Introduction
Sarcoidosis is an inflammatory disease characterized by the formation of noncaseating granulomas in one or multiple organ systems. Sarcoidosis most commonly affects the lungs and lymph nodes but can affect nearly any organ including the heart. There are no molecularly targeted therapies approved by the US Food and Drug Administration for the treatment of sarcoidosis, and prednisone, which is approved for pulmonary sarcoidosis, remains a mainstay of therapy. Cutaneous lesions have been described in approximately 25% to 33% of patients with sarcoidosis, and treatment options are limited; there are no US Food and Drug Administration–approved treatments for cutaneous sarcoidosis.1 In light of the safety profile of steroids, they are used infrequently for treatment of cutaneous sarcoidosis. Although tetracycline antibiotics, antimalarial drugs, methotrexate, thalidomide, tumor necrosis factor-alpha blockers, and other immunomodulatory agents can be utilized in sarcoidosis, including cutaneous sarcoidosis, treatment responses are often suboptimal.
Over the past few years, Janus kinase (JAK) inhibitors have emerged as a promising new treatment approach for patients with both cutaneous and internal organ sarcoidosis. Here, we describe a patient with long-standing cutaneous sarcoidosis treated with tofacitinib resulting in clearance of her skin. We also summarize the literature describing the use of JAK inhibitors to treat sarcoidosis.
Case report
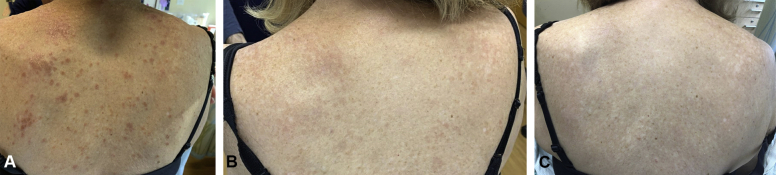
A 65-year-old woman with an 11-year history of cutaneous sarcoidosis presented for worsening skin involvement. Two skin biopsies were performed and showed well-formed, noncaseating granulomas consistent with sarcoidosis. Skin examination showed innumerable flesh colored to pink papules coalescing into plaques on her face, neck, shoulders, chest, back, and upper and lower extremities (Fig 1, A). There was no cervical, occipital, axillary, or inguinal lymphadenopathy. Pulmonary and cardiac review of systems was normal, and a chest x-ray and electrocardiogram were unremarkable.
Fig 1.
Clinical images of cutaneous involvement (A) at presentation, (B) after 3 months of tofacitinib, and (C) after 6 months of tofacitinib.
She had been taking hydroxychloroquine without any improvement. The hydroxychloroquine was discontinued, and the patient was treated with tofacitinib 5 mg twice daily. She reported improvement in her facial lesions within 2 weeks. After 3 months of treatment, her face and neck were clear, and only thin, faint papules persisted elsewhere (Fig 1, B). After 6 months of treatment, there were no longer any clinically apparent lesions (Fig 1, C). Her tofacitinib dose was reduced to 5 mg daily, and she continues to tolerate therapy without any adverse effects.
Discussion
Granuloma formation in sarcoidosis is immunologically complex. Multiple groups, including ours, have demonstrated that upregulation of JAK-signal transducer and activator of transcription (STAT)–dependent cytokine signaling, including interferon gamma activity, is involved in the pathogenesis of sarcoidosis. Early studies demonstrated constitutive activation of JAK-STAT signaling at the messenger RNA level in peripheral blood mononuclear cells and other tissue in patients with sarcoidosis.2,3 We previously analyzed cutaneous and pulmonary sarcoidosis tissue and found constitutive activation of STAT1 and STAT3 proteins in granulomas, which we hypothesized reflect the action of interferon gamma and interleukin 6 signaling, respectively.4, 5, 6 Additional JAK-STAT–dependent cytokines, including type I interferons, interleukin 2, interleukin 12, and interleukin 15 have also been implicated in other studies.7 Collectively, these findings provide the rationale for the use of JAK inhibitors as a molecularly targeted therapy to treat sarcoidosis.
