Highlights
-
•
Supplementation of exogenous enzymes in the general diet improves production characteristics in all stages of production.
-
•
Phytases are the most supplemented enzymes in all productive stages of pigs.
-
•
The inclusion of Phytases, used in the pig's diet showed an average dry matter digestibility (g/kg) 840.6 ± 25.5 in weaning, 862.5 ± 7.4 in growing and 802.0 ± 1.41 in finishing.
-
•
The inclusion of xylanases used in the pig's diet showed an average in dry matter digestibility (g/kg) 829.5 ± 7.14 in weaning and 759.1 ± 6.93 in finishing stages.
Keywords: Enzymes, Combined-enzymes, Mannanase, Phytase, Xylanase, Protease, Pig
Abstract
Supplementing exogenous enzymes in pig diets is an alternative solution to increase dietary energy and fiber digestibility to improve pig production performance at a low production cost and to reduce environmental impact with lower N and P excretions. The production stage, diet composition, enzyme source, amount and number of enzymes added, are factors to consider before using them. A database composed by 227 individual diets, resulting from 43 studies with 48 experimental records were divided in different production stages, with 19 records for weaning, 17 records for growing and 12 records for finishing. A descriptive statistical analysis of the chemical composition of the diets and enzyme doses was carried out. The data with normal distribution were analyzed calculating the mean, the minimum and maximum length, the standard deviation and the coefficient of variation. It was found that combined enzymes are the most widely reported enzyme combination in the supplementation of pigs at all stages of production. Phytases and Mannanases are commonly used at weaning and growing stages. Xylanases and Proteases have been reported to be used in all production stages. However, the highest yielding enzymes at weaning, growing and finishing stages were Phytases and Mannanases. Dietary supplementation of exogenous enzymes improves production characteristics at all stages of production. However, an improvement in growth performance and nutrient digestibility is not always observed. Future studies should focus on the interaction between production stages, composition of the diet, origin of the enzyme and the amount and number of enzymes added.
1. Introduction
Food ingredients included in pig diets, especially plant-based cereals, contain large amounts of non-starch polysaccharides (NSPs) (Adeola & Cowieson, 2011; Recharla et al., 2019). These NSPs are an important part of the plant ingredients (10–75%), and most of them are composed by arabinoxylans, cellulose and β-glucans (Choct, 2015). However, NSPs are poorly metabolized by pigs as they lack specific endogenous enzymes for their degradation (Jha & Berrocoso, 2015).
Supplementing exogenous enzymes as additives for pig diets hydrolyze NSPs, break the cell wall that encapsulates them, degrade anti-nutritional factors (protease inhibitors, antigenic proteins, non-protein amino acids) and perform the cleavage of glycolytic bonds that are not hydrolyzed by endogenous enzymatic activity (Kim et al., 2008; Lima, Da Silva, Araujo, Lima & Oliveira, 2007; Masey O'Neill, Smith & Bedford, 2014; Recharla et al., 2019), improving the digestibility of nutrients and thus can be used by the animal.
Most studies on animal diets seek strategies to improve feed efficiency, which are of particular interest to increase productive efficacy and reduce environmental impacts (Aarnink & Verstegen, 2007; Clark & Tilman, 2017). In this sense, exogenous enzymes improve feed efficiency and reduce feeding costs in the animal production industry (Adeola & Cowieson, 2011; Upadhaya, Park, Lee & Kim, 2016a), as pig feed accounts for 55–75% of total production costs (Nguyen, 2017).
Some exogenous enzymes included in diets for pigs are Phytases, Carbohydrases, Proteases and Lipases (Table 1) (Ravindran, 2013). In pigs, Phytase (myo-inositol hexakisphosphate phosphohydrolase) is a phosphohydrolytic enzyme that initiates the phosphate gradual removal from phytate (inositol hexakiphosphate), which is the main source of phosphorus found in cereal grains and oil seeds (Dersjant-Li, Awati, Schulze & Partridge, 2014).
Table 1.
Exogenous feed enzymes and target substrates.
| Enzyme | Target substrate |
|---|---|
| Phytases | Phytic acid |
| β-Glucanases | β-Glucan |
| Xylanases | Arabinoxylans |
| α-Galactosidases | Oligosaccharides |
| Amylase | Starch |
| Mannanases | Cell wall matrix (fiber components) |
| Cellulases | |
| Hemicellulases | |
| Pectinases | |
| Proteases | Proteins |
| Lipases | Lipids |
Adapted from Ravindran, 2013.
Of the world market for feed enzymes for monogastrics, it has been estimated that Phytases and Carbohydrases represent 90% and proteases and lipases 10% (Adeola & Cowieson, 2011). Therefore, the objective of the present systematic-review is to summarize the current knowledge on the use of exogenous enzymes in pig diets, to improve productive performance at weaning, growing and finishing stages with regard to their mode of action and effects. Also, this review aims at reporting the most efficient enzymes in pig productive performance and find the most supplemented exogenous enzymes in pig's diets at all productive stages (weaning, growing and finishing). The present systematic review evaluated productive variables that are improved with dietary supplementation of exogenous enzymes at each stage of production in pigs.
2. Materials and methods
2.1. Search strategy and selection criteria
Our search for information focused on studies reporting the use of exogenous enzymes in pig diets. A database was created from studies specifying the use of exogenous enzymes in pig diets and the articles used, covered the years 2000 – 2020. The publications were obtained from databases such as World Wide Science, ScienceDirect, Scopus, Springer Link, Wiley Online Library, Dialnet, SciELO, Science Research, PubMEd, Redalyc, Google Academic and ERIC. Obtaining information to find relevant publications was based on a chain of specific topics such as the various exogenous enzymes used in pigs.
The search string with the particular topic was supported by Boolean operators ("and", "or"), which served to specify the required information. All search terms within a string were checked for a “title, abstract and keyword”. The keywords used were: pigs, exogenous enzymes, action mode, effects, productive performance, treatment (control vs enzyme or combined-enzymes), pig production stage (weaning, growing and finishing), dosage of enzyme in the diet (g/kg), average daily gain weight (ADG kg/day), gain: feed ratio (G: F kg/kg), average daily feed intake (ADFI, kg/day) and digestibility of dry matter (DDM). Each of the obtained values was homogenized in the database to be able to be calculated: dosage of enzyme in the diet (g/kg), ADG (kg/day), G: F (kg/kg), ADFI (kg/day) and DDM (g/kg).
Only those studies reporting chemical composition, dosage of enzyme in the diet (g/kg), ADG (kg/day), G: F ratio (kg/kg), ADFI (kg/day) and DDM (%, g/kg) were included in the analysis. The publications that were eliminated or not considered in the present review, were because they did not have enough data and values that were required: pigs, exogenous enzymes, action mode, effects, productive performance, treatment (control vs enzyme or combined-enzymes), pig production stage (weaning, growing and finishing), dosage of enzyme in the diet (g/kg), ADG (kg/day), G: F ratio (kg/kg), ADFI (kg/day), and DDM (%, g/kg).
A total of 43 studies and with different enzymes doses were included in the database: Agyekum et al., 2015, Ao et al., 2010, Castro et al., 2011, Cho & Kim, 2013, Cho et al., 2017, Choe et al., 2017, Dersjant-Li, Plumstead, Awati & Remus, 2018, He et al., 2020, Jang, Kim, Jang, & Kim, 2020, Jo et al., 2012, Kiarie, Nyachoti, Slominski & Blank, 2007, Kim et al., 2004, Kim et al., 2008, Kim et al., 2013, Kim et al., 2017, Lan, Li & Kim, 2017, Lee et al., 2011, Lei, Cheong, Park & Kim, 2017, Li, Gabler, Loving, Gould & Patience, 2018, Lu et al., 2016, Lv et al., 2013, Martínez, Figueroa, Cordero, Sánchez & Martínez, 2017, Nguyen, Upadhaya, Lei, Yin & Kim, 2019, O'Shea et al., 2014, Olukosi, Sands & Adeola, 2007a, Omogbenigun, Nyachoti & Slominski, 2003, Omogbenigun, Nyachoti & Slominski, 2004, Owusu-Asiedu et al., 2012, Park et al., 2020, Recharla et al., 2019, Tsai, Dove, Bedford & Azain, 2019, Upadhaya et al., 2016a, 2016b, Woyengo, Dupe, Akinremi & Nyachoti, 2016, Yáñez, Landero, Owusu-Asiedu, Cervantes & Zijlstra, 2013, Yi et al., 2013, Yoon et al., 2010, Zeng et al., 2011, Zeng et al., 2014, Zeng et al., 2015, Zhang, Yang, Wang, Yang & Zhou, 2014, Zijlstra, Li, Owusu-Asiedu, Simmins, & Patience, 2004, Zuo et al., 2015. Experiments were treated individually even when published within one article. Experiments were treated individually even when published within one article.
2.2. Data extraction and analysis
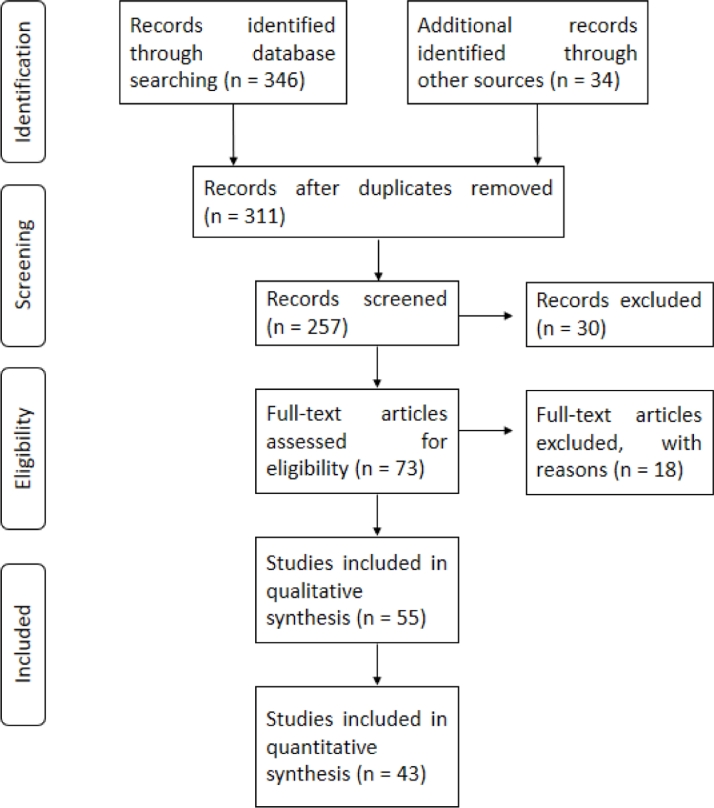
Our database consisted of 227 individual diets, resulting in 43 studies with 48 experimental records that were divided by production stages, with 19 records for weaning, 17 records for growing and 12 records for finishing (Figure 1). A statistical descriptive analysis of the chemical composition diets and enzyme doses was performed: enzymes, number of animals, dosage of enzyme in the diet, initial body weight, average daily feed intake, average daily gain weight, gain: feed ratio, digestibility of dry matter, to determine the effect of enzymes strains alone or in combined-enzymes on those variables. The datasets were analyzed for bifurcation by computing basic indices such as number of studies. The analysis was repeated for the length of each segment with statistical analyses such as mean, minimum, maximum length, standard deviation and coefficient of variation. The analysis was carried out using the SAS statistical software (SAS, 2004). The analysis to obtain the means was with Fisher's F test and the comparison of means was with Tukey's test.
Fig. 1.
PRISMA study flow diagram of the systematic review from initial search and screening to final selection of publications to be included in the study.
3. Results and discussion
The pig is a monogastric animal that does not produce endogenous enzymes capable of digesting dietary NSPs and this lead to increases in digesta viscosity, alterations in epithelial morphology of the intestine and reduced nutrient digestibility (Lindberg, 2014; Passos, Park, Ferket, von Heimendahl & Kim, 2015). Therefore, the purpose of exogenous enzymes is to improve pig productive performance by dietary means. Although the purpose of dietary supplementation of exogenous enzymes is to improve growth performance and nutrient digestibility, pigs receiving enzymes do not always show constant improvements (Barrera, Cervantes, Sauer, Araiza & Torrentera, 2004; Leek, Callan, Reilly, Beattie & O'Doherty, 2007; Olukosi et al., 2007a).
