Abstract
The field of nanotechnology has grown exponentially during the last few decades, due in part to the use of nanoparticles in many manufacturing processes, as well as their potential as clinical agents for treatment of diseases and for drug delivery. This has created several new avenues by which humans can be exposed to nanoparticles. Unfortunately, investigations assessing the toxicological impacts of nanoparticles (i.e. nanotoxicity), as well as their possible risks to human health and the environment, have not kept pace with the rapid rise in their use. This has created a gap-in-knowledge and a substantial need for more research. Studies are needed to help complete our understanding of the mechanisms of toxicity of nanoparticles, as well as the mechanisms mediating their distribution and accumulation in cells and tissues and their elimination from the body. This review summarizes our knowledge on nanoparticles, including their various applications, routes of exposure, their potential toxicity and risks to human health.
Keywords: Nanotoxicology, Nanoparticles, Toxicology, Cell death, Drug delivery
1. Introduction: What are nanoparticles?
The field of nanotechnology has expanded tremendously within the last few decades. This can be exemplified by the fact that federal spending on nanotechnology jumped from 500 million dollars in 2001 to 1.8 billion dollars in 2010 [1]. Nanoparticles (NPs) are defined as particles with diameters equal or smaller than 100 nm. NPs have special properties distinct from larger particles in the macro-scale size [2]. These properties include their small size and relatively large surface area, which facilitates their use in numerous areas including electronics, cosmetics and medicine. Currently, there is a growing application of NPs for both diagnostic and therapeutic purposes [3]. This review aims to introduce the reader to different types of NPs, their preparation and their various applications. The review then focuses on factors dictating the toxicity of NPs with a special emphasis on their target-organ toxicity and factors dictating their mechanisms of action. The review finishes with a description of current ongoing efforts to understand the mechanism mediating the toxicity of NPs and assess the risk they pose to humans.
2. Different types of nanoparticles
2.1. Solid nanoparticles (Iron oxide, Gadolinium, Manganese, Gold, Silver, and Platinum)
Iron-oxide nanoparticles are prepared by conjugating biocompatible polymers to an inorganic core of magnetite (Fe3O4) or maghemite (Fe2O3) via an anchoring group, such as an amine or carboxylic acid group. The stability of these NPs can be increased by forming a polymer-based shell around their cores. Iron oxide NPs are being used in various fields including use of sensors in combination with magnetic resonance imaging (MRI). The physical characteristics of iron oxide NPs, such as size distribution and surface chemistry, are largely affected by the method of preparation, which is true for all NPs. Iron oxide NPs are prepared using various techniques, including coprecipitation and microemulsions [4]. One limitation of iron oxide NPs is that they tend to aggregate due to magnetic, van der Waals, or hydrophilic/hydrophobic interactions. One approach to reduce their aggregation is changing their surface properties using PEGylation, which is the attachment of polyethylene glycol (PEG) to their surface [5].
Gadolinium (Gd3+) has been used as a clinical contrast agent in various MRI procedures such as brain tumor imaging and angiography [6,7]. The increased use of gadolinium NPs is due to their high relaxivity (long electronic relaxation times owing to their seven unpaired electrons), improved biodistribution, and their passive uptake in the tumor via the enhanced permeability and retention effect (EPR), which results from defects in the vasculature and lack of functional lymphatics. These features allow for a more guided and personalized therapy [8,9]. In addition, these NPs were shown to be efficient radio-sensitizers in different radio-resistant cell lines [9].
Gold nanoparticles (AuNPs) have been used in cancer diagnosis, photodynamic cancer therapy, radiotherapy, and in targeted gene therapy [10,11]. AuNPs are also used as drug-delivery scaffolds and were shown to have reduced toxicity as compared to other NPs [12]. AuNPs were also used for targeted therapy by conjugating them to targeting molecules, and have shown considerable potential for the delivery of various cancer drugs [13,14]. The use of AuNPs for drug delivery depends on their size, shape, surface characteristics, and their stability. Furthermore, these NPs can be used to develop theranostic systems where diagnosis, therapeutic, and imaging can be combined for improved and targeted therapies [15,16]. One challenge with the use of AuNPs, at least their use in humans, is that they have slow rates of elimination.
In contrast to AuNPs, the use of silver NPs (AgNPs) in medical applications has been more limited, although some studies have been recently published. Wang et al. developed Ag-hybridized-silica-NPs to use as immunosensors for prostate specific antigen (PSA) in human serum of patients, which allowed the detection of PSA over a wide concentration range from 0.05 to 50 ng/ml [17]. In another study, Swanner and his co-workers used AgNPs as a therapeutic agent and investigated their cytotoxic effects on triple-negative breast cancer. Their results showed that exposure of breast cancer cells to AgNPs caused dose-dependent toxicity and cell death [18]. AgNPs have been also used for the treatment of microbial infections. Chaudhari et al. (2016) used silver coated single walled carbon nanotubes (SWCNTs-Ag) for the delivery of antimicrobial peptides (APs) and showed higher potency compared to the APs alone. This study demonstrated the ability of these nanoparticles to target microbial pathogens while remaining non-toxic to human cells [19].
