Abstract
Biallelic 4-hydroxyphenylpyruvate dioxygenase-like protein (HPDL) variants were recently reported as a cause of progressive and incurable neurodegenerative diseases ranging from neonatal-onset leukoencephalopathy with severe neurodevelopmental delay to spastic paraplegia. Although the physiological function of HPDL remains unknown, its subcellular localization in the mitochondria has been reported. Here, we report a case of HPDL-related neurological disease that was clinically and neuroimaging compatible with Leigh syndrome, previously unreported, and was treated with a ketogenic diet.
Keywords: HPDL, Leukoencephalopathy, Leigh syndrome, Ketogenic diet, Mitochondria
Abbreviations: HPDL, 4-hydroxyphenylpyruvate dioxygenase-like protein; LS, Leigh syndrome; MRS, Magnetic resonance spectroscopy; PDHC, Pyruvate dehydrogenase complex
1. Introduction
Leigh syndrome (LS), which is the most common presentation of mitochondrial disease phenotype in children, presents with hyperlactatemia and a lesion in the basal ganglia and brainstem [1]. Over 75 disease-associated genes have been identified till date [2]. Recently, biallelic 4-hydroxyphenylpyruvate dioxygenase-like protein (HPDL) variants were reported as a cause of neurodegenerative diseases, which range from neonatal-onset mitochondrial leukoencephalopathy with severe neurodevelopmental delay to juvenile onset hereditary spastic paraplegia [3], [4], [5], [6]. Neuroradiological findings associated with HPDL variants were also extremely diverse. These ranged from abnormalities in the white matter and striatum to no pathological findings. However, there are no previous reports of lesions in the basal ganglia and brainstem resembling LS. Here, we report the case of a pediatric patient who developed leukoencephalopathy resembling LS. We detected novel compound heterozygous HPDL variants in the patient and treated the patient with a ketogenic diet.
2. Case presentation
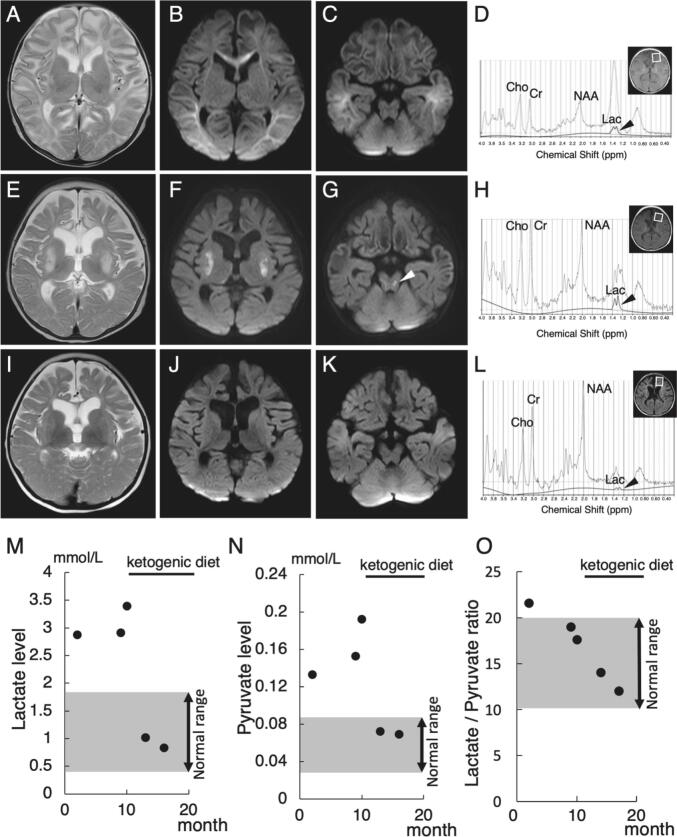
The patient was an 18-month-old child who was born to non-consanguineous parents at term after a normal pregnancy and delivery. Family history was non-contributory. From the fifth week, the patient exhibited poor feeding. At the age of eight weeks, the patient developed intractable cyanotic apnea. Brain MRI revealed a diffuse bilaterally symmetric, abnormal intensity in the white matter, predominantly in the frontal lobes (Fig. 1A). Diffusion-weighted images showed low signal intensity in the frontotemporal area, and high intensity in the occipital area (Fig. 1B and C). Single-voxel proton magnetic resonance spectroscopy (MRS) of the frontal white matter showed an abnormally high lactate peak (Fig. 1D). The patient received mechanical ventilation and vitamin cofactor therapy. The apnea resolved after treatment with phenobarbital and levetiracetam. The patient's plasma lactate and pyruvate levels and the lactate/pyruvate (L/P) ratio showed remained slightly higher than the normal levels (Fig. 1M−O). Cerebrospinal fluid studies showed a normal cell count, normal total protein level, and slightly elevated levels of lactate (2.85 mmol/l), pyruvate (0.13 mmol/l) and the L/P ratio (21.9). Urine organic acid, plasma amino acid, and dried blood spot acylcarnitine profiles were normal. Oral vitamin supplementation was continued.
