Abstract
The characterization of the population exposed to pesticides and the use of effective biomarkers to evaluate potential health effects are determinant to identify vulnerable groups, understanding the causality of diverse pathologies and propose prevention policies. This is particularly important in countries where intensive agricultural practices had an explosive expansion in last decades.
The aim of this study was assessing the usefulness of two exposure indexes questionnaire-based: Intensity Level of the pesticide Exposure (ILE) and Cumulative Exposure Index (CEI) and their scales, in terrestrial applicators of pesticide from the Province of Córdoba (Argentina). The analysis was performed contrasting ILE and CEI results with perceived symptomatology, in addition to effect and exposure biomarkers. A cross-sectional study was designed to compare pesticides body burdens and effect biomarkers between subjects occupationally (OE) and non-occupationally exposed (NOE) to pesticides. Prevalence of perceived symptomatology and genotoxicity damage was higher in the OE group. The exposure condition was the only variable explaining these differences. Significant associations were found between CEI and neurologic symptomatology (p < 0.05) and between ILE and plasmatic cholinesterase (p < 0.1). However, residues of HCB, β-HCH, α-endosulfan, pp'DDE, endrin, β-endosulfan, pp'DDT, endosulfan sulfate and mirex were found in blood samples from both groups. To our knowledge, this is the first report on pesticides body burdens in occupational exposure settings in Argentina. So far, our current results indicate that the occupational condition affects the health of the workers. Significant associations found between symptomatology and biomarkers with scales of CEI and ILE suggest their usefulness to verify different levels of exposure. Further research is necessary to propose these indexes as an affordable tool for occupational health surveillance in areas with difficult access to health care centres.
Keywords: Exposure indexes, Occupational health, Cholinesterases biomarkers, Genotoxicity biomarkers, Biomonitoring
Exposure indexes; occupational health; cholinesterases biomarkers; genotoxicity biomarkers; biomonitoring
1. Introduction
Populations are exposed to pesticides in different ways. Diet and household use of pesticides are the main ways of exposure to the general urban population. Inhalation of polluted air is an important exposure route for populations close to intensive agricultural areas, using high amounts of pesticides and phytosanitary products. Inhalation and skin absorption are also relevant exposure pathways for occupationally exposed populations (Yusa et al., 2015). Indeed, people who perform hazardous agricultural activities (e.g. load, mix or application of pesticides) have the major chances of suffering chronic toxicity risks due to their continuous exposure to diverse pesticides (Hofmann et al., 2009).
In Epidemiology, the assessment of the risk derived from the exposure to pesticides has been a challenge due to its inherent complexity. The estimation of the exposure is important to differentiate the level of risk associated, being important discriminating between individuals with low, medium or high levels of exposure (Alavanja et al., 2004). In the Province of Córdoba (Argentina), Lantieri et al. (2009) elaborated a self-administered questionnaire, adapted to the local work conditions, referred to the use and application of pesticides. This work enabled the characterization of a population group, constituted by persons working as terrestrial applicators of pesticides. It also allowed identifying which factors influenced their exposure. From this questionnaire, Lantieri et al. (2011) developed two algorithms: Intensity Level of the pesticide Exposure (ILE); and Cumulative Exposure Index (CEI), as well. Both indexes enable evaluating present and past exposures to pesticides by the terrestrial applicators of pesticides. Furthermore, scales were established with the purpose of classification of these workers into low, medium, and high exposure levels.
However, the evaluation of the exposure, defined from observational studies (indexes and scales), needs to be validated with biological responses (Butinof et al., 2017); namely, biomarkers that correlate with different levels of the scale.
Human biomonitoring has become a relevant tool to assess the exposure to pesticides (Yusa et al., 2015). Nevertheless, the analysis of contaminants in biological matrices is not enough to assess the health risks. Suitable biomonitoring studies need selecting the most appropriate biomarkers, which allow the early detection of both adverse effects and sub-clinical damage. Thus, the study of biomarkers has been a subject of big interest in recent years, because of their usefulness to evaluate human exposure to pesticides, promoting intervention and prevention strategies to reduce harmful health effects (Lionetto et al., 2019).
Regarding different types of pesticides, it is recognized that organophosphate (OP) and carbamates cause toxicity through the inhibition of the acetylcholinesterase (Fukuto, 1990). Conversely, there is a lack of appropriate biomarkers for other pesticides. Moreover, the possible responses resulting from the interaction between different types of pesticides are unknown in many cases (Hernandez et al., 2013). So far, genotoxicological biomonitoring is useful to assess genetic risk upon exposure to multiple chemicals. Thus, genotoxicity studies have focused on micronuclei (MN), comet assay (CE), sister chromatids exchanges (SCE), and chromosome aberrations (CA) as suitable biomarkers (Bolognesi, 2003).
The fast growth of the global population has triggered the need of intensive food production in the world, including intensive crops and animal production. However, most intensive farming is carried out in developing countries, where the less political restrictions, permissive regulations and lack of controls lead to the extensive use of pesticides and significant environmental pollution (Arrebola et al., 2012; Khan et al., 2010; Ruiz-Suárez et al., 2014; Wang et al., 2013). This situation causes severe health issues to farmers and exposed population (Elahi et al., 2019), being South America among less developed regions in the world facing this problem. Argentina is one of the major crop producers in Latin America. Since 1990, the intensification of agriculture has been accompanied by an increasing volume of pesticides application, from 140 million kg in 1998 to 340 million kg in 2016 (Cámara de Sanidad Agropecuaria Fertilizantes (CASAFE), 2016). In the Province of Córdoba, the land used for farming have been increased in last twenty years, duplicating the area dedicated to soya, and triplicating the area dedicated to maize and wheat (MAGyP, 2018). As a consequence of this situation, increased exposure to different pesticides and their mixtures, low or deficient use of personal protective equipment and phytosanitary prescription, and use of non-appropriated machinery during land fumigation has been reported (Butinof et al., 2014; Lantieri et al., 2009). These studies also showed a high prevalence of health issues related to unsafe work conditions in this area.
Persistent organochlorine (OC) pesticides were widely used before the contemporaneous non-persistent pesticides, including OP, carbamates, pyrethroids (PYR) and triazines. At present, OC pesticides are mostly banned, but they are still of concern because of their persistence in the environment and in human tissues as well (Dardiotis et al., 2020; Freire et al., 2017; Tsyganko, 2019). Contemporaneous pesticides, instead, do not tend to bio-accumulate and are usually metabolized and excreted in urine rapidly after exposure (Egeghy et al., 2011). However, due to the intensive use of pesticides, the population is continuously exposed to them, which can be confirmed by their detection in blood, including some of their metabolites (Barr et al., 2002).
Within this context, this work aims to validate the exposure indexes (ILE and CEI) and their scales through the combined analysis of perceived symptomatology and biomarkers determined in biological samples of farm-workers, particularly, ground pesticide applicators. We hypothesized that ILE and CEI ranges will correlate with both perceived symptomatology and biomarkers, enabling a robust method to evaluate the occupational health of exposed population in the future.
2. Materials and method
2.1. Study area and population
Pesticide exposure was evaluated in three of the most important agricultural geographical areas of the Province of Cordoba: South, Centre and North East (Marcos Juarez, Unión and San Justo County). Farm workers and people from surrounding areas were invited to participate in the study.
Thus, including two populations: a first group with individuals occupationally exposed to pesticides (OE; ground pesticide applicators), and a control group with individuals non-occupationally exposed (NOE) to pesticides. For each OE who agreed to participate, at least 1 NOE was invited and selected from the general population of the surrounding area, being the same gender and within a range of ±5 years of age with respect to the corresponding OE individual.
2.2. Inclusion/exclusion criteria
All subjects, both OE and NOE, had to fulfil the inclusion/exclusion criteria. All individuals were over 18 years of age, having residence in the study area during last 5 years, at least. Individuals under pharmacological therapy, chemotherapy/radiotherapy treatment, recent surgeries, and viral diseases were excluded. As smoking, alcoholism and drug addiction are genotoxic agents, subjects reporting these habits were excluded from the study. As pesticides have been linked with various chronic disorders, individuals presenting diabetes, neurological disorders, liver dysfunction or any other chronic condition were also excluded from the studied population, to avoid any interference with the biochemical parameters measured. No occupationally exposed subjects that previously worked as applicators of pesticides or being in regular contact with pesticides were also excluded from the study. Occupationally exposed individuals should have attended the mandatory courses for obtaining the applicator license, completed the enrolment questionnaire, and worked mixing, loading, fumigation, and/or repairing sprayer machinery for at least last two years.
