Abstract
Methyltetrahydrofolate reductase (MTHFR) is a key enzyme in folate metabolism, and its single nucleotide polymorphism (SNP) site C677T may be associated with gastrointestinal cancer. However, the relationship between MTHFR C677T polymorphism and gastrointestinal tumor markers carcinoma embryonic antigen (CEA), carbohydrate antigen 199 (CA199) and carbohydrate antigen 724 (CA724) in Helicobacter pylori (H. pylori) infection is not specified. This study aims to identify the association between MTHFR C677T polymorphism and gastrointestinal tumor markers (CEA, CA199 and CA724) in H. pylori infection. The relationship between MTHFR C677T polymorphism and gastrointestinal tumor markers in 58 patients with H. pylori infection and 94 non-infected patients was studied. We found that TT genotype was a susceptibility factor of H. pylori infection, which was also associated with increased CEA and CA724 levels. Moreover, there was a negative additive interaction between MTHFR gene C677T polymorphism and CEA levels in H.pylori infection. Meanwhile, there were significant differences in CEA levels between MTHFR C677T polymorphism and H.pylori infection. The presence of T allele led to a decrease in CEA levels when 13C urea breath test (13C-UBT) was positive, while the presence of T allele led to an increase in CEA levels when 13C-UBT was negative. Therefore, we suggest that healthy people should take MTHFR C677T polymorphism screening, combined with 13C-UBT and gastrointestinal tumor markers detection, which can screen out the susceptible population of H. pylori, and help to detect gastrointestinal cancer in the early stage.
Keywords: CA199, CA724, CEA, Gastrointestinal cancer, Helicobacter pylori, MTHFR C677T polymorphism
Introduction
Even though the knowledge of gastrointestinal cancer is getting more in depth, together with the large amount of funds and resources invested in the research and development of effective treatment for gastrointestinal cancer,1 in the past few decades, the mortality of gastrointestinal cancer has hardly decreased. So far, gastric cancer and colorectal cancer are still the top ten leading causes of cancer-related deaths in the world.2,3 Therefore, some scholars and clinical workers also focus on the early screening and diagnosis of gastrointestinal cancer.4,5 Regarding the 5-year survival rate, patients with early diagnosis and treatment have a higher survival rate than those diagnosed in advanced stage.6,7
The etiology of gastrointestinal cancer is multifactorial and co-regulated by different factors.8,9 Besides the external factors including environmental factors, dietary habits and socio-economic status,10 it is also related to individual factors, such as age, gender and genetic susceptibility.9,11 It has been confirmed that H.pylori infection is associated with a variety of gastrointestinal diseases and gastrointestinal cancer.12,13
MTHFR is a key enzyme in folate metabolism, which resides on chromosome 1 location p36.3, and it appears to be polymorphic such as the gene site C677T, one of the most studied and clinically important variants in exon 4.14 When the C allele gene at 677 is replaced by T allele, valine is replaced by alanine, which leads to the decrease of enzyme activity. The enzyme activities of heterozygous MTHFR C677T genotype and homozygous MTHFR T677T genotype were 60% and 30% of that of wild-type MTHFR C677C genotype, respectively.15,16 With the decrease of enzyme activity, folate metabolism is also affected, showing a higher degree of genomic DNA hypomethylation.17 As one of the characteristics of protooncogenes, hypomethylation leads to gastrointestinal cancer.18,19 However, the interaction between MTHFR C677T polymorphism and H.pylori in gastrointestinal cancers remains unclear.
As important indicators of gastrointestinal cancer screening, the increase of CEA, CA199 and CA724 indicates the occurrence of gastrointestinal cancer.20,21 Therefore, this study analyzes the independent and mutual relationships between MTHFR C677T polymorphism and gastrointestinal tumor markers (CEA, CA199 and CA724) with or without H.pylori infection. We aim to explore the significance of MTHFR C677T polymorphism, CEA, CA199, CA724 and H.pylori infection in early screening for gastrointestinal cancer.
Materials and methods
Study population
Data and blood sample were collected from January 2016 to October 2020 in the Health Management Center of the First Affiliated Hospital of Chongqing Medical University (CQMU). Inclusion criteria: subjects aged >18 without cancer history, and underwent gastrointestinal tumor markers (CEA, CA199 and CA724) test and (13C-UBT) during health checkup. Exclusion criteria: patients diagnosed with cancer before, or patients received or were under treatment for H.pylori eradication, or subjects with ethnic minorities or incomplete information. Finally, a total of 152 subjects were included in the study, of which 58 (38.20%) patients were 13C-UBT positive and 94 (61.8%) were 13C-UBT negative. All participants had signed the informed consent before health checkup, and this study has been approved by the ethics committee of the clinical college of CQMU (NO.2020-089).