Fig. 1.
Brain MRI and MRS findings and serum lactate and pyruvate levels. MRI at two months shows extensive cerebral white matter abnormalities especially in the frontal lobes [A]. Diffusion-weighted [B, C] images show low signal intensity in the fronto-temporal area, high intensity in the occipital area and no abnormal intensity signals in the brainstem at two months. At six months, axial T2-weighted [E] and diffusion-weighted [F] images demonstrate abnormal intensity signals in bilateral putamen, diffuse brain atrophy, and ventriculomegaly. Diffusion-weighted [G] images show abnormal intensity signals in the brainstem at six months (white arrow head). At eighteen months of age, bilateral lesions in the putamen with areas of restricted diffusion are seen at six months were absent in axial T2-weighted [I] and diffusion-weighted [J] images. Myelination was delayed for the patient's biological age. No significant progression of cerebral atrophy was observed compared to that at 6 months. Diffusion-weighted imaging [K] shows no abnormal intensity signals in the brainstem at eighteen months. MRS [D] (PRESS, TR/TR = 35/2000 msec, NEX = 128) in frontal white matter at two months, which was quantitatively analyzed with LCModel, shows prominent peak at around 1.3 ppm, which composed of macromolecules and lactate (broad line, 1.99 mmol/l) (black arrow head). Thin lines indicated the original spectra including all metabolites. Bold lines were the fitting curves for lactate. In MRS [H] at six months, the peak of lactate shows a high peak, similar to that at two months (1.80 mmol/l) (black arrow head). MRS [L] at eighteen months shows the reduced level of lactate (0.39 mmol/l) (black arrow head). Blood lactate [M], pyruvate [N] levels are slightly high before treatment with a ketogenic diet and thereafter, normalized. The lactate/pyruvate ratio [O] is slightly higher than the normal levels at two month and thereafter, decreased.
At 6 months of age, the patient again developed intractable cyanotic apnea and received the same treatment as before. MRI revealed new signal hyperintensities in the bilateral putamen and brainstem resembling LS (Fig. 1E–G), as well as reduced white matter volume and progressive cerebral atrophy. MRS showed an abnormal lactate peak (Fig. 1H). After acute therapy, the patient showed spastic quadriplegia, poor head control, mild strabismus, loss of ocular pursuit, and social smile. The patient had a slight increase in serum lactate (2.88–3.40 mmol/l) and pyruvate (0.13–0.19 mmol/l) levels despite the patient's stable condition (Fig. 1M and N). The L/P ratio was at the upper limit of normal range (Fig. 1O). These findings suggest that the patient had mitochondrial dysfunction. However, the mitochondrial respiratory chain activity in fibroblasts was unremarkable.
The patient started ketogenic dietary treatment at the age of 10 months. After 3 months, the serum lactate and pyruvate levels normalized (Fig. 1M, N). The L/P ratio was also decreased (Fig. 1O). The patient recovered the abilities to support the head, to socially smile, and to follow objects with the patient's eyes. On MRI, the bilateral putamen and brainstem lesions were no longer recognized at 18 months (Fig. 1I–K). Myelination progressed in the occipital lobe, although it was still delayed for the patient's age. The progression of cerebral atrophy appeared to decrease compared to the MRI findings at 6 months. MRS revealed a decrease in lactate levels (Fig. 1L). Chromosomal analysis and panel exome analysis of mitochondrial diseases were unremarkable. Whole exome sequencing identified compound heterozygous mutations in the HPDL gene: NM_032756.4:c.149_151del, p.(Gly50del) and c.537G > A, p.(Trp179*). The first variant given was a three base pair deletion of p.(Gly50del) and had small in-frame indels. The same codon p.Gly50Asp is listed twice in ClinVar as pathogenic [3], [6]. The second variant p.(Trp179*) is expected to lead truncating or missense mutations downstream. Therefore, according to the interpretation of the American College of Medical Genetics and Genomics criteria, these variations are likely pathogenic and pathogenic mutations, respectively.