2.3. Ethical considerations
All enrolled participants were invited to sign a written informed consent after an exhaustive explanation of the objectives of the research. The research proposal and all its instruments, were approved by the Ethics Review Committee of Hospital Nacional de Clínicas, and is registered in the Ethics Committee of Health Investigations of the Province of Cordoba (RePIS N°1582 y 044/10).
2.4. Exposure assessment
2.4.1. Exposure history
At the enrolment, all the subjects were requested to answer a medical history survey, including socio-demographic data and symptomatology related to pesticides exposure: general, dermatological, neurological, ocular, cardiorespiratory, gastric, and urinary symptoms (Supplementary Material- SM-Table SM1).
Ground applicators were also requested to complete the self-administered questionnaire consisting of 7 modules already published in Lantieri et al. (2009). Information related to the use of pesticides, tasks performed (mixing, loading, application and repair of machinery), age and time (days and hours per year) performing the task, the technology used to apply, number of hectares applied per year, distance from home to the nearest crop, and work practices (personal protective equipment (PPE) used to perform each task) were used in the present study.
2.4.2. Exposure indexes
Exposure indexes were calculated in occupationally exposed individuals using the data obtained from the self-administrated questionnaire and the algorithms developed by Lantieri et al. (2011). Briefly, ILE (Eq. 1) measures instantaneous worker's intensity exposure to pesticides, while CEI (Eq. 2) considers the duration of the exposure, incorporating the ILE information.
| (1) |
| (2) |
where:
mix: is the dichotomous response of mixing pesticides;
meth: is the category of the methodology used to apply pesticides;
PPE: is the category of the personal protective equipment used to do the job;
repair: is the dichotomous response of the repairing sprayer equipment;
house_dist: is the distance between the applicator's house and the nearest crop cultivation area;
Ha/year: is the number of hectares worked per year (55 is the amount of hectares fumigated with a simple load).
Categories corresponding to the methodology used to apply pesticides and PPE were described by Lantieri et al. (2011). The pesticide exposure scales for the applicators were estimated using the Bootstrap and Monte Carlo resampling methods, including the cut-off points or percentiles that delimit the scales. The cut-off points served as benchmarks to classify subjects into one out of three exposure categories: low, medium, and high.
2.5. Sample collection
Samples of venous blood were collected after an overnight fasting period and cool preserved until reach the laboratory (4–8 °C). Plasma, from EDTA treated tubes, was separated by centrifugation (10 min–1,500 x g), and used to measure acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity upon arrival the laboratory. Afterward, samples were stored at -80 °C until pesticide analysis. Plasma from heparin treated tubes was used to analyse cholesterol and triglycerides levels according to the kit manufacturer instruction (GT Laboratory, Santa Fe, Argentina). Heparinised blood samples were also used to evaluate MN, CE, SCE and CA. Sampling dates were coincident with the seed time in the Province of Córdoba (Argentina).
2.6. Effect biomarkers
Plasmatic AChE and BChE activities were determined by spectrophotometric assay (Ellman et al., 1961) using commercial kits (Roche Diagnostics GmbH, Mannheim, Germany; and Wienner laboratories, Rosario, Argentina, respectively), according to the manufacturer's instructions. The determination of cholinesterase activity was widely used to assess pesticides exposure. However, erythrocyte AChE is questionable in terms of analytical reproducibility (Wilson et al., 2002). Therefore, we decided to assess the plasmatic activity of the enzyme as a more reliable measure. Micronuclei, CE, SCE and CA techniques were performed according to Bolognesi et al. (1993), Singh et al. (1988), Perry and Wolff (1974), and Moorhead et al. (1960), respectively. Each biomarker measurement was carried out in triplicate.
2.7. Exposure biomarkers
Pesticides standards were obtained from Sigma-Aldrich (Argentina) certified assay: 99%. Pesticide Mix Standard (PMS) was prepared by dissolving individual pesticides in isooctane (Merck, Germany) including: α, β and γ (lindane) hexachlorocyclohexane (HCH), hexachlorobenzene (HCB), heptachlor and heptachlor endo, 1,1-dichloro-2,2-di(4-chlorophenyl)ethylene (p,p'DDE), 1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane (p,p'DDT), α and β-endosulfan, endosulfan sulfate, endrin, methoxychlor, mirex, chlorpyrifos ethyl and methyl, λ cyhalothrin, permethrin, β-cyfluthrin, cypermethrin, and deltamethrin.
2.7.1. Extraction procedure
Plasma from donors with unknown exposure to pesticides, calibration standards (CS) and quality controls (QC) were spiked with PMS. The procedure was performed as described in Corrion et al. (2005), with minor changes. Briefly, frozen plasma samples (-80 °C) were thawed at room temperature and vortexed to ensure homogeneity. Then, protein-free plasma was obtained starting with 500 μL plasma, adding 1 mL of water: methanol (75:25 v/v), and vortexed for 15 s. Pesticides were extracted from this protein-free plasma by adding 3 mL hexane, vortexed for 30 s and agitated using an orbital shaker for 1 h. Samples were then centrifuged (1,500 x g - 30 min). Finally, the supernatant was transferred to a clean tube and dried under a gentle stream of nitrogen. The concentrate was reconstituted in 100 μL of isooctane and transferred to a vial containing a glass insert.
2.7.2. Instrumentation
Analyses were performed using a Gas Chromatograph in tandem with a Mass Spectrometer (GC-MS, Shimadzu QP2010 Ultra) equipped with an autosampler. Analytical separation was achieved on a SLB 5ms capillary column (60 m × 0.25 mm x 0.25 μm film thickness) obtained from Supelco (Bellefonte, PA, USA). Data analysis was processed using LabSolutions software (GCMS solution Version 4.44). Optimal chromatographic conditions were established to separate the analytes of interest. Working conditions were: column oven temperature 80 °C, injector temperature 250 °C; injection mode splitless, column temperature program starting at 80 °C (1 min), raising 40 °C/min until reach 125 °C, raising 10 °C/min until reach 150 °C, raising 1.5 °C/min until reach 175 °C, raising 10 °C/min until reach 230 °C, raising 2 °C/min until 240 °C and a final ramp 30 °C/min to 300 °C (21 min). The carrier gas used was helium (purity 99.999%). After chromatographic separation, compounds were analyzed in the tandem mass spectrometer operated in the negative chemical ionization (NCI) mode in the MS. Identification of each analyte was based on the retention time of the peaks and the abundances of ratio of target ions and fragments. The GC-MS analytical parameters are shown in the supplementary material (Table SM2).
2.7.3. Method validation
Validation of the method was carried out according to the Guideline on bioanalytical method validation of the Europe Medicines Agency (EMA, 2011). Selectivity, lower limit of quantification (LLOQ), linearity, accuracy, and precision were evaluated to demonstrate the reliability of the method for the determination of pesticides in plasma.
2.8. Statistical analysis
Means or median and standard deviations or quantiles were used to describe baseline characteristics of the population. The outcomes were obtained using the full data set (excluding subjects who violate inclusion/exclusion criteria). Student's t test was used to compare continuous variables between OE and NOE subjects. Chi2 test was used to observed possible association between exposure condition and categorical variables. Mann-Whitney U test was used to compare median values. Correlations between biomarkers and indexes were calculated as Pearson Coefficient. p-values less than 0.05 and 0.1 were considered significant and borderline significant, respectively. Generalized Linear Models (GLM) were used to identify the main factors influencing the perceived symptomatology, the response of both effect and exposure biomarkers in the studied population. First, we evaluated unadjusted regression using the exposure condition as the predictor variable (NOE subjects = 0; OE subjects = 1) for each dependent variable (perceived symptomatology; cholinesterase activity, genotoxicity biomarkers and the concentration of pesticides). We also evaluated unadjusted regression using each dependent variable against the concentration of the pesticides. Then, several adjusted regressions (with age, body mass index-BMI, cholesterol and triglycerides levels, AChE and BChE activity, educational level, marital status, the presence of asthma and allergies in the linear predictor) were fitted to elucidate the effect of these multiple variables on each selected dependent variable.
3. Results
A total of 123 individuals were accepted to participate in the study. After considering inclusion/exclusion criteria, 100 subjects, all males, were finally included. Among those, 47 subjects were terrestrial applicators of pesticides, and 53 subjects were selected as their respective controls.