Genotyping of MTHFR C667T polymorphism
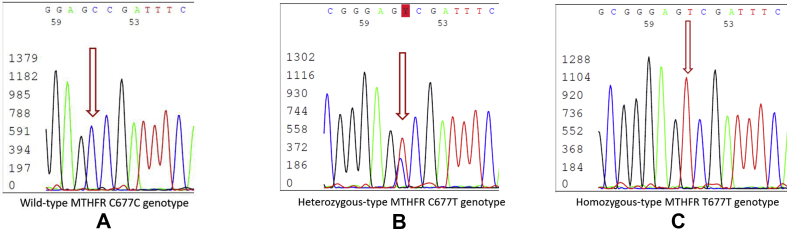
An amount of 3–5 ml peripheral venous blood was collected and placed in the EDTA anticoagulant tube for genotyping. Genomic DNA (batch number: q5502) was extracted from 200 μL anticoagulant peripheral blood with the adoption of whole blood genomic DNA rapid extraction kit (Tiangen Biology Inc.). DNA sequencing for determining the results of MTHFR C667T polymorphism was read after PCR amplification and purification (Fig. 1).22
Figure 1.
MTHFR C677T polymorphism sequencing map. (A) MTHFR C677T wild-type: CC genotype. (B) MTHFR C677T heterozygous: CT genotype. (C) MTHFR C677T homozygous: TT genotype. The ordinate represents was the signal intensity of gene, and the abscissa represents the position of base.
13C-UBT
In the morning fasting state, all subjects used 13C-UBT bottom gas collection bag (Shenzhen CNUO Haidway Biotechnology Co., LTD., China) to collect the exhaled gas before taking medicine, and then took 75 mg of 13C capsule orally. After 30 min, the matching sample gas collection bags were used to collect the breath after taking the medicine. The breath test tester (HCBT-01, Shenzhen CNUO Haidway Biotechnology Co., LTD., China) was used to calculate the 13C-UBT exceeding the baseline δ value. Diagnostic criteria: Delta over baseline (DOB) ≥ 4 was positive, and DOB < 4 was negative. Diagnostic accuracy:23 sensitivity was 98%, and specificity was 98%.24
Detection methods of human body index and biochemical index
The weight and height of the subjects were measured by a computer human scale (SK-X80/TCS-160D-W/H, Shenzhen Shuangjia Electronics Co., LTD., China). Blood pressure was measured by pulse wave medical sphygmomanometer (RBP-9001, Shenzhen Ruiguang Kangtai Technology Co., LTD.) during quiet sitting.25
All subjects fasted for more than 8 h before blood samples collection, and peripheral venous blood was collected for analysis under the fasting state from 7:30 AM to 10:00 AM.25 CEA, CA199 and CA724 were tested by chemiluminescence immunoassay on an automatic chemiluminescence immunoassay analyzer (Cobas 8000 e602, Roche Diagnostics Inc). White blood cell (WBC), Red blood cell count (RBC) and hemoglobin (HB) were analyzed on a five classification hematology analyzer (XE-2100, Sysmex Inc.). All blood biochemical tests have passed ISO15189 certification (No. ML00036).
Statistical analysis
IBM SPSS statistics 23 (IBM, Chicago, USA) was used to conduct the Chi-square goodness-of-fit test for calculating the Hardy–Weinberg equilibrium, and Chi-square test or Fisher's exact test was used to compare categorical variables between groups. Univariate logistic regression was used to analyze the distribution of MTHFR C677T polymorphism and 13C-UBT results. Multivariate logistic regression with forward LR variable selection was used to calculate the odd ratio (OR) and 95% confidence interval (CI) to evaluate the association between 13C-UBT positive and risk factors. Multivariate analysis of variance was used to analyze the interaction between MTHFRC677T polymorphism and 13C-UBT results on gastrointestinal tumor markers. Bootstrap method was used to calculate 95% CI, and SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used to calculate the statistical interaction between categorical variables and continuous variables by logistic regression.26 The synergy index S (S) = 1 and the relative excess risk of interaction (RERI) = 0 were defined as no interaction, S > 1 and RERI > 0 were defined as positive additive interactions, and S < 1 and RERI < 0 were defined as negative additive interactions. The attributable proportion of interaction (AP) was defined as the proportion of all cases attributable to the interactions between two factors. P < 0.05 was considered statistically significant.
Results
Anthropometric measurements, blood biochemical indexes and genotype of MTHFR C677T polymorphism in 13C-UBT positive and negative groups
The positive rate of 13C-UBT in males was 43.4%, which was higher than that in females (26.1%, P = 0.044). The positive rate of 13C-UBT in the group aged >60 (71.4%) was significantly higher than that in the other age groups (P = 0.027). In 13C-UBT positive group, CEA and CA724 levels were significantly higher than those in 13C-UBT negative group (both P < 0.05), but there was no significant difference of CA199 levels between the two groups. The diastolic blood pressure (DBP) and systolic blood pressure (SBD) in 13C-UBT positive group were higher than those in13C-UBT negative group (P = 0.017 and P = 0.023, respectively). The mean WBC of these two groups was within the normal range, and that of the 13C-UBT positive group (6.00 ± 1.34 10ˆ9/L) was higher than the 13C-UBT negative group (5.54 ± 1.42 10ˆ9/L, P = 0.045), Table 1.
