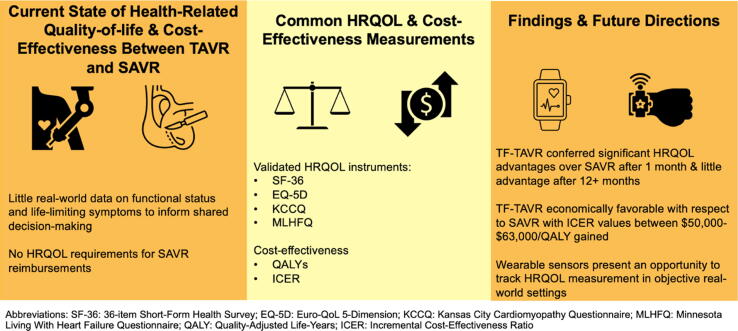
Highlights
-
•
Post-TAVR HRQOL shows more rapid short-term improvement than SAVR within trials.
-
•
Higher TAVR use requires better real-world TAVR/SAVR cost-effectiveness comparisons.
-
•
Wearable devices should be used in real-world settings to compare TAVR/SAVR HRQOL.
Keywords: TAVR, SAVR, Aortic Stenosis, Valvular heart disease, Wearable Devices
Abstract
Background
Aortic stenosis is a prevalent valvular heart disease that is treated primarily by surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR), which are common treatments for addressing symptoms secondary to valvular heart disease. This narrative review article focuses on the existing literature comparing recovery and cost-effectiveness for SAVR and TAVR.
Methods
Major databases were searched for relevant literature discussing HRQOL and cost-effectiveness of TAVR and SAVR. We also searched for studies analyzing the use of wearable devices to monitor post-discharge recovery patterns.
Results
The literature focusing on quality-of-life following TAVR and SAVR has been limited primarily to single-center observational studies and randomized controlled trials. Studies focused on TAVR report consistent and rapid improvement relative to baseline status. Common HRQOL instruments (SF-36, EQ-5D, KCCQ, MLHFQ) have been used to document that TF-TAVR is advantageous over SAVR at 1-month follow-up, with the benefits leveling off following 1 year. TF-TAVR is economically favorable relative to SAVR, with estimated incremental cost-effectiveness ratio values ranging from $50,000 to $63,000/QALY gained. TA-TAVR has not been reported to be advantageous from an HRQOL or cost-effectiveness perspective.
Conclusions
While real-world experiences are less described, large-scale trials have advanced our understanding of recovery and cost-effectiveness of aortic valve replacement treatment strategies. Future work should focus on scalable wearable device technology, such as smartwatches and heart-rate monitors, to facilitate real-world evaluation of TAVR and SAVR to support clinical decision-making and outcomes ascertainment.
1. Introduction
Aortic stenosis (AS) is the most widespread valvular heart condition in the developed world, with moderate to severe symptomatic AS impacting nearly 250,000 people in the U.S. alone [1], [2]. Although surgical aortic valve replacement (SAVR) had long been the principal treatment for AS prior to 2011, transcatheter aortic valve replacement (TAVR) has increasingly been adopted, particularly for high or prohibitive surgical risk populations [3], [4], [5]. TAVR has subsequently become an approved treatment option for low- and moderate-surgical risk populations, as meta-analyses have demonstrated reductions in early and midterm mortality, stroke, atrial fibrillation, acute kidney injury and major bleeding [6], [7], [8]. Based on high-quality randomized controlled trials, TAVR is now approved for the treatment of severe symptomatic aortic stenosis across the spectrum of surgical risk [9], [10], [11], [12]. As a percentage of total aortic valve replacement (AVR) procedures in the U.S., TAVR grew by 59% from 2012 to 2018 [13].
The primary outcomes for most TAVR and SAVR studies include complications and death. Nonetheless, patients often present for these procedures not only for extension of life, but also to alleviate life-limiting symptoms. While survival trajectories after SAVR or TAVR are well documented, less is known about the health-related quality of life (HRQOL) benefits. Assessments of the latter have often been conducted within the setting of trials, thus limiting the generalizability of these findings to real-world settings. Discrepancies in HRQOL data collection between SAVR and TAVR prevent comparisons of their relative efficacies in relieving life-limiting symptoms; reimbursement for TAVR is in part based on HRQOL assessments, but this is not the case for SAVR. Furthermore, most longitudinal HRQOL, functional status, and cost-effectiveness data are limited to a few select studies. Real-world baseline and longitudinal HRQOL and functional health assessments would allow patients and physicians to make data-driven decisions between TAVR and SAVR, as well as establish post-discharge monitoring to optimize patients’ recovery and offer insights into the mechanism of functional health trajectories. Modern wearable device technology presents a unique opportunity to predict objective post-discharge recovery patterns for AS patients by collecting physiological parameters and patient-reported data in real time (see Table 1).