Socio-demographic characteristics and personal health history are shown in Table 1. No differences in age, height and marital status were observed. Difference in weight was observed between groups with a higher number of OE subjects within the obesity category of the BMI classification. Educational level was significantly lower in OE than in NOE group. However, every subject in OE reported to have writing and reading skills and approximately half of this group has completed high school or university studies. According to the personal health history of chronic disorders, differences for dermatological allergies (Pearson chi2 4.6456 and p = 0.031) were found, showing that the frequency of these diseases is higher in the OE group. None of the enrolled subjects declared to have been diagnosed with cancer.
Table 1.
Socio-demographic characteristics and personal health history of subjects occupationally (n = 47) and non-occupationally (n = 53) exposed to pesticides from the Province of Córdoba.
| Non-occupationally exposed |
Occupationally exposed |
p-value | |
|---|---|---|---|
| Mean ± SD or % | Mean ± SD or % | ||
| Socio-demographic characteristics | |||
| Gender | Male | Male | |
| Agea | 38 ± 10 | 40 ± 10 | 0.4170 |
| Height (m)a | 1.76 ± 0.07 | 1.74 ± 0.09 | 0.2506 |
| Weight (kg)a | 79.17 ± 9.22 | 91.29 ± 16.37 | 0.0001 |
| BMIb,c | <0.0001 | ||
| Normal | 48 | 11 | |
| Overweight | 50 | 37 | |
| Obesity | 2 | 52 | |
| Educational levelc.d | <0.0001 | ||
| Elementary | 2 | 37 | |
| Middle | 10 | 15 | |
| High school | 23 | 35 | |
| University | 65 | 13 | |
| Marital statusc,e | 0.3810 | ||
| Married | 58 | 68 | |
| Divorced or separated | 0 | 2 | |
| Widower | 2 | 0 | |
| Single | 40 | 30 | |
| n | n | Pearson chi2 | p-value | |
|---|---|---|---|---|
| Personal health historyc | ||||
| Diabetes | 0 | 4 | 3.8114 | 0.051 |
| Hypertension | 1 | 3 | 0.8738 | 0.350 |
| Asthma | 1 | 3 | 0.8738 | 0.350 |
| Respiratory allergies | 6 | 6 | 0.0062 | 0.937 |
| Dermatological allergies | 1 | 7 | 4.6456 | 0.031 |
| Dermatitis | 2 | 2 | 0.0068 | 0.934 |
| Pneumonia | 4 | 7 | 0.6517 | 0.419 |
| Lung diseases | 2 | 3 | 0.1326 | 0.716 |
| Heart diseases | 2 | 2 | 0.0019 | 0.965 |
| Cancer | 0 | 0 | - | - |
| Kidney pathology | 2 | 2 | 0.0019 | 0.965 |
| Liver pathology | 13 | 15 | <0.0001 | 0.997 |
| Thyroid disease | 1 | 0 | 1.0936 | 0.296 |
p value of the comparisons of continue variables between non-occupationally and occupationally exposed groups was calculated using t-test.
BMI (bodymassindex, kg/m2).
Association between exposure and categorical variables was calculated using chi2 test.
Educational level was categorized as: Elementary (1), Middle (2), High school (3), University (4).
Marital status was categorized as: Married (1), Divorced or separated (2), Widower (3), Single (4).
Biochemical parameters and perceived symptomatology are shown in Table 2. No difference in cholesterol levels, but differences in triglyceride levels were observed between groups.
Table 2.
Biochemical parameters, effect biomarkers and perceived symptomatology of subjects occupationally (n = 47) and non-occupationally (n = 53) exposed to pesticides from the Province of Córdoba.
| Non-occupationally exposed |
Occupationally exposed |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Biochemical parametersa | |||
| Cholesterol level (mg/dL) | 184 ± 33 | 193 ± 73 | 0.4230 |
| Triglycerides level (mg/dL) | 99 ± 51 | 170 ± 218 | 0.0230 |
| Effect biomarkersa | |||
| BChE (U/L) | 4,872 ± 857 | 5,525 ± 1,196 | 0.0021 |
| AChE (U/L) | 3,152 ± 851 | 3,656 ± 1,006 | 0.0079 |
| ICH | 6.52 ± 0.75 | 9.71 ± 2.17 | <0.0001 |
| AC | 0.17 ± 0.27 | 1.11 ± 0.89 | <0.0001 |
| MN | 3.83 ± 0.71 | 6.66 ± 1.34 | <0.0001 |
| CE | 123.52 ± 3.95 | 146.61 ± 33.58 | <0.0001 |
| n | n | Pearson chi2 | p-value | |
|---|---|---|---|---|
| Perceived symptomatologyb | ||||
| General | 15 | 32 | 16.2825 | 0.003 |
| Dermatological | 12 | 25 | 11.2105 | 0.011 |
| Neurological | 16 | 29 | 13.4057 | 0.009 |
| Ocular | 11 | 27 | 14.6016 | 0.002 |
| Cardiorespiratory | 11 | 20 | 7.7287 | 0.102 |
| Gastric | 15 | 16 | 3.0859 | 0.544 |
| Urinary | 4 | 13 | 7.3774 | 0.025 |
p value of the comparisons of continue variables between non-occupationally and occupationally exposed groups was calculated using t-test.
Association between exposure and categorical variables was calculated using chi2 test.
Regarding perceived symptomatology, a significant higher prevalence of symptomatology in the OE, particularly for general, dermatological, neurological, ocular and urinary symptomatology was found. Unadjusted regression (Table 3) also shows differences between groups of subjects with an increase in the number of perceived symptoms per unit of the predictor variable: exposure condition, for the above mention symptomatology and, also, for cardiorespiratory symptoms. Adjusted regression, shows that the exposure condition is the only predictor variable explaining the significant differences observed between groups for general, dermatological, neurological and cardiorespiratory symptomatology. Conversely, the exposure condition did not show significant association with ocular, gastrointestinal and urinary symptomatology.
Table 3.
Effect of the exposure condition and confounding variables on perceived symptomatology and genotoxicity biomarkers in subjects occupationally (n = 47) and non-occupationally (n = 53) exposed to pesticides from the Córdoba province. Only statistical significant results (p < 0.05) are shown.
| Dependent variable | Predictor variable | Meana |
Adjusted meanb |
||
|---|---|---|---|---|---|
| Coef. | p-value | Coef. | p-value | ||
| General symptomatologyc | |||||
| Exposure condition | 0.9876 | <0.0001 | 0.9646 | 0.024 | |
| Dermatological symptomatologyc | |||||
| Exposure condition | 1.0209 | 0.002 | 1.4159 | 0.009 | |
| Neurological symptomatologyc | |||||
| Exposure condition | 0.9609 | <0.0001 | 0.9983 | 0.042 | |
| Ocular symptomatologyc | |||||
| Exposure condition | 1.1088 | <0.0001 | - | - | |
| Cardiorespiratory symptomatologyc | |||||
| Exposure condition | 1.0364 | 0.001 | 1.0950 | 0.028 | |
| Urinary symptomatologyc | |||||
| Exposure condition | 1.3729 | 0.015 | - | - | |
| SCEd | |||||
| Exposure condition | 3.1832 | <0.001 | 2.6855 | <0.001 | |
| CAc | |||||
| Exposure condition | 1.8498 | <0.001 | 1.8904 | 0.001 | |
| MNd | |||||
| Exposure condition | 2.8232 | <0.001 | 2.0486 | <0.001 | |
| Cholesterol level | 0.0071 | 0.007 | |||
| Presence of allergies | 1.0179 | 0.012 | |||
| CEc | |||||
| Exposure condition | 0.1713 | <0.001 | 0.1223 | <0.001 | |
| Presence of allergies | 0.1186 | 0.001 | |||
Predictor variable: exposure condition (NOE subjects = 0; OE subjects = 1).
Predictor variables: exposure condition, age, BMI (normal = 1; overweight = 2; obesity = 3), cholesterol and triglycerides levels, AChE and BChE activity, educational level (elementary = 1; middle = 2; high school = 3; university = 4), marital status (married = 1; divorced or separated = 2; widower = 3; single = 4), presence of asthma and allergies (no = 0; yes = 1) and the concentration of pesticides.
p-value obtained by applying Link function: Poisson log.
p-value obtained by applying Link function: Gaussian identity.