Table 1.
The characteristics of anthropometric measurements, biochemical indexes and MTHFR C677T genotype in 13C-UBT positive and negative groups.
| Variable | 13C-UBT positive group (n = 58,%) | 13C-UBT negative group (n = 94,%) | P value |
|---|---|---|---|
| Gender | 0.044 | ||
| Male | 46 (43.4) | 60 (56.6) | |
| Female | 12 (26.1) | 34 (73.9) | |
| Age, mean,(SD) | 51.34 (11.50) | 48.95 (9.66) | 0.169 |
| Age subgroup | 0.027 | ||
| <40 | 8 (36.4) | 14 (63.6) | |
| 40–60 | 40 (34.5) | 76 (65.5) | |
| >60 | 10 (71.4) | 4 (28.6) | |
| DBP(mmHg), mean,(SD) | 79.89 (12.29) | 75.14 (11.36) | 0.017 |
| SBP(mmHg), mean,(SD) | 127.49 (20.23) | 120.37 (17.35) | 0.023 |
| CEA(ng/ml), mean,(SD) | 2.08 (1.04) | 1.69 (0.74) | 0.008 |
| CA199(U/ml),median,(P25,P75) | 9.20 (5.80,15.25) | 9.65 (6.48,13.50) | 0.764 |
| CA724(U/ml),median,(P25,P75) | 3.70 (1.60,5.93) | 2.10 (1.08,4.23) | 0.004 |
| WBC(10^9/L), mean,(SD) | 6.00 (1.34) | 5.54 (1.42) | 0.045 |
| RBC(10^12/L), mean,(SD) | 5.05 (0.55) | 4.94 (0.58) | 0.244 |
| Hb(g/L), mean,(SD) | 153.12 (12.88) | 150.11 (15.48) | 0.216 |
| MTHFR C677T genotype | 0.091 | ||
| CC | 22 (39.3) | 34 (60.7) | |
| CT | 22 (31.0) | 49 (69.0) | |
| TT | 14 (56.0) | 11 (44.0) | |
Abbreviations: SD standard deviation, P25 and P75 the 25% percentile and the 75% percentile of the percentiles, DBP diastolic blood pressure, SBP systolic blood pressure, CEA carcinoma embryonic antigen, CA199 carbohydrate antigen199, CA724 carbohydrate antigen724, WBC white blood cell, RBC red blood cell, Hb haemoglobin, 13C-UBT 13C urea breath test.
P value < 0.05 was considered statistically significant.
The genotypes of MTHFR of all studied subjects were CC (36.84%), CT (46.71%) and TT (16.45%). The genotypes distribution of 13C-UBT positive group (χ2 = 2.980, P = 0.225) and 13C-UBT negative group (χ2 = 1.116, P = 0.572) were accorded with Hardy–Weinberg equilibrium test. By comparing three genotypes frequencies between 13C-UBT positive group and negative group, we found that the TT genotype was more frequent than CC and CT genotype in 13C-UBT positive group (P = 0.028), Table 2.
Table 2.
Genotype frequencies of MTHFR C677T polymorphism between 13C-UBT positive and negative groups.
| MTHFR C677T Polymorphism | 13C-UBT positive group (n = 58,%) | 13C-UBT negative group (n = 94,%) | OR (95%CI) | P value |
|---|---|---|---|---|
| CC vs. CT+TT | 0.827 | |||
| CC | 22 (39.3) | 34 (60.7) | 1.078 (0.548–2.122) | |
| CT+TT | 36 (37.5) | 60 (62.5) | Reference | |
| CT vs. CC+TT | 0.090 | |||
| CT | 22 (31.0) | 49 (69.0) | 0.561 (0.288–1.094) | |
| CC+TT | 36 (44.4) | 45 (55.6) | Reference | |
| TT vs. CC+CT | 0.028 | |||
| TT | 15 (57.7) | 11 (42.3) | 2.632 (1.113–6.225) | |
| CC+CT | 43 (34.1) | 83 (65.9) | Reference | |
Abbreviations: OR odds ratio, 95%CI 95% confidence interval, 13C-UBT 13C urea breath test.
P value < 0.05 was considered statistically significant.
Risk factors of 13C-UBT positive filtrated by univariate and multiple logistic regression analysis
Univariate analysis showed that gender, age, DBP, SBP, CEA, CA724, WBC and TT, CC and CT genotype were risk factors for 13C-UBT positive (P < 0.05). After multivariate analysis, only CEA, CA724 and TT, CC and CT genotype were the risk factors for 13C-UBT positive (P < 0.05), Table 3.
Table 3.