In this review, we found that in pig diets, the most supplemented enzymes at all productive stages (weaning, growing and finishing) are Phytases, Carbohydrases (Mannanases, Xylanase), Proteases and Combined-enzymes. In the next sections we will discuss and describe the function of each enzyme.
3.1. Phytases
Adeola and Cowieson (2011), reported that the best-selling enzymes are Phytases with 60% of the sale market, Carbohydrases with 30% and Proteases and Lipases with 10%. After the introduction of phytate-degrading enzymes in 1991, the use of microbial Phytases had a great boost, so their inclusion in pigs surpassed NSPs enzymes, which was predictable since phytate is present in diets with Phytases and they represent a viable alternative source of P and reduce its excretion (Selle & Ravindran, 2008). For this reason, its sale in the market had surpassed the use of other enzymes.
Adeola and Cowieson (2011), mentioned that Phytase inclusion level greater than 2500 FYT/kg of feed characterizes a high Phytase inclusion dose. Efficacy depends on various factors, such as pig growth stage, type of diet and source of Phytase (Jongbloed, Van Diepen, Kemme & Broz, 2004). Increasing the level of enzyme inclusion does not necessarily represent a linear improvement in nutrient utilization (Da Silva et al., 2019).
Phytases are supplemented in the same way at all productive stages, as well as Mannanases, with a higher use at weaning and growing pig stages (Tables 5 – 7), acting on the hydrolysis of phytate (myo–inositol 1,2,3,4,5,6–hexakis [dihydrogen] phosphate) to release the phosphate from this complex, improving the digestibility of phosphorus (P), calcium, amino acids, energy and reduced inorganic P excretion into the environment (De Faria et al., 2015; Dersjant-Li et al., 2014; EFSA, 2012). The most used Phytases in animal feed are histidine acid phosphatases (HAPs), followed by other classes of Phytase such as Phytase of helix β (BPPhy or alkaline Phytase), purple acid Phytase and protein tyrosine phosphatase (Lei, Weaver, Mullaney, Ullah & Azain, 2012). Improved availability of phosphorus and other minerals in pig's diets with the use of Phytase, reduces soil contamination (Sefer et al., 2012). Phytase in pig diets is generally added at 2.5 g/kg, but less than 50% of the Phytate in the diet is hydrolyzed (Dersjant-Li, Schuh, Weallean, Awati & Dusel, 2017; Selle, Cowieson & Ravindran, 2009). In the present review, the inclusion of Phytases (g/kg diet as DM) vary among productive stages (Table 5, Table 6, Table 7), with 2.50 ± 5.45, 2.57 ± 3.83, and 1.34 ± 0.88 g/kg diet at weaning, growing, and finishing stages, respectively (Table 8). Similar effects (P > 0.05) between studies were found to ADFI (kg/d), ADG (kg/d), G: F ratio while DDM showed 840.6 ± 25.5 g/kg at weaning, 862.5 ± 7.4 at growing and 802.0 ± 1.41 at finishing stages. The average daily gain with phytases supplementation at weaning stage was 11.9% higher and at growing stage was 7.3% higher compared to the control group. While at finishing stage, this effect becomes negative (−15.4%) possibly due to an improved efficiency of P utilization in younger pigs. At weaning stage, Zeng et al. (2014), reported on average an increase in ADG of 10.76%, an ADFI of 6.89% and a G: F of 3.50%, with phytase supplementation at 0.5–20 g/kg, this effect was higher than the present results. Yáñez et al. (2013), reported similar results to the present study on average an increase in ADG of 7.29%, a G: F of 7.46% and a decrease in ADFI of −1.36%, with phytase supplementation at 0.1 g/kg. In the growing stage, Zeng et al. (2011), reported on average an increase in ADG of 5.88%, a ADFI of 3.65% and a DDM of 0.13% with a Phytase supplementation at 0.25–2 g/kg, which is lower than the present study. On the contrary, at finishing stage, Olukosi et al. (2007a), reported on average an increase in ADG of 11.95%, a ADFI of 0.86%, a G: F of 7.69% and a decrease in DDM of 0.95%, with Phytase supplementation at 0.5–1 g/kg. This variation in production responses may be due to the amount of calcium/phosphorus in the diet and its interaction with other factors, as well as the concentration of phytases in the diet as a function of the pig's production stage, so the amount of P vs. enzyme supplemented in the diet should be reviewed. The amount of P vs. enzyme supplemented in the diet should be checked in order to observe optimal performance.
Table 5.
Effects of supplementing exogenous enzymes in pig diets at weaning productive stage on animal performance.
| Variable | Phytase | Xylanase | Protease | Combinationof exogenous enzymes | Control | |
|---|---|---|---|---|---|---|
| Studies | n | 14 | 9 | 4 | 23 | 31 |
| Number of animals | Mean ± SD | 55.86 ± 94.81 | 31.44 ± 35.76 | 51.25 ± 17.50 | 28.13 ± 21.64 | 47.03 ± 67.23 |
| Min – Max | 8 – 279 | 8 – 115 | 25 – 60 | 6 – 115 | 6 – 279 | |
| CV (%) | 169.74 | 113.72 | 34.15 | 76.94 | 142.94 | |
| Dosage of Enzyme in the diet, (g/kg) | Mean ± SD | 2.50 ± 5.45 | 0.054 ± 0.022 | 0.09 ± 0.05 | 0.80 ± 0.75 | |
| Min – Max | 0.07 – 15.68 | 0.018 – 0.076 | 0.03 – 0.14 | 0.06 – 2.85 | ||
| CV (%) | 217.68 | 41.25 | 53.89 | 93.55 | ||
| Dosage of Enzyme in the diet, (g/d) | Mean ± SD | 3.28 ± 7.09 | 0.06 ± 0.024 | 0.02 ± 0.08 | 1.06 ± 0.98 | |
| Min – Max | 0.10 – 20.0 | 0.03 – 0.10 | 0.10 – 0.30 | 0.1 – 4.00 | ||
| CV (%) | 216.31 | 39.97 | 40.83 | 95.12 | ||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | Mean ± SD | 0.46 ± 1.00 | 0.01 ± 0.005 | 0.02 ± 0.01 | 0.20 ± 0.19 | |
| Min – Max | 0.014 – 2.89 | 0.004 – 0.017 | 0.008 – 0.033 | 0.01 – 0.58 | ||
| CV (%) | 217.81 | 41.81 | 50.37 | 94.02 | ||
| Initial body weight, (kg) | Mean ± SD | 9.04 ± 1.63 | 7.73 ± 0.48 | 6.47 ± 0.40 | 7.16 ± 1.65 | 7.72 ± 1.65 |
| Min – Max | 6.41 – 11.90 | 6.47 – 7.98 | 6.27 – 7.06 | 5.36 – 9.94 | 5.36 – 11.90 | |
| CV (%) | 18.00 | 6.25 | 6.11 | 22.98 | 21.39 | |
| Animal performance | ||||||
| Average Daily Feed intake, (kg/d) | Mean ± SD | 0.72 ± 0.14 | 0.96 ± 0.45 | 0.43 ± 0.19 | 0.82 ± 0.35 | 0.69 ± 0.29 |
| Min – Max | 0.45 – 0.95 | 1.52 – 47.07 | 0.71 – 42.94 | 0.41 – 1.35 | 0.31 – 1.46 | |
| CV (%) | 18.78 | 47.07 | 42.94 | 42.42 | 41.75 | |
| Average Daily Gain, (kg/d) | Mean ± SD | 0.48 ± 0.08 | 0.55 ± 0.19 | 0.30 ± 0.02 | 0.45 ± 0.14 | 0.41 ± 0.15 |
| Min – Max | 0.30 – 0.54 | 0.29 – 0.79 | 0.28 – 0.32 | 0.23 – 0.60 | 0.22 – 0.78 | |
| CV (%) | 16.55 | 34.71 | 6.62 | 30.19 | 37.67 | |
| Gain: Feed, (kg/kg) | Mean ± SD | 0.67 ± 0.66 | 1.17 ± 0.54 | 0.62 ± 0.06 | 0.65 ± 0.29 | |
| Min – Max | 0.53 – 0.75 | 0.76 – 1.78 | 0.49 – 0.76 | 0.30 – 1.90 | ||
| CV (%) | 9.83 | 46.43 | 10.02 | 43.85 | ||
| Digestibility of Dry Matter, (g/kg) | Mean ± SD | 840.6 ± 25.5 | 829.5 ± 7.14 | 882.7 ± 12.20 | 716.6 ± 133.9 | 728.1 ± 238.1 |
| Min – Max | 810 – 869 | 821.8 – 835.9 | 869.2 – 892.9 | 510.0 – 874.4 | 0 – 896.7 | |
| CV (%) | 3.39 | 0.86 | 1.38 | 18.68 | 31.74 |
SD: Standard deviation; Minimum: Min; Maximum: Max; CV: Coefficient of variation.
Table 7.
Effects of supplementing exogenous enzymes in pig diets at finishing productive stage on animal performance.
| Variable | Phytase | Xylanase | Mannanase | Protease | Combination ofexogenous enzymes | Control | |
|---|---|---|---|---|---|---|---|
| Studies | n | 5 | 4 | 7 | 5 | 11 | 23 |
| Number of animals | Mean ± SD | 120.8 ± 144.7 | 39.5 ± 9.85 | 29.43 ± 16.28 | 41.60 ± 6.69 | 717.27 ± 9.69 | 49.82 ± 73.87 |
| Min – Max | 8 – 279 | 30 - 48 | 16 – 52 | 32 – 48 | 8 – 32 | 8 – 279 | |
| CV (%) | 119.78 | 24.93 | 55.31 | 16.09 | 56.08 | 148.26 | |
| Dosage of Enzyme in the diet, (g/kg) | Mean ± SD | 1.34 ± 0.88 | 1.74 ± 2.90 | 1.14 ± 0.33 | 0.84 ± 0.48 | 2.25 ± 1.87 | |
| Min - Max | 0.54 – 2.79 | 0.21 – 6.08 | 0.53 – 1.61 | 0.405 – 1.37 | 0.525 – 6.795 | ||
| CV (%) | 65.93 | 167.0 | 28.66 | 56.66 | 83.07 | ||
| Dosage of Enzyme in the diet, (g/d) | Mean ± SD | 0.70 ± 0.27 | 1.10 ± 1.93 | 0.43 ± 0.13 | 0.32 ± 0.16 | 1.15 ± 1.19 | |
| Min – Max | 0.50 – 1.0 | 0.10 – 4.0 | 0.20 – 0.60 | 0.20 – 0.50 | 0.40 – 4.50 | ||
| CV (%) | 39.12 | 175.81 | 29.25 | 51.35 | 103.09 | ||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | Mean ± SD | 0.11 ± 0.07 | 0.16 ± 0.28 | 0.043 ± 0.013 | 0.045 ± 0.019 | 0.16 ± 0.17 | |
| Min – Max | 0.055 – 0.22 | 0.019 – 0.58 | 0.018 – 0.055 | 0.024 – 0.065 | 0.053 – 0.647 | ||
| CV (%) | 59.89 | 171.89 | 31.42 | 43.51 | 108.41 | ||
| Initial body weight, (kg) | Mean ± SD | 37.72 ± 32.53 | 27.08 ± 4.73 | 82.34 ± 14.25 | 49.40 ± 16.96 | 44.00 ± 22.07 | 53.37 ± 24.15 |
| Min – Max | 10 – 75.8 | 23 33.9 | 56.15 – 92.7 | 28.78 – 68.45 | 10 – 69.1 | 10 – 92.7 | |
| CV (%) | 86.24 | 17.45 | 17.30 | 34.33 | 50.14 | 17.30 | |
| Animal performance | |||||||
| Average Daily Feed lntake, (kg/d) | Mean ± SD | 0.39 ± 0.084 | 0.41 ± 0.05 | 0.30 ± 0.037 | 0.33 ± 0.03 | 0.40 ± 0.05 | 0.34 ± 0.07 |
| Min – Max | 0.30 – 0.49 | 0.38 – 0.47 | 0.265 – 0.383 | 0.312 – 0.374 | 0.329 – 0.50 | 0.26 – 0.50 | |
| CV (%) | 21.45 | 12.05 | 12.49 | 9.05 | 13.50 | 20.57 | |
| Average Daily Gain, (kg/d) | Mean ± SD | 0.68 ± 0.18 | 0.80 ± 0.06 | 0.80 ± 0.02 | 0.88 ± 0.05 | 0.80 ± 0.20 | 0.80 ± 0.14 |
| Min – Max | 0.483 – 0.84 | 0.724 – 0.865 | 0.772 – 0.837 | 0.822 – 0.937 | 0.392 – 1.05 | 0.398 – 1.06 | |
| CV (%) | 26.17 | 7.31 | 2.66 | 5.93 | 25.59 | 17.86 | |
| Gain: Feed, (kg/kg) | Mean ± SD | 0.39 ± 0.08 | 0.41 ± 0.05 | 0.030 ± 0.04 | 0.33 ± 0.03 | 0.397 ± 0.05 | 0.34 ± 0.07 |
| Min – Max | 0.301 – 0.49 | 0.381 – 0.47 | 0.265 – 0.383 | 0.312 – 0.374 | 0.329 – 0.50 | 0.26 – 0.50 | |
| CV (%) | 21.45 | 12.05 | 12.49 | 9.05 | 13.50 | 20.57 | |
| Digestibility of Dry Matter, (g/kg) | Mean ± SD | 802.0 ± 1.41 | 759.1 ± 6.93 | 836.5 ± 34.62 | 722.9 ± 3.47 | 811.5 ± 29.58 | 791.1 ± 45.35 |
| Min–Max | 801–803 | 754.2 – 764 | 763.5 – 861.2 | 720.4 –725.3 | 777.2 –845.9 | 725.2 – 849.4 | |
| CV (%) | 0.18 | 0.91 | 4.14 | 0.48 | 3.65 | 5.73 |
SD: Standard deviation; Minimum: Min; Maximum: Max; CV: Coefficient of variation.