3.1. Exposure assessment
The ILE and CEI indexes were calculated for the OE group, finding that almost half of the population was within the medium level of exposure (Table 4). From the enrolment questionnaire, we also observed characteristics related to the conditioning factors of exposure (Table 4), with half of the subjects living at a distance lower than 500 m to the nearest crop cultivation. The average time working in the occupation was 15 ± 12 years (min. 2, max. 50 years), with a large number of subjects with more than 20 years carrying out this activity. Workers sprayed 13,767 ± 16,075 has (min. 150, max. 75,000 has) per year. The majority of the subjects used self-propelled machinery, having a pressurized cabin equipped with an activated carbon filter. The number of subjects who carry out the tasks of loading/mixing and application of pesticides with adequate PPE turned out to be low (32% and 21%, respectively), being even lower in those subjects repairing the machinery used to fumigate (9%).
Table 4.
Exposure levels and conditioning factors in subjects occupationally exposed to pesticides.
| Exposure level (percentile) | ILEa | % | CEIb | % |
|---|---|---|---|---|
| Low (0–25) | 0–1.24 | 25 | 0–46.99 | 26 |
| Medium (25–75) | >1.24–3.82 | 50 | >46.99–234.01 | 50 |
| High (75–100) | >3.82 | 25 | >234.01 | 24 |
| Conditioning factors | n (%) |
|---|---|
| Distance from home to the nearest crop | |
| <100 m | 10 (24) |
| >100 m < 500 m | 11 (26) |
| >500 m | 21 (50) |
| Labor seniority | |
| ≤5 years | 8 (22) |
| 6–10 years | 11 (31) |
| 11–20 years | 8 (22) |
| >20 years | 9 (25) |
| Hectares sprayed per year | |
| ≤5000 has | 11 (42) |
| >5000 ≤ 15000 has | 6 (23) |
| >15000 has | 9 (35) |
| Technology used to applyc | |
| Self-propelled machinery | 36 (80) |
| Without activated charcoal filter | 8 (18) |
| With activated charcoal filter | 30 (68) |
| Tractor | 9 (20) |
| Without cabin | - |
| With cabin | 9 (22) |
| Without activated charcoal filter | 8 (20) |
| With activated charcoal filter | - |
| Backpacks | 7 (16) |
| Mix/load of pesticides | |
| Partially protected | 26 (68) |
| Protected | 12 (32) |
| Application of pesticides | |
| Unprotected | 11 (30) |
| Partially protected | 18 (49) |
| Protected | 8 (21) |
| Repair of machinery | |
| Unprotected | 7 (21) |
| Partially protected | 23 (70) |
| Protected | 3 (9) |
ILE valid n = 44.
CEI valid n = 42.
More than one possible answer.
3.2. Effect biomarkers
Results of effect biomarkers are also shown in Table 2. Statistical differences for all the biomarkers evaluated between OE and NOE were found. Cholinesterase activity was lower within the NOE and we could not find a multiple regression model that correctly fit and explain these results. Genotoxicity biomarkers showed higher levels of genetic damage in the OE population for all the biomarkers evaluated. Unadjusted regression (Table 3) also shows an increased number of genotoxic alterations per unit of the predictor variable: exposure condition, for all the biomarkers. Adjusted regression (Table 3) showed that the exposure condition was the only predictor variable influencing the differences between groups for SCE and CA biomarkers, and the most important variable influencing the CE and MN results.
3.3. Exposure biomarkers
Table SM3 shows the method validation performance obtained. Residues of HCB, β-HCH, α-endosulfan, pp'DDE, endrin, β-endosulfan, pp'DDT, endosulfan sulfate, and mirex (μg/L) were found in the population samples (Table 5). Detection frequencies (DF) were calculated using concentrations found above the LLOQ. The most frequently detected pesticide was p,p'DDE, followed by β-HCH, HCB, and α-endosulfan. The other pesticides detected were found in less frequency (<10%). No residue of OP and PYR were found. No statistical differences in the median concentration of pesticides were found between groups. Neither unadjusted nor adjusted GLMs showed differences between groups.
Table 5.
Exposure biomarkers (μg/L) in subjects occupationally (n = 47) and non-occupationally (n = 53) exposed to pesticides from the Córdoba province. Results expressed as geometric means (GM), confidence interval (CI), median, range and 95th percentile (p95). Also detection frequencies (DF) are informed.
| Analite | Non-occupationally exposed |
Occupationally exposed |
p-valuea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF (%) | GM | CI | Median | Range | p95 | DF (%) | GM | CI | Median | Range | p95 | ||
| HCB | 18 | 0.170 | 0.106–0.272 | 0.161 | 0.115–0.225 | 0.260 | 27 | 0.206 | 0.141–0.304 | 0.179 | 0.143–0.279 | 0.292 | 0.2885 |
| β-HCH | 16 | 0.427 | 0.142–1.288 | 0.27 | 0.185–0.734 | 0.973 | 35 | 0.378 | 0.262–0.546 | 0.352 | 0.230–0.511 | 1.108 | 0.3848 |
| α-endosulfan | 18 | 0.366 | 0.160–0.835 | 0.225 | 0.215–0.723 | 1.828 | 16 | 0.398 | 0.208–0.760 | 0.345 | 0.285–0.787 | 0.856 | 0.4094 |
| pp'DDE | 29 | 0.422 | 0.240–0.743 | 0.267 | 0.196–0.911 | 1.575 | 43 | 0.323 | 0.232–0.450 | 0.320 | 0.176–0.432 | 0.611 | 0.5016 |
| Endrin | 2 | 0.356 | - | 0.356 | - | - | - | - | - | - | - | - | - |
| β-endosulfan | 6 | 0.257 | 0.071–0.928 | 0.316 | 0.143–0.376 | 0.376 | 3 | 72.149 | - | 72.149 | - | - | 0.1797 |
| pp'DDT | 4 | 0.197 | <0.001–2603.939 | 0.254 | 0.093–0.415 | 0.415 | 3 | 0.109 | - | 0.109 | - | - | 1.000 |
| Endosulfan sulfate | - | - | - | - | - | - | 3 | 5.017 | - | 5.017 | - | - | - |
| Mirex | 4 | 0.227 | 0.010–5.089 | 0.234 | 0.178–0.290 | - | 11 | 0.306 | 0.104–0.898 | 0.252 | 0.195–0.548 | 0.805 | 0.6434 |
Mann-Whitney U tests for comparisons between non-occupationally and occupationally exposed groups. Only results > LLOQ were used.
Regarding perceived symptomatology, unadjusted regression (Table 6) showed a positive association between neurologic symptoms and higher concentrations of β-HCH and pp'DDE. Also, higher reports of cardiorespiratory symptoms were positively associated with higher levels of mirex. Considering effect biomarkers, higher levels of SCE, CA, and MN were positively associated with higher concentrations of β-endosulfan and higher levels of CE were positively associated with higher concentration of HCB.
Table 6.
Effect of pesticides concentration on perceived symptomatology and effect biomarkers in the population from the Province of Córdoba. Only significant results (p < 0.05 or p < 0.01) are shown.
| Dependent variable | Predictor variablea | Unadjusted mean |
|
|---|---|---|---|
| Coef. | p-value | ||
| Neurological symptomatologyb | |||
| β-HCH | 0.2041 | 0.023 | |
| p,p'DDE | 0.2835 | 0.076 | |
| Cardiorespiratory symptomatologyb | |||
| Mirex | 3.2618 | 0.015 | |
| ICHc | |||
| β-endosulfán | 0.0356 | <0.001 | |
| CAc | |||
| β-endosulfán | 0.0116 | 0.012 | |
| MNc | |||
| β-endosulfán | 0.0220 | <0.001 | |
| CEb | |||
| HCB | 0.4930 | <0.001 | |
Predictor variable: concentration of pesticides.
p-value obtained by applying Link function: Poisson log.
p-value obtained by applying Link function: Gaussian identity.