Univariate and multivariate analysis of risk factors in 13C-UBT positive and negative groups.
| variable | Univariate Logistic Regression |
Multivariate Logistic Regression |
||
|---|---|---|---|---|
| OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Gander Male vs. Female | 2.172 (1.014–4.654) | 0.046 | ||
| Age <40 vs. 60 | 0.229 (0.054–0.973) | 0.046 | ||
| 40–60 vs. >60 | 0.211 (0.062–0.714) | 0.012 | ||
| DBP | 1.035 (1.005–1.066) | 0.020 | 1.032 (1.000–1.064) | 0.052 |
| SBP | 1.021 (1.002–1.040) | 0.027 | ||
| CEA | 1.674 (1.126–2.487) | 0.011 | 1.746 (1.128–2.700) | 0.012 |
| CA724 | 1.077 (1.012–1.145) | 0.019 | 1.096 (1.024–1.173) | 0.009 |
| WBC | 1.272 (1.001–1.617) | 0.049 | ||
| MTHFR C677T vs. MTHFR C677 C/T | 2.632 (1.113–6.225) | 0.028 | 2.678 (1.044–6.874) | 0.040 |
Abbreviations: OR odds ratio, 95%CI 95% confidence interval, DBP diastolic blood pressure, SBP systolic blood pressure, CEA carcinoma embryonic antigen, CA724 carbohydrate antigen724, WBC white blood cell.
P value < 0.05 was considered statistically significant.
MTHFR C677T polymorphism and CEA levels had negative additive interaction effect on 13C-UBT positive
By analyzing the additive interactions effect between MTHFR C677T polymorphism and DBP, CEA, CA724 on 13C-UBT results, only MTHFR C677T polymorphism had negative additive interaction with CEA levels (P = 0.026). The RERI was −1.407 (95% CI = −3.737–0.277) and S was 0.468 (95% CI = 0.072–0.910). The interaction between MTHFR C677T polymorphism and CEA was 63.1% (95% CI = −1.941–0.079) without considering other factors, Table 4.
Table 4.
The additive interactions between MTHFR C677T polymorphism and risk factors with13C-UBT results.
| Exposure | OR (95% CI) | RERI (95% CI) | AP (95% CI) | S (95% CI) | P value |
|---|---|---|---|---|---|
| MTHFR C677T polymorphism | 9.628 (0.261–355.538) | 0.155 (−0.016–7.964) | 0.015 (−0.04–0.040) | 1.019 (0.994–1.045) | 0.333 |
| DBP | 1.090 (0.993–1.197) | ||||
| MTHFR C677T polymorphism & DBP | 0.973 (0.929–1.019) | ||||
| MTHFR C677T polymorphism | 11.020 (2.852–42.584) | −1.407 (−3.737~-0.277) | −0.631 (−1.941~-0.079) | 0.468 (0.072–0.910) | 0.026 |
| CEA | 14.711 (3.712–58.304) | ||||
| MTHFR C677T polymorphism & CEA | 0.326 (0.168–0.634) | ||||
| MTHFR C677T polymorphism | 1.044 (0.548–1.988) | 0.041 (−0.024–0.153) | 0.323 (−0.022–0.152) | 1.032 (−1.089–3.199) | 0.136 |
| CA724 | 1.005 (0.837–1.206) | ||||
| MTHFR C677T polymorphism & CEA724 | 1.04 (0.942–1.146) |
Abbreviations: OR odds ratio, 95%CI 95% confidence interval, S synergy index, RERI relative excess risk of interaction, AP attributable proportion, DBP diastolic blood pressure, CEA carcinoma embryonic antigen, CA724 carbohydrate antigen724.
S < 1 and RERI <0 were defined as negative additive interactions. The absolute value of AP was the proportion of all 13C-UBT positive patients that can be attributed to the interaction of the two factors. P value < 0.05 was considered statistically significant.
Statistical interaction between MTHFR C677T polymorphism and 13C-UBT results in CEA, CA199 and CA724 levels
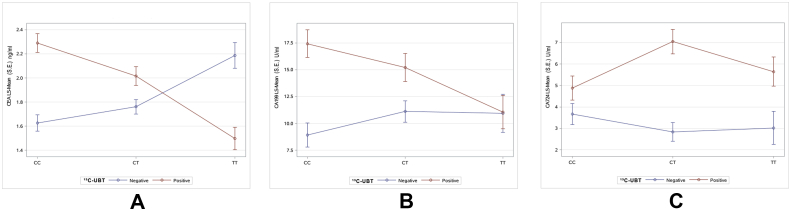
After correcting the influence of other factors, the results of factorial analysis showed that the CEA levels of male (2.13 ± 0.11 ng/ml) was significantly higher than those of female (1.66 ± 0.14 ng/ml, P = 0.002). Meanwhile, the CEA levels of the group aged >60 (2.41 ± 0.22 ng/ml) were significantly higher than those of other groups (aged <40: 1.51 ± 0.18 ng/ml, aged 40–60: 1.78 ± 0.09 ng/ml, P = 0.009), Table 5. There was a significant interaction between MTHFR C677T polymorphism and 13C-UBT results in CEA levels. The presence of T allele led to a decrease in CEA levels when 13C-UBT was positive, while the presence of T allele led to an increase in CEA levels when 13C-UBT was negative (P = 0.004), Table 5, Fig. 2A. The CA199 levels of female (15.63 ± 2.30 U/ml) were significantly higher than those of male (9.25 ± 1.84 U/ml, P = 0.010), and the CA724 levels of UBT positive (5.85 ± 0.93 U/ml) were significantly higher than those of negative (3.18 ± 0.92 U/ml, P = 0.020). However, there was no significant interaction between MTHFR C677T polymorphism and UBT results in CA199 and CA724 levels, Table 5 and Fig. 2B and C.