Table 6.
Effects of supplementing exogenous enzymes in pig diets at growing productive stage on animal performance.
| Variable | Phytase | Mannanase | Protease | Combination ofexogenous enzymes | Control | |
|---|---|---|---|---|---|---|
| Studies | n | 15 | 18 | 3 | 16 | 34 |
| Number of animals | Mean ± SD | 54.27 ± 93.24 | 35.67 ± 10.31 | 39.33 ± 7.51 | 34.50 ± 15.31 | 46.65 ± 61.06 |
| Min – Max | 8 – 279 | 24 – 52 | 35 – 48 | 8 – 48 | 8 – 279 | |
| CV (%) | 171.82 | 28.91 | 19.08 | 44.38 | 130.90 | |
| Dosage of Enzyme in the diet, (g/kg) | Mean ± SD | 2.57 ± 3.83 | 1.05 ± 0.55 | 0.27 ± 0.13 | 1.08 ± 0.82 | |
| Min – Max | 0.23 – 11.88 | 0.33 – 2.83 | 0.19 – 0.42 | 0.037 – 2.48 | ||
| CV (%) | 149.45 | 52.71 | 48.42 | 75.69 | ||
| Dosage of Enzyme in the diet, (g/d) | Mean ± SD | 2.52 ± 4.12 | 0.52 ± 0.30 | 0.15 ± 0.04 | 0.61 ± 0.53 | |
| Min – Max | 0.25 – 12.50 | 0.20 – 1.60 | 0.125 – 0.20 | 0.05 – 2.00 | ||
| CV (%) | 163.33 | 58.73 | 28.87 | 86.78 | ||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | Mean ± SD | 0.29 ± 0.43 | 0.07 ± 0.03 | 0.023 ± 0.009 | 0.08 ± 0.65 | |
| Min – Max | 0.03 – 1.33 | 0.03 – 0.161 | 0.018 – 0.033 | 0.009 – 0.226 | ||
| CV (%) | 147.44 | 43.49 | 39.78 | 80.87 | ||
| Initial body weight, (kg) | Mean ± SD | 18.68 ± 5.65 | 40.37 ± 15.20 | 25.66 ± 2.70 | 29.49 ± 20.77 | |
| Min – Max | 10.32 – 30.58 | 23.50 – 60.50 | 24.09 – 28.78 | 5.40 – 56.90 | ||
| CV (%) | 30.26 | 37.66 | 10.52 | 50.44 | ||
| Animal performance | ||||||
| Average Daily Feed lntake, (kg/d) | Mean ± SD | 1.17 ± 0.42 | 2.08 ± 0.50 | 1.74 ± 0.32 | 1.66 ± 0.85 | 1.75 ± 0.63 |
| Min – Max | 0.90 – 2.12 | 1.58 – 2.86 | 1.55 – 2.10 | 0.73 – 2.76 | 0.69 – 2.83 | |
| CV (%) | 35.74 | 23.87 | 18.22 | 51.32 | 36.06 | |
| Average Daily Gain, (kg/d) | Mean ± SD | 0.62 ± 0.16 | 0.80 ± 0.09 | 0.81 ± 0.12 | 0.73 ± 0.21 | 0.73 ± 0.18 |
| Min – Max | 0.44 – 0.99 | 0.69 – 0.96 | 0.72 – 0.94 | 0.39 – 0.95 | 0.36 – 1.03 | |
| CV (%) | 26.35 | 10.62 | 15.06 | 28.95 | 24.19 | |
| Gain: Feed, (kg/kg) | Mean ± SD | 0.52 ± 0.04 | 0.40 ± 0.06 | 0.47 ± 0.02 | 0.50 ± 0.14 | 0.43 ± 0.08 |
| Min – Max | 0.45 – 0.58 | 0.32 – 0.50 | 0.45 – 0.49 | 0.34 – 0.72 | 0.32 – 0.60 | |
| CV (%) | 8.49 | 16.27 | 4.54 | 27.29 | 18.71 | |
| Digestibility of Dry Matter, (%) | Mean ± SD | 862.5 ± 7.4 | 827.4 ± 24.5 | 754.2 ± 3.61 | 785.3 ± 59.8 | 804.7 ± 46.8 |
| Min – Max | 853 – 871 | 791 – 866.9 | 751.6 – 756.7 | 672 – 860 | 660 – 870 | |
| CV (%) | 0.85 | 2.96 | 0.48 | 7.61 | 5.81 |
SD: Standard deviation; Minimum: Min; Maximum: Max; CV: Coefficient of variation.
Table 8.
Effects of supplementing Phytases exogenous enzymes in pig diets at different growing stages on animal performance.
| Variable | Weaning | Growing | Finishing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Phytases | P-value | % Increment | Control | Phytases | P-value | % Increment | Control | Phytases | P-value | % Increment | |
| Number of animals | 309 | 287 | 367 | 367 | 359 | 359 | ||||||
| Dosage of Enzyme in the diet, (g/kg) | 0.01 ± 2.51 | 8.22 ± 2.49 | 0.03 | 0.01 ± 2.43 | 3.32 ± 2.50 | 0.04 | 0.005 ± 3.97 | 0.75 ± 3.91 | 0.03 | |||
| Dosage of Enzyme in the diet, (g/d) | 0.01 ± 2.00 | 6.29 ± 2.00 | 0.01 | 0.001 ± 2.00 | 3.51 ± 2.01 | 0.03 | 0.001 ± 3.17 | 1.30 ± 3.14 | 0.02 | |||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | 0.001 ± 0.34 | 1.16 ± 0.33 | 0.001 | 0.001 ± 0.34 | 0.40 ± 0.33 | 0.02 | 0.002 ± 0.53 | 0.14 ± 0.54 | 0.01 | |||
| Initial body weight, (kg) | 9.11 ± 4.99 | 9.10 ± 4.98 | 0.83 | −0.1 | 20.69 ± 5.99 | 20.72 ± 5.98 | 0.82 | 0.1 | 44.95 ± 9.46 | 39.90 ± 9.43 | 0.73 | −11.2 |
| Average Daily Feed intake, (kg/d) | 0.71 ± 0.21 | 0.71 ± 0.98 | 0.93 | 0.0 | 1.44 ± 0.21 | 1.47 ± 0.21 | 0.85 | 2.1 | 2.06 ± 0.35 | 1.97 ± 0.34 | 0.84 | −4.3 |
| Average Daily Gain, (kg/d) | 0.42 ± 0.08 | 0.47 ± 0.07 | 0.93 | 11.9 | 0.68 ± 0.07 | 0.73 ± 0.08 | 0.54 | 7.3 | 0.78 ± 0.12 | 0.66 ± 0.12 | 0.36 | −15.4 |
| Gain: Feed, (kg/kg) | 0.65 ± 0.04 | 0.64 ± 0.04 | 0.96 | −1.53 | 0.47 ± 0.05 | 0.50 ± 0.05 | 0.65 | 6.4 | 0.40 ± 0.06 | 0.38 ± 0.12 | 0.75 | −5.0 |
| Digestibility of Dry Matter (g/kg) | 839.5 ± 18.6 | 829.5 ± 18.6 | 0.53 | −1.19 | 865.5 ± 18.5 | 858.0 ± 18.6 | 0.63 | −0.1 | 821.0 ± 26.3 | 803.0 ± 26.2 | 0.02 | −2.2 |
% Increment, compared with the control diet.
3.2. Carbohydrases
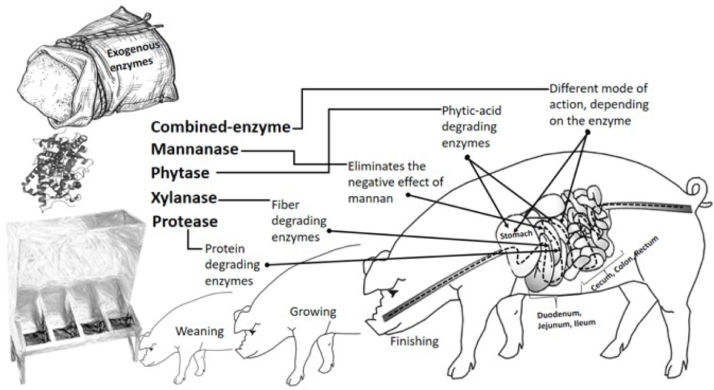
Carbohydrases are enzymes that catalyze the breakdown of complex carbohydrates into oligosaccharides, disaccharides, monosaccharides and are used as a method to help overcome the limitations of pigs to effectively utilize non-starch polysaccharides (NSPs) such as arabinoxylans and β-glucans (Campbell & Bedford, 1992). These enzymes hydrolyze plant cell wall components such as xylan, mannan and beta-glucan and assist in the release of nutritional constituents such as proteins, starch, lipids and other minerals that are trapped within the cell wall matrix (Li, Sauer, Huang & Gabert, 1996; Meng & Slominski, 2005; Nortey, Patience, Simmins, Trottier & Zijlstra, 2007). After hydrolysis of the NSPs and digestibility of the trapped nutrients, the resulting products are readily accessible to the gut microflora, which can have multiple beneficial effects on the gastrointestinal functionality of the animals (Yin, Zhang, Huang & Yin, 2010). Fiber-degrading enzymes should be applied to fibrous diets to improve efficient production of swine, especially considering low fiber digestibility of fiber-rich ingredients (Zhao, Zhang, Liu, Wang & Zhang, 2020). Carbohydrases work best in young pigs, due to their intestinal incapacity (Patience & DeRouchey, 2010), and to the negative effects caused by high fiber levels, thus improving growth performance (Tsai et al., 2017). This supplementation favors nutrient digestion at the most proximal portion of the digestive tract (Mathlouthi, Lalles, Lepercq, Juste & Larbier, 2002), (Figure 2). Limitations imposed by intestinal incapacity make Carbohydrases supplementation an essential dietary intervention in young pigs, but the use in sows is still scarce (Adeola & Cowieson, 2011).
Fig. 2.
Mode of action of exogenous enzymes in the production stages of the pig.
3.2.1. Mannanases
Mannanases use is due to the fact that the tract of pigs lacks the enzymes that target the links β−1,4-manosyl and α−1,6–galactosyl, so nutrient utilization and growth performance are limited and supplementation with β–mannanase or enzyme complex with β–mannanase has the potential to improve them, in addition to eliminating the negative effect of mannan (Ao et al., 2011; Jo et al., 2012; Kim et al., 2013, 2017; Pettey, Carter, Senne & Shriver, 2002; Veum & Odle, 2001).