3.4. Indexes and scales validation
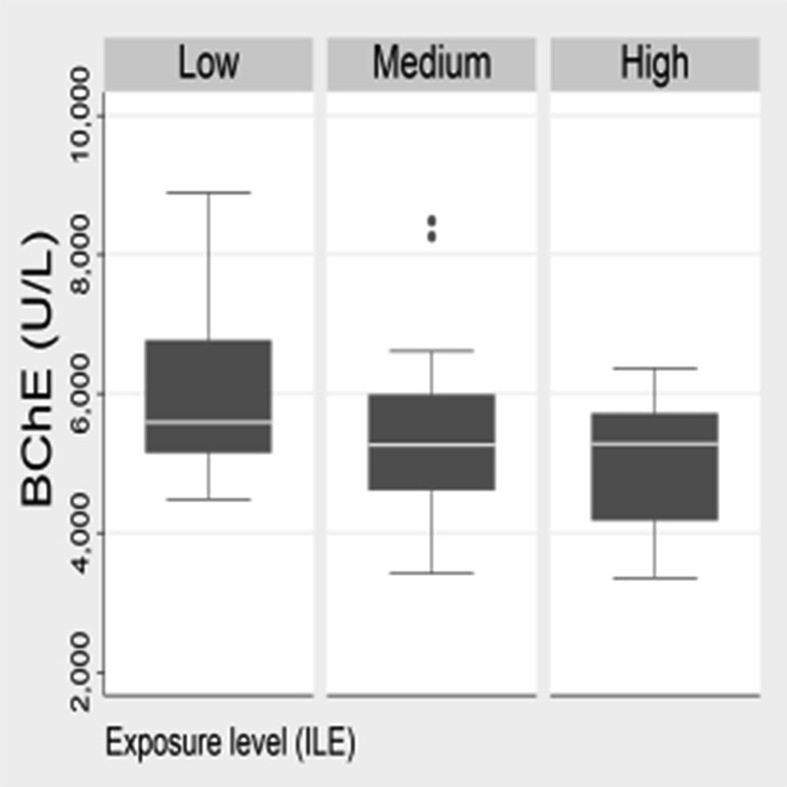
A positive significant association was found between exposure levels of CEI and neurologic symptomatology (Pearson coef. = 0.3462, p = 0.0247). This finding indicates the usefulness of the questionnaires about perceived symptomatology to interpret different levels of exposure. Negative borderline significant p-value (p < 0.1) was obtained between the exposure levels of ILE and plasmatic cholinesterase activities (AChE: Pearson coef. = -0.2828, p = 0.0629; BChE: Pearson coef. = -0.2774, p = 0.0683; Figure 1a and b). Although cholinesterase activities were within normal ranges, a drop in the enzyme activity was observed in subjects with higher levels of exposure. A larger number of subjects should be evaluated to fully elucidate this result. No significant associations were found between the indexes and genotoxicity, and exposure biomarkers.
Figure 1.
Butirylcholinesterase activity (BChE in U/L) by categories of exposure level according to Intensity Level of the pesticide Exposure (ILE) for the subjects occupationally exposed.
4. Discussion
Despite agricultural workers are who deal with the highest levels of exposure to pesticides because of their occupational setting (Hofmann et al., 2009), there are no previous studies evaluating exposure levels and their association with biomarkers response or the possible health consequences for this population in South America. Furthermore, to our knowledge, there are no previous reports on pesticides body burdens in occupational exposure settings in our country. There are only few studies for the general population. For instance, Filippi et al. (2021) demonstrated that the population living in an important agricultural area of Argentina is exposed to organophosphate and pyrethroid pesticides as a consequence of environmental exposure to these compounds. Also Bressán et al. (2021) report glyphosate presence in 20 % of urine samples from subjects inhabiting an Argentinean small rural village surrounded by crops fields.
Worldwide, most of the studies evaluating any type of occupational exposure, either using indirect (e.g. job title, self-report exposure or job history, job, crop or task exposure matrix, algorithms, index, score) or direct (biomarkers) measures, are performed in high income countries. Moreover, studies including both types of evaluation of the exposure as an approach for counterbalancing the weaknesses of one with the strengths of another, are scarce (Ohlander et al., 2020).
Furthermore, while acute effects of human exposure to pesticides are well known, the toxicological effects of chronic and low-dose pesticides exposure on human health remain unclear. However, due to the wide use of pesticides, great efforts are being made to elucidate how pesticides could be associated with chronic diseases (Gangemi et al., 2016; Mostafalou and Abdollahi.; 2013).
In the present work, we designed a cross-sectional study to evaluate the health conditions of people who perform agricultural activities from different perspectives: personal health history, perceived symptomatology, biochemical parameters and pesticides body burdens. We inquired whether the subjects had a previous diagnose of any symptomatology-related disease and we found that the OE group had a higher frequency of dermatological allergies than NOE. We also found a significant difference in weight between groups, with a higher proportion of OE within the obesity classification according to the body mass index (BMI ≥30 kg/m2). Also, triglyceride levels were statistically higher in the OE group than in NOE, with a mean value above the normal value (0–150 mg/dL). These results reinforce the recent concern on the role of occupational exposure to pesticides in the development of chronic disorders, particularly diabetes and obesity (Araoud et al., 2012; Park et al., 2019).
Many studies describe symptomatology, mainly neurological symptoms, related to pesticide exposure (Bedi et al., 2015; Khan et al., 2010). According to our results, the OE population has a significant higher prevalence of symptomatology than NOE not only for neurological, but also for general, dermatological, ocular, cardiorespiratory, and urinary symptomatology. The occupational condition was the only predictor variable that explains the differences between groups for almost every symptomatology.
Nevertheless, in the epidemiological assessment of exposure, being able to effectively differentiate levels of exposure to pesticides is a critical factor. There are various possible approaches for the estimation of the exposure levels. In this study, we used the algorithms ILE and CEI to calculate the level of exposure to pesticides in the OE group. Accordingly, we then classified subjects into one of three categories of the exposure scales. We found almost the same distribution of subjects for both indexes, with half of the population within the medium level of exposure. Previous observational studies carried on in larger cohorts of OE populations (terrestrial applicators of pesticides and horticulturist) from the Province of Córdoba showed a similar distribution of the subjects for both indexes (Butinof et al., 2014; Lantieri et al., 2011). Koutros et al. (2013) classified subjects into quartiles of exposure and also found that 50% of the subjects were within the middle (Q2 y Q3) level of exposure for all the pesticides specific exposure levels.
However, the classification of the subjects into the scales of the indexes needs to be validated with biomarkers responses that correlate with the different levels of exposure. For that reason, we measured several effect and exposure biomarkers to evaluate the usefulness of the indexes and to prevent misclassification of the exposed subjects.
Responses of AChE and BChE in plasma for both OE and NOE showed activities within the normal range (1700–5778 and 3200–9000 U/L, respectively). However, the lowest enzymatic activities were found in the NOE group. A previous case-control study, carried out in Argentina, reported similar results, with higher cholinesterase activities in subjects within the OE group than in NOE, but without significant differences (Lerda and Masiero, 1990). Worldwide, many case/control studies describe no association between chronic low-doses exposure to pesticides and a drop in the cholinesterase activity (Adad et al., 2015; Benedetti et al., 2017; Oliveira et al., 2019). These results could be explained by induction of compensatory synthesis of the enzyme after long-term exposure to pesticides (Hernandez et al., 2005; Keller et al., 2001). Moreover, applicators of pesticides are exposed to complex mixtures of pesticides, not only to anticholinergic pesticides. Therefore, possible interactions between pesticides would be considered. Furthermore, to correctly interpret results, the baseline cholinesterase activity of the subjects before the exposure should be known, due to possible differential susceptibility among individuals (Dulaurent et al., 2006; Strelitz et al., 2014). Argentinian regulation (Law 24557, resolution 37/2010) establishes an annual measure of AChE in erythrocytes in OE subjects. In accordance with regulations from developed countries, a single annual measure of the enzyme activity should not be useful in terms of health issues (Nassar and Ribeiro, 2020).
The evaluation of genetic risk is useful to assess exposure to pesticide mixtures (Bolognesi, 2003). Our results showed higher levels of genetic damage in the OE population than in the NOE for all the genetic biomarkers evaluated. The occupational condition was the only, or the main, predictor variable influencing these differences. Increased genetic and epigenetic alterations are widely described in the OE population regarding the NOE population (Benedetti et al., 2017; Martinez-Valenzuela et al., 2017; Oliviera et al., 2019). These findings confirm the fact that pesticides, used by the OE subjects, are capable to induce genotoxic changes in this population, in addition to the cumulative effect that these alterations could produce, triggering chronic diseases.
Regarding the presence of agrochemicals in plasma, low detection frequencies (DF) for the studied pesticides studied were found (max. 43% for pp'DDE). Our current results are comparable to findings in Belgium, Bolivia, India, Saudi Arabia, Mexico, Brazil, Spain and China (Al-Daghri et al., 2019; Arrebola et al., 2012; Bedi et al., 2015; Bhatnagar et al., 2004; Delgado et al., 2002; Li et al., 2018; Pirard et al., 2018; Ruiz-Suárez et al., 2014; Turci et al., 2010). Generally, DF in the OE subjects was higher than in NOE subjects, but no statistical differences were found between median concentrations of the pesticides of the groups (Table 5).