To date, there have been 11 other reports, comprising a total of 21 patients with sarcoidosis, that have demonstrated benefit from JAK inhibition (Table I). Nine studies used oral JAK inhibitors, including tofacitinib (JAK1/2/3), ruxolitinib (JAK1/2), and baricitinib (JAK1/2), and 2 studies used topical (compounded) 2% tofacitinib ointment.4,5,7, 8, 9, 10, 11, 12, 13, 14, 15 Key details of these reports are summarized in Table I. Two of the 3 ruxolitinib reports, which were among the earlier reports, were based on observations made in patients taking the medication for coexisting polycythemia vera, with incidental improvement in sarcoidosis. Patients included men and women with varied skin phototypes and disease durations ranging from 10 weeks to 25 years. Those with cutaneous disease experienced complete responses to systemic JAK inhibition in 83% of the reports and partial responses in the rest. Partial responses in cutaneous involvement were seen in the two reported patients treated with topical tofacitinib. Regarding assessment of systemic disease, complete responses of internal organ sarcoidosis have been observed in 27% of patients treated with a JAK inhibitor and partial improvement occurred in the others. Kerkemeyer et al8 showed, in a prospective series of 5 patients with pulmonary sarcoidosis treated with tofacitinib, that of 3 patients who completed the study, all had improvement in respiratory symptoms and were able to successfully taper their systemic glucocorticoids.
Table I.
A summary of the reported use of Janus kinase inhibitors to treat cutaneous and systemic sarcoidosis
| Drug | JAKs | Dose | n | Δ Cutaneous? | Δ Systemic? | Disease duration | Fitzpatrick type | Sex | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Oral JAK inhibiton | ||||||||||
| Tofacitinib | JAK1/2/3 | 5 mg twice daily | 1 | CR | N/A | 8 y | II | F | United States | Damsky et al4 |
| Tofacitinib | JAK1/2/3 | 5 mg twice daily | 3 | CR (3) | N/A | 6-25 y | VI | 2 F, 1 M | United States | Damsky et al5 |
| Tofacitinib | JAK1/2/3 | 5-10 mg twice daily | 1 | CR | CR | 21 y | I | F | United States | Damsky et al6 |
| Tofacitinib | JAK1/2/3 | 2.5-16 mg daily | 5 | CR/NCR (3), PR (2) | PR (3), N/A (2) | Not reported | White | F | Australia | Kerkemeyer et al8 |
| Tofacitinib | JAK1/2/3 | 5 mg twice daily | 5 | N/A | PR (3), N/A (2) | 1-5 y | 4 Caucasian, 1 African American | 4 M, 1 F | United States | Friedman et al9 |
| Ruxolitinib | JAK1/2 | 5 mg twice daily | 1 | CR | NCR | 18 y | European anscestry | F | France | Rotenberg et al10 |
| Ruxolitinib | JAK1/2 | 10 mg twice daily | 1 | CR | PR | 1 y | VI | F | United States | Wei et al11 |
| Ruxolitinib | JAK1/2 | 20 mg daily | 1 | N/A | CR | 5 y | Not reported | F | France | Levraut et al12 |
| Baricitinib | JAK1/2 | 4 mg daily | 1 | N/A | CR | 10 wk | Not reported | F | Brazil | Scheinberg et al13 |
| Topical JAK inhibition | ||||||||||
| Tofacitinib | JAK1/2/3 | 2% ointment twice daily | 1 | PR | N/A | 5 y | II | F | United States | Singh et al14 |
| Tofacitinib | JAK1/2/3 | 2% ointment twice daily | 1 | PR | N/A | 9 y | VI | M | United States | Alam et al15 |
CR, Complete response; F, female; JAK, Janus kinase; M, male; N/A, not applicable; NCR, near complete response; PR, partial response.
In conclusion, there is significant unmet medical need in the treatment of sarcoidosis. The response of our patient's widespread cutaneous sarcoidosis to tofacitinib is consistent with the efficacy that has been previously reported. Taken together, there seems to be great promise of JAK inhibitors for the treatment of sarcoidosis, although prospective, controlled studies are needed to more completely evaluate the efficacy and safety of this therapeutic approach.
Conflicts of interest
Dr King has served on advisory boards and/or is a consultant and/or is a clinical trial investigator for AbbVie, Aclaris Therapeutics Inc, AltruBio Inc, Almirall, Arena Pharmaceuticals, Bioniz Therapeutics, Bristol-Meyers Squibb, Concert Pharmaceuticals Inc, Dermavant Sciences Inc, Eli Lilly and Company, Incyte Corp, LEO Pharma, Otsuka/Visterra Inc, Pfizer Inc, Regeneron, Sanofi Genzyme, TWi Biotechnology Inc, and Viela Bio. He is on speaker bureaus for Pfizer Inc, Regeneron, and Sanofi Genzyme. Dr Damsky has received research funding from Pfizer, is a consultant for Eli Lilly and Twi Biotechnology, and receives licensing fees from EMD/Sigma/Millipore in unrelated work. Author Talty has no conflicts of interest to declare.