The most widely used Carbohydrases found in the present review are Mannanases ( Table 5, Table 6, Table 7), which have become biotechnologically important since they target the hydrolysis of complex polysaccharides of plant tissues into simple molecules such as oligosaccharides, manose (Dhawan & Kaur, 2007), and Xylanases that enhance energy use by the pig (Nortey et al., 2007).
The inclusion of Mannanases used in the pigs diet at any productive stage (Table 6 and Table 7), showed a DDM (g/kg) of 827.4 ± 24.5 and 836.5 ± 34.62 at growing and finishing stages, respectively. However, when these data are expressed in percentage, it is observed that compared to control groups, there is an increase in the average daily gain of 3.8% and 1.3%, at weaning and at growing stages, respectively, and an improved G:F ratio (2.7%) at growing stage which can be explained by improved efficiency of energy utilization.
At growing stage, some authors have reported productive variables in pig performance with the use of Mannanases, Lv et al. (2013), reported on average an increase in ADG of 16.96%, a G:F of 22.19%, a DDM of 2.78% and a decrease in ADFI of −4.98%, with Mannanase supplementation, which is higher than the present study at 0.2–0.6 g/kg Table 9. A lower response was reported by Kim et al. (2017), on average an increase in ADG of 7.16%, a ADFI of 2.61% and a DDM of 2.33%, with Mannanase supplementation at 0.4–1.6 g/kg . At finishing stage, Similar to the present results, Yoon et al. (2010), reported on average an increase in ADG of 2.96%, in G: F of 6.46%, in DDM of 0.99% and a decrease in ADFI of −3.16%, with Mannanase supplementation at 0.2–0–6 g/kg (Table 4).
Table 9.
Effects of supplementing Mannanase exogenous enzymes in pig diets at different growing stages on animal performance.
| Variable | Weaning | Growing | Finishing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Mannanase | P-value | % increment | Control | Mannanase | P-value | % increment | Control | Mannanase | P-value | % increment | |
| Number of animals | 32 | 32 | 243 | 215 | 106 | 70 | ||||||
| Dosage of Enzyme in the diet, (mg/kg) | 0.001 ± 0.02 | 0.45 ± 0.02 | 0.03 | 0.002 ± 0.02 | 0.47 ± 0.02 | 0.04 | ||||||
| Dosage of Enzyme in the diet, (g/d) | 0.001 ± 0.19 | 1.31 ± 0.18 | 0.04 | 0.002 ± 0.27 | 1.20 ± 0.27 | 0.02 | ||||||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | 0.01 ± 0.01 | 0.08 ± 0.01 | 0.45 | 0.001 ± 0.01 | 0.05 ± 0.01 | 0.68 | ||||||
| Initial body weight, (kg) | 6.96 ± 15.6 | 6.96 ± 0.05 | 0.98 | 0.0 | 41.1 ± 6.39 | 40.6 ± 6.38 | 0.93 | 1.2 | 70.6 ± 9.04 | 72.7 ± 9.05 | 0.94 | 2.9 |
| Average Daily Feed lntake, (kg/d) | 0.56 ± 0.05 | 0.54 ± 0.05 | 0.82 | −3.6 | 2.12 ± 0.20 | 2.09 ± 0.20 | 0.94 | −1.4 | 2.58 ± 0.28 | 2.60 ± 0.28 | 0.96 | 0.8 |
| Average Daily Gain, (kg/d) | 0.26 ± 0.09 | 0.27 ± 0.08 | 0.89 | 3.8 | 0.78 ± 0.04 | 0.79 ± 0.04 | 0.95 | 1.2 | 0.80 ± 0.05 | 0.80 ± 0.05 | 0.98 | 0.0 |
| Gain: Feed, (kg/kg) | 0.37 ± 0.02 | 0.38 ± 0.02 | 0.97 | 2.7 | 0.32 ± 0.03 | 0.33 ± 0.03 | 0.98 | 3.1 | ||||
| Digestibility of Dry Matter (g/kg) | 808.9 ± 10.6 | 821.7 ± 0.04 | 0.75 | 1.6 | 806.2 ± 14.9 | 810.3 ± 14.9 | 0.78 | 0.5 | ||||
% increment, compared with the control diet.
Table 4.
Mode of action and main effects of enzymes used in pig diets at finishing stage.
| Enzyme | Mode of Action | Main Effects | Reference |
|---|---|---|---|
| Mannanase | Improves ATTD of GE, N, DM and CP. | -Increased ADG, G: F, ADFI and blood glucose concentration. -Decreased fat thickness of rib number 10. |
Yoon et al., 2010; Cho & Kim, 2013; Kim et al., 2013 |
| Phytase | Improves digestibility of P. | -Increases ADG and Ca. -Decreased P excretion. |
Olukosi et al., 2007 |
| Protease | Improves AID of GE. ATTD of CP. | -Increases ADG and GE. -Decreases ADFI. Fecal ammonia emission. |
Olukosi et al., 2007; O'Shea et al., 2014; Choe et al., 2017; Lei et al., 2017 |
| Xylanase | Improves AID of GE. ATTD of GE, N and DM. Digestibility of P. | -Increases ADG and GE. -Decreases P excretion. Manure odor emissions. Fat thickness of rib number 10. |
Olukosi et al., 2007; Cho & Kim, 2013; O'Shea et al., 2014; Cho et al., 2017 |
| Amylase | Improves the digestibility of P. | -Increases ADG. | Olukosi et al., 2007 |
| Galactosidase | Improves ATTD of DM and N. | -Increases ADG. | Kim et al., 2013 |
AID: Apparent ileal digestibility; DM: Dry matter; GE: Gross energy; CP: Crude protein; ATTD: Apparent total tract digestibility; P: Phosphorus; ADG: Average daily weight gain; G:F: Feed gain; ADFI: Average daily feed intake; Ca: Calcium; N: Nitrogen.
3.2.2. Xylanases
Xylanase is another carbohydrase used in pig diets, the inclusion covers all stages of production (Table 5, Table 6, Table 7), and is within the 80% of the best-selling Carbohydrases worldwide for use in monogastric diets (Adeola & Cowieson, 2011). Endo-1,4-β–Xylanase is produced by a genetically modified strain of Bacillus subtilis TD160 (229) (European Union Reference Laboratory for Feed Additives, 2014). Xylanase has the ability to hydrolyze the xylan content of 1,4–β–d–xyloside bonds (International Union of Biochemistry & Molecular Biology, 1992) as well as dried distiller's grains with soluble (DDGS) wheat and rapeseed meal to improve energy use by the pig (Nortey et al., 2007). The magnitude of effect of exogenous Xylanases depends on the nutritional value of the diet to which they are added (Cowieson & Bedford, 2009).
The inclusion of Xylanases used in the pigs diet at any productive stage (Table 5, Table 7), showed an average dry matter digestibility (g/kg) of 829.5 ± 7.14 at weaning and 759.1 ± 6.93 at finishing stages. Table 10 shows the effects of supplementing Xylanases exogenous enzymes in pig diets. Overall, no significant differences (P > 0.5) were observed. However, when these data are expressed in percentage, it was observed that the best response is at weaning stage, with an increase in the average daily gain of 2.5% compared to the control group. With regard to performance, at weaning stage, Lan et al. (2017), reported on average an increase in ADG of 3.88%, a ADFI of 0.34%, a G: F of 3.50% and a DDM of 2.25%, with Xylanase supplementation at 0.05–0.1 g/kg. At finishing stage, Cho et al. (2017), reported on average an increase in ADG of 1.81%, a ADFI of 0.18%, a G: F of 1.58% and a decrease in DDM of −0.62%, with Xylanase supplementation at 0.1 g/kg, which correspond to the present results.
Table 10.
Effects of supplementing Xylanases exogenous enzymes in pig diets at different growing stages on animal performance.
| Variable | Weaning | Growing | Finishing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Xylanases | P-value | % increment | Control | Xylanases | P-value | % increment | Control | Xylanases | P-value | % increment | |
| Number of animals | 98 | 98 | 49 | 49 | 228 | 228 | ||||||
| Dosage of Enzyme in the diet, (g/kg) | 0.001 ± 0.50 | 0.08 ± 0.49 | 0.05 | 0.001 ± 0.58 | 1.43 ± 0.58 | 0.02 | ||||||
| Dosage of Enzyme in the diet, (g/d) | 0.001 ± 0.74 | 0.05 ± 0.75 | 0.08 | 0.001 ± 0.86 | 2.24 ± 0.85 | 0.04 | ||||||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | 0.001 ± 0.07 | 0.01 ± 0.07 | 0.21 | 0.002 ± 0.08 | 0.21 ± 0.08 | 0.08 | ||||||
| Initial body weight, (kg) | 7.35 ± 2.35 | 7.36 ± 2.35 | 0.98 | 0.1 | 20.7 ± 4.07 | 19.5 ± 4.07 | 0.80 | −5.8 | 27.57 ± 2.35 | 27.53 ± 2.34 | 0.95 | −0.1 |
| Average Daily Feed lntake, (kg/d) | 0.59 ± 0.15 | 0.58 ± 0.15 | 0.97 | −1.7 | 2.11 ± 0.25 | 2.03 ± 0.25 | 0.91 | −3.8 | 1.93 ± 0.15 | 1.94 ± 0.14 | 0.86 | 0.5 |
| Average Daily Gain, (kg/d) | 0.40 ± 0.05 | 0.41 ± 0.05 | 0.91 | 2.5 | 1.03 ± 0.08 | 0.93 ± 0.09 | 0.88 | −9.7 | 0.82 ± 0.05 | 0.80 ± 0.04 | 0.75 | −2.4 |
| Gain: Feed, (kg/kg) | 1.14 ± 0.29 | 1.10 ± 0.28 | 0.94 | −3.5 | 0.44 ± 0.36 | 0.43 ± 0.36 | 0.97 | −2.3 | ||||
| Digestibility of Dry Matter (g/kg) | 765.3 ± 15.6 | 754.2 ± 15.6 | 0.92 | −1.5 | ||||||||
3.3. Proteases
The productive results when proteases used in the pig's diet at any productive stage (Table 6, Table 7), showed an average in DMD (g/kg) 882.7 ± 12.20, 754.2 ± 3.61, 722.9 ± 3.47, in weaning, growing and finishing stages respectively. The effects of supplementing Proteases in pig diets are shown in Table 11. Dietary inclusion of Proteases did not affect (P > 0.05) ADFI, ADG, G: F, and DDM at all productive stages. On another hand, the fact that no improvement in CP and AA digestibility is observed in Protease-treated soybean meal (SBM) compared with untreated SBM, is because pigs fed a diet containing pretreated SBM with Protease enzyme had no change in G: F ratio compared with pigs fed with untreated SBM (Rooke, Slessor, Fraser & Thomson, 1998). However, when these data are expressed in percentage, it is observed that there is a reduction of 2.7% in the ADFI at weaning stage, possibly due to a better utilization of protein, leading to a reduction in feed consumption, without affecting their productive parameters, when including proteases compared to the control group, showing an increase in the ADG of 45% and a better G:F ratio (56%). This effect decreased at growing stage, showing an increase in the ADG of 2. 5% and a better G:F ratio (4.5%) possibly due to a better efficiency of protein utilization at younger stages. In terms of performance, at weaning stage, Zuo et al. (2015), reported on average an increase in ADG of 6.31%, in ADFI of 5.62% and a decrease in DDM of −0.26%, with a Protease supplementation at 0.1–0.3 g/kg, which is lower to the present results. At finishing stage, Lei et al. (2017), reported on average a decrease in ADG of −0.11%, a ADFI of −1.90%, a DDM of −0.47 and an increase in G: F of 1.79%, with Protease supplementation at 0.5 g/kg, better utilization of protein (essential amino acids) leads to a reduction in feed intake, without affecting their productive parameters, with a better G:F ratio.
Table 11.