Organochlorine pesticides are persistent in the environment and due to their lipophilic characteristics they could be bio-accumulated in human tissues. Consumption of contaminated food is the main way of entry of these compounds into the human body. Therefore, residues of OC pesticides are still found in biological samples of the general population, even without occupational exposure (Cao et al., 2012). As many studies mention, factors such as age and gender may affect pesticides concentrations. Thus, we only compare our results with reports evaluating adults male subjects, or studies showing no gender differences in the pesticide concentration (Saoudi et al., 2014; Schettgen et al., 2015). The median concentration of HCB measured in this study for OE and NOE subjects was comparable with studies carried out in the general populations of Germany, Bolivia, Greece, India, and Italy (Arrebola et al., 2012; Bhatnagar et al., 2004; Deering et al., 2020; Koureas et al., 2016; Turci et al., 2010). The median plasma concentration of β-HCH obtained in the current study was similar to other studies carried out in Spain, Germany, and Italy (Deering et al., 2020; López et al., 2007; Turci et al., 2010), at least one order of magnitude lower than studies carried out in Mexico, India, and China (Bhatnagar et al., 2004; Li et al., 2018; Ruiz-Suárez et al., 2014), and one order of magnitude higher than a study carried on Swiss population (Bjermo et al., 2013). The median concentration of pp'DDE in this study was comparable with studies carried out in general population from Brazil, Germany, and Italy (Delgado et al., 2002; Schettgen et al., 2015; Turci et al., 2010), but much lower than studies carried on occupationally exposed population from Mexico and India (Bedi et al., 2015; Ruiz-Suárez et al., 2014). Although DF of pp'DDT was low, we used positive results to calculate the ratio pp'DDE/pp'DDT, looking to assess whether the exposure to pp'DDT is the result of the present (ratio <1) or past (ratio >1) exposure to pp'DDT (Qin et al., 2011). Our results (data not shown) confirm that the exposure to pp'DDT was in the past. The concentration of endosulfan in plasma is scarcely reported in the bibliography, with many studies informing median concentrations below the detection limit (<LOD). In Argentina, the use of endosulfan was banned in 2013 (Resolución SAGPyA 511/11). Thus, our findings could be related to this regulation, even when it is one of the most frequently found pesticide in fruits and vegetables of the Argentina domestic market (Mac Loughlin et al., 2018).
Worldwide, few studies have used algorithms and indexes to estimate the exposure of occupationally exposed populations. Association between the intensity of the exposure and the biomarkers measured was already described (Intayoung et al., 2021; Fuhrimann et al., 2020; Park et al., 2020). However, to our knowledge, none study has focused in differentiate and validate the classification of occupationally exposed subjects into different levels of risk/exposure. The usefulness of the scales proposed by the indexes was verified by the association found between CEI with neurologic symptomatology, and ILE with the cholinesterase activities as well. Both findings indicate the utility of the questionnaires about perceived symptomatology, in addition to the cholinesterase activity, to interpret the different levels of exposure. It is worth mentioning that, in spite of normal cholinesterase activities, a drop in the enzyme activity was observed in subjects with higher levels of exposure (Figure 1). Conversely, no significant association was found for CEI and ILE with genotoxicity and the presence of pesticides in plasma. This lack of correlation could be explained by the early onset of genotoxic damage, of measuring already prohibited pesticides and by the number of subjects evaluated.
The evaluation of currently used pesticides in plasma, but also in other biological samples with less invasive procedures as saliva or urine, could help for a future better validation of these indexes.
5. Conclusion
To our knowledge, this is the first report differentiating and validating the classification of occupationally exposed subjects according to their levels of exposures. Even when the levels of pesticides in plasma did not show significant differences between OE and NOE, the symptomatology and the effect biomarkers responses seem to be useful tools to assess the health condition of the exposed workers and the level of exposure to pesticides (indexes and scales).
Moreover, this is the first report on pesticides exposure in the studied area, which results help to establish baseline values of pesticides and effect biomarkers in human samples, enhancing the knowledge on occupational and environmental exposure conditions, leading to future preventive regulations. Furthermore, these surveillance tools here applied, adapted to the local work conditions, could be especially useful in areas where the access to health care centres and laboratories is difficult. Major efforts should be done to measure non legacy and current used pesticides in biological matrices, helping to elucidate the usefulness of the exposure indexes to propose them as a future surveillance tool. Further studies should address not only the exposure to insecticides but also to herbicides and fungicides that has been even less studied.
Declarations
Author contribution statement
Iohanna Filippi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Patricia Lucero, Rocío Inés Bonansea: Performed the experiments; Analyzed and interpreted the data.
Daniel Lerda, Ricardo Antonio Fernandez: Conceived and designed the experiments; Analyzed and interpreted the data.
Mariana Butinof: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Daniel Alberto Wunderlin: Contributed reagents, materials, analysis tools or data; Wrote the paper.
María Valeria Amé, Sonia Edith Muñoz: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Agencia Nacional de Promoción Científica y Técnica (FONCyT-PICT 2015-1784; PICT 2018-2505), Secretaría de Ciencia y Técnica (SECyT, UNC, Res. 411/2018) and Instituto Nacional de Cáncer (RM 1995/15).
Iohanna Filippi was supported by CONICET.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank every subject who voluntarily participate in the study and to every person who facilitates the work.
Contributor Information
María V. Amé, Email: valeria.ame@unc.edu.ar.
Sonia E. Muñoz, Email: smunoz@fcm.unc.edu.ar.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary material
References
- Adad L.M.M., Rodrigues de Andrade H.H., Kvitko K., Lehmann M., de Carvalho Melo Cavalcante A.A., Rodrigues Dihl R. Occupational exposure of workers to pesticides: toxicogenetics and susceptibility gene polymorphisms. Genet. Mol. Biol. 2015;38(3):308–315. doi: 10.1590/S1415-475738320140336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavanja M.C.R., Hoppin J.A., Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu. Rev. Publ. Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Al-Daghri N., Abd-Alrahman S.H., Wani K., Panigrahy A., McTernan P.G., Al-Attas O.S., Alokail M.S. Biomonitoring and risk assessment of organochlorine pesticides among Saudi adults. Arabian J. Chem. 2019;12:1795–1801. [Google Scholar]
- Araoud M., Neffeti F., Douki W., Hfaiedh H.B., Akrout M., Hassine M., Najjar M.F., Kenan I.A. Adverse effects of pesticides on biochemical and haematological parameters in Tunisian agricultural workers. J. Expo. Sci. Environ. Epidemiol. 2012;22:243–247. doi: 10.1038/jes.2012.11. [DOI] [PubMed] [Google Scholar]
- Arrebola J.P., Cuellar M., Claure E., Quevedo M., Antelo S.R., Mutch E., Ramirez E., Fernandez M.F., Olea N., Mercado L.A. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and adipose tissue from Bolivia. Environ. Res. 2012;112:40–47. doi: 10.1016/j.envres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Barr D.B., Barr J.R., Maggio V.L., Whitehead R.D., Sadowski M.A., Whyatt M., Needham L.L. A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high-resolution mass spectrometry. J. Chromatogr. B. 2002;778:99–111. doi: 10.1016/s0378-4347(01)00444-3. [DOI] [PubMed] [Google Scholar]