Platinum-based NPs (PtNPs) have also seen limited medical applications. However, Spain et al. have used PtNPs as electrochemical immunosensors by conjugating PtNPs to a recombinant PSA antibody for the detection of prostate cancer cells. Their technique was able to detect PSA at picomolar concentration levels [20].
2.2. Quantum and Cornell dots
Quantum dot NPs (QDNPs) are used in various electronic applications such as solar cells and light-emitting diodes (QD-LEDs) [21–23]. In addition, QDNPs have also been used in cancer cell imaging and assessment of the tumor microenvironment [24]. One unique property of QDs, as compared to most NPs, is that they have very small sizes ranging between 2 and 7 nm in diameter. Further, QDs can be combined with different semi-conductors and heavy metals such as CdSe, PbS and ZnS [25]. However, QDs have the drawback that they display high levels of toxicty both in vitro and in vivo. The addition of heavy metals with QDs has further limited their clinical utility due to concerns about the added toxicity associated with these metals. This combination has further limited the use of QDs in living systems, although these NPs have seen heavy use in the field of nanosensors and diagnostics [26].
Silica based Cornell dots (C dots) have been proposed as alternatives for QDNPs. These spherically shaped C dots have a silica based core in which fluorescent molecules are inserted and are usually surrounded by a PEGylated silica shell [27]. C dots can be targeted to tumors theoretically by conjugating them to monoclonal antibodies specific for certain cancer markers. Once bound to the targeted cells, C dots can be illuminated using a near-infrared light source, resulting in fluorescence and optical guidance during surgeries. It is also suggested that C dots can be used as nanoparticulate drug carriers by conjugating them to drugs used in cancer therapy. However, a challenge with these NPs is that they are quite stable in living systems, and as such may not be biodegradable [28].
2.3. Carbon-based nanoparticles
Carbon-based NPs, such as carbon nanotubes (CNTs) include single-walled (SWCNTs), multi-walled (MWCNTs), graphene quantum dots (GQDs), fullerenes (spherical, ellipsoidal, or tube-shaped) and nanodiamonds. These NPs are being increasingly used in various biomedical applications such as drug delivery, imaging and photothermal therapy [19,29,30]. Despite their promising applications owing to their high stability, specific surface chemistry and high drug loading capacity, the safety of CNTs is still questionable as they were shown to be toxic to healthy tissues especially in cases of chronic exposure [31–33].
2.4. Lipid-based NPs (liposomes)
Liposomes have been intensively studied since they were first described in 1965 [34]. In general, liposomes typically are composed of a phospholipid bilayer that entrap an aqueous core. The addition of various lipids, cholesterol, hydrophilic polymers (e.g., polyethylene glycol) have all been used to alter their stability, biodistrubtion, efficacy and toxicity. Owing to their various advantages, liposomes are considered to be one of the most successful drug-carrier systems to date. A number of liposome-based formulations have been approved by the FDA such as Doxil®, and many others are either in clinical or preclinical trials [35].
The choice of liposome preparation methods depends on many factors including the physicochemical properties of the material to be encapsulated, the liposomal components, effective concentration of the encapsulated substance, optimum desired size, stability, shelf-life of liposomes, batch-to-batch reproducibility and feasibility of large-scale production. Useful liposomes rarely form spontaneously; they typically form after supplying enough energy to a dispersion of lipids in a polar solvent, such as water [35,36]. Methods of preparing liposomes in the laboratory may involve drying down lipids from their organic solvents, followed by dispersion of lipids in aqueous media and purification of the resultant liposomes. Large scale manufacruring of liposomes involve different approaches, such as the solvent injection method. Robust anlaysis of the final product, size, stability, charge, encapsulation efficiency, rate and extent of release of encapsulated agents, are necessary to ensure batch to batch reproducibility and performance [37].
Liposomes can be made using various methods such as hydration of a thin lipid film (Bangham method), the reverse-phase evaporation method, and the ethanol injection method [37,38]. The size, lamellarity, and homogeneity of liposomes can be controlled using different techniques such as sonication, extrusion, and high-pressure homogenization [39,40]. The majority of liposomes are prepared using zwitterionic lipids. The addition of PEG to the distearoyl phosphatidylethanolamine (DSPE) confers a slight negative charge to the surface of the liposomes. Cationic liposomes are used as gene delivery carriers and have been used for clinical and non-clinical applications [41,42]. Early liposome formulations (conventional liposomes) exhibited short half-life’s and rapid systemic clearances. PEGylation of liposomes, addition of cholesterol and use of saturated high-phase transition lipids resulted in the generation of “Stealth™ liposomes” (SL), with increased stability and greater systemic circulation times by minimizing their opsonization by serum proteins and clearance by fixed and circulating macrophages [43–45]. In addition to their ability to stabilize drugs and enhance their biodistribution, the long systemic circulation time of Stealth™ liposomes allows them to accumulate passively in solid tumors due to the EPR effect [46,47]. Doxil® (Centocor Ortho Biotech, Horsham, PA), which encapsulate the anti-cancer drug doxorubicin, is an example of clinically used stealth liposomes. SL were also recently used to encapsulate the p21 activated kinase (PAK-1) inhibitor IPA-3, which was effective at decreasing the growth of human prostate cells in vitro and tumor (PC-3) xenografts in vivo, with no evidence of toxicity [48].
