Abstract
N6-methyladenosine (m6A) RNA methylation is an emerging area of epigenetics, which is a reversible and dynamic modification mediating by ‘writers’ (methylase, adding methyl groups, METTL3, METTL14, and WTAP), ‘erasers’ (demethylase, deleting methyl groups, FTO and ALKBH5), and ‘readers’ (YTHDF1-3, YTHDC1 and YTHDC2). Recent studies in human, animal models and cell levels have disclosed a critical role of m6A modification in regulating the homeostasis of metabolic processes and cardiovascular function. Evidence from these studies identify m6A as a candidate of biomarker and therapeutic target for metabolic abnormality and cardiovascular diseases (CVD). Comprehensive understanding of the complexity of m6A regulation in metabolic diseases and CVD will be helpful for us to understand the pathogenesis of CVD. In this review, we discuss the regulatory role of m6A in metabolic abnormality and CVD. We will emphasize the clinical relevance of m6A dysregulation in CVD.
Keywords: Cardiovascular disease, FTO, Heart failure, Metabolic syndrome, METTL3, Myocardial infarction, N6-methyladenosine, RNA epigenetics
Introduction
Recently, epigenetics has been demonstrated to be closely related to the onset and development of metabolic abnormality and cardiovascular diseases (CVD).1, 2, 3, 4, 5 Epigenetics is a reversible, dynamic modification on the levels of DNA, histone and RNA, mainly including DNA methylation, histone acetylation, RNA modification and chromatin rearrangement.6 Among these modifications in epigenetics, DNA methylation and histone modification have been well studied, while RNA modification is an emerging field of epigenetics.
There are more than one hundred posttranscriptional modifications of RNA in regulating its stability, decay, translation and splicing.7 5′ cap modification plays an important role in RNA metabolism and was drew attention by the early studies.8 mRNA 7-methylguanylate (m7G) capping is forming by the action of 5′ triphosphatese, guanylyltransferase, and guanine-7 methyltransferase, and is of great important in mRNA translation and cell viability.9 N6-methyladenosine (m6A) is recognized as most abundant internal post-transcriptional modification in eukaryocytes and is essential for the regulation of various cellular processes and many diseases.10,11 Following the identification of m6A, multiple post-transcriptional modifications were discovered in mRNA, including 5-methylcytosine (m5C), pseudouridine (Ψ), N1-methyladenosine (m1A), 5-hydroxymethylcytosine (hm5C) and N6, 2′-O-dimethyladenosine (m6Am).8 Despite these chemical modifications have been identified for decades, the development of researches in the modifications is slow and we know little about their biological functions. In recent years, due to the rapid development of highly specific antibodies and the high-throughput sequencing technologies,12,13 researches on the RNA epigenetic modifications especially m6A RNA methylation have made great progress in disclosing the potential mechanism of human diseases, such as cancers,14 neurological disorders,15 metabolic abnormality and CVD.16,17
RNA m6A methylation
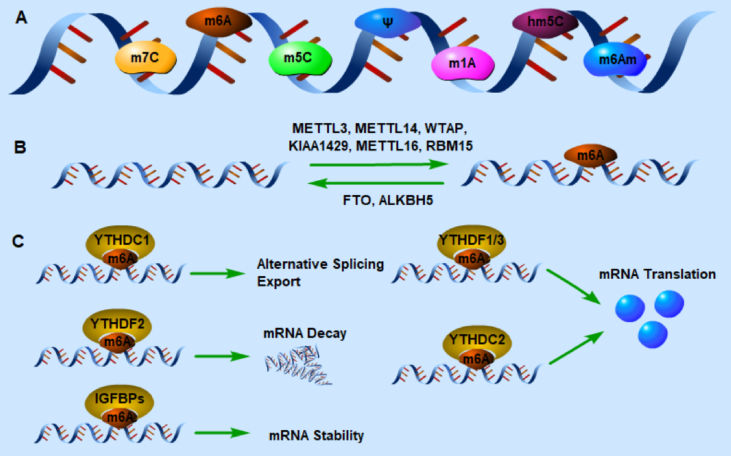
As a widely studied chemical modification, m6A methylation is an epigenetic modification of adding a methyl group which provided by S-adenosyl methionine (SAM) to the N6 site of adenosine, which was identified in 1970s.18, 19, 20, 21 It is a reversible and dynamic modification in RNA level, including mRNA, micro-RNA, lncRNA, circ-RNA, transfer RNA (tRNA), ribosomal RNA (rRNA) and small nuclear RNA (snRNA).22 It is estimated that about 0.1%–0.4% of adenosine in RNA is modified by m6A in eukaryocytes, and 2–3 m6A-modified sites per transcript.22 It regulates RNA methylation by ‘writers’ (methylase, adding methyl groups) and ‘erasers’ (demethylase, deleting methyl groups). And the altered methylation is recognized by readers, then exerts a regulatory function in RNA stability, decay, translation and nuclear export (Fig. 1).
Figure 1.
Regulation of gene expression by RNA modifications. (A) Chemical modification of eukaryotic mRNA. (B & C) Mechanism of m6A modification. Installation of methyl groups in m6A modification is accomplished by writers: METTL3, METTL14, WTAP, KIAA1429, METTL16 and RBM15. And the reversal of m6A methylation is mediated by erasers: FTO and ALKBH5. The altered m6A transcripts are recognized by m6A readers, and then leading to different effects on methylated mRNAs.
Writers
Installation of methyl groups in m6A methylation is accomplished by a highly conserved RNA methyltransferase complex, including methyltransferase-like 3 (METTL3), METTL14, and Wilms tumor suppressor-1-associated protein (WTAP) in the core of the complex.23, 24, 25, 26 METTL3 and METTL14 contain a SAM-binding motif, and tend to catalyze the m6A in a consensus motif of RRACH (where R = G or A, and H = A, C or U), which usually occurs in 3′UTR and transcription start site.12,13 In this complicated methyltransferase complex, METTL3 and METTL14 form stable heterodimers, in which METTL3 is the first methyltransferase discovered as a catalytic subunit, and METTL14 promotes the binding with RNA as an RNA-binding platform.24 WTAP is a regulatory subunit to recruit METTL3- METTL14 complex to bind to mRNA.26 Subsequently, many methylases have been found, such as KIAA1429,25 METTL16,27 and RBM15.28
Erasers
The reversal of m6A methylation is mediated by the only two found demethylases FTO (ALKBH9) and ALKBH5, the so-called ‘erasers’ of m6A. Both of them belong to the AlkB family, and they catalyze the demethylation of m6A in a Fe (II)- and α-ketoglutarate dependent manner.22 FTO was initially found as an obesity-related gene, and it was first discovered to remove m6A modification of mRNA effectively in vitro in 2011.29 Subsequent studies have shown that FTO oxidizes m6A to two intermediates, N6-hydroxymethyladenosine (hm6A) and N6-formyladenosine (f6A).30 Due to FTO is a dioxygenase, its enzymatic efficiency is expected to weaken under hypoxic or ischemic conditions,16 such as myocardial ischemia or infarction. It is also reported that FTO not only removes m6A methylation, but also reverses m6Am modification in cells, which stabilizes the 5′ cap structure of mRNA.31 This indicates that FTO is also the eraser of m6Am, and plays a role on the stability of mRNA. Shortly after FTO was validated, ALKBH5 was identified as the second mammalian m6A demethylase. Different from FTO, ALKBH5 directly reverses m6A to adenosine, thus no intermediate can be detected.32
Readers
It has been demonstrated that the YTH domain can selectively bind to the m6A site in RNA, so the protein with a YTH domain has specific function of recognizing the altered m6A methylation.33 The proteins that can recognize m6A modified and contain the YTH domain include YTHDF1–3, YTHDC1, and YTHDC2. It is validated that YTHDF2 binds to mRNA to accelerated its degradation.34 In contrast, YTHDF1 and YTHDF3 enhance translation efficiency by recruiting translation initiation factors in HeLa cells.35,36 YTHDC1 has a variety of regulatory functions, including regulating mRNA splicing by recruiting specific splicing factors,37 accelerating mRNA nuclear export38 and promoting the decay of specific transcripts.39 Recent studies have shown that YTHDC2 can improve the translation efficiency of hypoxia-inducible factor-1alpha (HIF-1α) mRNA and regulate spermatogenesis through its helicase action.40,41 Meyer et al reported that eukaryotic initiation factor 3 (eIF3), a component of 43S translation initiation complex, could directly bind to the mRNA m6A sites at 5′UTR, which played an important role in translation initiation.42 Heterogeneous nuclear ribonucleoprotein C (HNRNPC) is a rich nuclear RNA binding protein, which is known to participate in the processing of pre-mRNA.43 It has been found that m6A can regulate the binding of HNRNPC-RNA, thus affecting the abundance and alternative splicing of target genes.44 Another kind of reader, insulin-like growth factor 2 mRNA-binding protein 1–3 (IGF2BP1–3), stabilizes target mRNA in a m6A dependent manner.45 A recent study confirmed that Proline rich coiled-coil 2 A (Prrc2a) was an m6A reader, and the results showed that Prrc2a stabilized the m6A modified mRNA that needed for myelination.46
m6A in metabolic and cardiovascular diseases
There are many risk factors for causing CVD. The occurrence of CVD is the result of long-term interaction of the risk factors. Metabolic disease, which results from disrupted metabolic processes of proteins, fats, carbohydrates and other substances,47 is a great threaten to cardiovascular health, especially hypertension, dyslipidemia, diabetes mellitus, atherosclerosis, obesity and nonalcoholic fatty liver disease (NAFLD). The role of m6A modification in metabolic and CVD has been explored (Table 1, Fig. 2 and 3).