Effects of supplementing Proteases exogenous enzymes in pig diets at different growing stages on animal performance.
| Variable | Weaning | Growing | Finishing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Proteases | P-value | % increment | Control | Proteases | P-value | % increment | Control | Proteases | P-value | % increment | |
| Number of animals | 23 | 23 | 83 | 83 | 168 | 168 | ||||||
| Dosage of Enzyme in the diet, (g/kg) | 0.001 ± 0.06 | 0.25 ± 0.06 | 0.01 | 0.01 ± 0.05 | 0.16 ± 0.05 | 0.03 | 0.001 ± 0.04 | 0.28 ± 0.04 | 0.01 | |||
| Dosage of Enzyme in the diet, (g/d) | 0.001 ± 0.18 | 0.12 ± 0.17 | 0.14 | 0.005 ± 0.17 | 0.31 ± 0.18 | 0.04 | 0.001 ± 0.12 | 0.71 ± 0.12 | 0.02 | |||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | 0.002± 0.01 | 0.03 ± 0.01 | 0.84 | 0.001 ± 0.01 | 0.03 ± 0.01 | 0.90 | 0.001 ± 0.01 | 0.04 ± 0.01 | 0.02 | |||
| Initial body weight, (kg) | 7.1 ± 16.3 | 7.1 ± 16.3 | 0.98 | 0.0 | 29.2 ± 11.6 | 26.5 ± 11.6 | 0.70 | −9.2 | 41.3 ± 8.2 | 47.3 ± 8.1 | 0.78 | 14.5 |
| Average Daily Feed lntake, (kg/d) | 0.72 ± 0.43 | 0.71 ± 0.43 | 0.94 | −2.7 | 1.86 ± 0.30 | 1.83 ± 0.30 | 0.93 | −1.6 | 2.72 ± 0.21 | 2.55 ± 0.21 | 0.91 | −6.2 |
| Average Daily Gain, (kg/d) | 0.22 ± 0.08 | 0.32 ± 0.07 | 0.54 | 45.4 | 0.81 ± 0.05 | 0.83 ± 0.05 | 0.90 | 2.5 | 0.88 ± 0.04 | 0.88 ± 0.05 | 0.99 | 0.0 |
| Gain: Feed, (kg/kg) | 0.30 ± 0.03 | 0.47 ± 0.07 | 0.21 | 56.7 | 0.44 ± 0.02 | 0.46 ± 0.02 | 0.87 | 4.5 | 0.31 ± 0.02 | 0.34 ± 0.02 | 0.88 | 9.7 |
% increment compared with the control group.
The mode of action of protease in its productive stages of the pig will improve the digestibility of the nutrients (Table 5, Table 6, Table 7 )., as well as the intestinal fermentation capacity and the longer transit time (Choe et al., 2017; Lei et al., 2017; Nguyen et al., 2019; Tactacan, Cho, Cho & Kim, 2016; Zuo et al., 2015). Pigs have the ability to produce digestive proteases such as pepsin, trypsin, chymotrypsin and carboxypeptidases that digest proteins included in the diet. A fraction of these proteins included in the feed that is intake it, are excreted in the feces, which means that an exogenous protease can improve the use of the proteins (Lemme, Ravindran & Bryden, 2004; Parsons, Castanon & Han, 1997).
3.4. Combined enzymes
The pig industry will continue to seek cost-effective alternative food ingredients, such as cereal co-products from the biofuel and milling industries (Kiarie & Nyachoti, 2009). In the present review, from 50 different diets, the most used combined enzymes in pig diets were Phytases (34 diets), Mannanases (25 diets), Xylanases (13 diets) and Proteases (12 diets).
Carbohydrases mixture can produce a greater benefit than each of the individually acting enzymes (Juanpere, Perez-Vendrell, Angulo & Brufau, 2005; Meng, Slominski, Nyachoti, Campbell & Guenter, 2005; Olukosi, Cowieson & Adeola, 2007b). Understanding how enzymes work together to hydrolyse their respective substrates and knowing the mode of action of the combination used in animal diets, maximizes productive efficiency (Adeola & Cowieson, 2011), although the benefits of the enzyme combination also depend on the composition of the diet (Meng & Slominski, 2005). At weaning stage, Yi et al. (2013), reported an average increase in ADG of 13.50%, a ADFI of 2.49%, a G: F of 10% and a DDM of 1.36%, with Amylase + Protease + Xylanase supplementation at 0.1–0.15 g/kg. Kim et al. (2004), reported on average a decrease in ADG of −5.25% and in ADFI of −2.44%, with Glucanase + Xylanase + Amylase + Pectinase + Protease supplementation at 0.5–1.5 g/kg (Table 2). At growing stage, Owusu-Asiedu et al. (2012), reported on average an increase in ADG of 2%, a decrease in ADFI of −12.04% and an increase in G: F of 16.66% and DDM of 1.8%, with a supplementation of Xylanase + Glucanase of 0.05–0.1 g/kg. Ao et al. (2010), reported on average an increase in ADG of 2.71%, in G: F of 4.23%, in DDM of 1.46% and a decrease in ADFI of −1.35%, with Galactosidase + Mannanase supplementation at 1–2 g/kg (Table 3). At finishing stage, Olukosi et al. (2007a), reported a decrease in ADG of −34%, in ADFI of −21.05%, in G: F of −13.63% and an increase in DDM of 1.15%, with Xylanase + Amylase + Protease supplementation at 0.5 g/kg. O'Shea et al. (2014), reported on average a decrease in ADG of −14.15% and in ADFI of −10.61%, with Protease + Xylanase supplementation at 0.4 g/kg.
Table 2.
Mode of action and main effects of enzymes used in pig diets at weaning stage.
| Enzyme | Action Mode | Main Effects | Reference |
|---|---|---|---|
| Mannanase | Improves the viscosity of the ileal digesta. | Increases AID of DM and NSPs. Lactobacillus and lactate count. | Kiarie et al., 2007 |
| Phytase | Improves AID of DM, GE, CP, starch, NSPs, Ca, P, inositol hexaphosphate (IP6), some AA (leucine, lysine, phenylalanine, alanine, cysteine, isoleucine, threonine, asparagine and serine) and phytate. ATTD of DM, GE, CP, starch, NSPs, phytate, Ca, P, Na, K, Mg, and Zn as well as in the retention of Mg and Zn. | -Increases ADG, ADFI and G: F ratio. Bone strength and plasma phosphorous concentrations. -Decreases fecal P excretion. Concentration of calcium in plasma, as well as the activity of alkaline phosphatase in plasma and bone. |
Omogbenigun et al., 2004; Zeng et al., 2011; Yáñez et al., 2013, 2014 |
| Protease | Improves AID of DM, GE, CP, starch, NSPs, phytate, isoleucine, valine and aspartic acid. ATTD of DM, GE, CP, starch, NSPs, phytate, and utilization of P. Nutrient digestibility and modification of microbial communities in the posterior intestine. Viscosity of the stomach digesta. Acetic, propionic and butyric acid concentrations in the cecum and colon. Volatile fatty acid concentrations and proportion of bacteria in the large intestine. Intestinal fermentation capacity and longer transit time. | -Increases ADG, ADFI and G: F ratio. Treponema and Barnesiella bacteria in the intestine. Population of Lactobacillus spp. and Bacillus spp. in the cecum. Amylase, lipase and protease in the small intestine. -Decreases fecal P excretion. Bacterias Prevotella, Butyricicoccus, Ruminococcus and Succinivibrio. E. coli population in the colon. Populations of Salmonella spp. and Escherichia coli spp. in the feces. NH3 emission in feces and blood creatinine level. |
Omogbenigun et al., 2004; Yi et al., 2013; Zhang et al., 2014; Zuo et al., 2015; Tactacan et al., 2016; Recharla et al., 2019 |
| Xylanase | Improves ileal and stomach viscosity. Acetic, propionic and butyric acid concentrations in the cecum and colon. Volatile fatty acid concentrations and proportion of bacteria in the large intestine. ATTD of DM, NDF, ADF, CP, GE, starch, NSPs, phytate, and utilization of P. AID of DM, GE, CP, starch, NSPs, phytate, isoleucine, valine, and aspartic acid. Individual sugars (arabinose, xylose, mannose and glucose). Nutrient digestibility and modification of microbial communities in the posterior intestine. | -Increases ADG, ADFI, G: F ratio and FCR. Treponema and Barnesiella bacteria in the intestine. Population of Lactobacillus spp. and Bacillus spp. in the cecum. Amylase, lipase, lactate and protease in the small intestine. -Decreases fecal P excretion. Bacterias Prevotella, Butyricicoccus, Ruminococcus and Succinivibrio. E. coli population in the colon. Populations of Salmonella spp. and E. coli spp. in the feces. Lactobacillus spp. and bacterial metabolites in the stomach. Blood urea nitrogen concentration and fecal emission of NH3 and H2S. |
Omogbenigun et al., 2004; Zijlstra, Li, Owusu-Asiedu, Simmins, & Patience, 2004; ; Kiarie et al., 2007; Owusu-Asiedu, Simmins, Brufau, Lizardo & Péron, 2010; Yi et al., 2013; Zhang et al., 2014; Lan et al., 2017; Li et al., 2018; Recharla et al., 2019 |
| Glucanase | Improves the viscosity of the ileal digesta. AID of DM, GE, CP, starch, NSPs, phytate, isoleucine, valine, and aspartic acid. ATTD of DM, ADF, GE, CP, starch, NSPs, phytate and utilization of P. Nutrient digestibility and modification of microbial communities in the posterior intestine. Individual sugars (arabinose, xylose, mannose and glucose). | -Increased Lactobacillus and lactate count. ADG, ADFI, G: F ratio and FCR. Treponema and Barnesiella bacteria in the intestine. -Decreases fecal P excretion. Bacterias Prevotella, Butyricicoccus, Ruminococcus and Succinivibrio. |
Omogbenigun et al., 2004; Zijlstra, Li, Owusu-Asiedu, Simmins, & Patience, 2004; Kiarie et al., 2007; Owusu-Asiedu et al., 2010; Li et al., 2018; Recharla et al., 2019 |
| Amylase | Improves AID of DM, GE, CP, starch, NSPs and phytate. ATTD of DM, GE, CP, starch, NSPs, phytate, and utilization of P. Nutrient digestibility and modification of microbial communities in the posterior intestine. Viscosity of the stomach digesta. Acetic, propionic and butyric acid concentrations in the cecum and colon. Volatile fatty acid concentrations and proportion of bacteria in the large intestine. | -Increases ADG, ADFI and G: Fratio. Treponema and Barnesiella bacteria in the intestine. Population of Lactobacillus spp. and Bacillus spp. in the cecum. -Decreases fecal P excretion. Bacterias Prevotella, Butyricicoccus, Ruminococcus and Succinivibrio. E. coli population in the colon. Populations of Salmonella spp. and E. coli spp. in the feces. |
Omogbenigun et al., 2004; Yi et al., 2013; Zhang et al., 2014; Recharla et al., 2019 |
| Invertase | Improves AID of DM, GE, CP, starch, NSPs and phytate. ATTD of DM, GE, CP, starch, NSPs, phytate, and utilization of P. | -Increases ADG and G: F.ratio -Decreased fecal P excretion. |
Omogbenigun et al., 2004 |
| Cellulase | Improves the viscosity of the ileal digesta and the integrity of the intestinal barrier. | -Increases ADG, AID of DM and NSPs. ATTD of ADF. Lactobacillus and lactate count. | Kiarie et al., 2007; Li et al., 2018 |
| Pectinase, Galactanase |
Improves the viscosity of the ileal digesta. | -Increases AID of DM and NSPs. Lactobacillus and lactate count. | Kiarie et al., 2007 |
AID: Apparent ileal digestibility; DM: Dry matter; NSPs: Non-starch polysaccharides; GE: Gross energy; CP: Crude protein; ATTD: Apparent total tract digestibility; P: Phosphorus; ADG: Average daily weight gain; G:F ratio: Gain Feed ratio; ADF: Acid detergent fiber; NDF: Neutral detergent fiber; FCR: Feed conversion ratio; ADFI: Average daily feed intake; Ca: Calcium; AA: Amino acids; Na: Sodium; K: Potassium; Mg: Magnesium; Zn: Zinc; N: Nitrogen; BUN: Blood urea nitrogen.
Table 3.