3. Discussion
This is the first report of a patient with HPDL variants who developed leukoencephalopathy with bilateral putamen and brainstem lesions resembling LS. This patient's initial MRI showed diffuse white matter damages, as seen in previously reported cases of HPDL variants [3]. However, lesions resembling LS emerged later. Previous reports also showed that HPDL variants cause pathology reminiscent of mitochondrial diseases, such as hyperlactatemia [3], [4], [5], [6]. Although the physiological function of HPDL remains unknown, its subcellular localization in the mitochondria has been reported [3], [4], [5], [6]. Clinically, most HPDL-associated disorders with neonatal encephalopathy show diffuse white matter damage at the onset, as seen in this case, resulting in a severe phenotype [3]. However, no clear genotype-phenotype correlation was observed. The LS-like MRI findings with increased lactate in this case further suggest that the disease-causing HPDL variants may be associated with abnormal mitochondrial function.
After ketogenic dietary treatment, the patient's clinical symptoms and biochemical findings improved. Furthermore, lesions resembling LS disappeared, and myelination began to progress on MRI. Although the natural history of cases with HPDL variants has not been established, we found this clinical recovery to be remarkable. As a characteristic biochemical finding, this patient showed mild hyperpyruvinemia, as also reported in another case with a severe phenotype harboring HPDL variants [3]. The elevation of the blood pyruvate and the low L/P ratio are also recognized in the patients of mitochondrial diseases with pyruvate dehydrogenase complex (PDHC) deficiency, which is a mitochondrial disorder representing LS and the ketogenic diet proved to be beneficial [7], [8], although the levels of them in this case are higher than that of the typical patient with PDHC deficiency. In PDHC deficiency, this is because pyruvate derived from glycolysis cannot be metabolized through the tricarboxylic acid cycle. Ketone bodies supplied by a ketogenic diet serve as an alternative energy substrate to glucose, thereby suppressing seizure and apnea, and extending longevity [9]. The absence of abnormalities in the mitochondrial respiratory chain activity is also common in both diseases [3]. These common features led us to attempt ketogenic dietary treatment in this patient. The levels of plasma lactate, pyruvate and the L/P ratio and the lactate levels on MRS decreased after starting ketogenic diet. The clinical improvement observed in this patient suggested that HPDL may be involved in the pyruvate metabolic pathway in the mitochondria. Despite not having measured PDHC activity in this case, whether PDHC activity was inhibited in cells might be given to a new insight into the function analysis of HPDL. The progression of LS is often episodic [2]. Therefore, the efficacy of a ketogenic diet in HPDL-associated disorders should be further validated clinically and biologically, since HPDL-associated disorders have been reported to be progressive and incurable.
4. Conclusions
This study provides new insights into the variations of neuroimaging in patients with HPDL variants and suggests the possibility of treatment with a ketogenic diet.
Declaration of interest
None.
Acknowledgments
Acknowledgement
We thank Dr. Syunji Mugikura and Daisuke Ito for technical assistance in the MRS analysis of this article.