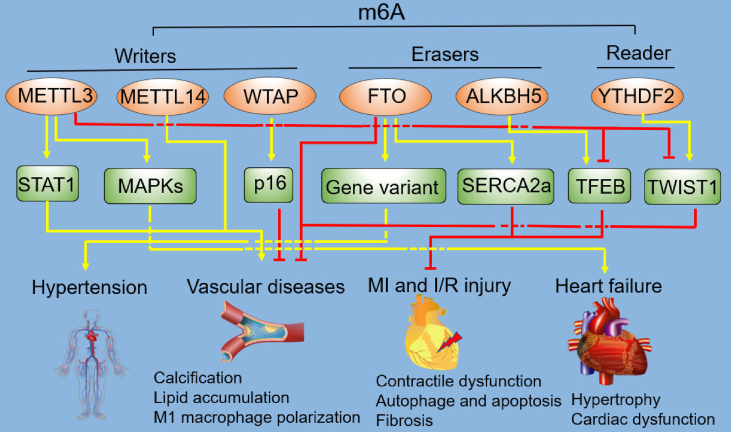
Figure 3.
Regulation of cardiovascular diseases (CVD) by m6A. As a novel regulator of CVD, m6A plays various roles in the development of hypertension, vascular diseases, MI and I/R injury, and heart failure, via different pathways. The dashed lines mean the nodes are non-intersect. m6A, N6-methyladenosine; METTL3, methyltransferase-like 3; METTL14, methyltransferase-like 14; WTAP, Wilms' tumor 1-associating protein; FTO, fat mass and obesity associated protein; ALKBH5, Alk B homologue 5; YTHDF2, YTH domain family protein 2; STAT1, signal transducer and activator of transcription 1; MAPKs, mitogen-activated protein kinases; SERCA2a, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a; TFEB, transcription factor EB; TWIST1, twist-related protein 1; MI, myocardial infarction; I/R, ischemia/reperfusion.
Table 1.
The role of m6A in metabolic and cardiovascular diseases.
| Metabolic and cardiovascular diseases | m6A regulators | Biological function | Mechanism | PMID | References |
|---|---|---|---|---|---|
| Hypertension | FTO | – | Gene variant | 24641884 | 53 |
| m6A | Influences pulmonary hypertension | Influences the circRNA-miRNA-mRNA co-expression network | 31931709 | 50 | |
| m6A | – | m6A-SNPs | 31175347, 19057520 | 51,52 | |
| Diabetes mellitus | FTO | Influences glucose metabolism | Induces mRNA expression of FOXO1, G6PC, and DGAT2 | 30137347 | 61 |
| FTO | Loss of FTO protects mice from glucose intolerance and insulin resistance | Loss of FTO increases AKT phosphorylation in endothelial cells and skeletal muscle | 31801409 | 70 | |
| FTO | Influences insulin resistance and adipose tissue inflammation | Silencing FTO induces the transformation of macrophages into M1-type pro-inflammatory macrophages | 31709454 | 71 | |
| METTL 14 | Decreases β-cell proliferation and insulin degranulation | Influences insulin/IGF1-AKT-PDX1 pathway | 31867565 | 67 | |
| METTL3 | Inhibits hepatic insulin sensitivity | METTL3 silence decreases the m6A methylated and total mRNA level of Fasn | 31405565 | 69 | |
| Obesity | FTO | Loss of endothelial FTO antagonizes obesity-induced metabolic and vascular dysfunction | FTO deficiency upregulates L-Pgds and prostaglandin D2 levels | 31801409 | 70 |
| FTO | Increases in skeletal muscle mass | – | 31572457 | 84 | |
| FTO | FTO depletion blocks adipogenesis | FTO regulates splicing of RUNX1T1 | 25412662 | 91 | |
| FTO | Promotes adipogenesis | Inhibits of the Wnt/β-catenin signaling | 28267420 | 130 | |
| FTO | FTO deficiency affects browning of white adipose tissue | – | 27827997 | 131 | |
| FTO | Regulates mitotic clonal expansion | Enhances RUNX1T1 | 25881961 | 92 | |
| METTL3 | Inhibits adipogenesis | – | 25725156 | 93 | |
| METTL3 | METTL3 knockdown promotes mitotic clonal expansion and adipogenesis | Promotes CCND1 expression | 31434544 | 94 | |
| YTHDF2 | Prolongs cell cycle progression and suppresses adipogenesis | YTHDF2 inhibits CCNA2 and CDK2 | 30305247 | 87 | |
| YTHDF2 | Inhibits autophagy and adipogenesis | Inhibits Atg5 and Atg7 | 31451060 | 90 | |
| YTHDF2 | Inhibits adipogenesis | Inactivates JAK2-STAT3-C/EBPβ signaling | 31295563 | 97 | |
| YTHDF1 | Promotes adipogenesis | Targets MTCH2 | 30339471 | 99 | |
| Nonalcoholic fatty liver disease | FTO | Enhances oxidative stress and lipogenesis | – | 23329013 | 105 |
| FTO | – | The expression of FTO is significantly correlated to FOXO1 | 25382334 | 106 | |
| FTO | Induces lipid accumulation | – | 32116145 | 108 | |
| Vascular diseases | METTL14 | METTL14 de-expression decreases the calcification and enhances the vascular repair function | – | 31697949 | 110 |
| FTO | Enhances angiogenesis | – | 29997116 | 16 | |
| METTL3 | Promotes osteogenic differentiation | Inhibits twist-related protein 1 through a YTHDF2-dependent pathway | 31761339 | 118 | |
| METTL3 | Facilitates M1 macrophage polarization | Methylates STAT1 mRNA and upregulates its expression | 31365297 | 114 | |
| WTAP | Inhibits vascular smooth muscle cell proliferation and migration | Regulates p16 via m6A modification | 31986407 | 116 | |
| FTO | Attenuates lipid accumulation in macrophage foam cells and alleviates atherosclerosis | – | 28253220 | 111 | |
| Myocardial infarction and ischemia-reperfusion injury | m6A | – | m6A-SNPs | 30221544 | 120 |
| FTO | Improves cardiac contractile function | Selectively demethylates cardiac contractile transcripts | 29997116 | 16 | |
| METTL3 | Silencing METTL3 enhances autophagic flux and inhibits apoptosis | METTL3 methylates TFEB at two m6A residues in the 3′-UTR | 30870073 | 122 | |
| ALKBH5 | Inhibition of ALKBH5 inhibits autophagic flux and enhances apoptosis | TFEB binds to the ALKBH5 promoter and activates its transcription | 30870073 | 122 | |
| Heart failure | FTO | Knockout of FTO impairs cardiac function | – | 31849158 | 127 |
| METTL3 | Controls cardiac homeostasis and hypertrophy | Enhances the levels of MAP3K6, MAP4K5 and MAPK14 | 30586742 | 17 | |
| FTO | Regulates cardiac function | Selectively demethylates cardiac contractile transcripts | 29997116 | 16 |
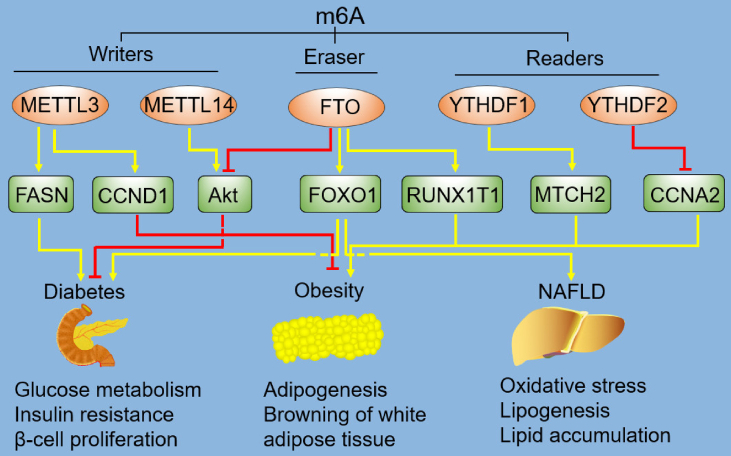
Figure 2.