2.5. Nanoemulsions
Nanoemulsions (NE) are one of the most common NPs used in pharmaceutical applications. These systems are formed of two immiscible liquids, usually oil and water, and are used as nanocarriers of drugs in nanodroplets, where oil droplets are dispersed in water, or water droplets are dispersed in oil. These dispersions are typically stabilized using surfactants [49,50]. NE are categorized into anionic, cationic and neutral nanoemulsions based on the surfactant used. They are prepared using different techniques including high pressure homogenization, low energy emulsification, microfluidization, and solvent evaporation [51]. Major applications of NE include treatment of cancer and delivery of vaccines; however, a major limitation of these carriers is that they are thermodynamically unstable especially for emulsions > 500 nm. In contrast, nanoemulsions with small droplets, in the range of 20–200 nm, are more thermodynamically stable [49]. Long term thermodynamic stability is still a major limitation of NE [51].
NE have the ability to protect drugs from hydrolysis and enzymatic degradation, resulting in increased drug stability and increased circulation half-life. NE are reported to be suitable for multiple routes of administration including parenteral, oral, transdermal and ocular [52]. Similar to many other NPs, NE are subject to opsonization, and phagocytosis by circulatory and fixed macrophages. NE may also be transported into organs such as the liver and the spleen because of their perforated blood vessels, which makes NE great drug carriers if these organs are the target site of the treatment [53].
2.6. Polymer based NPs
Polymer based NPs have been used increasingly in recent years. Two of the more commonly used are poly-lactic glycolic acid (PLGA) and chitosan. These NPs are both biocompatible and biodegradable and were both approved by the FDA for clinical use. Polymer based NPs can be used as carriers for proteins, genes and drugs [54,55]. The encapsulated entity can be incorporated in a shell or matrix of polymer, or attached to the surface. PEGylated polymeric NPs have been shown to have prolonged blood circulation and improved uptake of the encapsulated entity at the target site [56].
3. Nanoparticle applications and uses
Nanoparticles have been increasingly used during the last decade in areas such as the chemical industry, food technology, electronics, skin creams, sports equipment, cosmetics and biomedicine (Table 1). This review will primarily focus on the use of NPs in biomedical applications, which has seen less use of NPs, despite a huge amount of funding and studies towards this goal. The reader is referred to other reviews that focus on other applications of NPs [57–59].
Table 1.
Classes of nanoparticles, their applications and their potential toxicity.
| Nanoparticles | Applications | Potential Toxicity effects | References |
|---|---|---|---|
| Quantum dots | Imaging, diagnosis, electronic applications | Inflammation, liver damage | 30, 31, 32, 33,34 |
| Gold | Photodynamic cancer therapy, radiotheray, gene therapy | liver damage, activation of hepatic macrophages | 20, 21, 22, 23, 24 |
| Silver | Drug delivery, diagnosis, imaging | Kidney damage, inflammation, mineralization | 27 |
| Platinum | Diagnosis | liver, kidney damage | 28, 29 |
| Titanium dioxide, Zinc oxide | Dermatological drug delivery, food additive, paints, food packaging | Inflammation, liver damage, DNA damage | 91, 99, 147 |
| Carbon nanotube | Drug delivery, imaging, photothermal therapy | Inflammation, DNA damage | 37, 38, 39, 40, 146 |
| Liposomes | Drug delivery | Skin | 42, 43, 47, 69 |
| Polymeric (PLGA) | Drug/protein/gene delivery | inflammation | 53, 54, 55 |
3.1. NP applications in drug delivery
Nanoparticles have been suggested to be useful in the prevention, diagnosis and treatment of different diseases such as cancer and cardiovascular disease [60,61]. Numerous NPs are being tested for drug delivery, including liposomes, iron oxide NPs, and polymeric NPs, and some are being tested in preclinical and clinical trials with the goal to improve site-specific delivery of encapsulated compounds [62–64]. In theory, the encapsulation of drugs into NPs permits greater and more specific uptake of drugs into diseased cells such as cancer cells, as compared to normal/healthy cells. This would limit the off-target toxicity of the encapsulated drug, a major limitation for the use of many chemotherapeutics.
Encapsulation of drugs within NPs can improve their stability by minimizing preciptation of poorly souble compounds (i.e., reducing the need for toxic cosolvents) and altering their rates of metabolism and clearance. Additionally, NPs may also improve drug efficacy by promoting slower and sustained drug release and improving drug delivery [65–67]. For example, the extended circulation of doxorubicin-encapsulated liposomes was shown to have a 300-fold increase in efficacy, compared to free doxorubicin, in the treatment of metastatic cancer and Kaposi’s sarcoma [68,69].
Encapsulating a drug into a basic NP alone does not result in specific cell targeting. Typically, an additional moiety must be included to enhance specific cell delivery. As such, NPs can be actively targeted to cells by conjugating them to specific ligands capable of binding to specific membrane proteins, such as overexpressed folate receptors in breast cancer cells and other folate receptor positive cancer cells [70]. Quantum dot NPs were recently used by Bwatanglang et al. who used “folic-acid functionalized chitosan-encapsulated QDs” and reported enhanced binding and internalization following their binding to folate receptors in MCF-7 and MDA-MB-231 breast cancer cells [71]. Gold NPs (AuNPs) were also suggested to be useful for the treatment of breast cancer by targeting cellular mitochondria and by inducing apoptosis [72]. Despite their promising efficacy as drug carriers, there are less NPs in clinical use than one would predict, partly because of toxicity by mechanisms not fully understood. This is especially true for studies where NPs are being administered chronically [3].