Table 1.
Studies of SAVR HRQOL.
| Author (year) | Study design | Sample size | Risk category | Validated HRQOL instrument(s) | Follow-up timeframe |
|---|---|---|---|---|---|
| Klomp et al. (2016) | Prospective Observational | 762 | High or prohibitive | SF-36a | 30 days, 1 year |
| Kurfirst et al. (2014) | Prospective Observational | 310 | High | SF-36 | 1 year |
| Grady et al. (2011) | Prospective Observational | 816 | N/A | SF-36 | 3, 6, 12, 24, 36 months |
| Sundt et al. (2000) | Retrospective Observational | 133 | N/A | SF-36 | 5 years |
| Oliveira et al. (2012) | Retrospective Observational | 114 | N/A | None (self-reported) | Mean of 47.2 months |
| Gavalski et al. (2020) | Retrospective Observational | 84 | N/A | EQ-5Db | Mean of 22.4 months |
| Tseng et al. (1997) | Retrospective Observational | 247 | N/A | SF-36 | Mean of 4.1 years |
SF-36 = 36-item Short Form Health Survey.
EQ-5D = Euro-QoL 5-Dimension.
Fig. 1 the purpose of this narrative review is to describe the existing literature underpinning the recovery and comparative cost-effectiveness of TAVR and SAVR. We also examine the potential for wearable device technology to help researchers predict recovery trajectories more accurately by transmitting real-time, real-world physiologic and activity data. Importantly, patients may undergo procedures that may not align with their expectations for long-term success due in part to inadequate information concerning real-world, treatment-specific functional recovery trajectories. Findings related to these treatment options are imperative to support treatment decision-making for the nearly 90,000 U.S. patients undergoing TAVR or SAVR annually [13].
Fig. 1.
Comparisons of Validated HRQOL Instruments and Cost-Effectiveness for TAVR and SAVR.
2. Methods
The databases PubMed, Google Scholar, UpToDate, and Web of Science were searched for studies in which HRQOL and/or functional status for TAVR and SAVR patients were the primary outcomes of interest. Observational studies and randomized controlled trials were included. Literature (observational studies, randomized controlled trials, meta-analyses) spanning all surgical risk categories were included. Other criteria included those leveraging validated HRQOL instruments and/or cost-effectiveness comparisons between TAVR and SAVR. Studies that focused only on outcomes as endpoints and/or did not include AVR as a procedure type were excluded. With respect to TAVR/SAVR HRQOL and cost-effectiveness comparisons, most studies were found based on their relation to the pivotal Placement of Aortic Transcatheter Valves (PARTNER) and CoreValve randomized controlled trials [9], [10], [11], [12] (see Table 2).
Table 2.
Comparisons of TAVR & SAVR.
| Author (year) | Group | Study design | Sample size | Risk category | Validated HRQOL instrument(s) | Follow-up timeframe |
|---|---|---|---|---|---|---|
| Reynolds et al. (2012) | PARTNER Cohort A | RCT | 628 | High | KCCQa, SF-12, EQ-5D | 1, 6, 12 months |
| Gada et al. (2015) | PARTNER Cohort A | RCT | 875 | High | KCCQ, SF-12, EQ-5D | 1, 6, 12 months |
| Baron et al. (2017) | PARTNER 2 Cohort A | RCT | 1,833 | Intermediate | KCCQ, SF-12, EQ-5D | 1, 12, 24 months |
| Makkar et al. (2020) | PARTNER 2 Cohort A | RCT | 2,032 | Intermediate | KCCQ | 5 years |
| Baron et al. (2019) | PARTNER 3 | RCT | 943 | Low | KCCQ, SF-12, EQ-5D | 1, 6, 12 months |
| Arnold et al. (2015) | CoreValve US Pivotal Trial | RCT | 795 | High | KCCQ, SF-12, EQ-5D | 1, 6, 12 months |
| Straiton et al. (2018) | N/A | Meta-analysis | 2,775 | High | KCCQ, SF-12, EQ-5D, MLHFQb | 1–12 months |
| Ando et al. (2018) | N/A | Meta-analysis | 4,125 | N/A | KCCQ, SF-12, EQ-5D | 30 days, 1 year |
| Arnold et al. (2017) | N/A | Observational | 7,014 | High | KCCQ | 30 days, 1 year |
KCCQ = Kansas City Cardiomyopathy Questionnaire.
Minnesota Living with Heart Failure Questionnaire.