Regulation of metabolic disorders by m6A. The components of m6A methylation includes writers, erasers, and readers, and they regulate the development of diabetes, obesity and NAFLD by various down-stream targets. The dashed lines mean the nodes are non-intersect. m6A, N6-methyladenosine; METTL3, methyltransferase-like 3; METTL14, methyltransferase-like 14; FTO, fat mass and obesity associated protein; YTHDF1, YTH domain family protein 1; YTHDF2, YTH domain family protein 2; FASN, fatty acid synthase; CCND1, cyclin D1; Akt, also known as protein kinase B; FOXO1, forkhead box protein O1; RUNX1T1, runt-related transcription factor 1; MTCH2, mitochondrial carrier homology 2; CCNA2, cyclin A2; NAFLD, nonalcoholic fatty liver disease.
Hypertension
Hypertension is a major risk factor for CVD, and researchers have recently disclosed that epitranscriptomic mechanism of m6A plays a critical role in hypertension.48 The global level of m6A methylation is reduced in spontaneously hypertensive rat pericytes49 and hypoxia mediated pulmonary hypertension,50 and the m6A is distributed mainly in the coding sequence region, 3′UTR and 5′UTR of mRNAs.49 m6A circXpo6 and m6A circTmtc3 are downregulated in pulmonary hypertension, moreover, m6A connects with circRNA-miRNA-mRNA network to affect the development of pulmonary hypertension.50 Mo et al found that 1236 m6A-associated single-nucleotide polymorphisms (m6A-SNPs) were related to blood pressure (BP), especially diastolic BP, and approximately 10% of these BP-associated m6A-SNPs were associated with coronary artery disease or stroke.51 m6A-SNPs rs56001051, rs9847953, rs197922, and rs740406 were strongly associated with BP-related genes expression in Chinese individuals.51 Meyer et al also found that m6A-SNPs (Lys67Arg, rs197922) was associated with hypertension and BP in Whites in the Atherosclerosis Risk in Communities Study and in the Women's Genome Health Study.52 A meta-analysis comprising 57,464 hypertensive patients and 41,256 controls demonstrated that FTO variant was related to hypertension in both European and Asian populations.53 However, FTO gene rs9939609 variant has positive associations with BMI and neck circumference, but does not have an effect on BP in hypertension patients.54 Patients with gestation-associated arterial hypertension were showed to have higher incidence of heterozygotic genotype AT (FTO gene) than healthy puerperants.55 These results reveal that m6A is closely related to hypertension, further study its epitranscriptomic mechanisms will provide us more concepts of the cause and treatment of hypertension.
Diabetes mellitus
Patients with diabetes develop a distinct form of atherosclerosis due to endothelial injury, which lead to myocardial ischemia and ultimately to heart failure. Type 2 diabetes (T2D) is characterized by deficient insulin, insulin resistance and hyperglycemia. FTO rs9939609 and rs9940128 variants are closely related to hyperglycemia, insulin resistance and diabetes mellitus in several populations.56, 57, 58, 59, 60
Dysfunction of glucose metabolism is one of main characteristics of diabetes mellitus. As an m6A demethylase, FTO post-transcriptionally modulates glucose metabolism via m6A-dependent pathways. It is reported that m6A mRNA methylation involves in glucose metabolism through hepatic gluconeogenesis.61,62 FTO, METTL3, METTL14, and WTAP are upregulated in T2D patients.61 The global level of m6A methylation is decreased in T2D and mainly contributed by FTO rather than ALKBH5.63 Furthermore, FTO specially demethylates some genes and enhances their expression in protein level, such as forkhead box protein O1 (FOXO1), glucose-6-phosphate (G6P) and diacylglycerol O-acyltransferase 2 (DGAT2), which are related to increased blood glucose in patients.61 As an essential transcription factor for mediating gluconeogenesis through G6P, FOXO1 was identified as a direct substrate of FTO.62 And entacapone, a potential FTO inhibitor, exerts the glucose-lowering function by acting on the FTO-FOXO1 pathway.62 In addition, Zhou et al observed the expression of activating transcription factor 4 (ATF4) was increased in FTO overexpression transgenic mice.64 Further research showed that ATF4 was able to increase glucose production by modulating G6P.65
The intact β-cell in pancreatic islet is essential for glucose homeostasis.66 MeRIP-seq indicated a decreased m6A level in T2D human islets.67 Dysfunction of islet, such as reduced β-cell proliferation and insulin degranulation, was observed in β-cell specific knockout of METTL14 in mice, which accelerated the occurrence of diabetes.67 It is reported that METTL3/14 are responsible for the functional maturity of neonatal β cells, and depletion of METTL3/14 results in hypo-insulinemia and hyperglycemia.68 In addition, the role of m6A modification in insulin resistance was also explored. The insulin sensitivity is enhanced in hepatocyte-specific METTL3 knock-out mice fed a high-fat diet, by targeting fatty acid synthase (Fasn) in an m6A-dependent manner.69 Moreover, silence of endothelial FTO was demonstrated to alleviate glucose intolerance and insulin resistance induced by high-fat diet, via up-regulating AKT phosphorylation in endotheliocytes and skeletal muscle.70 Contradictorily, Hu et al validated that losing FTO induced the transformation of macrophages into M1-type pro-inflammatory macrophages to aggravate insulin resistance in T2D mice.71 Mussa et al identified FTO as a potential biomarker and novel therapeutic target for hypoglycemia-associated autonomic failure, a complication of diabetes.72 These results disclose that m6A is emerging as a regulator of diabetes mellitus.