Mode of action and main effects of enzymes used in pig diets at growing stage.
| Enzyme | Action Mode | Main Effects | Reference |
|---|---|---|---|
| Mannanase | Improves AID of AA. ATTD of DM, NDF, ADF, GE, CP, Ca, mannose, galactose, phosphorus, blood glucose concentration and BUN. Digestibility of N. | -Increases ADG, ADFI and G: F. -Decrease in the population of fecal coliforms and NH3. |
Ao et al., 2010; Jo et al., 2012; Kim et al., 2013; Lv et al., 2013; Yoon et al., 2010; (Kim et al., 2017); Upadhaya et al., 2016a |
| Phytase | Improves AID of DM lysine, threonine, serine, isoleucine, asparagine and valine. ATTD of P, Ca, DM, GE, leucine, lysine, phenylalanine, alanine and cysteine. | -Increases ADG, FCR and G: F. -Decreases ADFI and fecal P excretion. Plasma calcium concentration, as well as plasma and bone alkaline phosphatase activity. |
Nortey et al., 2007; Kim et al., 2008; Zeng et al., 2011; Woyengo et al., 2016 |
| Protease | Improves ATTD of DM, GE, CP and BUN. Nutrient digestibility. | -Increases ADG, G: F. Blood creatinine levels. -Decreases the emission of ammonia gasses, blood norepinephrine levels and the emission of harmful gasses. |
Jo et al., 2012; Nguyen et al., 2019 |
| Xylanase | Improves AID of DM, AA, Isoleucine, P and CP. Nutrient transport. Digestibility of N. | -Increased G: F, FCR and glucose. -Decreases ADFI. |
Nortey et al., 2007; Kim et al., 2008; Ao et al., 2010; Owusu-Asiedu et al., 2012; Agyekum et al., 2015 |
| Mannosidase | Improves AID of AA. Digestibility of N. | -Increases ADG, BUN and glucose. | Ao et al., 2010 |
| Galactosidase | Improves AID of the DM and AA. Digestibility of N. | -Increases ADG, BUN and glucose | Ao et al., 2010 |
| Galactomannase | Improves AID of the DM, and AA. Digestibility of N. | -Increased glucose. | Ao et al., 2010 |
| Amylase | Improves ATTD of the DM, GE, CP, and BUN. | -Increased ADG and G: F. | Jo et al., 2012 |
| Glucanase | Improves AID of DM, AA and CP. Nutrient transport. Digestibility of N. | Increased G: F and glucose. | Agyekum et al., 2015; Ao et al., 2010; Owusu-Asiedu et al., 2012 |
AID: Apparent ileal digestibility; DM: Dry matter; GE: Gross energy; CP: Crude protein; ATTD: Apparent total tract digestibility; P: Phosphorus; ADG: Average daily weight gain; G:F ratio: Gain Feed ratio; ADF: Acid detergent fiber; NDF: Neutral detergent fiber; FCR: Feed conversion ratio; ADFI: Average daily feed intake; Ca: Calcium; AA: Amino acids; N: Nitrogen; BUN: Blood urea nitrogen.
The inclusion of combined-enzymes, used in the pig diet at any productive stage (Tables 5 – 7), showed an average in dry matter digestibility (g/kg) of 716.6 ± 133.9, 785.3 ± 59.8 and 811.5 ± 29.58 at weaning, growing and finishing productive stages, respectively, which target different antinutritional compounds in food to obtain the maximum benefit from the enzyme (Adeola & Cowieson, 2011). The effects of supplementing combined enzymes (Table 12) had no effect (P > 0.05) on ADFI, ADG, G: F, and DDM at all productive stages in the present study. However, it was observed that there is a reduction of 1.4% in the AFDI at weaning stage, when including combined-enzymes, resulting in an increase in the ADG of 4.9% and a better G: F ratio (1.6%). This effect was more visible at finishing stage, showing a better G: F ratio (8.0%) possibly due to a higher ADFI (4.6%) compared to the control group. The enzyme combination has led to better nutrient utilization, showing in all studies a reduction in ADFI, and a slight increase in DDM.
Table 12.
Effects of supplementing combination of exogenous enzymes in pig diets at different growing stages on animal performance.
| Variable | Weaning | Growing | Finishing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Multi-enzyme | P-value | % increment | Control | Multi-enzyme | P-value | % increment | Control | Multi-enzyme | P-value | % increment | |
| Number of animals | 283 | 283 | 172 | 172 | 140 | 140 | ||||||
| Dosage of Enzyme in the diet, (g/kg) | 1.32 ± 0.31 | 0.001 ± 0.31 | 0.03 | 0.78 ± 0.33 | 0.001 ± 0.33 | 0.01 | 1.32 ± 0.36 | 0.001 ± 0.36 | 0.02 | |||
| Dosage of Enzyme in the diet, (g/d) | 0.94 ± 0.37 | 0.01 ± 0.36 | 0.03 | 1.30 ± 0.39 | 0.002 ± 0.39 | 0.02 | 2.43 ± 0.42 | 0.001 ± 0.42 | 0.03 | |||
| Dosage of Enzyme in the diet, (g/kg LW0.75) | 0.22 ± 0.05 | 0.001 ± 0.05 | 0.34 | 0.11 ± 0.05 | 0.001 ± 0.05 | 0.31 | 0.17 ± 0.06 | 0.001 ± 0.05 | 0.04 | |||
| Initial body weight, (kg) | 7.03 ± 4.90 | 7.19 ± 4.91 | 0.83 | 2.3 | 24.93 ± 7.76 | 25.00 ± 7.75 | 0.99 | 0.3 | 48.69 ± 5.76 | 48.55 ± 5.76 | 0.99 | −0.3 |
| Average Daily Feed lntake, (kg/d) | 0.72 ± 0.18 | 0.71 ± 0.18 | 0.54 | −1.4 | 1.56 ± 0.19 | 1.59 ± 0.18 | 0.90 | 1.9 | 2.18 ± 0.21 | 2.28 ± 0.21 | 0.98 | 4.6 |
| Average Daily Gain, (kg/d) | 0.41 ± 0.05 | 0.43 ± 0.05 | 0.94 | 4.9 | 0.71 ± 0.09 | 0.70 ± 0.09 | 0.97 | 1.4 | 0.86 ± 0.04 | 0.83 ± 0.04 | 0.89 | −3.5 |
| Gain: Feed, (kg/kg) | 0.63 ± 0.03 | 0.62 ± 0.03 | 0.96 | 1.6 | 0.51 ± 0.03 | 0.51 ± 0.03 | 0.98 | 0.0 | 0.37 ± 0.04 | 0.40 ± 0.05 | 0.90 | 8.1 |
| Digestibility of Dry Matter (g/kg) | 733.8 ± 49.40 | 702.5 ± 49.4 | 0.24 | −4.3 | 794.7 ± 54.1 | 784.2 ± 54.1 | 0.51 | −1.3 | 807.7 ± 69.9 | 799.7 ± 69.9 | 0.43 | −1.0 |
4. Conclusion
Nowadays, the use of combined enzymes in pig diets has been widely reported at all productive stages. Their use is due to the multiple enzymatic activities that can be carried out against antinutritive compounds in the diet, which can benefit animal performance. Phytases are the most supplemented enzymes at all productive stages of pigs, surpassing the use of Mannanases and Xylanases, as well as Proteases, although the latter are less frequently supplemented in pig diets. More studies are necessary to understand the interaction between diet composition, productive stage, origin of the enzyme, quantity, and number of added enzymes, since all those variables interfere with the mode of action and have specific effects at different productive stages. Although most research using exogenous enzyme supplementation in pig diets has shown to produce positive results compared to control diets, there not consistent improvements in growth, performance, and nutrient digestibility and this deserves further attention.
5. Author´s contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, MGR, LERJ, and E.V.-B.-P; methodology, MGR, EAA; software, MGR; LERJ; EAA; validation, MGR, E.V.-B.-P and LERJ; formal analysis, EAA, LERJ; investigation, EAA, LERJ, MGR; resources, MGR, JOA; data curation, MGR, EAA, LERJ; writing—original draft preparation, LERJ, MGR, E.V.-B.-P and EAA; writing—review and editing, LERJ, MGR, E.V.-B.-P; visualization, MGR; supervision MGR; project administration, MGR; funding acquisition, MGR. All authors have read and agreed to the published version of the manuscript”, please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
6. Funding
This project was supported by Universidad Autonoma del Estado de México (Project ID UAEMex4974/2020)and SEP-PRODEP UAEM CA193.
7. Availability of data and materials
At the request to the corresponding author
8. Ethics approval and consent to participate
Not applicable.
9. Consent for publication
Not applicable.
Ethical statements
The author's state that no animals were used in this study, as it is a review of previous work in pigs, with the addition of enzymes.
CRediT authorship contribution statement
Edgar Aranda-Aguirre: Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft. Lizbeth E. Robles-Jimenez: Conceptualization, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing. Jorge Osorio-Avalos: Resources. Einar Vargas-Bello-Pérez: Conceptualization, Validation, Writing – original draft, Writing – review & editing. Manuel Gonzalez-Ronquillo: Conceptualization, Methodology, Software, Validation, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration Competing of Interest
The authors declare that they no competing interest.
Acknowledgments
Miss L Robles Jimenez was granted with a Conacyt (Mexico) fellowship for her PhD studies.