Table 5.
The statistical interactions between MTHFR C677T polymorphism and13C-UBT positive/negative on CEA, CCA199 and CA724 levels.
| Variation sources | DOF | Anova sum of squares | Mean square | F value | Pr > F |
|---|---|---|---|---|---|
| CEA Level | |||||
| Gender | 1 | 6.40495752 | 6.40495752 | 9.86 | 0.002 |
| Age subgroup | 2 | 6.34313373 | 3.17156687 | 4.88 | 0.009 |
| 13C-UBT positive/negative | 1 | 0.15807094 | 0.15807094 | 0.24 | 0.623 |
| MTHFR C677T | 2 | 0.23877914 | 0.11938957 | 0.18 | 0.832 |
| MTHFR C677T∗13C-UBT positive/negative | 2 | 7.59083895 | 3.79541948 | 5.85 | 0.004 |
| CA199 Level | |||||
| Gender | 1 | 1206.172895 | 1206.172895 | 6.71 | 0.010 |
| Age subgroup | 2 | 290.846119 | 145.423060 | 0.81 | 0.447 |
| 13C-UBT positive/negative | 1 | 476.913076 | 476.913076 | 2.65 | 0.106 |
| MTHFR C677T | 2 | 87.775419 | 43.887709 | 0.24 | 0.784 |
| MTHFR C677T∗13C-UBT positive/negative | 2 | 319.821314 | 159.910657 | 0.89 | 0.413 |
| CA724 Level | |||||
| Gender | 1 | 5.1593504 | 5.1593504 | 0.15 | 0.699 |
| Age subgroup | 2 | 26.1993490 | 13.0996745 | 0.38 | 0.684 |
| 13C-UBT positive/negative | 1 | 190.8321394 | 190.8321394 | 5.55 | 0.020 |
| MTHFR C677T | 2 | 14.2538377 | 7.1269189 | 0.21 | 0.813 |
| MTHFR C677T∗13C-UBT positive/negative | 2 | 62.2266539 | 31.1133270 | 0.90 | 0.407 |
Abbreviations: DOF degree of freedom, CEA carcinoma embryonic antigen, CA199 carbohydrate antigen 199, CA724 carbohydrate antigen 724. 13C-UBT 13C urea breath test.
Prob > F means P value, < 0.05 means significant, rejecting the original hypothesis.
Figure 2.
Least Squares means line plot of interaction between MTHFR C677T polymorphism and 13C-UBT results on CEA, CA199, CA724 levels. Main effects of MTHFR C677T polymorphism subgroup was CC genotype, CT genotype and TT genotype. Main effects of 13C-UBT results subgroup was 13C-UBT positive (red line) and 13C-UBT negative (blue line). When the red line and the blue line crossed, there was statistical interaction between MTHFR C677T polymorphism and 13C-UBT results on CEA, CA199, CA724 levels. (A) MTHFR C677T polymorphism and 13C-UBT results had statistical interaction on CEA levels (P = 0.004). (B) MTHFR C677T polymorphism and 13C-UBT results had not statistical interaction on CA199 levels (P = 0.413). (C) MTHFR C677T polymorphism and 13C-UBT results had not statistical interaction on CA724 levels (P = 0.407), but the mean levels of CA724 in 13C-UBT positive (red line) was significantly higher than that in 13C-UBT negative (blue line) (P = 0.020).
Discussion
In our study, the infection rate of H.pylori was 38.20%, which was lower than 47% reported by Nagy P, Johansson S, et al.27 This may due to the different inclusion criteria of the two studies. MTHFR C677T polymorphism detection and gastrointestinal tumor markers were not routine screening items.28 People who underwent the screening were likely to be in better socio-economic condition and had reduced infection rates.29 Meanwhile, the infection rate of H.pylori in men was significantly higher than that in women. With the increase of age, the infection rate also increased, which was similar to the previous results.12,30
In our study, CC and CT genotypes were predominant in the Han population in Chongqing, which was similar to the results of several studies in Asia.19,31 TT genotype was more likely to be associated with H.pylori infection than CC and CT genotypes (Table 2). This might because of the T SNP mutation, leading to the decrease of MTHFR enzyme activity and affecting the methylation of human gastric mucosa cells, which results in the integrity of gastric mucosa and fosters the colonization of H.pylori.32 And H.pylori infection will further affect folic acid absorption and metabolism,33,34 leading to the damage of gastric mucosa and inducing local chronic inflammation.13,35 This was consistent with the results of our previous study that WBC in H.pylori infected subjects was higher than that in uninfected subjects23 (Table 1).