- Bedi J.S., Gill J.P.S., Kaur P., Sharma A., Aulakh R.S. Evaluation of pesticide residues in human blood samples from Punjab (India) Vet. World. 2015;8(1):66–71. doi: 10.14202/vetworld.2015.66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti D., Lopes Alderete B., Telles de Souza C., Ferraz Dias J., Niekraszewicz L., Cappetta M., Martínez-López W., Da Silva J. DNA damage and epigenetic alteration in soybean farmers exposed to complex mixture of pesicides. Mutagenesis. 2017:1–9. doi: 10.1093/mutage/gex035. 00. [DOI] [PubMed] [Google Scholar]
- Bhatnagar V.K., Kashyap R., Zaidi S.S.A., Kulkarni P.K., Saiyed H.N. Levels of DDT, HCH, and HCB residues in human blood in Ahmedabad, India. Bull. Environ. Contam. Toxicol. 2004;72:261–265. doi: 10.1007/s00128-003-9049-9. [DOI] [PubMed] [Google Scholar]
- Bjermo H., Darnerud P.O., Lignell S., Pearson M., Rantakokko P., Nälsén C., Barbieri H.E., Kiviranta H., Lindroos A.K., Glynn A. Fish intake and breastfeeding time are associated with serum concentrations of organochlorines in a Swedish population. Environ. Int. 2013;51:88–96. doi: 10.1016/j.envint.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Bolognesi C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat. Res. 2003;543:251–272. doi: 10.1016/s1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Bolognesi C., Parrini M., Bonassi S., Ianello G., Salanitto A. Cytogenetic analysis of a human population occupationally exposed to pesticides. Mutat. Res. 1993;285:239–249. doi: 10.1016/0027-5107(93)90112-s. [DOI] [PubMed] [Google Scholar]
- Bressán I.G., Llesuy S.F., Rodriguez C., Ferloni A., Dawidowski A.R., Figar S.B., Giménez M.I. Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the determination of glyphosate in human urine after pre-column derivatization with 9-fluorenylmethoxycarbonyl chloride. J. Chromatogr. B. 2021;1171:122616. doi: 10.1016/j.jchromb.2021.122616. [DOI] [PubMed] [Google Scholar]
- Butinof M., Fernández R., Lantieri M.J., Stimolo M.I., Blanco M., Machado A.L., Franchini G., Gieco M., Portilla M., Eandi M., Sastre A., Diaz M.P. In: Pesticides –toxic Aspects. London. Larramendy M., Soloneski S., editors. 2014. Pesticides and agricultural work environments in Argentina; pp. 105–134. [Google Scholar]
- Butinof M., Fernández R., Muñoz S.E., Lerda D., Blanco M., Lantieri M.J., Antolini L., Gieco M., Ortiz P., Filippi I., Franchini G., Eandi E., Montedoro F., Díaz M.P. Valoración de la exposición a plaguicidas en cultivos extensivos de Argentina y su potential impacto sobre la salud. Rev. Argentina Salud Públ. 2017;8(33):8–15. [Google Scholar]
- Cao L.L., Yan C.H., Yu X.D., Tian Y., Zou X.Y., Lu D.S., Shen X.M. Determination of polychlorinated biphenyls and organochlorine pesticides in human serum by gas chromatography with micro-electron capture detector. J. Chromatogr. Sci. 2012;50:145–150. doi: 10.1093/chromsci/bmr031. [DOI] [PubMed] [Google Scholar]
- Cámara de Sanidad Agropecuaria y Fertilizantes CASAFE . 2016. Datos del Mercado Argentino de Fitosanitario. Argentina.https://www.casafe.org/pdf/2018/ESTADISTICAS/Informe-Mercado-Fitosanitarios-2016.pdf (accessed 10.02.21) [Google Scholar]
- Corrion M.L., Ostrea E.M., Bielawski D.M., Posecion N.C., Seagraves J.J. Detection of prenatal exposure to several classes of environmental toxicants and their metabolites by gas chromatography–mass spectrometry in maternal and umbilical cord blood. J. Chromatogr. B. 2005;822:221–229. doi: 10.1016/j.jchromb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Dardiotis E., Aloizou A.M., Sakalakis E., Siokas V., Koureas M., Xiromerisiou G., Petinaki E., Wilks M., Tsatsakis A., Hadjichristodoulou C., Stefanis L., Hadjigeorgiou G.M. Organochlorine pesticide levels in Greek patients with Parkinson’s disease. Toxicol. Rep. 2020;7:596–601. doi: 10.1016/j.toxrep.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering K., Spiegel E., Quaisser C., Nowak D., Rakete S., Garí M., O’Reillya S.B. Exposure assessment of toxic metals and organochlorine pesticides among employees of a natural history museum. Environ. Res. 2020;184:109271. doi: 10.1016/j.envres.2020.109271. [DOI] [PubMed] [Google Scholar]
- Delgado I.F., Barreto H.H.C., Kussumi T.A., Baptista Alleluia I., Baggio C.A., Paumgartten F.J.R. Serum levels of organochlorine pesticides and polychlorinated biphenyls among inhabitants of Greater Metropolitan Rio de Janeiro, Brazil. Cad. Saúde Pública, Rio de Janeiro. 2002;18(2):519–524. doi: 10.1590/s0102-311x2002000200017. [DOI] [PubMed] [Google Scholar]
- Dulaurent S., Saint-Marcoux F., Marquet P., Lachatre G. Simultaneous determination of six dialkylphosphates in urine by liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2006;831:223–229. doi: 10.1016/j.jchromb.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Egeghy P.P., Cohen Hubal E.A., Tulve N.S., Melnyk L.J., Morgan M.K., Fortmann R.C., Sheldon L.S. Review of pesticide urinary biomarker measurements from selected US EPA children’s observational exposure studies. Int. J. Environ. Res. Publ. Health. 2011;8:1727–1754. doi: 10.3390/ijerph8051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi E., Weijun C., Zhang H., Nazeer M. Agricultural intensification and damages to human health in relation to agrochemicals: application of artificial intelligence. Land Use Pol. 2019;83:461–474. [Google Scholar]
- Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency EMA Guideline on bioanalytical method validation. Comm. Med. Prod. Human Use. 2011;44:1–23. [Google Scholar]
- Freire C., Koifman R.J., Koifman S. Serum levels of organochlorine pesticides in blood donors: a biomonitoring survey in the North of Brazil, 2010–2011. Sci. Total Environ. 2017;598:722–732. doi: 10.1016/j.scitotenv.2017.04.128. [DOI] [PubMed] [Google Scholar]
- Filippi I., Bravo N., Grimalt J.O., Butinof M., Lerda D., Fernández R.D., Muñoz S.E., Amé M.V. Pilot study of exposure of the male population to organophosphate and pyrethroid pesticides in a region of high agricultural activity (Córdoba, Argentina) Environ. Sci. Pollut. Control Ser. 2021 doi: 10.1007/s11356-021-14397-1. [DOI] [PubMed] [Google Scholar]
- Fukuto T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Persp. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrimann S., Staudacher P., Lindh C., van Wendel de Joode B., Mora A.M., Winkler M.S., Kromhout H. Variability and predictors of weekly pesticide exposure in applicators from organic, sustainable and conventional smallholder farms in Costa Rica. Occup. Environ. Med. 2020;77:40–47. doi: 10.1136/oemed-2019-105884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi S., Gofita E., Costa C., Teodoro M., Briguglio G., Nikitovic D., Tzanakakis G., Tsatsakis A.M., Wilks M.F., Spandidos D.A., Fenga C. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (Review) Int. J. Mol. Med. 2016;38:1012–1020. doi: 10.3892/ijmm.2016.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A.F., Parrón T., Tsatsakis A.M., Requena M., Alarcón R., López-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Hernández A.F., López O., Rodrigo L., Gil F., Pena G., Serrano J.L., Parrón T., Álvarez J.C., Lorente J.A., Pla A. Changes in erythrocyte enzymes in humans long-term exposed to pesticides Influence of several markers of individual susceptibility. Toxicol. Lett. 2005;159:13–21. doi: 10.1016/j.toxlet.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hofmann J.N., Keifer M.C., Furlong C.E., De Roos A.J., Farin F.M., Fenske R.A., van Belle G., Checkoway H. Serum cholinesterase inhibition in relation to paraoxonase-1 (PON1) status among organophosphate-exposed agricultural pesticide handlers. Environ. Health Persp. 2009;17(9):1402–1408. doi: 10.1289/ehp.0900682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intayoung U., Wunnapuk K., Kohsuwan K., Sapbamrer R., Khacha-ananda S. Effect of occupational exposure to herbicides on oxidative stress in sprayers. Saf. Health Work. 2021;12:127–132. doi: 10.1016/j.shaw.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M., Robitzki A., Layer P.G. Anticholinesterase treatment of chicken retinal cells increases acetylcholinesterase protein independently of protein kinase C. Neurosci. Lett. 2001;309:21–24. doi: 10.1016/s0304-3940(01)02013-4. [DOI] [PubMed] [Google Scholar]