References
- Aarnink A.J.A., Verstegen M.W.A. Nutrition, key factor to reduce environmental load from pig production. Livestock Science. 2007;109(1–3):194–203. doi: 10.1016/j.livsci.2007.01.112. [DOI] [Google Scholar]
- Adeola O., Cowieson A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve on ruminant animal production. Journal of Animal Science. 2011;89(10):3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Agyekum A.K., Sands J.S., Regassa A., Kiarie E., Weihrauch D., Kim W.K. Effect of supplementing a fibrous diet with a xylanase and β-glucanase blend on growth performance, intestinal glucose uptake, and transport-associated gene expression in growing pigs. Journal of Animal Science. 2015;93(7):3483–3493. doi: 10.2527/jas.2015-9027. [DOI] [PubMed] [Google Scholar]
- Ao X., Meng Q.W., Yan L., Kim H.J., Hong S.M., Cho J.H. Effects of non-starch polysaccharide-degrading enzymes on nutrient digestibility, growth performance and blood profiles of growing pigs fed a diet based on corn and soybean meal. Asian-Australasian Journal of Animal Science. 2010;23(12):1632–1638. doi: 10.5713/ajas.2010.10123. [DOI] [Google Scholar]
- Ao X., Zhou T.X., Meng Q.W., Lee J.H., Jang H.D., Cho J.H. Effects of a carbohydrase cocktail supplementation on the growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs fed palm kernel meal. Livestock Science. 2011;137(1–3):238–243. doi: 10.1016/j.livsci.2010.11.014. [DOI] [Google Scholar]
- Barrera M.A., Cervantes M., Sauer W.C., Araiza A.B., Torrentera N. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. Journal of Animal Sciences. 2004;82(7):1997–2003. doi: 10.2527/2004.8271997x. [DOI] [PubMed] [Google Scholar]
- Campbell G.L., Bedford M.R. Enzyme application for monogastric feeds: A review. Canadian Journal of Animal Science. 1992;72:449–466. doi: 10.4141/cjas92-058. [DOI] [Google Scholar]
- Castro A.M.A., Rosales G.S., Angeles M.L., Varela B.D., Landín M.G., Ibargüengoytia C.J.A. Fitasa y enzimas fibrolíticas en dietas para cerdos con diferentes sustratos. Revista Mexicana de Ciencias Pecuarias. 2011;2(2):117–135. [Google Scholar]
- Cho J.H., Kim I.H. Effects of beta mannanase and xylanase supplementation in low energy density diets on performances, nutrient digestibility, blood profiles and meat quality in finishing pigs. Asian Journal of animal and Veterinary Advances. 2013;8(4):622–630. doi: 10.3923/ajava.2013.622.630. [DOI] [Google Scholar]
- Cho J.H., Park J.H., Lee D.H., Lee J.M., Song T.H., Kim I.H. Effects of xylanase supplementation on growth performance, digestibility, fecal gas emission, and meat quality in growing-finishing pigs. Canadian Journal of Animal Science. 2017;97(1):95–100. doi: 10.1139/cjas-2015-0198. [DOI] [Google Scholar]
- Choct M. Feed non-starch polysaccharides for monogastric animals: Classification and function. Animal Production Science. 2015;55(12):1360–1366. doi: 10.1071/AN15276. [DOI] [Google Scholar]
- Choe J., Kim K.S., Kim H.B., Park S., Kim J., Kim S. Effect of protease on growth performance and carcass characteristics of growing-finishing pigs. South African Journal of Animal Science. 2017;47(5):697–703. doi: 10.4314/sajas.v47i5.13. [DOI] [Google Scholar]
- Clark M., Tilman D. Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environmental Research Letters, 064016. 2017;12(6):1–11. doi: 10.1088/1748-9326/aa6cd5. [DOI] [Google Scholar]
- Cowieson A.J., Bedford M.R. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: Complementary mode of action? World´s Poultry Science Journal. 2009;65(4):609–624. doi: 10.1017/S0043933909000427. [DOI] [Google Scholar]
- Da Silva C.A., Callegari M.A., Dias C.P., Bridi A.M., Pierozan C.R., Foppa L. Increasing doses of phytase from citrobacter braakii in diets with reduced inorganic phosphorus and calcium improve growth performance and lean meat of growing and finishing pigs. PloS one, e0217490. 2019;14(5):1–13. doi: 10.1371/journal.pone.0217490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Faria H.G., Thomaz M.C., Ruiz U.D.S., Robles-Huaynate R.A., Watanabe P.H., De Melo G.M.P. Effects of phytase on pig diets digestibilities, bone mineral deposition, performance and manure production. Semina: Ciências Agrárias Londrina. 2015;36(6):4519–4530. doi: 10.5433/1679-0359.2015v36n6Supl2p4519. [DOI] [Google Scholar]
- Dersjant-Li Y., Awati A., Schulze H., Partridge G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. Journal of the Science of Food and Agriculture. 2014;95(5):878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li Y., Plumstead P., Awati A., Remus J. Productive performance of commercial growing and finishing pigs supplemented with a buttiauxella phytase as a total replacement of inorganic phosphate. Animal Nutrition Journal. 2018;4(4):351–357. doi: 10.1016/j.aninu.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li Y., Schuh K., Weallean A.L., Awati A., Dusel G. Effect of a buttiauxella phytase on production performance in growing/finishing pigs fed a European-type diet without inclusion of inorganic phosphorus. Journal of Applied Animal Nutrition. 2017;5(4):1–7. doi: 10.1017/JAN.2017.3. [DOI] [Google Scholar]
- Dhawan S., Kaur J. Microbial mannanases: An overview of production and applications. Critical Reviews in Biotechnology. 2007;27(4):197–216. doi: 10.1080/07388550701775919. [DOI] [PubMed] [Google Scholar]
- European Union Reference Laboratory for Feed Additives (EURL-FA). (2014). Geel, Belgium. https://ec.europa.eu/food/sites/food/files/safety/docs/oc_eurl_wp_2015_feed_additives_en.pdf.
- EFSA Scientific opinion on the safety and efficacy of ronozyme ®HiPhos M/L (6-phytase) as a feed additive for poultry and pigs. EFSA panel on genetically modified organisms (GMO) EFSA Journal. 2012;10(1):1–12. doi: 10.2903/j.efsa.2012.2527. [DOI] [Google Scholar]
- He X., Yu B., He J., Huang Z., Mao X., Zheng P. Effects of xylanase on growth performance, nutrients digestibility and intestinal health in weaned piglets. Livestock Science, 103940. 2020;233:1–7. doi: 10.1016/j.livsci.2020.103940. [DOI] [Google Scholar]
- International Union of Biochemistry and Molecular Biology . Academic Press; New York: 1992. Enzyme nomenclature: Recommendations of the nomenclature committee of international union of biochemistry and molecular biology on the nomenclature and classification of enzymes. ISBN: 9781483298689. [Google Scholar]
- Jang J.C., Kim K.H., Jang Y.D., Kim Y.Y. Effects of dietary β-mannanase supplementation on growth performance, apparent total tract digestibility, intestinal integrity, and immune responses in weaning pigs. Animals. 2020;10(4):1–10. doi: 10.3390/ani10040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal : An International Journal of Animal Bioscience. 2015;9(9):1441–1452. doi: 10.1017/S1751731115000919. 10.1017%2FS1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J.K., Ingale S.L., Kim J.S., Kim Y.W., Kim K.H., Lohakare J.D. Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. Journal of Animal Science. 2012;90(9):3041–3048. doi: 10.2527/jas.2010-3430. [DOI] [PubMed] [Google Scholar]
- Jongbloed A.W., Van Diepen J.Th.M., Kemme P.A., Broz J. Efficacy of microbial phytase on mineral digestibility in diets for gestating and lactating sows. Livestock Production Science. 2004;91(1–2):143–155. doi: 10.1016/j.livprodsci.2004.07.017. [DOI] [Google Scholar]
- Juanpere J., Perez-Vendrell A.M., Angulo E., Brufau J. Assessment of potential interactions between phytase and glycosidase enzyme supplementation on nutrient digestibility in broilers. Poulty Science. 2005;84(4):571–580. doi: 10.1093/ps/84.4.571. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Nyachoti C.M. Alternative feed ingredients in swine diets. In Proceedings of the 32nd Saskatchewan Pork Industry Symposium, Saskatoon: Saskatchewan Pork Development Board. 2009;1(1):29–38. [Google Scholar]
- Kiarie E., Nyachoti C.M., Slominski B.A., Blank G. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme1, 2. Journal of Animal Science. 2007;85(11):2982–2993. doi: 10.2527/jas.2006-481. [DOI] [PubMed] [Google Scholar]
- Kim B.G., Tian J.Z., Lim J.S., Kil D.Y., Jeon H.Y., Chung Y.K. Influences of enzyme complex supplementation on growth, ileal and apparent fecal digestibility and morphology of small intestine in pigs. Asian-Australasian Journal of Animal Sciences. 2004;17(12):1729–1735. doi: 10.5713/ajas.2004.1729. [DOI] [Google Scholar]
- Kim J.C., Sands J.S., Mullan B.P., Pluske J.R. Performance and total-tract digestibility responses to exogenous xylanase and phytase in diets for growing pigs. Animal Feed Science and Technology. 2008;142(1–2):163–172. 10.1016%2Fj.anifeedsci.2007.07.004. [Google Scholar]
- Kim J.S., Ingale S.L., Hosseindoust A.R., Lee S.H., Lee J.H., Chae B.J. Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal : An International Journal of Animal Bioscience. 2017;11(2):202–208. doi: 10.1017/s1751731116001385. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Ingale S.L., Lee S.H., Kim K.H., Kim J.S., Lee J.H. Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs. Animal Feed Science and Technology. 2013;186(1–2):64–70. doi: 10.1016/j.anifeedsci.2013.08.008. [DOI] [Google Scholar]
- Lan R., Li T., Kim I. Effects of xylanase supplementation on growth performance, nutrient digestibility, blood parameters, fecal microbiota, fecal score and fecal noxious gas emission of weaning pigs fed corn-soybean meal-based diet. Animal Science Journal. 2017;88(9):1398–1405. doi: 10.1111/asj.12771. [DOI] [PubMed] [Google Scholar]
- Lee S.D., Jung H.J., Cho K.H., Park J.C., Kim I.C., Seong P.N. Effects of corn-dried distiller's grains with solubles and enzyme premix supplements on growth performance, carcass characteristics and meat quality parameters in finishing pigs. Journal of Animal Science. 2011;82(3):461–467. doi: 10.1111/j.1740-0929.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- Leek A.B.G., Callan J.J., Reilly P., Beattie V.E., O'Doherty J.V. Apparent component digestibility and manure ammonia emission in finishing pigs fed diets based on barley, maize or wheat prepared without or with exogenous non-starch polysaccharide enzymes. Animal Feed Science and Technology. 2007;135(1–2):86–99. doi: 10.1016/j.anifeedsci.2006.03.024. [DOI] [Google Scholar]
- Lei X.G., Weaver J.D., Mullaney E., Ullah A.H., Azain M.J. Phytase, a new life for an “old” enzyme. Annual Review of Animal Biosciences. 2012;1:283–309. doi: 10.1146/annurev-animal-031412-103717. [DOI] [PubMed] [Google Scholar]
- Lei X.J., Cheong J.Y., Park J.H., Kim I.H. Supplementation of protease, alone and in combination with fructooligosaccharide to low protein diet for finishing pigs. Animal Science Journal. 2017;88(12):1987–1993. doi: 10.1111/asj.12849. [DOI] [PubMed] [Google Scholar]
- Lemme A., Ravindran V., Bryden W.L. Ileal digestibility of amino acids in feed ingredients for broilers. World´s Poultry Science Journal. 2004;60(4):423–437. doi: 10.1079/WPS200426. [DOI] [Google Scholar]
- Li Q., Gabler N.K., Loving C.L., Gould S.A., Patience J.F. A dietary carbohydrase blend improved intestinal barrier function and growth rate in weaned pigs fed higher fiber diets. Journal of Animal Science. 2018;96(12):5233–5243. doi: 10.1093/jas/sky383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Sauer W.C., Huang S.X., Gabert V.M. Effect of β-glucanase supplementation to hulless barley- or wheat-soybean meal diets on the digestibilities of energy, protein,β-glucans, and amino acids in young pigs. Journal of Animal Science. 1996;74(7):1649–1656. doi: 10.2527/1996.7471649x. [DOI] [PubMed] [Google Scholar]
- Lima M.R., Da Silva J.H.V., Araujo J.A., Lima C.B., Oliveira E.R.A. Enzimas exógenas na alimentação de aves. Acta Veterinaria Brasilica. 2007;1(4):99–110. doi: 10.21708/avb.2007.1.4.485. [DOI] [Google Scholar]
- Lindberg J.E. Fiber effects in nutrition and gut health in pigs. Journal of Animal Science and Biotechnology. 2014;1,5(1):2–7. doi: 10.1186/2049-1891-5-15. 15.10.1186/2049-1891-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Preynat A., Legrand-Defretin V., Geraert P.A., Adeola O., Ajuwon K.M. Effects of dietary supplementation of exogenous multi-enzyme mixture containing carbohydrases and phytase on growth performance, energy and nutrient digestibility, and selected mucosal gene expression in the small intestine of weanling pigs fed nutrient deficient diets. Canadian Journal of Animal Science. 2016;96(2):243–251. doi: 10.1139/cjas-2015-0078. [DOI] [Google Scholar]