3.2. NP application in gene therapy
NPs are used as delivery devices in gene therapy to replace either missing or diseased genes. Gopalan et al. showed the efficacy of cationic lipid diethyl oleyl-1, 2-bis (oleoyloxy)-3-(trimethyl ammonio) propane (DOPT) and cholesterol, (DOTAP:Chol) NPs as gene carriers for the treatment of lung cancer using the tumor suppressor gene (FUS1) in vitro and in vivo [73]. A study published in the same year showed the efficacy of poly-lactic glycolic acid, PLGA-mediated gene delivery of the wild-type p53 gene to alter breast cancer cell growth [74]. Another study in the following year used PEgylated polymeric NPs (DNA-containing poly (ethylene glycol)-modified (PEGylated) gelatin NPs) to deliver the tumor necrosis factor gene (TNFα) to solid tumors and showed that these NPs were effective, biocompatible, and had a long circulation time after their systemic administration into Lewis lung carcinoma (LLC) bearing mice [75]. In a more recent study, Guo and his group used AuNPs as non-viral gene carriers for the delivery of small-interfering RNA (siRNA) to decrease cancer-associated gene expression in prostate cancer cells [76]. Although the use of NPs for gene therapy is a promising approach for the treatment of diseases such as cancer, viral infections and some inherited diseases, the application of cationic NP has been known to illict strong immunological responses and the overall safety and effectiveness of these strategies are still under investigation.
3.3. NP application in imaging and diagnosis
Molecular imaging is a powerful tool to detect and quantify cellular and molecular changes in vitro and in vivo. NP-based probes usually have high photostability and wide ranges of absorption coefficients making them suitable for detection as well as diagnosis of cellular changes [77]. NPs can also be further conjugated with antibodies and other molecules for more efficiently targeted approaches [2]. In a 2003 study, Wu and his group used QD—based immunofluorescent labeling of the breast cancer marker Her2 and showed superior efficacy when compared to conventional fluorophores in animal models [78]. In the following year, Gao and his team showed the accumulation of fluorescent QDNPs in cancer cells when administered to human prostate cancer xenograft bearing nude mice [79].
Iron oxide NPs have been used to track and evaluate inflammation, diagnose cancers, and to determine stem cell progression. Zhu et al. used these NPs in conjunction with magnetic resonance imaging (MRI) to track prostate specific membrane antigen (PSMA) and showed specific uptake of these NPs into PMSA expressing cells [80]. Other NPs used for this purpose include those reported by Wang et al. who developed a “silver-Ag-hybridized-silica-NP-based electrochemical immunosensor” for detecting PSA in human serum [17]. AgNPs were also used by Swanner et al. to radiosensitize triple-negative breast cancer cells [18], and near-infrared QDs and 89Zr dual-labeled NPs were recently used as self-illuminating NPs for imaging lymph nodes and prostate cancer tumors [81]. Other NPs recently developed to detect PSA include CNT-based biosensors [82]. Finally, Misra et al. used a carbon NP-DNA complex (CNPLex) to transfect a green fluorescent protein (GFP) reporter gene containing plasmid DNA (pDNA) pEGFP-N1 into breast cancer MCF-7 and MDA-MB-231 cells and showed high efficiency of these NPs to deliver the plasmid into the cytoplasm of the transfected cells [83].
Liposome-based NPs can also be used for combined treatment and imaging of cancer cells. Yeh et al. recently used peptide-conjugated liposomes to deliver both doxorubicin and vinorelbine to cancer cells [84]. Another study used a liposomal-based approach to target breast cancer cells and ultrasound stimuli to facilitate the release of doxorubicin, which correlated with tumor regression in a breast cancer mouse model [85].
4. Routes of exposures to NPs
NPs have been suggested to be useful in the diagnosis of disease, medical imaging, drug delivery, gene therapy, in industrial applications and many other areas. However, the toxicological effects of these NPs on human (and animal) health after exposure are not fully understood. This lack of understanding results in an ethical obligation to take necessary precautions regarding their use to minimize both occupational and environmental exposure [2]. The remainder of this review focuses on the toxic effects of NPs exposure on human health.
While this review focses on toxicity, it should be pointed out that the primary reason for the lack of NPs’ use in the clinic is a lack of activity, as opposed to toxicty. This lack of activity is most likely attributed to an inability to change the pharmacokinetics of the encapsulated drugs, as well as the rapid clearance of NPs by the liver, spleen and other organs [86]. Nevertheless, there do exist some NPs whose toxicty is known to be a limitation, inluding cationic NPs. These NPs can biodegrade somewhat rapidly, and both the parents and the biodegradable products penetrate deeply into tissues and cells, due in part to their small size. This results in plasma membrane destabilization and cell death, tissue damage, and organ dysfunction [87,88].