Obesity
Patients with obesity are more susceptible to CVD and tend to a worse outcome.73,74 It results from an interaction between the genetic traits and environmental factors, including high-fat diet and lacking of exercise. Epigenetic mechanisms such as m6A modification are essential for the development of obesity.
FTO, as an obesity-related protein before identified as an m6A demethylase, is instinctively related to obesity. Sequence variants of FTO were observed in obesity in many populations, such as European, East Asian, African, Arab and Brazilian populations.75, 76, 77, 78, 79, 80, 81 A study of the connection of parental diet during pregnancy with obesity in offspring by Kaspi et al showed that parental low-fat diet affected the obesity phenotype by altering the expression of FTO and METTL3 in the offspring.82 Wang et al found that METTL3 played an important role in postnatal maturation of brown adipose tissue (BAT), and BAT-specific knockout of METTL3 leaded to the development of high-fat diet-induced obesity.83 Moreover, increased expression of FTO is related to elevated skeletal muscle mass in overweight male adolescents,84 whereas inhibited FTO activity by entacapone decreases body weight and lowers fasting blood glucose in diet-induced obese mice.62 Similarly, Wang et al found that FTO was involved in the skeletal muscle differentiation by regulating mitochondrial function via mTOR-PGC-1α axis.85 Furthermore, treatment with FTO inhibitor increase myogenic tone in obesity, which contributed to prevent the development of obesity-induced hypertension.70 Interestingly, AMPK, the known energy sensor, is found to exert regulatory function of lipid accumulation in skeletal muscle by influencing FTO and m6A modification.86 Therefore, it is believable that the translational study of controlling lipid accumulation in skeletal muscle by using m6A related medicines will be valuable.86
Mechanistically, m6A participates in the development of obesity by influencing the process of adipogenesis and lipid metabolism. Depletion of FTO reduces cyclin A2 (CCNA2) and cyclin dependent kinase 2 (CDK2), the vital regulators of mitotic clonal expansion, thus inhibits the cell cycle progression of preadipocytes and adipogenesis.87,88 FTO regulates the expression of CCNA2 and CDK2 via m6A-YTHDF2 dependent mechanism.87,88 Zinc finger protein (Zfp217), a regulator of adipogenesis, was also validated to regulate adipogenesis in an FTO-YTHDF2 dependent manner.89 This FTO-YTHDF2 axis was also demonstrated to facilitate autophagosome formation and autophagy and thus promoted adipogenesis.90 Moreover, FTO-dependent m6A demethylation transforms the mRNA splicing of RUNX1T1 (an adipogenic regulatory factor) into the pro-adipogenic short isoform to increase adipocyte proliferation.91,92 Interestingly, it was reported that METTL3 and FTO played opposite roles in adipogenesis.93 METTL3 enhances the m6A methylation of cyclin D1 (CCND1) mRNA, which recognized by YTHDF2 and thus degraded, leading to blocked cell-cycle progression and adipogenesis inhibition.94 CCAAT enhancer binding protein β (C/EBPβ) is a pivotal transcriptional factor regulating adipocyte differentiation in the early stage.95 Knockdown of METTL3 promotes adipogenesis by activating JAK1-STAT5-C/EBPβ signaling through m6A-YTHDF2-dependent mechanism.96 Similarly, Wu et al disclosed that FTO deficiency suppressed adipogenesis in porcine and mouse via JAK2-STAT3-C/EBPβ pathway, and YTHDF2 facilitated JAK2 mRNA decay in an m6A dependent manner in this process.97 Moreover, YTHDF2 recognizes methylated FAM134B (Family with Sequence Similarity 134, Member B) mRNA and leads to its decay and thus reduces its protein abundance, resulting in adipogenesis in porcine adipocytes.98 YTHDF1 facilitates translation of mitochondrial carrier homology 2 (MTCH2) mRNA to promote adipogenesis.99 Taken together, m6A plays a vital role in adipogenesis and obesity.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis, which caused by metabolic disorders of de novo lipogenesis, fatty acid uptake, fatty acid oxidation, and triglycerides export.100 NAFLD increases the risk of CVD, such as atherosclerosis, cardiomyopathy, and arrhythmia, by the pathological mechanisms of systemic inflammation, endothelial dysfunction, insulin resistance, oxidative stress, and altered lipid metabolism.101, 102, 103 Recently, m6A methylation has been reported to play an important role in the development of NAFLD.
A decreased global level of m6A and increased FTO expression are detected in NAFLD.104, 105, 106 Exposure of endocrine disrupting chemicals (EDCs) is related to induction of NAFLD, global m6A level and expression of m6A modulators are alerted when exposure by EDCs in zebrafish.107 FTO knockdown significantly mitigates dexamethasone-induced fatty liver (a mouse model of NAFLD).108 Moreover, it is reported that exenatide therapy ameliorates lipid accumulation and inflammatory changes in NAFLD by decreasing FTO expression in a PI3K-dependent mechanism.109
Atherosclerosis
The role of m6A methylation in the development and progression of vascular calcification,110 obesity-induced vascular dysfunction,70 atherosclerosis111 and angiogenesis16 has been explored. Atherosclerosis is the most prevalent disease threatening the vasculature, and is characteristic by lipid deposition and fiber cap formation.112 Mo and colleagues found that overexpression of FTO by adeno-associated virus serotype 9 (AAV9) significantly reduced the lipidic profiles including plasma total cholesterol and LDL cholesterol, resulting in preventing the formation of atherosclerotic plaques.111 However, Kruger et al demonstrated that loss of endothelial FTO prevented obesity-induced vascular dysfunction by using endothelial FTO-deficient mice.70 It may be reasonable that FTO exerts different effects in different cell types. But there is no doubt that FTO is capable of affecting vascular homeostasis properties.
It is increasingly recognized that inflammation and immunity contribute mainly to the pathological development of atherosclerosis.113 m6A writer METTL3 is markedly upregulated following the M1 polarization of mouse macrophages, and knockdown of METTL3 inhibits M1, but leaded to M2, macrophage polarization.114 METTL3 exerts pro-inflammatory effect via METTL3-STAT1 axis.114 Hu et al also reported that loss of FTO resulted in the transformation of macrophages into M1-type pro-inflammatory macrophages.71 These findings imply that METTL3 inhibitor or FTO agonist may be potential anti-inflammatory targets. Vascular smooth muscle cells (VSMCs) are the predominant cell type in the arterial wall, and the abnormal proliferation and migration of VSMCs leads to intimal hyperplasia, resulting in arterial restenosis and increasing the risk of atherosclerosis.115 Zhu et al showed that epigenetic modifications in VSMCs such as m6A played a critical role in atherosclerotic lesion restenosis.116 The expression of m6A writer WTAP is reduced in balloon catheter-injured rat carotid artery.116 Total Panax notoginseng saponin up-regulates the reduced WTAP-p16 signaling to repress intimal hyperplasia,116 which provides a concept that we can use the existing medicines or novel inhibitors to regulate m6A modification to treat some diseases such as CVD.
Vascular calcification is characterized by increased stiffness of vascular wall and decreased compliance due to ectopic deposits of calcium phosphate, and has been proved to increase cardiovascular events and mortality.117 Recent evidence indicates that the global m6A methylation is increased in calcific arteries and in human artery smooth muscle cells induced by indoxyl sulfate, and METTL14 is increased in these settings.110 Overexpression of METTL14 by adenovirus increases osteoblast conversion of smooth muscle cells.110 Similarly, METTL3 also plays a positive role in promoting osteogenic differentiation of human aortic valve interstitial cells via targeting twist-related protein 1 (TWIST1) in a YTHDF2-dependent manner.118 These results provide novel mechanistic insights into osteogenic differentiation and the onset of vascular calcification, further researches are needed to validate the diagnostics and therapeutics value of m6A in vascular calcification.