- Lv J.N., Chen Y.Q., Guo X.J., Piao X.S., Cao Y.H., Dong B. Effects of supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian-Australasian Journal of Animal Sciences. 2013;26(4):579–587. doi: 10.5713/ajas.2012.12612. 10.5713%2Fajas.2012.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A.J.A., Figueroa V.J.L., Cordero M.J.L., Sánchez T.E.M.T., Martínez A.M. Starting pig diets including wheat bran and supplemented with xylanase. Ecosystems and Agricultural Resources Magazine. 2017;4(10):73–80. [Google Scholar]
- Masey O'Neill H.V., Smith J.A., Bedford M.R. Multicarbohydrase enzymes for non-ruminants. Asian-Australasian Journal Animal Sciences. 2014;27(2):290–301. doi: 10.5713/ajas.2013.13261. 10.5713%2Fajas.2013.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathlouthi N., Lalles J.P., Lepercq P., Juste C., Larbier M. Xylanase and beta-glucanase supplementation improve conjugated bile acid fraction in intestinal contents and increase villus size of small intestine wall in broiler chickens fed a ryebased diet. Journal of Animal Science. 2002;80(11):2773–2779. doi: 10.2527/2002.80112773x. [DOI] [PubMed] [Google Scholar]
- Meng X., Slominski B.A. Nutritive values of corn, soybean meal, canola meal, and peas for broiler chickens as affected by a multicarbohydrase preparation of cell wall degrading enzymes. Poultry Science. 2005;84(8):1242–1251. doi: 10.1093/ps/84.8.1242. [DOI] [PubMed] [Google Scholar]
- Meng X., Slominski B.A., Nyachoti C.M., Campbell L.D., Guenter W. Degradation of cell wall polysacxharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poultry Science. 2005;84(1):37–47. doi: 10.1093/ps/84.1.37. [DOI] [PubMed] [Google Scholar]
- Nguyen D.H, Park, J. W., & Kim, I. H. Effect of crumbled diet on growth performance, market day age and meat quality of growing-finishing pigs. J. Appl. Anim. Res. 2017;45:396–399. doi: 10.1080/09712119.2016.1206904. [DOI] [Google Scholar]
- Nguyen D.H., Upadhaya S.D., Lei X.J., Yin J., Kim I.H. Influence of dietary protease supplementation to corn-soybean meal based high and low energy diets on growth performance, nutrient digestibility, blood profiles, and gas emission in growing pigs. Canadian Journal of Animal Science. 2019;99(3):482–488. doi: 10.1139/cjas-2017-0104. [DOI] [Google Scholar]
- Nortey T.N., Patience J.F., Simmins P.H., Trottier N.L., Zijlstra R.T. Effects of individual or combined xylanase and phytase supplementation on energy, amino acid, and phosphorus digestibility and growth performance of grower swine fed wheat-based diets containing wheat millrun. Journal of Animal Science. 2007;85(6):1432–1443. doi: 10.2527/jas.2006-613. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Cowieson A.J., Adeola O. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poultry Science. 2007;86(1):77–86. doi: 10.1093/ps/86.1.77. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Sands J.S., Adeola O. Supplementation of carbohydrases or phytase individually or in combination to diets for weanling and growing-finishing pigs. Journal of Animal Science. 2007;85(7):1702–1711. doi: 10.2527/jas.2006-709. [DOI] [PubMed] [Google Scholar]
- Omogbenigun F.O., Nyachoti C.M., Slominski B.A. The effect of supplementing microbial phytase and organic acids to a corn-soybean based diet fed to early-weaned pigs. Journal of Animal Science. 2003;81(7):1806–1813. doi: 10.2527/2003.8171806x. [DOI] [PubMed] [Google Scholar]
- Omogbenigun F.O., Nyachoti C.M., Slominski B.A. Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. Journal of Animal Science. 2004;82(4):1053–1061. doi: 10.2527/2004.8241053x. [DOI] [PubMed] [Google Scholar]
- O'Shea C.J., Mc Alpine P.O., Solan P., Curran T., Varley P.F., Walsh A.M. The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower-finisher pigs. Animal Feed Science and Technology. 2014;189:88–97. doi: 10.1016/j.anifeedsci.2013.11.012. [DOI] [Google Scholar]
- Owusu-Asiedu A., Kiarie E., Péron A., Woyengo T.A., Simmins P.H., Nyachoti C.M. Growth performance and nutrient digestibilities in nursery pigs receiving varying doses of xylanase and β-glucanase blend in pelleted wheat- and barley-based diets. Journal of Animal Science. 2012;4:92–94. doi: 10.2527/jas.51323. [DOI] [PubMed] [Google Scholar]
- Owusu-Asiedu A., Simmins P.H., Brufau J., Lizardo R., Péron A. Effect of xylanase and β-glucanase on growth performance and nutrient digestibility in piglets fed wheat-barley-based diets. Livestock Science. 2010;134(1):76–78. doi: 10.1016/j.livsci.2010.06.102. [DOI] [Google Scholar]
- Park S., Lee J.J., Yang B.M., Cho J.H., Kim S., Kang J. Dietary protease improves growth performance, nutrient digestibility, and intestinal morphology of weaned pigs. Journal of Animal Science and Technology. 2020;62(1):21–30. doi: 10.5187/jast.2020.62.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C.M., Castanon F., Han Y. Protein and amino acid acid digestibility of meat and bone meal. Poultry Science. 1997;76(2):361–368. doi: 10.1093/ps/76.2.361. [DOI] [PubMed] [Google Scholar]
- Passos A.A., Park I., Ferket P., von Heimendahl E., Kim S.W. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Animal Nutrition. 2015;1(1):19–23. doi: 10.1016/j.aninu.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience, J.F., .& DeRouchey, J.M. (2010). Feed additives for swine - enzymes and phytase. Animal Science white papers, technical reports, and fact sheets. 13.
- Pettey L.A., Carter S.D., Senne B.W., Shriver J.A. Effects of beta-mannanase addition to corn-soybean meal diets on growth performance, carcass traits, and nutrient digestibility of weanling and growing-finishing pigs. Journal of Animal Science. 2002;80(4):1012–1019. doi: 10.2527/2002.8041012x. [DOI] [PubMed] [Google Scholar]
- Ravindran V. Feed enzymes: The science, practice, and metabolic realities. Journal of Applied Poultry Research. 2013;22(3):628–636. doi: 10.3382/japr.2013-00739. [DOI] [Google Scholar]
- Recharla N., Kim D., Ramani S., Song M., Park J., Balasubramanian B. Dietary multi-enzyme complex improves in vitro nutrient digestibility and hind gut microbial fermentation of pigs. PloS one, e02117459. 2019;14(5):1–19. doi: 10.1371/journal.pone.0217459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke J.A., Slessor M., Fraser H., Thomson J.R. Growth performance and gut function of piglets weaned at four weeks of age and fed protease-treated soya-bean meal. Animal Feed Science and Technology. 1998;70(3):175–190. doi: 10.1016/S0377-8401(97)00083-7. [DOI] [Google Scholar]
- SAS . 7th ed. SAS. Inst. Inc. Cary; North Carolina. USA.: 2004. Statistical analysis system, user’s guide. statistical. version. [Google Scholar]
- Sefer D., Petrujkic B., Markovic R., Grdovic S., Nestorovic B., Bogosavljevic V. Effect of phytase supplementation on growing pigs performance. Acta Veterinaria Beograd. 2012;62(5–6):627–639. [Google Scholar]
- Selle P.H., Cowieson A.J., Ravindran V. Consequences of calcium interactions with phytase and phytase for poultry and pigs. Livestock Science. 2009;124(1):126–141. [Google Scholar]
- Selle P.H., Ravindran V. Phytate-degrading enzymes in pig nutrition. Livestock Science. 2008;113(2):99–122. doi: 10.1016/j.livsci.2007.05.014. [DOI] [Google Scholar]
- Tactacan G.B., Cho S.Y., Cho J.H., Kim I.H. Performance responses, nutrient digestibility, blood characteristics, and measures of gastrointestinal health in weanling pigs fed protease enzyme. Asian Australasian Journal of Animal Science. 2016;29(7):998–1003. doi: 10.5713/ajas.15.0886. https://dx.doi.org/10.5713%2Fajas.15.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T., Dove C.R., Cline P.M., Owusu-Asiedu A., Walsh M.C., Azain M. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livestock Science. 2017;197:4–52. doi: 10.1016/j.livsci.2017.01.008. [DOI] [Google Scholar]
- Tsai T.C., Dove R., Bedford M.R., Azain M.J. Effect of phytase on phosphorous balance in 20 kg barrows fed low or adequate phosphorous diets. Animal Nutrition Journal. 2019;6(1):9–15. doi: 10.1016/j.aninu.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S.D., Park J.W., Lee J.H., Kim I.H. Efficacy of β-mannanase supplementation to corn–soya bean meal-based diets on growth performance, nutrient digestibility, blood urea nitrogen, faecal coliform and lactic acid bacteria and faecal noxious gas emission in growing pigs. Archives of Animal Nutrition. 2016;70(1):33–43. doi: 10.1080/1745039x.2015.1117697. [DOI] [PubMed] [Google Scholar]
- Upadhaya S.D., Yun H.M., Kim I.H. Influence of low or high density corn and soybean meal-based diets and protease supplementation on growth performance, apparent digestibility, blood characteristics and noxious gas emission of finishing pigs. Animal Feed Science and Technology. 2016;216:281–287. doi: 10.1016/j.anifeedsci.2016.04.003. [DOI] [Google Scholar]
- Veum T.L., Odle J. In: Swine nutrition. Lewis A.J., Southern L.L.., editors. CRC Press; New York, USA: 2001. Feeding neonatal pigs; pp. 671–690. [Google Scholar]
- Woyengo T.A., Dupe V.I.G.E., Akinremi O.O., Nyachoti C.M. Performance and nutrient digestibility in growing pigs fed wheat dried distillers’ grain with solubles-containing diets supplemented with phytase and multi-carbohydrase. Animal Science Journal. 2016;87(4):570–577. doi: 10.1111/asj.12461. [DOI] [PubMed] [Google Scholar]
- Yáñez J.L., Landero J.L., Owusu-Asiedu A., Cervantes M., Zijlstra R.T. Growth performance, diet nutrient digestibility, and bone mineralization in weaned pigs fed pelleted diets containing thermostable phytase. Journal of Animal Science. 2013;91(2):745–754. doi: 10.2527/jas.2011-4949. https://doi-org.eres.qnl.qa/10.2527/jas.2011-4949. [DOI] [PubMed] [Google Scholar]
- Yi J.Q., Piao X.S., Li Z.C., Zhang H.Y., Chen Y., Li Q.Y. The effects of enzyme complex on performance, intestinal health and nutrient digestibility of weaned pigs. Asian Australasian Journal of Animal Science. 2013;26(8):1181–1188. doi: 10.5713/ajas.2013.13129. https://dx.doi.org/10.5713%2Fajas.2013.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F., Zhang Z., Huang J., Yin Y. Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. British Journal of Nutrition. 2010;103(10):1404–1412. doi: 10.1017/S0007114509993321. [DOI] [PubMed] [Google Scholar]
- Yoon S.Y., Yang Y.X., Shinde P.L., Choi J.Y., Kim J.S., Kim Y.W. Effects of mannanase and distillers dried grain with solubles on growth performance, nutrient digestibility, and carcass characteristics of grower-finisher pigs. Journal of Animal Science. 2010;88(1):181–191. doi: 10.2527/jas.2008-1741. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Li Q., Tian Q., Zhao P., Xu X., Yu S. Super high dosing with a novel buttiauxella phytase continuously improves growth performance, nutrient digestibility, and mineral status of weaned pigs. Biological Trace Element Research. 2015;168(1):103–109. doi: 10.1007/s12011-015-0319-2. [DOI] [PubMed] [Google Scholar]
- Zeng Z.K., Piao X.S., Wang D., Li P.F., Xue L.F., Salmon L. Effect of microbial phytase on performance, nutrient absorption and excretion in weaned pigs and apparent ileal nutrient digestibility in growing pigs. Asian-Australasian Journal Animal Science. 2011;24(8):1164–1172. doi: 10.5713/ajas.2013.13370. [DOI] [Google Scholar]
- Zeng Z.K., Wang D., Piao X.S., Li P.F., Zhang H.Y., Shi C.X. Effects of adding super dose phytase to the phosphorus-deficient diets of young pigs on growth performance, bone quality, minerals and amino acids digestibilities. Asian Australasian Journal Animal Science. 2014;27(2):237–246. doi: 10.5713/ajas.2013.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.G., Yang Z.B., Wang Y., Yang W.R., Zhou H.J. Effects of dietary supplementation of multi-enzyme on growth performance, nutrient digestibility, small intestinal digestive enzyme activities, and large intestinal selected microbiota in weanling pigs. Journal of Animal Science. 2014;92(5):2063–2069. doi: 10.2527/jas.2013-6672. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang G., Liu L., Wang J., Zhang S. Effects of fibre-degrading enzymes in combination with different fibre sources on ileal and total tract nutrient digestibility and fermentation products in pigs. Archives of Animal Nutrition. 2020;74(4):309–324. doi: 10.1080/1745039X.2020.1766333. [DOI] [PubMed] [Google Scholar]
- Zijlstra R.T., Li S., Owusu-Asiedu A., Simmins P.H., Patience J.F. Effect of carbohydrase supplementation of wheat- and canola-meal-based diets on growth performance and nutrient digestibility in group-housed weaned pigs. Canadian Journal of Animal Science. 2004;84(4):689–696. doi: 10.4141/A03-127. [DOI] [Google Scholar]
- Zuo J., Ling B., Long L., Li T., Lahaye L., Yang C. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Animal Nutrition. 2015;1(4):276–282. doi: 10.1016/j.aninu.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
At the request to the corresponding author