. Study protocol for the G-SPIRIT trial: a randomised, placebo-controlled, double-blinded phase III trial of granulocyte colony-stimulating factor-mediated neuroprotection for acute spinal cord injury. BMJ Open. 2018;8(5) doi: 10.1136/bmjopen-2017-019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derakhshanrad N., Saberi H., Yekaninejad M.S., Joghataei M.T., Sheikhrezaei A. Granulocyte-colony stimulating factor administration for neurological improvement in patients with postrehabilitation chronic incomplete traumatic spinal cord injuries: a double-blind randomized controlled clinical trial. J Neurosurg Spine. 2018;29(1):97–107. doi: 10.3171/2017.11.SPINE17769. [DOI] [PubMed] [Google Scholar]
- 8.Fehlings M.G., Kim K.D., Aarabi B., Rizzo M., Bond L.M., McKerracher L. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: design of the SPinal cord injury Rho INhibition InvestiGation (SPRING) clinical trial. J Neurotrauma. 2018;35(9):1049–1056. doi: 10.1089/neu.2017.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman R.G., Fehlings M.G., Frankowski R.F., Burau K.D., Chow D.S., Tator C. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31(3):239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura K., Nagoshi N., Tsuji O., Matsumoto M., Okano H., Nakamura M. Application of hepatocyte growth factor for acute spinal cord injury: the road from basic studies to human treatment. Int J Mol Sci. 2019;20(5) doi: 10.3390/ijms20051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghobrial G.M., Anderson K.D., Dididze M., Martinez-Barrizonte J., Sunn G.H., Gant K.L. Human neural stem cell transplantation in chronic cervical spinal cord injury: functional outcomes at 12 Months in a phase II clinical trial. Neurosurgery. 2017;64(CN_suppl_1):87–91. doi: 10.1093/neuros/nyx242. [DOI] [PubMed] [Google Scholar]
- 12.Curtis E., Martin J.R., Gabel B., Sidhu N., Rzesiewicz T.K., Mandeville R. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22(6):941–950. doi: 10.1016/j.stem.2018.05.014. e6. [DOI] [PubMed] [Google Scholar]
- 13.Tabakow P., Jarmundowicz W., Czapiga B., Fortuna W., Miedzybrodzki R., Czyz M. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22(9):1591–1612. doi: 10.3727/096368912X663532. [DOI] [PubMed] [Google Scholar]
- 14.Oh S.K., Choi K.H., Yoo J.Y., Kim D.Y., Kim S.J., Jeon S.R. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78(3):436–447. doi: 10.1227/NEU.0000000000001056. discussion 47.10.1227/NEU.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 15.Cusimano M., Biziato D., Brambilla E., Donega M., Alfaro-Cervello C., Snider S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135(Pt 2):447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Sugai K., Fukuzawa R., Shofuda T., Fukusumi H., Kawabata S., Nishiyama Y. Pathological classification of human iPSC-derived neural stem/progenitor cells towards safety assessment of transplantation therapy for CNS diseases. Mol Brain. 2016;9(1):85. doi: 10.1186/s13041-016-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi Y., Okada Y., Itakura G., Iwai H., Nishimura S., Yasuda A. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS one. 2012 doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuji O., Sugai K., Yamaguchi R., Tashiro S., Nagoshi N., Kohyama J. Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem cells. 2019;37(1):6–13. doi: 10.1002/stem.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 21.Okita K., Yamakawa T., Matsumura Y., Sato Y., Amano N., Watanabe A. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem cells. 2013;31(3):458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 22.Morizane A., Doi D., Takahashi J. Neural induction with a dopaminergic phenotype from human pluripotent stem cells through a feeder-free floating aggregation culture. Methods Mol Biol. 2013;1018:11–19. doi: 10.1007/978-1-62703-444-9_2. [DOI] [PubMed] [Google Scholar]
- 23.Kanemura Y., Mori H., Kobayashi S., Islam O., Kodama E., Yamamoto A. Evaluation of in vitro proliferative activity of human fetal neural stem/progenitor cells using indirect measurements of viable cells based on cellular metabolic activity. J Neurosci Res. 2002;69(6):869–879. doi: 10.1002/jnr.10377. [DOI] [PubMed] [Google Scholar]
- 24.Shibata T. Current and future molecular profiling of cancer by next-generation sequencing. Jpn J Clin Oncol. 2015;45(10):895–899. doi: 10.1093/jjco/hyv122. [DOI] [PubMed] [Google Scholar]