3. Results
3.1. SAVR
While HRQOL is a component of data elements reported through the TVT registry, consistent, longitudinal use of HRQOL metrics is not the standard of care for SAVR outside of select retrospective and prospective analyses [14]. Nonetheless, these studies offer some insights into the recovery trajectories of SAVR patients’ functional recovery and HRQOL. The Medical Outcomes Survey Short-Form 36 (SF-36) is among the most widely used HRQOL instruments to assess many different patient populations [15], [16], [17], [18], [19]. Other instruments used were the Barthel Index (to measure functional status), self-reported perception of quality of life (QoL), and the EuroQoL (EQ-5D) [20], [21]. With few exceptions, these studies focused on longitudinal recovery of at least one year after measuring baseline. Additionally, most studies placed more emphasis on patient age, rather than on surgical risk category (i.e., low, moderate, high/prohibitive), as a predictor of QoL outcomes.
Tseng et al. and Sundt et al. conducted two of the earliest studies related to functional outcomes and QoL following SAVR [18], [22]. These sought to evaluate whether SAVR was appropriate for elderly patients (patients aged > 70 and > 80, respectively), assessing operative and late mortality as well as QoL using the SF-36. Tseng et al. found that compared with age-matched population norms, SAVR patients scored as well or better in all SF-36 categories except mental health [22]. Sundt et al. reported similar findings; five of the eight SF-36 categories showed higher scores for AVR than age-matched cohorts [18]. This study also reported that among 5-year survivors, the median New York Heart Association (NYHA) functional class fell from 3 to 1, revealing a marked improvement in functional status. Because these studies examined quality of life at a single time point postoperatively, they did not measure longitudinal change in HRQOL from baseline; however, both observed SF-36 scores higher than their age-matched comparison group in at least 5 of the 8 categories.
More recent SAVR-HRQOL studies show improvements at multiple time points in postoperative HRQOL across different study populations. Using an aggregated physical component summary (PCS) and mental component summary (MCS), Grady and colleagues measured HRQOL using the SF-36 in a population of patients undergoing isolated cardiac operations [17]. PCS and MCS measurements were taken at baseline and after 3 and 6 months, then every 6 months thereafter through 3 years postoperatively. In the context of the general US population, baseline PCS was lower; postoperative PCS improved to approximately the general population level through 6 months but declined moderately through 36 months. Baseline MCS was higher than the general population and improved through 3 months, remaining steady thereafter [17]. This study’s main finding was that improvement in HRQOL after cardiac surgery occurs mostly within the first 3–6 months, then remains constant for up to 3 years. Higher BMI and older age were the greatest predictors of PCS decline after 6 months.
Kurfirst et al. conducted a similar study but stratified outcomes by two age (in years) groups: patients 70 or younger and patients older than 70 [16]. Consistent with the findings in Grady’s study, improvement in HRQOL was observed early after surgery and remained relatively constant after 1 year. The greatest predictor was preoperative HRQOL; higher preoperative HRQOL was strongly associated with a lack of postoperative improvement. Importantly, baseline HRQOL was found to be higher in the younger group, but greater improvements from baseline were observed in the older group, suggesting that older patients could obtain greater relative benefits from SAVR than younger patients [16]. Klomp et al. also sought to examine the effect of patient age on HRQOL recovery after SAVR but found that age was not associated with PCS or MCS 1 year after surgery [15]. Although PCS and MCS were observed to be lower after 30 days, after 1 year both categories had significantly improved to a level expected for the age-matched reference population. Higher preoperative HRQOL was again predictive of postoperative non-improvement.
3.2. TAVR
The three landmark PARTNER trials led to substudies analyzing the comparative HRQOL benefits of SAVR and TAVR. Major PARTNER substudies of HRQOL have used the disease-specific Kansas City Cardiomyopathy Questionnaire (KCCQ) and its short-form, KCCQ-12 [9], [10], [11]. These reliable and commonly used tools measure quality of life in patients with heart failure (HF) [23]. The KCCQ-12 is a 12-item, 4-domain validated instrument that assesses symptom frequency, social and physical limitations, and quality of life impairment due to HF symptoms [23]. The four domains describe patients’ health status on a scale of 0–100 in 25-point ranges [24]. Although there is no exact association between KCCQ and NYHA classification, the most common functional status tool used in clinical practice and trials, patients with higher KCCQ scores generally have lower associated NYHA classes [24].
PARTNER substudies comparing SAVR and balloon-expandable TAVR have assessed HRQOL at consistently similar postoperative time horizons (e.g. 1- , 6- and 12-month time frames) [25], [26]. An early study by Reynolds et al. that measured HRQOL among high-risk patients compared both TAVR with a transapical approach (TA-TAVR) and with a transfemoral approach (TF-TAVR) with SAVR and found that improvements in HRQOL after one year were similar [26]. TF-TAVR patients showed clinically relevant improvements in HRQOL after 1-month follow-up compared with SAVR, but TA-TAVR did not result in any advantage over SAVR [26]. A later study by Gada et al. sought to understand whether improvements in the TA-TAVR method had led to better HRQOL outcomes [25]. Comparing TA-TAVR PARTNER patients in the nonrandomized continued access registry with patients from the PARTNER RCT, at 1-, 6- and 12-month follow-up, there was still no advantage over SAVR [25]. While it is important to note that TA-TAVR utilization has since decreased dramatically, these results confirm that among patients who are still candidates SAVR candidates, TA-TAVR may not provide significant HRQOL benefits [27], [28].