Myocardial infarction and ischemia-reperfusion injury
Myocardial infarction (MI) is myocardial necrosis caused by acute and persistent ischemia and hypoxia of coronary artery. The enzymatic activity of FTO may be attenuated under hypoxic or ischemic conditions such as MI,16 because it is a dioxygenase that oxidatively demethylates m6A-containing mRNAs.29 Not only that, but the expression of FTO was decreased in failing heart due to MI both in human and mice.16 So, increased m6A modification levels have been observed in mice after 4 weeks of MI.16 Overexpression of FTO by AAV9 exerts various cardioprotective function in mice after MI. It not only improves cardiac contractile mechanics by targeting Ca2+-ATPase pump SERCA2a, but also induces angiogenesis in the ischemic conditions.16 It is known that angiogenesis is crucial to the recovery of heart function after MI.119 The cardiac remodeling such as fibrosis after MI will worsen the heart function and lead to heart failure. FTO is reported to significantly reduce fibrosis and scar area in mouse models of MI.16 A study investigating about 185,000 CAD patients and controls, and found that 304 out of 4390 m6A-SNPs were associated with CAD.120
The recovery of blood flow after myocardial ischemia can cause additional damage to cardiomyocytes through induction of oxidative stress and release of oxidative free radicals.121 The resulting myocardial ischemia-reperfusion (IR) injury can lead to MI and heart failure. Song et al reported that m6A mRNA methylation was increased in hypoxia/reoxygenation (H/R)-treated cardiomyocytes and IR-treated mice.122 Silencing METTL3 can attenuate IR injury by enhancing autophagic flux and inhibiting apoptosis in H/R-treated cardiomyocytes.122 While ALKBH5 has an opposite effect during myocardial IR.122 The lncRNA MALAT1 induces inflammation response through regulating PTGS2 by targeting miR-26b in myocardial IR injury,123 so Yang et al assumed that m6A modification to MALAT1 may be essential for myocardial IR injury, and may act as a potential therapeutic target.124 Saxena et al put forward a hypothesis that optimizing cardiac ischemic preconditioning by regulating m6A modification levels of cardioprotective mRNAs (such as eNOS, SOD, and HO-1) may result in rendering ischemic cardiac preconditioning more robust and reducing infarct size.125 However, these need to be demonstrated by experimental evidence.
Cardiac hypertrophy and heart failure
Heart failure is the deadly end stage of various CVDs, commonly caused by myocardial infarction, hypertension, degenerative valve disease and dilated cardiomyopathy.126 The main pathologic basis of heart failure is pathologic hypertrophy of the myocardium and increased fibrotic scar tissue, and blocking the myocardial remodeling is the key to the treatment of heart failure. Recently, m6A mRNA methylation has been reported to be involved in mediating these structural changes in the failing heart.
The global level of m6A modification is increased in isolated primary cardiomyocytes responded to hypertrophic stimulation and hypertrophic myocardium, by the evidence from m6A methylation RNA immunoprecipitation followed by next-generation sequencing.17,127 Genes involves in regulating kinases and intracellular signaling pathways are enriched by m6A analysis.17 Inhibition of the m6A RNA methylase METTL3 blocks the ability of cardiomyocytes to undergo hypertrophy when stimulated to grow, while increased expression of the METTL3 is sufficient to promote cardiomyocyte hypertrophy both in vitro and in vivo.17 Upregulated m6A methylation leads to compensated cardiac hypertrophy while decreased m6A drives eccentric cardiomyocyte remodeling and dysfunction, highlighting the critical significance of this novel epitranscriptomic mechanism and its potential therapeutic target in cardiac hypertrophy.17
The m6A methylation is also increased in heart failure.16,127 And the decreased FTO mainly contributes to the increased m6A in human and mouse failing hearts post MI, because the loss of FTO is continuous in the development of heart failure.16 FTO-deficient mice by cardiomyocyte restricted knockout or AAV9 are showed impaired cardiac function under stress stimuli,16,127 while overexpression of FTO improves the heart function and postpones the development of heart failure.16 It is also reported that patients with the FTO TT genotype (SNP rs17817449) exhibits a significantly increased risk for organ rejection when undergoing heart transplantation due to end-stage heart failure.128 FTO demethylates cardiac contractile genes such as SERCA2A, MYH6/7, RYR2 in a m6A dependent manner, and increases their protein expression to enhance cardiac contraction.16 Moreover, overexpression of FTO is capable of reversing cardiac fibrosis and inducing angiogenesis in the heart failure post MI.16 In summary, m6A is considered to be critical in the development of heart failure,129 and more researches are need to demonstrate its therapeutic effect.
Future perspective
In recent years, due to the rapid development of highly specific antibodies of m6A and the high-throughput sequencing technologies, the m6A RNA methylation research has made great progress in disclosing the potential mechanism of the onset and development of CVD. However, there are many essential issues that need to be addressed.
It is noted that FTO was reported to enhance cardiac constriction in heart failure after MI,16 whereas Kruger et al revealed that loss of endothelial FTO prevented obesity-induced vascular dysfunction.70 And it cannot be ignored that FTO is initially found as an obesity-related gene, and plays a role in promoting adipogenesis as a demethylase. These results indicate that the role of FTO in CVD is different in different cell types, different issues, and different pathological conditions. Thus, the clinical translation of FTO is likely to require to design tissue-specific or cell-specific agonist or inhibitor.
Song et al122 and Ruan et al123 have investigated the interaction of autophagy and lncRNA with m6A, respectively. However, the connection of m6A with many regulatory mechanisms related to CVD remains unknown, such as DNA methylation, histone deacetylation, or non-coding RNA. That suggests the mechanism of m6A regulates the biological process needs to be identified.
More profound experimental, translational and clinical research evidence is needed to validate the m6A methylation as the diagnostic biomarkers and therapeutic targets for CVD. In the oncology field, m6A has been identified to be related to circulating tumor cells, a biomarker for monitoring and preventing the development of metastatic diseases. Thus, the association of m6A with traditional biomarkers of CVD such as myocardial enzyme in MI, pro-BNP in heart failure, is needed to be assessed.
Conclusion
In summary, a better understanding of the modifications regulated by m6A methylation under different pathologic conditions is valuable in the exploration of novel biomarkers and therapeutic targets for CVDs. And more profound experimental, translational and clinical research are needed to map the complete picture of m6A modification in CVDs.
Conflict of Interests
The authors have no conflicts to declare.
Funding
This work was supported by funding from the Innovative Research Groups of the National Natural Science Foundation of China (81521001), Major Research Plan of the National Natural Science Foundation of China (91639104), a grant to Aijun Sun from the National Science Fund for Distinguished Young Scholars (81725002), National Natural Science Foundation of China (81800348) and Youth Fund of Zhongshan Hospital, Fudan University 2018ZSQN04.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Aijun Sun, Email: sun.aijun@zs-hospital.sh.cn.
Junbo Ge, Email: jbge@zs-hospital.sh.cn.