Footnotes
Funding sources: There were no funding sources for this work. Author Talty is supported by National Institutes of Health (F30CA254246-01A1). Dr Damsky is supported by a career development award from the Dermatology Foundation.
IRB approval status: Not applicable.
References
- 1.Yanardaǧ H., Pamuk O.N., Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97(8):978–982. doi: 10.1016/s0954-6111(03)00127-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhou T., Casanova N., Pouladi N. Identification of JAK-STAT signaling involvement in sarcoidosis severity via a novel microRNA-regulated peripheral blood mononuclear cell gene signature. Sci Rep. 2017;7(1):4237. doi: 10.1038/s41598-017-04109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Zhao X., Wang J., Zong M., Yang H. Bioinformatics analysis of gene expression profile data to screen key genes involved in pulmonary sarcoidosis. Gene. 2017;596:98–104. doi: 10.1016/j.gene.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Damsky W., Thakral D., Emeagwali N., Galan A., King B. Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis. N Engl J Med. 2018;379(26):2540–2546. doi: 10.1056/NEJMoa1805958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damsky W., Thakral D., McGeary M.K., Leventhal J., Galan A., King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82(3):612–621. doi: 10.1016/j.jaad.2019.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damsky W., Young B.D., Sloan B., Miller E.J., Obando J.A., King B. Treatment of multiorgan sarcoidosis with tofacitinib. ACR Open Rheumatol. 2020;2(2):106–109. doi: 10.1002/acr2.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbankowski T., Hoser G., Domagała-Kulawik J. Th1/Th2/Th17-related cytokines in the bronchoalveolar lavage fluid of patients with sarcoidosis: association with smoking. Pol Arch Med Wewn. 2012;122(7-8):320–325. [PubMed] [Google Scholar]
- 8.Kerkemeyer K.L., Meah N., Sinclair R.D. Tofacitinib for cutaneous and pulmonary sarcoidosis: A case series. J Am Acad Dermatol. 2021;84(2):581–583. doi: 10.1016/j.jaad.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Friedman M.A., Le B., Stevens J. Tofacitinib as a steroid-sparing therapy in pulmonary sarcoidosis, an open-label prospective proof-of-concept study. Lung. 2021;199(2):147–153. doi: 10.1007/s00408-021-00436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotenberg C., Besnard V., Brillet P.Y., Giraudier S., Nunes H., Valeyre D. Dramatic response of refractory sarcoidosis under ruxolitinib in a patient with associated JAK2-mutated polycythemia. Eur Respir J. 2018;52(6):1801482. doi: 10.1183/13993003.01482-2018. [DOI] [PubMed] [Google Scholar]
- 11.Wei J.J., Kallenbach L.R., Kreider M., Leung T.H., Rosenbach M. Resolution of cutaneous sarcoidosis after Janus kinase inhibitor therapy for concomitant polycythemia vera. JAAD Case Rep. 2019;5(4):360–361. doi: 10.1016/j.jdcr.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levraut M., Martis N., Viau P., Suarez F., Queyrel V. Refractory sarcoidosis-like systemic granulomatosis responding to ruxolitinib. Ann Rheum Dis. 2019;78(11):1606–1607. doi: 10.1136/annrheumdis-2019-215387. [DOI] [PubMed] [Google Scholar]
- 13.Scheinberg M., Maluf F., Wagner J. Steroid-resistant sarcoidosis treated with baricitinib. Ann Rheum Dis. 2020;79(9):1259–1260. doi: 10.1136/annrheumdis-2020-217271. [DOI] [PubMed] [Google Scholar]
- 14.Singh K., Wang A., Heald P. Treatment of angiolupoid sarcoidosis with tofacitinib ointment 2% and pulsed dye laser therapy. JAAD Case Rep. 2021;7:122–124. doi: 10.1016/j.jdcr.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam M., Fang V., Rosenbach M. Treatment of cutaneous sarcoidosis with tofacitinib 2% ointment and extra virgin olive oil. JAAD Case Rep. 2021;9:1–3. doi: 10.1016/j.jdcr.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]