Quality-of-life studies of the PARTNER 2 cohort A clinical trials (intermediate surgical risk) showed similar longitudinal results to the high-risk patients: short-term (i.e. 1-month) HRQOL benefits were significantly better for TF-TAVR than for SAVR patients, but 1- and 2-year health status benefits were no different [10]. Additionally, the short-term advantage did not apply to TA-TAVR patients [29]. A landmark follow-up study published in 2020 reported no difference in functional status or HRQOL between TAVR and SAVR after 5 years [30]. Results from the PARTNER 3 trials (low surgical risk) reported similar results but with some variation from high- and intermediate-risk patients. Baron and colleagues reported that TAVR resulted in greater generic and disease-specific QoL improvements in the short term, but in contrast to previous studies, TAVR also resulted in a modest yet still significant advantage over SAVR at 6 and 12 months [8]. The CoreValve US Pivotal Trial established higher survival rates in high-risk patients receiving self-expanding TAVR compared with SAVR and much like the PARTNER results, CoreValve substudies also revealed that improvements in generic and disease-specific HRQOL among TAVR patients were vascular access specific [12], [31].
Several studies outside of the PARTNER and CoreValve cohorts have further established that TAVR patients experience significant gains in QoL and functional recovery, with older patients and those needing a permanent pacemaker seeing smaller improvements [32], [33]. Recent meta-analyses have documented that HRQOL and functional capacity improve after TAVR, despite better baseline functioning in recent studies and that non-TF TAVR patients’ trajectories are similar to those of SAVR patients [34], [35]. Two studies found that predictive models of poor outcome after TAVR developed in clinical trials perform well in real-world settings; however, poor outcomes are still observed frequently [14], [36]. This finding highlights the need for more consistent, real-world assessments of functional recovery and HRQOL to more accurately predict patients’ for experiencing improved QoL.
3.3. Cost-effectiveness
As is the case with HRQOL, TAVR/SAVR cost-effectiveness data are mostly limited to a few select studies. In the PARTNER Cohort B trial, patients at prohibitive surgical risk were randomized to either TAVR or medical therapy [37]. Reynolds et. al found that TAVR led to a gain of 1.3 more quality-adjusted life years (QALYs) than the control, resulting in an estimated incremental cost-effectiveness ratio (ICER) of $62,889 per QALY gained. Although 1-year follow-up costs were lower for TAVR than for medical therapy, TAVR costs more than standard medical therapy due to increased life expectancy. The relative cost of TAVR was comparable to that of other medical interventions, such as hemodialysis, which is estimated at about $71,000 per QALY [38]. The finding of $62,889 per QALY gained was very close to the $61,889 figure generally considered to be an acceptable ICER value within the US healthcare system [37].
Reynolds and colleagues also conducted the first study to examine the comparative cost-effectiveness of TAVR and SAVR [39]. In the PARTNER Cohort A (high surgical risk) population, it was found that cost-effectiveness varied by TAVR access site: TF-TAVR was economically favorable, with an ICER < $50,000 per QALY gained, but TA-TAVR was associated with higher costs and lower QALYS, making it economically inferior to SAVR. Thus, the TF-TAVR finding is within the cost range considered high economic value for cardiovascular therapy in the U.S [40]. In the CoreValve U.S. High Risk Pivotal Trial, TAVR was associated with an ICER of about $55,000/QALY and $43,000/life-year (LY), reflecting TAVR’s high economic value with respect to SAVR [41]. Baron et al. compared the economic impact of both the second-generation SAPIEN XT (XT-TAVR) Valve and the third-generation SAPIEN 3 (S3-TAVR) valves with SAVR [42]. Although procedural costs were higher for both XT-TAVR and S3-TAVR, index hospitalization non-procedural costs, physician fees and follow-up medical care costs were lower, while quality-adjusted life expectancy was higher, making both types of TAVR valves economically favorable over SAVR [42].
Measures used to derive cost effectiveness estimates are displayed in Table 3. The cost estimates, using payments from Medicare to institutions or physicians, of TAVR and SAVR were determined based on unit prices of procedure devices and other ancillary costs, while follow-up hospital care and index hospital costs were determined using Medicare cost-to-charge ratios. Physician fees and outpatient costs were calculated using the Medicare fee schedule [37], [39], [41], [42].
Table 3.