Recent studies have shown that MTHFR C677T polymorphism may lead to abnormal DNA and/or RNA synthesis, and can repair abnormalities and chromosome damage in gastric mucosa infected by H.pylori,36 resulting in dysfunction of MTHFR enzyme and then affecting methylation29,32 and making conditions for other carcinogenic factors to cause abnormal methylation of protooncogenes,17 thus leading to the occurrence and development of gastrointestinal cancer.37 In our study, H.pylori infection was associated with increased CEA and CA724 levels but not associated with CA199 levels (Table 3), which was similar to the study of Xu MY, Cao B, et al.30 Meanwhile, TT genotype was more closely related to H.pylori infection than CC and CT genotypes (Table 3). Therefore, our study suggested that TT genotype was a risk factor for H.pylori infection in Chinese Han population in Chongqing.
In our study, MTHFR C677T polymorphism had a negative additive interaction with CEA in H.pylori infection (Table 4). Although the CEA levels of all subjects were within the normal range, it still indicated that different genotypes infected with H.pylori would cause different degrees of elevated CEA levels. Further analysis showed that the presence of T allele led to a decrease in CEA levels for H.pylori infection, while the presence of T allele led to an increase in CEA levels for H.pylori non-infection, suggesting that the TT genotype was a risk factor for H.pylori infection. However, when H.pylori was negative, the TT genotype was a risk factor for elevated CEA levels. This is similar to a study that TT genotype increases moderate to severe atrophic gastritis and gastric mucosal intestinal metaplasia in Chinese Han population without H.pylori infection.38 Although many studies have shown that MTHFR C677T polymorphism was associated with gastrointestinal cancer, the specific relationship was not clear.18,38,39 We speculated that the specificity of MTFHR C677T polymorphism for gastrointestinal cancer during H.pylori infection might be related to the site of cancer origin.19,40 The previous studies have reported that there was association between MTHFR C677T polymorphism and gastric cancer in Chongqing, and CT and TT genotypes were protective factors for gastric cardia cancer, and TT genotype was a risk factor for gastric body cancer.41 In addition, our research also found that H.pylori infection was still a risk factor for increased CA724 levels. CA724 was suggested for management of gastrointestinal cancer as early as two decades.21 Therefore, we hypothesized that MTHFR C677T polymorphism might have different effects on cancers in different parts of the gastrointestinal tract in the case of H.pylori infection.41,42 But further animal and clinical trials are required to prove this.
Our study confirmed that H.pylori infection was related to gender and age.38 Our study also found that the blood pressure (BP) in patients with H.pylori infection were higher than those without H.pylori infection. It was suggested that H.pylori infection might be a risk factor for cardiovascular and cerebrovascular diseases,43,44 although the BP was in the normal range. Previous studies have also shown that H.pylori infection was positively correlated with the prevalence of hypertension in Chinese adults.45 Therefore, further experiments are required to explore whether H.pylori infection and MTHFR C677T polymorphism have an interaction effect on cardiovascular and cerebrovascular diseases.
All subjects included in this study were from a healthy population, so it was not possible to assess the effects of MTHFR C677T polymorphism and H.pylori on different gastrointestinal benign and malignant diseases. Yet, it indicates that the screening of MTHFR C677T polymorphism, 13C-UBT and gastrointestinal tumor markers (CEA, CA199 and CA724) in healthy population can detect the susceptible population of H.pylori and gastrointestinal cancers early. Next, we plan to investigate the specificity and sensitivity of MTHFRC677T polymorphism, 13C-UBT combined with gastrointestinal tumor markers test in H. pylori susceptible population and early screening of gastrointestinal cancer. We also plan to further study the influence of MTHFR C677T polymorphism and H.pylori infection on benign and malignant gastrointestinal lesions.
Conclusions
Based on the results of this study, we found that TT genotype was a susceptibility factor for H.pylori infection. But in the presentation of H.pylori infection, MTHFR C677T polymorphism might have different effects on different parts of gastrointestinal cancer. The screening of MTHFR C677T polymorphism, combined with the screening of 13C-UBT and gastrointestinal tumor markers can detect the susceptible population of H. pylori and gastrointestinal cancers.
Conflict of Interests
Authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Xiaoxing Wu, Email: xiaoxwu@hospital.cqmu.edu.cn.
Bin Peng, Email: Pengbin@cqmu.edu.cn.