References
- 1.van der Harst P., de Windt L.J., Chambers J.C. Translational perspective on epigenetics in cardiovascular disease. J Am Coll Cardiol. 2017;70(5):590–606. doi: 10.1016/j.jacc.2017.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong J., Agha G., Baccarelli A.A. The role of DNA methylation in cardiovascular risk and disease: methodological aspects, study design, and data analysis for epidemiological studies. Circ Res. 2016;118(1):119–131. doi: 10.1161/CIRCRESAHA.115.305206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuznetsova T., Prange K.H.M., Glass C.K., de Winther M.P.J. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. 2020;17(4):216–228. doi: 10.1038/s41569-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha G., Mendelson M.M., Ward-Caviness C.K. Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease. Circulation. 2019;140(8):645–657. doi: 10.1161/CIRCULATIONAHA.118.039357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P., Ge J., Li H. Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease. Nat Rev Cardiol. 2020;17(2):96–115. doi: 10.1038/s41569-019-0235-9. [DOI] [PubMed] [Google Scholar]
- 6.Ng R.K., Gurdon J.B. Epigenetic inheritance of cell differentiation status. Cell Cycle. 2008;7(9):1173–1177. doi: 10.4161/cc.7.9.5791. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert W.V., Bell T.A., Schaening C. Messenger RNA modifications: form, distribution, and function. Science. 2016;352(6292):1408–1412. doi: 10.1126/science.aad8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling V.H. Regulation of mRNA cap methylation. Biochem J. 2009;425(2):295–302. doi: 10.1042/BJ20091352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N., Pan T. N6-methyladenosine-encoded epitranscriptomics. Nat Struct Mol Biol. 2016;23(2):98–102. doi: 10.1038/nsmb.3162. [DOI] [PubMed] [Google Scholar]
- 12.Dominissini D., Moshitch-Moshkovitz S., Schwartz S. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149(7):1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choe J., Lin S., Zhang W. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561(7724):556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon K.J., Ringeling F.R., Vissers C. Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell. 2017;171(4):877–889. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathiyalagan P., Adamiak M., Mayourian J. FTO-dependent N(6)-methyladenosine regulates cardiac function during remodeling and repair. Circulation. 2019;139(4):518–532. doi: 10.1161/CIRCULATIONAHA.118.033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorn L.E., Lasman L., Chen J. The N(6)-methyladenosine mRNA methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139(4):533–545. doi: 10.1161/CIRCULATIONAHA.118.036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry R.P., Kelley D.E., Friderici K., Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5′ terminus. Cell. 1975;4(4):387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubin D.T., Taylor R.H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2(10):1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams J.M., Cory S. Modified nucleosides and bizarre 5′-termini in mouse myeloma mRNA. Nature. 1975;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m⁶A RNA methylation. Nat Rev Genet. 2014;15(5):293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Yue Y., Han D. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Feng J., Xue Y. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz S., Mumbach M.R., Jovanovic M. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8(1):284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ping X.L., Sun B.F., Wang L. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pendleton K.E., Chen B., Liu K. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169(5):824–835. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patil D.P., Chen C.K., Pickering B.F. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia G., Fu Y., Zhao X. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y., Jia G., Pang X. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun. 2013;4 doi: 10.1038/ncomms2822. e1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauer J., Luo X., Blanjoie A. Reversible methylation of m(6)A(m) in the 5′ cap controls mRNA stability. Nature. 2017;541(7637):371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng G., Dahl J.A., Niu Y. Sprouts of RNA epigenetics: the discovery of mammalian RNA demethylases. RNA Biol. 2013;10(6):915–918. doi: 10.4161/rna.24711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theler D., Dominguez C., Blatter M., Boudet J., Allain F.H. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res. 2014;42(22):13911–13919. doi: 10.1093/nar/gku1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Lu Z., Gomez A. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Zhao B.S., Roundtree I.A. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H., Wang X., Lu Z. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao W., Adhikari S., Dahal U. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Roundtree I.A., Luo G.Z., Zhang Z. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017;6 doi: 10.7554/eLife.31311. e31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shima H., Matsumoto M., Ishigami Y. S-adenosylmethionine synthesis is regulated by selective N(6)-adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep. 2017;21(12):3354–3363. doi: 10.1016/j.celrep.2017.11.092. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe A., Tanikawa K., Tsunetomi M. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016;376(1):34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Hsu P.J., Zhu Y., Ma H. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27(9):1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer K.D., Patil D.P., Zhou J. 5′ UTR m(6)A promotes cap-independent translation. Cell. 2015;163(4):999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cieniková Z., Damberger F.F., Hall J., Allain F.H., Maris C. Structural and mechanistic insights into poly(uridine) tract recognition by the hnRNP C RNA recognition motif. J Am Chem Soc. 2014;136(41):14536–14544. doi: 10.1021/ja507690d. [DOI] [PubMed] [Google Scholar]
- 44.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H., Weng H., Sun W. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu R., Li A., Sun B. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29(1):23–41. doi: 10.1038/s41422-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi S., Kong N., Feng C. Drug delivery strategies for the treatment of metabolic diseases. Adv Healthc Mater. 2019;8(12) doi: 10.1002/adhm.201801655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paramasivam A., Vijayashree Priyadharsini J., Raghunandhakumar S. N6-adenosine methylation (m6A): a promising new molecular target in hypertension and cardiovascular diseases. Hypertens Res. 2020;43(2):153–154. doi: 10.1038/s41440-019-0338-z. [DOI] [PubMed] [Google Scholar]
- 49.Wu Q., Yuan X., Han R., Zhang H., Xiu R. Epitranscriptomic mechanisms of N6-methyladenosine methylation regulating mammalian hypertension development by determined spontaneously hypertensive rats pericytes. Epigenomics. 2019;11(12):1359–1370. doi: 10.2217/epi-2019-0148. [DOI] [PubMed] [Google Scholar]
- 50.Su H., Wang G., Wu L., Ma X., Ying K., Zhang R. Transcriptome-wide map of m(6)A circRNAs identified in a rat model of hypoxia mediated pulmonary hypertension. BMC Genomics. 2020;21(1) doi: 10.1186/s12864-020-6462-y. e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mo X.B., Lei S.F., Zhang Y.H., Zhang H. Examination of the associations between m(6)A-associated single-nucleotide polymorphisms and blood pressure. Hypertens Res. 2019;42(10):1582–1589. doi: 10.1038/s41440-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 52.Meyer T.E., Shiffman D., Morrison A.C. GOSR2 Lys67Arg is associated with hypertension in whites. Am J Hypertens. 2009;22(2):163–168. doi: 10.1038/ajh.2008.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He D., Fu M., Miao S., Hotta K., Chandak G.R., Xi B. FTO gene variant and risk of hypertension: a meta-analysis of 57,464 hypertensive cases and 41,256 controls. Metabolism. 2014;63(5):633–639. doi: 10.1016/j.metabol.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Marcadenti A., Fuchs F.D., Matte U., Sperb F., Moreira L.B., Fuchs S.C. Effects of FTO RS9939906 and MC4R RS17782313 on obesity, type 2 diabetes mellitus and blood pressure in patients with hypertension. Cardiovasc Diabetol. 2013;12 doi: 10.1186/1475-2840-12-103. e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zotova T.Y., Lapaev N.N., Azova M.M. Distribution of polymorphisms of the Renin-angiotensin system genes (ACE, AGT, and AGTR1), ITGB3, and FTO in pregnant patients with hypertensive disorders. Bull Exp Biol Med. 2019;167(1):74–78. doi: 10.1007/s10517-019-04464-6. [DOI] [PubMed] [Google Scholar]