Cost-effectiveness analyses.
| Author (year) | Group | Study design | Procedural Expenditures (Resource-based Cost Accounting) | Inpatient Expenditures (Medicare cost-charge ratio) Expenditures (index and follow-up) | Physician Fees (Medicare fee schedule) | Sample size | Risk category | Follow-up Timeframe |
|---|---|---|---|---|---|---|---|---|
| Reynolds et al. (2012) | PARTNER Cohort B | RCT | Yes (TAVR & Medical Therapy) | Yes | Yes | 234 | Prohibitive | 1 year |
| Reynolds et al. (2012) | PARTNER Cohort A | RCT | Yes (TAVR & SAVR) | Yes | Yes | 647 | High | 1 year |
| Reynolds et al. (2016) | CoreValve US Pivotal Trial | RCT | Yes (TAVR & SAVR) | Yes | Yes | 795 | High | 1 year |
| Baron et al. (2019) | PARTNER 2 Cohort A | RCT | Yes (TAVR & SAVR) | Yes | Yes | 3,110 | Intermediate | 2 years for XT-TAVR, 1 year for S3-TAVR |
One important limitation of TAVR cost-effectiveness studies is the implicit assumption that TAVR substitutes for SAVR on a one-for-one basis among low- to moderate-surgical risk patients. This assumption may have been true during the early diffusion of TAVR, but the rapid growth in TAVR utilization among low- to moderate-risk patients suggests that TAVR is expanding the number of patients receiving AVR treatments. Thus, the real-world cost-effectiveness of TAVR may be overestimated in low- to moderate-risk populations.
3.4. Wearable-device technology
To properly inform patient-physician treatment decision-making, further comparisons of HRQOL and cost-effectiveness in real-world settings are needed. To that end, wearable electronic accessories (e.g. smartwatches, pedometers, heart rate monitors) that leverage biosensors to obtain activity, movement, and physiologic data constitute a promising way to apply the lessons learned from trials and observational studies to real-life scenarios. By obtaining accurate, longitudinal HRQOL data, wearable devices also have the potential to increase the accuracy of comparative cost-effectiveness data between TAVR and SAVR. Joshi and colleagues reviewed the functions of different available sensors that were used to measure vital signs [43]. Common measurements included heart rate, respiratory rate, temperature, body posture and activity levels. Most sensors were unobtrusive and contained EHR integration. However, common pitfalls included short battery life, false positives, and an inability to measure all vital signs. Still, the results of this review were encouraging and indicate that wearable technology presents a practical method of detecting early signs of patient deterioration and monitoring functional recovery.
In the context of TAVR and SAVR specifically, wearable device technology has been used for remote monitoring, measuring physical activity, and evaluating rates of atrial fibrillation following cardiac surgery [44], [45], [46]. Hermans et al. identified the need for managing the increasing strain on healthcare resource and developed a framework for remote postprocedural ECG observation [44]. Their proposal differs from usual telemetry systems by allowing for out-of-hospital monitoring through leveraging automatic detection algorithms and continuous home-to-hospital data. This level of healthcare decentralization is still largely hypothetical; however, a recent pilot study evaluated the effects of wearable activity trackers on physical activity amongst elderly patients at higher risk of CVD [47]. An ongoing prospective study by Lorenzoni et al. is currently in the process of using a commercial smartwatch to address the lack of recovery trajectory data for TAVR/SAVR patients [48]. This study will track a variety of physical activity measurements and assess HRQOL using the SF-36 for follow-up periods of 1, 3, 6 and 12 months with the aim of demonstrating the efficacy of wearable devices in assessing longitudinal functional recovery [48].
Wearable sensors may serve as a promising tool to support home-based cardiac rehabilitation (HBCR), as it has the potential to increase participation by reducing barriers to access. Pedometers have been used to track TAVR patients’ steps during a 12-week rehabilitation program that contributed to improved functional status and SF-36 physical functioning scores [49]. Trials have also demonstrated that the use of wearable heart rate monitors, in conjunction with remote telemetry monitoring, phone applications, and text messaging can lead to equivalent (or better) results relative to center-based rehabilitation [50].
4. Discussion
The overview of current literature on TAVR/SAVR HRQOL and functional recovery presented in this review reveals a growing need to evaluate post-discharge recovery trajectories in real-world settings. Several studies have shown promising results within large trials, but real-world HRQOL assessments are not collected reliably enough to inform treatment-specific decision-making [14]. Dae Hyun Kim identifies “time lag between practice and QoL prediction research” as one of the key barriers to incorporating QoL into treatment decision-making [51]. Given the rapid growth of consumer wearable devices and the increasing need for longitudinal data within TAVR and SAVR, wearable devices present an opportunity to address this time lag by collecting patient information in real time. Comparing baseline with post-procedural HRQOL and functional status in a real-world setting would allow physicians and patients to make more informed decisions between TAVR and SAVR and identify opportunities to improve post-procedural care through real-time monitoring and notifications. To that end, future studies should evaluate opportunities to engage patients longitudinally through patient-reported outcomes ascertainment. This approach could involve transmitting patient data from validated wearable instruments to a centralized database via Smartphone apps. Possible data could include heart rate, blood pressure, step count, patient-perceived level of effort and HRQOL (using the KCCQ-12 and SF-12 at pre-specified intervals). Tracking these data over the course of a year or longer would allow researchers to predict recovery trajectories more accurately, thus giving future TAVR/SAVR candidates and their clinical care providers with information necessary to make better-informed decisions.