While the mecahnisms of toxicty of catioinic NPs are fairly well understood, there remains a lack of complete understanding of the mechanisms mediating the toxic effects of other NPs [3]. Further, there is a relatively lack of information correlating NP-induced toxicty to exposure in humans. Studies that have been conducted demonstrate a high pulmonary inflammatory responses for some NPs. Further, many NPs can cause oxidative stress and interact with cellular macromolecules, mostly because of their increased surface area to size ratio [89,90]. Other biophysical features of NPs, such as size and surface properties also enhance their distribution, accumulation and uptake via various pathways and may affect various cellular and organ functions [91,92].
4.1. Different routes of exposure to nanoparticles
Exposure to NPs may occur through ingestion, injection, inhalation, and skin contact (Fig. 1). Similar to most chemicals, the potential toxicities on the different organ systems are dependent on the exposure routes. Some of these exposures are unintentional such as in pulmonary inhalation during occupational exposure that can cause lung inflammatory reactions and even fibrosis and necrosis of lung tissues [93,94]. Other exposures are intentional, such as application of skin products or ingestion.
Fig. 1.
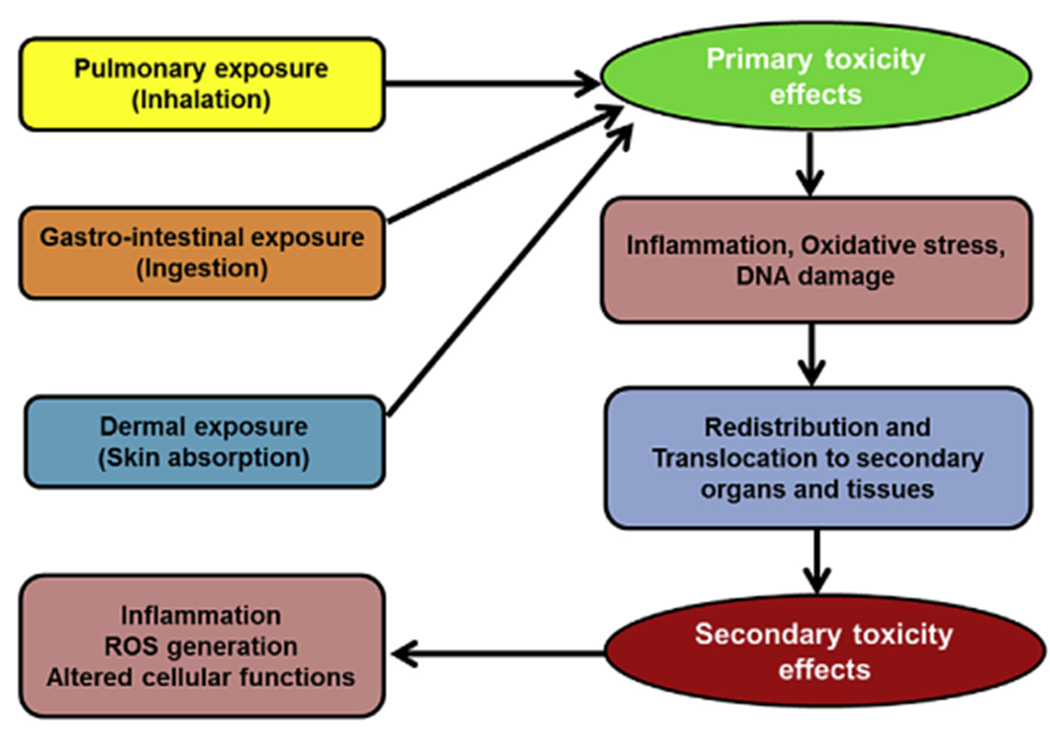
Exposure routes of nanoparticles and their associated toxicological effects.
4.2. Exposure through the skin
The skin is the largest organ in the human body and is an effective barrier against many potentially toxic chemicals. The skin is also a major route of administration and exposure to different cosmetics, sunscreens and components of surgical implants containing NPs. However, the permeability of the skin to these NPs is still not fully understood [95]. The rapidly growing use of NPs-based cosmetics has resulted in increased dermal exposure, which is followed by un-intended penetration into the blood circulation. The penetration of NPs through the skin can happen via the transcellular pathway and/or via the follicular and sweat gland penetration pathways [96–98]. Titanium dioxide nanoparticles (TiO2 NPs) are an example of NPs widely used in dermatological applications. These NPs have been shown in some models to indirectly damage the skin by causing cell death. There is also little evidence that some NPs can induce select organ toxicity due to their absorption, distribution and subsequent tissue accumulation following their skin permeation [99]. These effects, of course, are dependent on the dose of the NPs used.
The skin can also be a major route of unintentional exposure to NPs that are generated during welding fumes emissions, during waste incineration from natural gases and oil production, and during NP manufacturing. Once these NPs penetrate the skin, they can cause various toxic effects including DNA damage and apoptosis [100–103].
4.3. Exposure through inhalation
NPs can be intentionally and unintentionally inhaled resulting in their penetration into the lungs and interaction with the epithelium layer. The resulting inflammation can ultimately take NPs to nearby lymph nodes [104–106]. The factors that influence the inhalation of NPs and their effects on lungs include their physical (primarily their size) and chemical properties, their inhaled quantity, their rate of deposition in the lungs, and their rate of elimination [107]. Different studies have suggested that NPs can alter the function of alveolar macrophages and may also mediate the genesis of lung inflammation and lung cancer [108–110].