- 56.Khoshi A., Bajestani M.K., Shakeri H., Goodarzi G., Azizi F. Association of Omentin rs2274907 and FTO rs9939609 gene polymorphisms with insulin resistance in Iranian individuals with newly diagnosed type 2 diabetes. Lipids Health Dis. 2019;18(1) doi: 10.1186/s12944-019-1085-5. e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hjort R., Löfvenborg J.E., Ahlqvist E. Interaction between overweight and genotypes of HLA, TCF7L2, and FTO in relation to the risk of latent autoimmune diabetes in adults and type 2 diabetes. J Clin Endocrinol Metab. 2019;104(10):4815–4826. doi: 10.1210/jc.2019-00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasser F.A., Algenabi A.A., Hadi N.R., Hussein M.K., Fatima G., Al-Aubaidy H.A. The association of the common fat mass and obesity associated gene polymorphisms with type 2 diabetes in obese Iraqi population. Diabetes Metab Syndr. 2019;13(4):2451–2455. doi: 10.1016/j.dsx.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 59.Naaz K., Kumar A., Choudhury I. Assessment of FTO gene polymorphism and its association with type 2 diabetes mellitus in North Indian populations. Indian J Clin Biochem. 2019;34(4):479–484. doi: 10.1007/s12291-018-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beysel S., Pinarli F.A., Eyerci N. HNF1A gene p.I27L is associated with co-existing preeclampsia in gestational diabetes mellitus. Gynecol Endocrinol. 2020;36(6):530–534. doi: 10.1080/09513590.2019.1698023. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y., Shen F., Huang W. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(3):665–673. doi: 10.1210/jc.2018-00619. [DOI] [PubMed] [Google Scholar]
- 62.Peng S., Xiao W., Ju D. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci Transl Med. 2019;11(488) doi: 10.1126/scitranslmed.aau7116. eaau7116. [DOI] [PubMed] [Google Scholar]
- 63.Shen F., Huang W., Huang J.T. Decreased N(6)-methyladenosine in peripheral blood RNA from diabetic patients is associated with FTO expression rather than ALKBH5. J Clin Endocrinol Metab. 2015;100(1):E148–E154. doi: 10.1210/jc.2014-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J., Wan J., Shu X.E. N(6)-Methyladenosine guides mRNA alternative translation during integrated stress response. Mol Cell. 2018;69(4):636–647. doi: 10.1016/j.molcel.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li K., Zhang J., Yu J. MicroRNA-214 suppresses gluconeogenesis by targeting activating transcriptional factor 4. J Biol Chem. 2015;290(13):8185–8195. doi: 10.1074/jbc.M114.633990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Jesus D.F., Kulkarni R.N. Epigenetic modifiers of islet function and mass. Trends Endocrinol Metab. 2014;25(12):628–636. doi: 10.1016/j.tem.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 67.De Jesus D.F., Zhang Z., Kahraman S. m(6)A mRNA methylation regulates human beta-cell biology in physiological states and in type 2 diabetes. Nat Metab. 2019;1(8):765–774. doi: 10.1038/s42255-019-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Sun J., Lin Z. m(6)A mRNA methylation controls functional maturation in neonatal murine β cells. Diabetes. 2020;69(8):1708–1722. doi: 10.2337/db19-0906. [DOI] [PubMed] [Google Scholar]
- 69.Xie W., Ma L.L., Xu Y.Q., Wang B.H., Li S.M. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism. Biochem Biophys Res Commun. 2019;518(1):120–126. doi: 10.1016/j.bbrc.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 70.Krüger N., Biwer L.A., Good M.E. Loss of endothelial FTO antagonizes obesity-induced metabolic and vascular dysfunction. Circ Res. 2020;126(2):232–242. doi: 10.1161/CIRCRESAHA.119.315531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu F., Tong J., Deng B., Zheng J., Lu C. MiR-495 regulates macrophage M1/M2 polarization and insulin resistance in high-fat diet-fed mice via targeting FTO. Pflugers Arch. 2019;471(11–12):1529–1537. doi: 10.1007/s00424-019-02316-w. [DOI] [PubMed] [Google Scholar]
- 72.Mussa B.M., Taneera J., Mohammed A.K., Srivastava A., Mukhopadhyay D., Sulaiman N. Potential role of hypothalamic microRNAs in regulation of FOS and FTO expression in response to hypoglycemia. J Physiol Sci. 2019;69(6):981–991. doi: 10.1007/s12576-019-00718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y.C., McPherson K., Marsh T., Gortmaker S.L., Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 74.Hinnouho G.M., Czernichow S., Dugravot A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36(9):551–559. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dina C., Meyre D., Gallina S. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 76.Frayling T.M., Timpson N.J., Weedon M.N. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ningombam S.S., Chhungi V., Newmei M.K. Differential distribution and association of FTO rs9939609 gene polymorphism with obesity: a cross-sectional study among two tribal populations of India with East-Asian ancestry. Gene. 2018;647:198–204. doi: 10.1016/j.gene.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Yako Y.Y., Echouffo-Tcheugui J.B., Balti E.V. Genetic association studies of obesity in Africa: a systematic review. Obes Rev. 2015;16(3):259–272. doi: 10.1111/obr.12260. [DOI] [PubMed] [Google Scholar]
- 79.Hebbar P., Abu-Farha M., Mohammad A. FTO variant rs1421085 associates with increased body weight, soft lean mass, and total body water through interaction with ghrelin and apolipoproteins in Arab population. Front Genet. 2019;10 doi: 10.3389/fgene.2019.01411. e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klungland A., Dahl J.A. Dynamic RNA modifications in disease. Curr Opin Genet Dev. 2014;26:47–52. doi: 10.1016/j.gde.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 81.da Fonseca A.C.P., Abreu G.M., Zembrzuski V.M. The association of the fat mass and obesity-associated gene (FTO) rs9939609 polymorphism and the severe obesity in a Brazilian population. Diabetes Metab Syndr Obes. 2019;12:667–684. doi: 10.2147/DMSO.S199542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaspi A., Khurana I., Ziemann M. Diet during pregnancy is implicated in the regulation of hypothalamic RNA methylation and risk of obesity in offspring. Mol Nutr Food Res. 2018 doi: 10.1002/mnfr.201800134. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Gao M., Zhu F. METTL3 is essential for postnatal development of brown adipose tissue and energy expenditure in mice. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-15488-2. e1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doaei S., Kalantari N., Mohammadi N.K. Up-regulation of FTO gene expression was associated with increase in skeletal muscle mass in overweight male adolescents. Arch Med Sci. 2019;15(5):1133–1137. doi: 10.5114/aoms.2019.87239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Huang N., Yang M. FTO is required for myogenesis by positively regulating mTOR-PGC-1α pathway-mediated mitochondria biogenesis. Cell Death Dis. 2017;8(3) doi: 10.1038/cddis.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu W., Feng J., Jiang D. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N(6)-methyladenosine. Sci Rep. 2017;7 doi: 10.1038/srep41606. e41606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu R., Liu Y., Yao Y. FTO regulates adipogenesis by controlling cell cycle progression via m(6)A-YTHDF2 dependent mechanism. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(10):1323–1330. doi: 10.1016/j.bbalip.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Wu R., Yao Y., Jiang Q. Epigallocatechin gallate targets FTO and inhibits adipogenesis in an mRNA m(6)A-YTHDF2-dependent manner. Int J Obes (Lond) 2018;42(7):1378–1388. doi: 10.1038/s41366-018-0082-5. [DOI] [PubMed] [Google Scholar]