The studies included in this review should be considered in light of their limitations. First, current validated HRQOL instruments are subject to bias towards patients who are physically/mentally capable to complete these surveys [18]. Second, given this study was a narrative review, comparisons across studies did account for variation in sample size that would otherwise be addressed through a meta-analysis. Third, this study did not address all important outcomes that may impact procedural HRQOL, such as infectious endocarditis [52]. Fourth, while traditional cost-effectiveness comparisons have assumed that TAVR serves as a one-to-one replacement for SAVR in all but prohibitive risk patients, the recent expansion of TAVR into low- and moderate-risk individuals means that TAVR populations now include patients who would not have previously been referred for or treated with SAVR [53]. Future analyses should account for the diffusion of TAVR into these patient populations and evaluate relative cost-effectiveness accordingly.
5. Conclusion
The current state of AVR HRQOL literature demonstrates that further investigation is needed to properly inform shared decision-making between SAVR and TAVR. Quality-of-life analyses specific to SAVR are mostly observational and are generally not standardized in their approach to measuring patients’ recovery trajectories. While more recent studies comparing TAVR and SAVR are more standardized in their methods, comparisons are mostly limited to RCTs and call into question the generalizability of these findings, as HRQOL data in real-world settings are largely incomplete. Cost-effectiveness analyses are likewise limited to trials and are not necessarily indicative of the long-term comparative costs between the two procedures in real-world practice. Although not yet studied extensively in this setting, wearable device technology holds great promise for measuring physical activity and allowing physicians to track patients’ recovery trajectories in real time. The ability to collect longitudinal HRQOL data and compare to pre-interventional baseline data would allow providers to (a) quickly identify those patients who require further interventions and (b) supply real-world data to inform future decisions between SAVR and TAVR. Future studies comparing functional outcomes between TAVR and SAVR should make use of wearable devices and measure patients’ recovery trajectories in a real-world setting.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Michelle Hou, M.S.; Kayvan Najarian, Ph.D.; Karen Kim, M.D., M.S.
Disclosures
Dr. Sukul receives partial salary support from Blue Cross Blue Shield of Michigan. Dr. Thompson receives partial salary support from Blue Cross Blue Shield of Michigan as Co-Director of the Michigan Value Collaborative. Donald S. Likosky receives: (i) extramural support from the Agency for Healthcare Research and Quality (AHRQ, R01HS026003) and the National Institutes of Health (NHLBI, R01HL146619); (ii) partial salary support from Blue Cross Blue Shield of Michigan; and (iii) support as a consultant to the American Society of ExtraCorporeal Technology.
References
- 1.Carabello B.A., Paulus W.J. Aortic stenosis. Lancet. 2009;373(9667):956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 2.Moore M., Chen J., Mallow P.J., Rizzo J.A. The direct health-care burden of valvular heart disease: evidence from US national survey data. Clinicoecon Outcomes Res. 2016 Oct;18(8):613–627. doi: 10.2147/CEOR.S112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach D.S., Cimino N., Deeb G.M. Unoperated patients with severe aortic stenosis. J. Am. Coll. Cardiol. 2007;50:2018–2019. doi: 10.1016/j.jacc.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., van Es G.-A., Hahn R.T., Kirtane A.J., Krucoff M.W., Kodali S., Mack M.J., Mehran R., Rodés-Cabau J., Vranckx P., Webb J.G., Windecker S., Serruys P.W., Leon M.B. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Thoracic Cardiovascular Surg. 2013;145(1):6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., Rigolin V.H., Sundt T.M., Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25) doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 6.Gargiulo G. Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016;165:334–344. doi: 10.7326/M16-0060. [DOI] [PubMed] [Google Scholar]