The inhalation of some NPs is thought to cause inflammatory reactions associated with symptoms of asthma [111]. For example, the exposure of mice lungs to carbon-based NPs was shown to cause increased eosinophilic response [112]. There are also active investigations of pulmonary exposure to NPs in the workplace including, carbon nanotubes (CNTs), titanium dioxide, aluminum oxide, and silver NPs. These studies, and others, suggest that the major route of exposure is through handling tasks in secondary manufacturing industrial-scale facilities and that monitoring exposure is a crucial step in health risk managements [113,114]. These studies also suggest that in addition to size, the shape and flexibility of the NP may influence their toxicity.
4.4. Exposure through ingestion
NPs have been used heavily in the agriculture and food industry [115]. NPs use in the food industry has many purposes including changing/masking product’s taste and texture, improving packaging as well as changing the absorption profile of certain products [116]. The extensive use of NPs for oral ingestion has raised serious concerns about their safety to the human health. While many NPs, such as liposomes, are not usually absorbed by the gastrointestinal system; solid NP and micro- and nano-emulsions have been used to improve oral solubility and dissolution of encapsulated drugs. Once ingested some NPs are absorbed through the digestive system via M-cells, then transported to the lymphatic tissues [98,117]. Other ingested NPs can either be excreted if unstable, or accumulate in the digestive system, which can result in blockage of the gastrointestinal tract [118].
5. Absorption, distribution, metabolism, and excretion (ADME) of NPs
Once in the blood stream many NPs accumulate in different organs such as the liver, spleen, kidneys and lungs because of the unique architecture of their leaky blood vessels and in tumors because of the EPR effect [53,119]. The exact organ is dependent on the biophysical and chemical properties of the NPs, as well as the organ. In addition, aggregation of NPs may affect their ADME profile.
In general, the liver and spleen are the major sites for accumulation of many NPs regardless of the exposure route. QDs have been shown to aggregate into clusters in the lymph nodes once injected into mice [120]. Metal-containing NPs are able to form ions, which enter the blood circulation and accumulate in several tissues, including the liver [121]. Paek et al. suggested that even though zinc oxide NPs are found in the systemic circulation in both particulate and zinc ionic forms, they exist primarily in the ionic form in the tissues [122].
Silver NPs have been shown to accumulate primarily in the liver, lungs and the spleen after intravenous injections [123]. Gold NPs have been shown to accumulate rapidly in the liver and spleen after a single intravenous injection. The ability of NPs to accumulate in many tissues, as well as persist in the blood, results in their incomplete clearance and altered redistribution. This results in a slow elimination from the urine, feces and lungs [124].
Both urinary and fecal clearances of NPs have been shown to be size-dependent, as smaller NPs are cleared faster than larger ones [125]. The major pathway for cellular uptake appears to be endocytosis, and some have suggested that NPs transported into the cell by endocytosis can remain for weeks and even months [126,127]. The exact contribution of this accumulation on the mechanisms of cellular toxicity remains debatable and more research is needed to understand the contribution of endocytosis in the cellular toxicity of NPs.
6. Toxicological effects of NPs on target organs
Recent studies using various animal models showed that NPs accumulate in different organs and have the potential to cause different health risks (Fig. 2). Many of these studies have been done using shortterm exposure regimens, and there is a need for more studies assessing the long-term effects of NPs, such as after chronic exposure. Such exposure would model what is seen in workers handling NPs in manufacturing plants, as well as those involved in the storage, disposal and shipping of these NPs. We also need more studies on the toxicokinetics of NPs in blood and tissues [3]. While some studies assessing the toxicokinetics of NPs do exist, many of these quantify only the total drug, or payload, but do not quantify the NPs themselves, or determine the ratio of free to encapsulated drug. As a result, the actual pharmacokinetics of drug-NP systems are not well described [53,128]. Further, additional strategies are needed to address similar issues in tissues where many NPs accumulate, including the lungs, liver, spleen and kidneys [129].
Fig. 2.
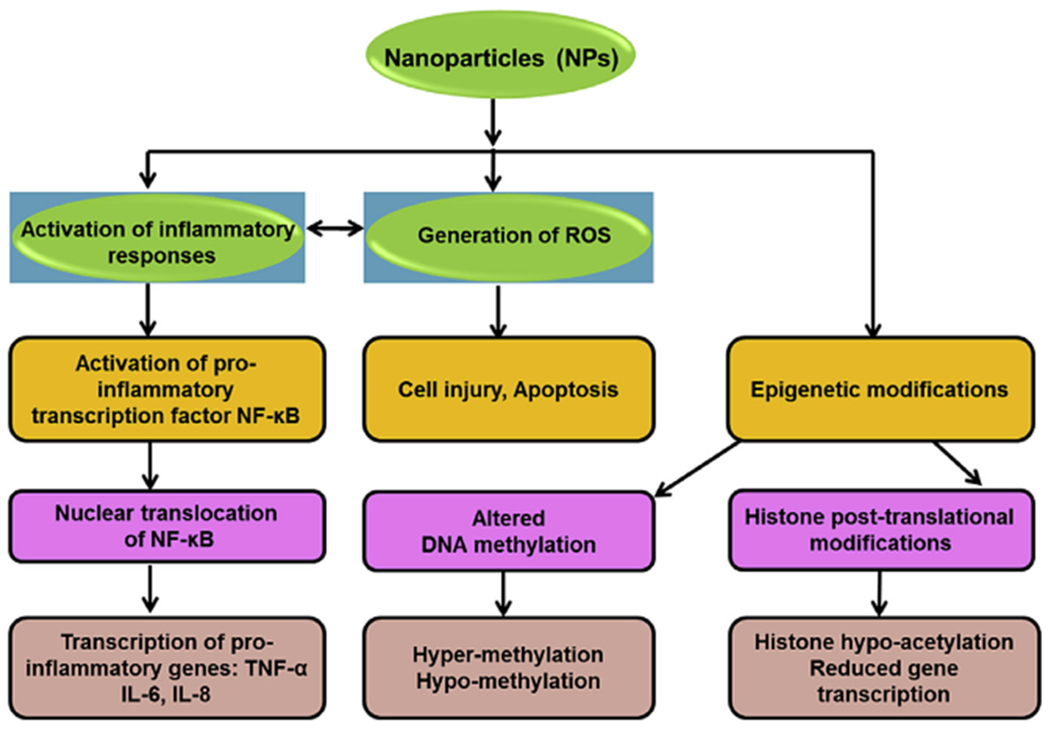
Mechanisms of cellular toxicity of nanoparticles.