Kun Qian, Email: hxjsqk@hotmail.com.
Wei Zhang, Email: cyzhangwei@hotmail.com.
Jiang Min, Email: hustminjiang@126.com.
Mingjun Zhang, Email: 708993302@qq.com.
Fanling Zeng, Email: zengfanling@hospital.cqmu.edu.cn.
Ziwei Wang, Email: wangziwei@hospital.cqmu.edu.cn.
References
- 1.Maruthappu M., Head M.G., Zhou C.D. Investments in cancer research awarded to UK institutions and the global burden of cancer 2000-2013: a systematic analysis. BMJ Open. 2017;7(4):e013936. doi: 10.1136/bmjopen-2016-013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Wolf A.M.D., Fontham E.T.H., Church T.R. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 5.Smith R.A., Andrews K.S., Brooks D. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297–316. doi: 10.3322/caac.21446. [DOI] [PubMed] [Google Scholar]
- 6.Bettegowda C., Sausen M., Leary R.J. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao Y.C., Fang W.L., Wang R.F. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer. 2019;22(2):255–263. doi: 10.1007/s10120-018-0860-8. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31(4):925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 9.Yusefi A.R., Bagheri Lankarani K., Bastani P., Radinmanesh M., Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev. 2018;19(3):591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohebbi M., Wolfe R., Jolley D., Forbes A.B., Mahmoodi M., Burton R.C. The spatial distribution of esophageal and gastric cancer in Caspian region of Iran: an ecological analysis of diet and socio-economic influences. Int J Health Geogr. 2011;10 doi: 10.1186/1476-072X-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YY, Derakhshan MH. Environmental and lifestyle risk factors of gastric cancer. Arch Iran Med. 2013;16(6):358–365. [PubMed] [Google Scholar]
- 12.Zamani M., Ebrahimtabar F., Zamani V. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi: 10.1111/apt.14561. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X.Y., Zhang P.Y., Aboul-Soud M.A. From inflammation to gastric cancer: role of Helicobacter pylori. Oncol Lett. 2017;13(2):543–548. doi: 10.3892/ol.2016.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan L., Li Y., Zhang Z., Sun Z., He Y., Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8(1):242–253. doi: 10.1038/s41398-018-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frosst P, Blom HJ, Milos R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetrahydrofolate reductase. Nat Genet. 1995;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 16.Campbell I.G., Baxter S.W., Eccles D.M., Choong D.Y. Methylenetetrahydrofolate reductase polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2002;4(6):R14. doi: 10.1186/bcr457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boccia S., Hung R., Ricciardi G. Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol. 2008;167(5):505–516. doi: 10.1093/aje/kwm344. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P., Rai V. MTHFR C677T polymorphism and risk of esophageal cancer: an updated meta-analysis. Egypt J Med Human Genetic. 2018;19:273–284. [Google Scholar]
- 19.Chen L., Lu N., Zhang B.H., Weng L.I., Lu J. Association between the MTHFR C677T polymorphism and gastric cancer susceptibility: a meta-analysis of 5,757 cases and 8,501 controls. Oncol Lett. 2015;10(2):1159–1165. doi: 10.3892/ol.2015.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y., Wang J., Zhou Y., Sheng S., Qian S.Y., Huo X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. 2018;8(1):e2732. doi: 10.1038/s41598-018-21048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XZ, Zhang WH, Chen HN. Associations between serum CA724 and HER2 overexpression among stage II–III resectable gastric cancer patients: an observational study. Oncotarget. 2016;7(17):23647–23657. doi: 10.18632/oncotarget.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi T., Liang Y.Q., Xia H.Y. Prevalence of the methylenetetrahydrofolate reductase 677C>T polymorphism in the pregnant women of Yunnan Province, China. Medicine (Baltimore) 2020;99(45):e22771. doi: 10.1097/MD.0000000000022771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Xiaoxing, Zhang Wei, Qian Kun, Jiang Min, Peng Bin. Relationship between the total white blood cells and the classification and helicobacter pylori infection. J Chongqing Med Univ. 2018;43:469–472. [Google Scholar]
- 24.Kato M., Ota H., Okuda M. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. 2019;24(4):e12597. doi: 10.1111/hel.12597. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Zhou Y., Zhang M., Wang Y., Qin B. The methylenetetrahydrofolate reductase genotype 677CT and non-alcoholic fatty liver disease have a synergistic effect on the increasing homocysteine levels in subjects from Chongqing, China. Genes Dis. 2019;6(1):88–95. doi: 10.1016/j.gendis.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderWeele T.J., Knol M.J. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 27.Nagy Peter, Johansson Saga, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:e8. doi: 10.1186/s13099-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese Society of Health Management TEBoJoMC. Zhang Qing. Expert consensus on the management of important results with abnormal values in health checkup (pilot edition) 2019;13:97–101. [Google Scholar]