- 7.Villablanca P.A. A meta-analysis and meta-regression of long-term outcomes of transcatheter versus surgical aortic valve replacement for severe aortic stenosis. Int. J. Cardiol. 2016;225:234–243. doi: 10.1016/j.ijcard.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Baron S.J. Health Status After Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2019;74:2833–2842. doi: 10.1016/j.jacc.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Smith C.R. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 10.Leon M.B. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 11.Mack M.J. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 12.Adams D.H. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 13.Bowdish M.E. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2020 Update on Outcomes and Research. Ann. Thorac. Surg. 2020;109:1646–1655. doi: 10.1016/j.athoracsur.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Arnold S.V., Spertus J.A., Vemulapalli S., Li Z., Matsouaka R.A., Baron S.J., Vora A.N., Mack M.J., Reynolds M.R., Rumsfeld J.S., Cohen D.J. Quality-of-Life Outcomes After Transcatheter Aortic Valve Replacement in an Unselected Population: A Report From the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2(4):409. doi: 10.1001/jamacardio.2016.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen Klomp W.W. Survival and quality of life after surgical aortic valve replacement in octogenarians. J. Cardiothorac. Surg. 2016;11:38. doi: 10.1186/s13019-016-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurfirst V. Health-related quality of life after cardiac surgery–the effects of age, preoperative conditions and postoperative complications. J. Cardiothorac. Surg. 2014;9:46. doi: 10.1186/1749-8090-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady K.L. Improvements in health-related quality of life before and after isolated cardiac operations. Ann. Thorac. Surg. 2011;91:777–783. doi: 10.1016/j.athoracsur.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Sundt, T. M. et al. Quality of life after aortic valve replacement at the age of >80 years. Circulation 102, III70–4 (2000). [DOI] [PubMed]
- 19.Failde I., Ramos I. Validity and reliability of the SF-36 Health Survey Questionnaire in patients with coronary artery disease. J. Clin. Epidemiol. 2000;53:359–365. doi: 10.1016/s0895-4356(99)00175-4. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira S.M. Long-term survival, autonomy, and quality of life of elderly patients undergoing aortic valve replacement. J. Card. Surg. 2012;27:20–23. doi: 10.1111/j.1540-8191.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 21.Gavalaki A. Outcomes and quality of life after aortic valve surgery in octogenarians. J. Card. Surg. 2020;35:341–344. doi: 10.1111/jocs.14377. [DOI] [PubMed] [Google Scholar]
- 22.Tseng E.E., Lee C.A., Cameron D.E., Stuart R.S., Greene P.S., Sussman M.S., Watkins L., Gardner T.J., Baumgartner W.A. Aortic valve replacement in the elderly. Risk factors and long-term results. Ann. Surg. 1997;225(6):793–804. doi: 10.1097/00000658-199706000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spertus J.A., Jones P.G. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual. Outcomes. 2015;8:469–476. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spertus J.A., Jones P.G., Sandhu A.T., Arnold S.V. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542. [DOI] [PubMed] [Google Scholar]
- 25.Gada H. Temporal Trends in Quality of Life Outcomes After Transapical Transcatheter Aortic Valve Replacement: A Placement of AoRTic TraNscathetER Valve (PARTNER) Trial Substudy. Circ. Cardiovasc. Qual. Outcomes. 2015;8:338–346. doi: 10.1161/CIRCOUTCOMES.114.001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds M.R. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A) J. Am. Coll. Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari E. Transfemoral versus transapical approach for transcatheter aortic valve implantation: hospital outcome and risk factor analysis. J. Cardiothorac. Surg. 2017;12:78. doi: 10.1186/s13019-017-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll J.D. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 29.Baron S.J., Arnold S.V., Wang K., Magnuson E.A., Chinnakondepali K., Makkar R., Herrmann H.C., Kodali S., Thourani V.H., Kapadia S., Svensson L., Brown D.L., Mack M.J., Smith C.R., Leon M.B., Cohen D.J. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMACardiol. 2017;2(8):837. doi: 10.1001/jamacardio.2017.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makkar R.R. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020;382:799–809. doi: 10.1056/NEJMoa1910555. [DOI] [PubMed] [Google Scholar]
- 31.Arnold S.V. Health Status After Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Increased Surgical Risk: Results From the CoreValve US Pivotal Trial. JACC Cardiovasc. Interv. 2015;8:1207–1217. doi: 10.1016/j.jcin.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalili H. Quality of Life Outcomes After Transcatheter Aortic Valve Replacement in Nonagenarians. J. Invasive Cardiol. 2020;32:375–379. doi: 10.25270/jic/20.00027. [DOI] [PubMed] [Google Scholar]
- 33.Bhardwaj A., Ramanan T., Sawant A.C., Sinibaldi E., Pham M., Khan S., Qureshi R., Agrawal N., Khalil C., Hansen R., Baldo S., Colern G., Corbelli J., Pershad A., Beck H., Iyer V. Quality of life outcomes in transcatheter aortic valve replacement patients requiring pacemaker implantation. J. Arrhythm. 2018;34(4):441–449. doi: 10.1002/joa3.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straiton, N., Jin, K., Bhindi, R. & Gallagher, R. Functional capacity and health-related quality of life outcomes post transcatheter aortic valve replacement: a systematic review and meta-analysis. Age Ageing 47, 478–482 (2018). [DOI] [PubMed]