6.1. Toxicological effects on the lungs
Studies have shown that respiratory exposure to metal containing NPs, such as cadmium-containing-QDs, caused inflammatory reactions and fibrosis in rat lungs [130–133]. However, in a recent study, injection of NPs in the lungs caused inflammatory reactions but did not cause fibrosis [134]. Several in vivo studies have suggested that these toxic effects are mostly localized in the lungs and that pulmonary exposure to NPs do not cause serious systemic toxic effects on extra-pulmonary tissues and organs [131,135]. Further studies are needed to resolve these controversies.
6.2. Toxicological effects on the liver
The liver is where most exogenous chemicals are metabolized. NPs are typically cleared by fixed and circulatory macrophages and their degradants, including encapsulated drugs, are commonly metabolized by the liver [136]. Kim and his group showed that AgNPs cause dose-dependent changes in the level of cholesterol and alkaline phosphatase leading to mild liver damage [137]. The authors suggested that these toxic effects may be attributed to the release and accumulation of metal ions from NPs. They also showed that NPs administered intravenously or intraperitoneally tend to accumulate in the liver [137]. Another study showed that intraperitoneal injection of zinc oxide NPs into mice increased the levels of alkaline phosphatase (ALP) and glutamic pyruvic transaminase (GPT), which correlated to liver dysfunction [138]. In addition, these NPs increased body weight, organ weight and induced a mild to severe inflammatory responses [138]. This study is in agreement with that from Bartneck et al. who showed that intravenously injected AuNPs accumulated in the liver, and induced activation of hepatic macrophages associated with liver damage [139].
6.3. Toxicological effects on the spleen
Studies have shown that NPs can infiltrate and accumulate in immune system cells and organs, such as the spleen [140,141]. Despite these studies, we still lack a comprehensive understanding of the mechanism mediating these interactions. However, studies do show that metal containing NPs induce inflammatory reactions and increased counts of neutrophils and lymphocytes in the blood [142]. Moreover, iron containing NPs were shown to cause oxidative stress and hepatic lipid peroxidation following the accumulation of iron in macrophages of the liver and spleen [143]. Non-metal NPs also can accumulate in macrophages [144]. Further, studies have shown that systemic exposure to select NPs may increase the levels of cytokines and chemokines, which initiate the migration of immune cells to the damaged sites including the spleen. The increased levels of these cytokines, including interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), granulocyte-macrophage colony-stimulating factor (GM-CSF, a glycoprotein secreted by macrophages), as well as the number of T cells, mast cells, natural killer cells, endothelial cells and fibroblasts, have been shown to be dose-dependent in animals treated with NPs [145].
6.4. Toxicological effects on the kidneys
The kidneys maintain the homeostasis of the body in part by controlling the excretion of metabolic wastes and the regulation of electrolyte composition. Small sized NPs can be cleared through the kidneys [146,147], and depending on their residence time during their excretion, NPs may cause nephrotoxicity [3]. In vivo studies showed that cadmium-containing silica NPs (SiNPs-Cd) induced renal inflammation and fibrosis after pulmonary exposure [148]. The renal inflammation persisted for up to 30 days post administration. Further, immunohistochemistry demonstrated increased expression of interleukin 6 (IL-6), transforming growth factor beta 1 (TGF-β1), fibronectin and vimentin, mainly in the cortex and medulla. It was hypothesized that these toxic effects correlated to the translocation of NPs from the systemic circulation to the kidneys. Silica-based NPs also have been shown to induce acute kidney damage in mice after a single intraperitoneal dose injection [149]. Specifically, these NPs caused renal fibrosis and tubule regeneration marked by the increased expression and translocation of NF-κB p65 to the nucleus. This finding is supported by in vitro studies showing that silica NPs are cytotoxic to normal rat kidney (NRK) cells, and caused increased expression of fibrosis markers [149]. Other studies have shown that AgNPs can cause tubular dilatation, mineralization and inflammation in rat kidneys and increase proteinurea [130]. Finally, Kim et al. (2010) demonstrated a gender-associated accumulation of AgNPs in the kidneys, which was two-fold higher in female rat kidneys compared to males [137].