- 29.Kouitcheu Mabeku L.B., Noundjeu Ngamga M.L., Leundji H. Potential risk factors and prevalence of Helicobacter pylori infection among adult patients with dyspepsia symptoms in Cameroon. BMC Infect Dis. 2018;18(1):e278. doi: 10.1186/s12879-018-3146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu M.Y., Cao B., Chen Y. Association between Helicobacter pylori infection and tumor markers: an observational retrospective study. BMJ Open. 2018;8(8):e022374. doi: 10.1136/bmjopen-2018-022374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie S.Z., Liu Z.Z., Yu J.H. Association between the MTHFR C677T polymorphism and risk of cancer: evidence from 446 case-control studies. Tumour Biol. 2015;36(11):8953–8972. doi: 10.1007/s13277-015-3648-z. [DOI] [PubMed] [Google Scholar]
- 32.Neves Filho E.H., Alves M.K., Lima V.P., Rabenhorst S.H. MTHFR C677T polymorphism and differential methylation status in gastric cancer: an association with Helicobacter pylori infection. Virchows Arch. 2010;457(6):627–633. doi: 10.1007/s00428-010-0996-3. [DOI] [PubMed] [Google Scholar]
- 33.Boyuk B., Ozgur A., Atalay H., Celebi A., Ekizoglu I., Aykurt E. Helicobacter pylori infection coexisting with intestinal metaplasia is not associated with colorectal neoplasms. Prz Gastroenterol. 2019;14(2):133–139. doi: 10.5114/pg.2019.85897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salgueiro J., Zubillaga M., Goldman C. Review article: is there a link between micronutrient malnutrition and Helicobacter pylori infection? Aliment Pharmacol Ther. 2004;20(10):1029–1034. doi: 10.1111/j.1365-2036.2004.02265.x. [DOI] [PubMed] [Google Scholar]
- 35.Tahara T., Arisawa T., Shibata T. Increased number of methylated CpG islands correlates with Helicobacter pylori infection, histological and serological severity of chronic gastritis. Eur J Gastroenterol Hepatol. 2009;21(6):613–619. doi: 10.1097/MEG.0b013e32830e28b2. [DOI] [PubMed] [Google Scholar]
- 36.Coppede F., Stoccoro A., Tannorella P., Gallo R., Nicoli V., Migliore L. Association of polymorphisms in genes involved in one-carbon metabolism with MTHFR methylation levels. Int J Mol Sci. 2019;20(15):e3754. doi: 10.3390/ijms20153754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saberi S., Zendehdel K., Jahangiri S. Impact of methylenetetrahydrofolate reductase C677T polymorphism on the risk of gastric cancer and its interaction with Helicobacter pylori infection. Iran Biomed J. 2012;16(4):179–184. doi: 10.6091/ibj.1102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong S., Ye F., Dang Y., Hua Y., Zhang G. Association of MTHFR C677T polymorphism with severity and localization of chronic atrophic gastritis patients without Helicobacter pylori infection: a case control study. BMC Cancer. 2020;20(1):e725. doi: 10.1186/s12885-020-07208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan L., Liu Z., Wei G. Genetic polymorphisms in folate-metabolizing genes associated with gastric cancer prognosis in northwest China subjects. J Cancer. 2020;11(21):6413–6420. doi: 10.7150/jca.46978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B., Fan S., Zhi X. Geographical and ethnic distribution of MTHFR gene polymorphisms and their associations with diseases among Chinese population. Clin Genet. 2017;92(3):243–258. doi: 10.1111/cge.12929. [DOI] [PubMed] [Google Scholar]
- 41.Si P.R., Fang D.C., Zhang H., Yang L.Q., Luo Y.H., Liao H.Y. [The relationship between methylenetetrahydrofolate reductase gene polymorphism and microsatellite instability in gastric cancer] Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(10):794–799. [PubMed] [Google Scholar]
- 42.Chen J., Yuan L., Duan Y.Q. Impact of methylenetetrahydrofolate reductase polymorphisms and folate intake on the risk of gastric cancer and their association with Helicobacter pylori infection and tumor site. Genet Mol Res. 2014;13(4):9718–9726. doi: 10.4238/2014.January.24.2. [DOI] [PubMed] [Google Scholar]
- 43.Zhao M., Wang X., He M. Homocysteine and stroke risk: modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke. 2017;48(5):1183–1190. doi: 10.1161/STROKEAHA.116.015324. [DOI] [PubMed] [Google Scholar]
- 44.Luo Z., Lu Z., Muhammad I. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17(1):e191. doi: 10.1186/s12944-018-0837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan Z., Hu L., Hu M., Lei X., Huang Y., Lv Y. Helicobacter pylori infection and prevalence of high blood pressure among Chinese adults. J Hum Hypertens. 2018;32(2):158–164. doi: 10.1038/s41371-017-0028-8. [DOI] [PubMed] [Google Scholar]