- 35.Ando T., Takagi H., Briasoulis A., Grines C.L., Afonso L. Comparison of Health Related Quality of Life in Transcatheter Versus Surgical Aortic Valve Replacement: A Meta-Analysis. Heart Lung Circ. 2019;28:1235–1245. doi: 10.1016/j.hlc.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Arnold S.V., Cohen D.J., Dai D., Jones P.G., Li F., Thomas L., Baron S.J., Frankel N.Z., Strong S., Matsouaka R.A., Edwards F.H., Brennan J.M. Predicting Quality of Life at 1 Year After Transcatheter Aortic Valve Replacement in a Real-World Population. Circ. Cardiovasc. Qual. Outcomes. 2018;11(10) doi: 10.1161/CIRCOUTCOMES.118.004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds M.R., Magnuson E.A., Wang K., Lei Y., Vilain K., Walczak J., Kodali S.K., Lasala J.M., O'Neill W.W., Davidson C.J., Smith C.R., Leon M.B., Cohen D.J. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B) Circulation. 2012;125(9):1102–1109. doi: 10.1161/CIRCULATIONAHA.111.054072. [DOI] [PubMed] [Google Scholar]
- 38.McFarlane P.A., Bayoumi A.M., Pierratos A., Redelmeier D.A. The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int. 2003;64:1004–1011. doi: 10.1046/j.1523-1755.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds M.R. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A) J. Am. Coll. Cardiol. 2012;60:2683–2692. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Anderson J.L. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:2304–2322. doi: 10.1016/j.jacc.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds M.R. Cost-Effectiveness of Transcatheter Aortic Valve Replacement With a Self-Expanding Prosthesis Versus Surgical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016;67:29–38. doi: 10.1016/j.jacc.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baron S.J., Wang K., House J.A., Magnuson E.A., Reynolds M.R., Makkar R., Herrmann H.C., Kodali S., Thourani V.H., Kapadia S., Svensson L., Mack M.J., Brown D.L., Russo M.J., Smith C.R., Webb J., Miller C., Leon M.B., Cohen D.J. Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Risk. Circulation. 2019;139(7):877–888. doi: 10.1161/CIRCULATIONAHA.118.035236. [DOI] [PubMed] [Google Scholar]
- 43.Joshi M. Wearable sensors to improve detection of patient deterioration. Expert Rev. Med. Devices. 2019;16:145–154. doi: 10.1080/17434440.2019.1563480. [DOI] [PubMed] [Google Scholar]
- 44.Hermans M.C., Van Mourik M.S., Hermens H.J., Baan Jr J., Vis M.M. Remote Monitoring of Patients Undergoing Transcatheter Aortic Valve Replacement: A Framework for Postprocedural Telemonitoring. JMIR Cardio. 2018;2(1):e9. doi: 10.2196/cardio.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y., Green P., Maurer M., Lazarte R., Kuzniecky J.R., Hung M.Y., Garcia M., Kodali S., Harris T. Relationship Between Accelerometer-Measured Activity and Self-Reported or Performance-Based Function in Older Adults with Severe Aortic Stenosis. Curr. Geriatr. Rep. 2015;4(4):377–384. doi: 10.1007/s13670-015-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funk, M., Richards, S. B., Desjardins, J., Bebon, C. & Wilcox, H. Incidence, timing, symptoms, and risk factors for atrial fibrillation after cardiac surgery. Am. J. Crit. Care 12, 424–33; quiz 434–5 (2003). [PubMed]
- 47.Roberts L.M. Wearable Technology To Reduce Sedentary Behavior And CVD Risk In Older Adults: A Pilot Randomized Clinical Trial. Clin. Interv. Aging. 2019;14:1817–1828. doi: 10.2147/CIA.S222655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorenzoni G. Using Wearable Devices to Monitor Physical Activity in Patients Undergoing Aortic Valve Replacement: Protocol for a Prospective Observational Study. JMIR Res. Protoc. 2020;9 doi: 10.2196/20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattal G.K., Park K.E., Winchester D.E. Home-Based Cardiac Rehabilitation (HBCR) In Post-TAVR Patients: A Prospective, Single-Center, Cohort Pilot Study. Cardiol. Ther. 2020;9(2):541–548. doi: 10.1007/s40119-020-00186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas R.J. Home-Based Cardiac Rehabilitation: A Scientific Statement From the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. doi: 10.1161/CIR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 51.Hyun K.D. Incorporating Quality of Life Prediction in Shared Decision Making About Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Qual. Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.005097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Palo M., Scicchitano P., Malvindi P.G., Paparella D. Endocarditis in Patients with Aortic Valve Prosthesis: Comparison between Surgical and Transcatheter Prosthesis. Antibiotics (Basel) 2021;10 doi: 10.3390/antibiotics10010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim K.M. Evolving trends in aortic valve replacement: A statewide experience. J. Card. Surg. 2018;33:424–430. doi: 10.1111/jocs.13740. [DOI] [PubMed] [Google Scholar]