- Khan D.A., Hashmi I., Mahjabeen W., Naqvi T.A. Monitoring health implications of pesticide exposure n factory workers in Pakistan. Environ. Monit. Assess. 2010;168:231–240. doi: 10.1007/s10661-009-1107-2. [DOI] [PubMed] [Google Scholar]
- Koureas M., Karagkouni F., Rakitskii V., Hadjichristodoulou C., Tsatsakis A., Tsakalof A. Serum levels of organochlorine pesticides in the general population of Thessaly, Greece, determined by HS-SPME GC–MS method. Environ. Res. 2016;148:318–321. doi: 10.1016/j.envres.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Koutros S., Beane Freeman L.E., Lubin J.H., Heltshe S.L., Andreotti G., Hughes Barry K., DellaValle C.T., Hoppin J.A., Sandler D.P., Lynch C.F., Blair A., Alavanja M.C.R. Risk of total and aggressive prostate cancer and pesticide use in the agricultural health study. Am. J. Epidemiol. 2013;177(1):59–74. doi: 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantieri M.J., Butinof M., Fernández R.A., Stimolo M.I., Blanco M., Díaz M.P. In: Pesticides in the Modern World - Effects of Pesticides Exposure, Croatia. Stoytcheva Margarita., editor. 2011. Work practices, exposure assessment and geographical analysis of pesticide applicators in Argentina; pp. 115–138. [Google Scholar]
- Lantieri M.J., Meyer P.R., Butinof M., Fernández R.A., Stimolo M.I., Díaz M.P. Exposición a plaguicidas en agroaplicadores terrestres de la provincia de Córdoba, Argentina: factores condicionantes. Agriscientia. 2009;26(2):43–54. [Google Scholar]
- Lerda D., Masiero B. Estudio Citogenético, Bioquímico y de la Función Reproductiva en Personas Expuestas a Plaguicidas. Acta Bioquímica Clínica Latinoamericana. 1990;24(3):247–255. [Google Scholar]
- Li J., Wang P., Shi S., Xue J. Background biomonitoring of residue levels of 137 pesticides in the blood plasma of the general population in Beijing. Environ. Monit. Assess. 2018;190:315. doi: 10.1007/s10661-018-6694-3. [DOI] [PubMed] [Google Scholar]
- Lionetto M.G., Caricato R., Giordano M.E. Pollution biomarkers in environmental and human biomonitoring. Open Biomark. J. 2019;9:1–9. [Google Scholar]
- López R., Goñi F., Etxandia A., Millán E. Determination of organochlorine pesticides and polychlorinated biphenyls in human serum using headspace solid-phase microextraction and gas chromatography-electron capture detection. J. Chromatogr. B. 2007;846:298–305. doi: 10.1016/j.jchromb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Mac Loughlin T.M., Peluso M.L., Etchegoyen M.A., Alonso L.L., de Castro M.C., Percudani M.C., Marino D.J.G. Pesticide residues in fruits and vegetables of the Argentine domestic market: occurrence and quality. Food Contr. 2018;93:129–138. [Google Scholar]
- Ministerio de Agricultura, Ganadería y Pesca (MAGyP) 2018. Estimaciones Agrícolas. Argentina.http://datosestimaciones.magyp.gob.ar/reportes.php?reporte=Estimaciones (accessed: 24.01.21) [Google Scholar]
- Martínez-Valenzuela C., Waliszewski S.M., Amador-Muñoz O., Meza E., Calderón-Segura M.E., Zenteno E., Huichapan-Martínez J., Caba M., Félix-Gastélum R., Longoria-Espinoza R. Aerial pesticide application causes DNA damage in pilots from Sinaloa, Mexico. Environ. Sci. Pollut. Res. 2017;24:2412–2420. doi: 10.1007/s11356-016-7974-5. [DOI] [PubMed] [Google Scholar]
- Moorhead P., Nowell P.C., Mellan W.J. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp. Cell Res. 1960;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- Mostafalou S., Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Nassar P.P.M., Ribeiro M.G. Considerations for cholinesterase biomonitoring in flower and ornamental plant greenhouse workers. Sci. Total Environ. 2020;711:135228. doi: 10.1016/j.scitotenv.2019.135228. [DOI] [PubMed] [Google Scholar]
- Oliveira A.F.B., Souza M.R., Benedetti D., Souza Scottia A., Smidt Piazza L., Hilario Garcia A.L., Ferraz Dias J., Niekraszewiczc L.A., Duarte A., Bauer D., Amaral L., Bassi Branco C.L., Melo Reis E., Rabaioli da Silva F., da Silva J. Investigation of pesticide exposure by genotoxicological, biochemical, genetic polymorphic and in silico analysis. Ecotoxicol. Environ. Saf. 2019;179:135–142. doi: 10.1016/j.ecoenv.2019.04.023. [DOI] [PubMed] [Google Scholar]
- Ohlander J., Fuhrimann S., Basinas I., Cherrie J.W., Galea K.S., Povey A.C., van Tongeren M., Harding A.H., Jones K., Vermeulen R., Kromhout H. Systematic review of methods used to assess exposure to pesticides in occupational epidemiology studies, 1993-2017. Occup. Environ. Med. 2020;77:357–367. doi: 10.1136/oemed-2019-105880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Choi J.R., Kim S.K., Lee S., Lee K., Kim J.Y., Oh S.S., Koh S.B. Increased risk of atherosclerosis associated with pesticide exposure in rural areas in Korea. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0232531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim S.K., Kim J.C., Lee K., Choi J.R., Chang S.J., Chung C.H., Parkh K.S., Oh S.S., Koh S.B. Exposure to pesticides and the prevalence of diabetes in a rural population in Korea. Neurotoxicology. 2019;70:12–18. doi: 10.1016/j.neuro.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Pirard C., Compere S., Firquet K., Charlier C. The current environmental levels of endocrine disruptors (mercury, cadmium, organochlorine pesticides and PCBs) in a Belgian adult population and their predictors of exposure. Int. J. Hyg Environ. Health. 2018;221:211–222. doi: 10.1016/j.ijheh.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Qin Y.Y., Leung C.K., Lin C.K., Leung A.O., Wang H.S., Giesy J.P., Wong M.H. Halogenated POPs and PAHs in blood plasma of Hong Kong residents. Environ. Sci. Technol. 2011;45(4):1630–1637. doi: 10.1021/es102444g. [DOI] [PubMed] [Google Scholar]
- Ruiz-Suárez L.E., Castro-Chan R.A., Rivero-Pérez N.E., Trejo-Acevedo A., Guillén-Navarro G.K., Geissen V., Bello-Mendoza R. Levels of organochlorine pesticides in blood plasma from residents of malaria-endemic communities in chiapas, Mexico. Int. J. Environ. Res. Publ. Health. 2014;11:10444–10460. doi: 10.3390/ijerph111010444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoudi A., Fréry N., Zeghnoun A., Bidondo M.L., Deschamps V., Göen T., Garnier R., Guldner L. Serum levels of organochlorine pesticides in the French adult population: the French National Nutrition and Health Study (ENNS), 2006–2007. Sci. Total Environ. 2014;472:1089–1099. doi: 10.1016/j.scitotenv.2013.11.044. [DOI] [PubMed] [Google Scholar]
- Schettgen T., Alt A., Esser A., Kraus T. Current data on the background burden to the persistentorganochlorine pollutants HCB, p,p-DDE as well as PCB 138, PCB 153 and PCB 180 in plasma of the general population in Germany. Int. J. Hyg Environ. Health. 2015;218:380–385. doi: 10.1016/j.ijheh.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Strelitz J., Engel L.S., Keifer M.C. Blood acetylcholinesterase and butyrylcholinesterase as biomarkers of cholinesterase depression among pesticide handlers. Occup. Environ. Med. 2014;71(12):842–847. doi: 10.1136/oemed-2014-102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsyganko V. Organochlorine pesticides in marine ecosystems of the far eastern seas of Russia (2000-2017) Water Res. 2019;161:43–53. doi: 10.1016/j.watres.2019.05.103. [DOI] [PubMed] [Google Scholar]
- Turci R., Balducci C., Brambilla G., Colosio C., Imbriani M., Mantovani A., Vellere F., Minoia C. A simple and fast method for the determination of selected organohalogenated compounds in serum samples from the general population. Toxicol. Lett. 2010;192:66–71. doi: 10.1016/j.toxlet.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Wang H.S., Chen Z.J., Wei W., Man Y.B., Giesy J.P., Du J., Zhang G., Wong C.K.C., Wong M.H. Concentrations of organochlorine pesticides (OCPs) in human blood plasma from Hong Kong: markers of exposure and sources from fish. Environ. Int. 2013;54:18–25. doi: 10.1016/j.envint.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Wilson B.W., Henderson J.D., Ramirez A., O’Malley M.A. Standardization of clinical cholinesterase measurements. Int. J. Toxicol. 2002;21:385–388. doi: 10.1080/10915810290096595. [DOI] [PubMed] [Google Scholar]
- Yusa V., Millet M., Coscolla C., Roca M. Analytical methods for human biomonitoring of pesticides. A review. Anal. Chim. Acta. 2015;891:15–31. doi: 10.1016/j.aca.2015.05.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.