- 25.Futreal P.A., Coin L., Marshall M., Down T., Hubbard T., Wooster R. A census of human cancer genes. Nat Rev Canc. 2004;4(3):177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sondka Z., Bamford S., Cole C.G., Ward S.A., Dunham I., Forbes S.A. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Canc. 2018;18(11):696–705. doi: 10.1038/s41568-018-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amberger J.S., Hamosh A. Searching online mendelian inheritance in man (OMIM): a knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinformatics. 2017;58:1 2 1–2 12. doi: 10.1002/cpbi.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y. Grafted human-induced pluripotent stem-cell–derived neurospheres promote motor functional recovery after spinal cord injury in mice. P Natl Acad Sci USA. 2011:1–6. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwai H., Shimada H., Nishimura S., Kobayashi Y., Itakura G., Hori K. Allogeneic neural stem/progenitor cells derived from embryonic stem cells promote functional recovery after transplantation into injured spinal cord of nonhuman primates. Stem Cells Transl Med. 2015;4(7):708–719. doi: 10.5966/sctm.2014-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asterias, Biotherapeutics . January 24, 2019. Asterias provides top line 12 Month data update for its OPC1 phase 1/2a clinical trial in severe spinal cord injury.https://www.globenewswire.com/news-release/2019/01/24/1704757/0/en/Asterias-Provides-Top-Line-12-Month-Data-Update-for-its-OPC1-Phase-1-2a-Clinical-Trial-in-Severe-Spinal-Cord-Injury.html (accessed May 20th 2021) [Google Scholar]
- 31.Sugita S., Iwasaki Y., Makabe K., Kimura T., Futagami T., Suegami S. Lack of T Cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Reports. 2016;7(4):619–634. doi: 10.1016/j.stemcr.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nori S., Okada Y., Nishimura S., Sasaki T., Itakura G., Kobayashi Y. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Reports. 2015;4(3):360–373. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanimoto Y., Yamasaki T., Nagoshi N., Nishiyama Y., Nori S., Nishimura S. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Transl Med. 2020;9(4):465–477. doi: 10.1002/sctm.19-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi T., Kawano O., Sakai H., Ideta R., Ueta T., Maeda T. The potential for functional recovery of upper extremely function following cervical spinal cord injury without major bone injury. Spinal Cord. 2013;51(11):819–822. doi: 10.1038/sc.2013.90. [DOI] [PubMed] [Google Scholar]
- 35.Catz A., Itzkovich M., Agranov E., Ring H., Tamir A. SCIM--spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35(12):850–856. doi: 10.1038/sj.sc.3100504. [DOI] [PubMed] [Google Scholar]
- 36.Bohannon R.W., Smith M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 37.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi J. Preparing for first human trial of induced pluripotent stem cell-derived cells for Parkinson's disease: an interview with Jun Takahashi. Regen Med. 2019;14(2):93–95. doi: 10.2217/rme-2018-0158. [DOI] [PubMed] [Google Scholar]
- 39.Iida T., Iwanami A., Sanosaka T., Kohyama J., Miyoshi H., Nagoshi N. Whole-genome DNA methylation analyses revealed epigenetic instability in tumorigenic human iPS cell-derived neural stem/progenitor cells. Stem cells. 2017;35(5):1316–1327. doi: 10.1002/stem.2581. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki M., Iwanami A., Nagoshi N., Kohyama J., Itakura G., Iwai H. Evaluation of the immunogenicity of human iPS cell-derived neural stem/progenitor cells in vitro. Stem Cell Res. 2017;19:128–138. doi: 10.1016/j.scr.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Okubo T., Iwanami A., Kohyama J., Itakura G., Kawabata S., Nishiyama Y. Pretreatment with a gamma-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injury. Stem Cell Reports. 2016;7(4):649–663. doi: 10.1016/j.stemcr.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenzweig E.S., Brock J.H., Lu P., Kumamaru H., Salegio E.A., Kadoya K. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24(4):484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports. 2013;1(4):283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strnadel J., Carromeu C., Bardy C., Navarro M., Platoshyn O., Glud A.N. Survival of syngeneic and allogeneic iPSC-derived neural precursors after spinal grafting in minipigs. Sci Transl Med. 2018;10(440) doi: 10.1126/scitranslmed.aam6651. [DOI] [PubMed] [Google Scholar]
- 46.Lebkowski J. GRNOPC1: the world's first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med. 2011;6(6 Suppl):11–13. doi: 10.2217/rme.11.77. [DOI] [PubMed] [Google Scholar]
- 47.Nori S., Ahuja C.S., Fehlings M.G. Translational advances in the management of acute spinal cord injury: what is new? What is hot? Neurosurgery. 2017;64(CN_suppl_1):119–128. doi: 10.1093/neuros/nyx217. [DOI] [PubMed] [Google Scholar]