References
- 1.Zeviani M., Bertagnolio B., Uziel G. Neurological presentations of mitochondrial diseases. J. Inherit. Metab. Dis. 1996;19:504–520. doi: 10.1007/BF01799111. [DOI] [PubMed] [Google Scholar]
- 2.Lake N.J., Compton A.G., Rahman S., Thorburn D.R. Leigh syndrome: one disorder, more than 75 monogenic causes. Ann. Neurol. 2016;79:190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 3.Husain R.A., Grimmel M., Wagner M., Hennings J.C., Marx C., Feichtinger R.G., Saadi A., Rostásy K., Radelfahr F., Bevot A., Döbler-Neumann M., Hartmann H., Colleaux L., Cordts I., Kobeleva X., Darvish H., Bakhtiari S., Kruer M.C., Besse A., Ng A.C., Chiang D., Bolduc F., Tafakhori A., Mane S., Ghasemi Firouzabadi S., Huebner A.K., Buchert R., Beck-Woedl S., Müller A.J., Laugwitz L., Nägele T., Wang Z.Q., Strom T.M., Sturm M., Meitinger T., Klockgether T., Riess O., Klopstock T., Brandl U., Hübner C.A., Deschauer M., Mayr J.A., Bonnen P.E., Krägeloh-Mann I., Wortmann S.B., Haack T.B. Bi-allelic HPDL variants cause a neurodegenerative disease ranging from neonatal encephalopathy to adolescent-onset spastic paraplegia. Am. J. Hum. Genet. 2020;107:364–373. doi: 10.1016/j.ajhg.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S.G., Lee S., Fabunan R., Chai G., Zaki M.S., Abdel-Salam G., Sultan T., Ben-Omran T., Alvi J.R., McEvoy-Venneri J., Stanley V., Patel A., Ross D., Ding J., Jain M., Pan D., Lübbert P., Kammerer B., Wiedemann N., Verhoeven-Duif N.M., Jans J.J., Murphy D., Toosi M.B., Ashrafzadeh F., Imannezhad S., Karimiani E.G., Ibrahim K., Waters E.R., Maroofian R., Gleeson J.G. Biallelic variants in HPDL, encoding 4-hydroxyphenylpyruvate dioxygenase-like protein, lead to an infantile neurodegenerative condition. Genet. Med. 2021;23:524–533. doi: 10.1038/s41436-020-01010-y. [DOI] [PubMed] [Google Scholar]
- 5.Morgan N.V., Yngvadottir B., O'Driscoll M., Clark G.R., Walsh D., Martin E., Tee L., Reid E., Titheradge H.L., Maher E.R. Evidence that autosomal recessive spastic cerebral palsy-1 (CPSQ1) is caused by a missense variant in HPDL. Brain Commun. 2021;3:fcab002. doi: 10.1093/braincomms/fcab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiessner M., Maroofian R., Ni M.Y., Pedroni A., Müller J.S., Stucka R., Beetz C., Efthymiou S., Santorelli F.M., Alfares A.A., Zhu C., Meszarosova A.Uhrova, Alehabib E., Bakhtiari S., Janecke A.R., Otero M.G., Chen J.Y.H., Peterson J.T., Strom T.M., Jonghe P.De, Deconinck T., Ridder W.De, Winter J.De, Pasquariello R., Ricca I., Alfadhel M., van de Warrenburg B.P., Portier R., Bergmann C., Firouzabadi S.Ghasemi, Jin S.C., Bilguvar K., Hamed S., Abdelhameed M., Haridy N.A., Maqbool S., Rahman F., Anwar N., Carmichael J., Pagnamenta A., Wood N.W., Mau-Them F.Tran, Haack T., Rocco M.Di, Ceccherini I., Iacomino M., Zara F., Salpietro V., Scala M., Rusmini M., Xu Y., Wang Y., Koh K., Nan H., Ishiura H., Tsuji S., Lambert L., Schmitt E., Lacaze E., Küpper H., Dredge D., Skraban C., Goldstein A., Willis M.J.H., Grand K., Graham J.M., Lewis R.A., Millan F., Duman Ö., Dündar N., Uyanik G., Schöls L., Nürnberg P., Nürnberg G., Bordes A.Catala, Seeman P., Kuchar M., Darvish H., Rebelo A., Bouçanova F., Medard J.J., Chrast R., Auer-Grumbach M., Alkuraya F.S., Shamseldin H., Tala S.Al, Varaghchi J.Rezazadeh, Najafi M., Deschner S., Gläser D., Hüttel W., Kruer M.C., Kamsteeg E.J., Takiyama Y., Züchner S., Baets J., Synofzik M., Suzuki Y., Schüle R., Horvath R., Houlden H., Bartesaghi L., Lee H.J., Ampatzis K., Pierson T.M., Senderek J., Genomics England Research Consortium, PREPARE Network Biallelic variants in HPDL cause pure and complicated hereditary spastic paraplegia. Brain. 2021;144:1422–1434. doi: 10.1093/brain/awab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel K.P., O'Brien T.W., Subramony S.H., Shuster J., Stacpoole P.W. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol. Genet. Metab. 2012;106:385–394. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Cui Y., Jiang D., Ma C.Y., Tse H.F., Hwu W.L., Lian Q. Management of Leigh syndrome: current status and new insights. Clin. Genet. 2021;93(108):1131–1140. doi: 10.1111/cge.13139. [DOI] [PubMed] [Google Scholar]
- 9.Wexler I.D., Hemalatha S.G., McConnell J., Buist N.R., Dahl H.H., Berry S.A., Cederbaum S.D., Patel M.S., Kerr D.S. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]