- 89.Song T., Yang Y., Wei H. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 2019;47(12):6130–6144. doi: 10.1093/nar/gkz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X., Wu R., Liu Y. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2020;16(7):1221–1235. doi: 10.1080/15548627.2019.1659617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao X., Yang Y., Sun B.F. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24(12):1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merkestein M., Laber S., McMurray F. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat Commun. 2015;6 doi: 10.1038/ncomms7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X., Zhu L., Chen J., Wang Y. mRNA m(6)A methylation downregulates adipogenesis in porcine adipocytes. Biochem Biophys Res Commun. 2015;459(2):201–207. doi: 10.1016/j.bbrc.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 94.Liu Q., Zhao Y., Wu R. ZFP217 regulates adipogenesis by controlling mitotic clonal expansion in a METTL3-m(6)A dependent manner. RNA Biol. 2019;16(12):1785–1793. doi: 10.1080/15476286.2019.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo L., Li X., Tang Q.Q. Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) beta. J Biol Chem. 2015;290(2):755–761. doi: 10.1074/jbc.R114.619957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao Y., Bi Z., Wu R. METTL3 inhibits BMSC adipogenic differentiation by targeting the JAK1/STAT5/C/EBPbeta pathway via an m(6)A-YTHDF2-dependent manner. FASEB J. 2019;33(6):7529–7544. doi: 10.1096/fj.201802644R. [DOI] [PubMed] [Google Scholar]
- 97.Wu R., Guo G., Bi Z. m(6)A methylation modulates adipogenesis through JAK2-STAT3-C/EBPbeta signaling. Biochim Biophys Acta Gene Regul Mech. 2019;1862(8):796–806. doi: 10.1016/j.bbagrm.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 98.Cai M., Liu Q., Jiang Q., Wu R., Wang X., Wang Y. Loss of m(6) A on FAM134B promotes adipogenesis in porcine adipocytes through m(6) A-YTHDF2-dependent way. IUBMB Life. 2019;71(5):580–586. doi: 10.1002/iub.1974. [DOI] [PubMed] [Google Scholar]
- 99.Jiang Q., Sun B., Liu Q. MTCH2 promotes adipogenesis in intramuscular preadipocytes via an m(6)A-YTHDF1-dependent mechanism. FASEB J. 2019;33(2):2971–2981. doi: 10.1096/fj.201801393RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu W., Cao H., Yan J., Huang R., Ying H. Micro-managers' of hepatic lipid metabolism and NAFLD. Wiley Interdiscip Rev RNA. 2015;6(5):581–593. doi: 10.1002/wrna.1295. [DOI] [PubMed] [Google Scholar]
- 101.Stahl E.P., Dhindsa D.S., Lee S.K., Sandesara P.B., Chalasani N.P., Sperling L.S. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(8):948–963. doi: 10.1016/j.jacc.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 102.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 103.Luo J., Xu L., Li J., Zhao S. Nonalcoholic fatty liver disease as a potential risk factor of cardiovascular disease. Eur J Gastroenterol Hepatol. 2015;27(3):193–199. doi: 10.1097/MEG.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 104.Chen J., Zhou X., Wu W., Wang X., Wang Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J Physiol Biochem. 2015;71(3):405–413. doi: 10.1007/s13105-015-0420-1. [DOI] [PubMed] [Google Scholar]
- 105.Guo J., Ren W., Li A. Fat mass and obesity-associated gene enhances oxidative stress and lipogenesis in nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(4):1004–1009. doi: 10.1007/s10620-012-2516-6. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J., Li S., Li J. Expression and significance of fat mass and obesity associated gene and forkhead transcription factor O1 in non-alcoholic fatty liver disease. Chin Med J (Engl) 2014;127(21):3771–3776. [PubMed] [Google Scholar]
- 107.Sun L., Ling Y., Jiang J. Differential mechanisms regarding triclosan vs. bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126318. e126318. [DOI] [PubMed] [Google Scholar]
- 108.Hu Y., Feng Y., Zhang L. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m(6)A on lipogenic mRNAs. RNA Biol. 2020;17(7):930–942. doi: 10.1080/15476286.2020.1736868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S., Wang X., Zhang J. Exenatide ameliorates hepatic steatosis and attenuates fat mass and FTO gene expression through PI3K signaling pathway in nonalcoholic fatty liver disease. Braz J Med Biol Res. 2018;51(8) doi: 10.1590/1414-431X20187299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J., Ning Y., Zhang H. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.117034. e117034. [DOI] [PubMed] [Google Scholar]
- 111.Mo C., Yang M., Han X. Fat mass and obesity-associated protein attenuates lipid accumulation in macrophage foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Hypertens. 2017;35(4):810–821. doi: 10.1097/HJH.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 112.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 113.Bäck M., Yurdagul A. Jr, Tabas I., Öörni K., Kovanen P.T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Y., Liu Z., Tang H. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am J Physiol Cell Physiol. 2019;317(4):c762–c775. doi: 10.1152/ajpcell.00212.2019. [DOI] [PubMed] [Google Scholar]
- 115.Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 116.Zhu B., Gong Y., Shen L. Total Panax notoginseng saponin inhibits vascular smooth muscle cell proliferation and migration and intimal hyperplasia by regulating WTAP/p16 signals via m(6)A modulation. Biomed Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109935. [DOI] [PubMed] [Google Scholar]
- 117.Bardeesi A.S.A., Gao J., Zhang K. A novel role of cellular interactions in vascular calcification. J Transl Med. 2017;15(1) doi: 10.1186/s12967-017-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou T., Han D., Liu J. Factors influencing osteogenic differentiation of human aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2019;158(4):1–65. doi: 10.1016/j.jtcvs.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 119.Ferrara N., Kerbel R.S. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 120.Mo X.B., Lei S.F., Zhang Y.H., Zhang H. Detection of m(6)A-associated SNPs as potential functional variants for coronary artery disease. Epigenomics. 2018;10(10):1279–1287. doi: 10.2217/epi-2018-0007. [DOI] [PubMed] [Google Scholar]
- 121.Binder A., Ali A., Chawla R., Aziz H.A., Abbate A., Jovin I.S. Myocardial protection from ischemia-reperfusion injury post coronary revascularization. Expert Rev Cardiovasc Ther. 2015;13(9):1045–1057. doi: 10.1586/14779072.2015.1070669. [DOI] [PubMed] [Google Scholar]
- 122.Song H., Feng X., Zhang H. METTL3 and ALKBH5 oppositely regulate m(6)A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15(8):1419–1437. doi: 10.1080/15548627.2019.1586246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruan Z., Wang S., Yu W., Deng F. LncRNA MALAT1 aggravates inflammation response through regulating PTGS2 by targeting miR-26b in myocardial ischemia-reperfusion injury. Int J Cardiol. 2019;288 doi: 10.1016/j.ijcard.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 124.Yang C., Fan Z., Yang J. m(6)A modification of LncRNA MALAT1: a novel therapeutic target for myocardial ischemia-reperfusion injury. Int J Cardiol. 2020;306(9) doi: 10.1016/j.ijcard.2019.11.140. [DOI] [PubMed] [Google Scholar]
- 125.Saxena R., Weintraub N.L., Tang Y. Optimizing cardiac ischemic preconditioning and postconditioning via epitranscriptional regulation. Med Hypotheses. 2020;135 doi: 10.1016/j.mehy.2019.109451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McMurray J.J., Pfeffer M.A. Heart failure. Lancet. 2005;365(9474):1877–1889. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 127.Berulava T., Buchholz E., Elerdashvili V. Changes in m6A RNA methylation contribute to heart failure progression by modulating translation. Eur J Heart Fail. 2020;22(1):54–66. doi: 10.1002/ejhf.1672. [DOI] [PubMed] [Google Scholar]
- 128.Hubacek J.A., Vymetalova J., Lanska V., Dlouha D. The fat mass and obesity related gene polymorphism influences the risk of rejection in heart transplant patients. Clin Transplant. 2018;32(12) doi: 10.1111/ctr.13443. [DOI] [PubMed] [Google Scholar]
- 129.Devaux Y., Nossent A.Y. A role for m6A RNA methylation in heart failure development? Eur J Heart Fail. 2020;22(1):67–69. doi: 10.1002/ejhf.1714. [DOI] [PubMed] [Google Scholar]
- 130.Chen X., Luo Y., Jia G., Liu G., Zhao H., Huang Z. FTO promotes adipogenesis through inhibition of the Wnt/β-catenin signaling pathway in porcine intramuscular preadipocytes. Anim Biotechnol. 2017;28(4):268–274. doi: 10.1080/10495398.2016.1273835. [DOI] [PubMed] [Google Scholar]
- 131.Ronkainen J., Mondini E., Cinti F. Fto-deficiency affects the gene and MicroRNA expression involved in Brown adipogenesis and browning of white adipose tissue in mice. Int J Mol Sci. 2016;17(11) doi: 10.3390/ijms17111851. e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]