6.5. Toxicological effects on the brain
It should be noted that most NPs do not typically cross the BBB, as such most NPs do not induce significant toxicty in this organ. That being said, there are some NPs that have been shown to accumulate in the brain, and these are typically relatively small NPs, which enter either by passive diffusion or by receptor-mediated endocytosis [150]. Others have reported that NPs may be taken up into the brain via transsynaptic transport mechanisms or enter by directly disrupting the BBB resulting in central nervous system toxicity. Metal containing NPs may accumulate in the brain using these mechanisms [151–153]. Further, long term intranasal administration of titanium dioxide nanoparticles (TiO2NPs) has been shown to cause hemorrhage in the brains of mice, in correlation with changes in the levels of trace elements, enzymes and neurotransmitters [154].
Poly (n-butylcyano-acrylate) (PBCA) nanoparticles coated with polysorbate 80 have been suggested to facilitate the delivery of drugs to the brain for the treatment of diseases of the central nervous system. As mentioned above, the mechanisms of action may be disruption of the BBB. In addition, these NPs have also been shown to increase the release of interleukin-8 (IL-8) in correlation with disruption of the BBB integrity [155]. Another study demonstrated that AgNPs can induce changes in the cerebral myelin integrity in mice after oral administration [156]. This was accompanied by changes in the expression of several myelin-specific proteins including CNP (2′, 3 ′-cyclic nucleotide phosphodiesterase), MAG (myelin-associated glycoprotein) and MOG (myelin/oligodendrocyte glycoprotein). Furthermore, AgNPs exposure correlated with increased body weight and temperature [156]. Other metal-containing NPs reported to induce oxidative stress in the brain include titanium dioxide (TiO2), zinc oxide (ZnO) and aluminum oxide (Al2O3) [157]. All of these NPs were shown to be localized in the cytoplasm and nucleus using transmission electron microscopy (TEM) and were also suggested to alter the level of dopamine and norepinephrine in the cerebral cortex [157]. It remains to be seen if these NPs are gaining access to brain by disruption of the BBB, or by other mechanisms.
6.6. Toxicity to other organs
NPs have been shown to target organs other than those discussed above. For example, zinc oxide NPs (ZnO NPs) administered to rats by oral gavage for three months caused submucosal edema and inflammatory cell infiltration in the stomach [157]. In addition these NPs caused inflammatory reactions and apoptosis in the pancreas [158].
The effect of NPs on the skin has been discussed above. Dermal administration of AgNPs resulted in reduced thickness of the epidermis and papillary layer and an increase in the number of Langerhans cells [159]. It is suggested that permeation of NPs into the outermost stratum corneum layers is dependent on the physicochemical properties of NPs and also on the hydrophilic pathways, including sweat pores, hair follicles, and the intercellular spaces between the corneocytes [95]. We should mention that even though NPs could damage the skin when administered dermally, there is little evidence of systemic and internal organs damage caused by dermally administered NPs [160]. Further, like all toxicants, the effects are concentration-dependent.
7. Mechanisms of cytotoxic effects of NPs
The potential toxicities of NPs on various target organs are mediated by different mechanisms. Some of these mechanisms include oxidative stress and generation of reactive oxygen species (ROS), DNA damage, alteration of protein structures and, disruption of membrane integrity. Some metal-containing NPs and silica NPs can cause oxidative stress, inflammation and DNA damage following their acute systemic exposure [161]. These toxicities are usually the result of two major factors, their surface area and reactivity in the target sites [162].
7.1. Oxidative stress
Several NPs have been shown to induce ROS and to inhibit the action of antioxidants. Metal or metal oxide NPs have been continuously shown to induce oxidative stress in the liver, spleen and kidneys [143,157,163]. The increased levels of ROS in these tissues is attributed to activation of specific stress-related cell signaling pathways, mitochondrial dysfunction, DNA damage that leads to cell cycle arrest and apoptosis [164]. The major route of entry into the cells includes endocytosis facilitated by binding to membrane receptors such as growth factor receptors, as well as integrins. The extent of endocytosis is dependent on the size of the NPs, and smaller NPs are more likely to be internalized than larger NPs. Therefore small NPs are more likely to cause cellular toxicities. Once inside the cells, NPs pass through the endosomal/lysosomal pathways and the different cytoplasmic networks [115,163].
NP-induced ROS production is known to result from mitochondrial dysfunction [163]. Other mechanisms mediating NP-induced oxidative stress include protein oxidation, alteration of catalases’ activities, and increased release of nitric acid and glutamic acid, as was shown in the brain of mice exposed to titanium dioxide NPs [165]. Ze et al. suggested that oxidative stress caused by these NPs may occur via the p38-Nrf-2 signaling pathway [154].
7.2. Inflammatory reactions
Studies have shown that NPs can increase the levels of a number of inflammatory cytokines [166–168]. NPs are usually taken up by macrophages in macrophage-rich organs, such as the liver and spleen, which induces the release of cytokines from macrophages. NPs can also bind to specific macrophage receptors with collagenous structure (MARCO) [169]. Once bound, NPs are internalized and translocated inside the macrophages via macropinocytosis or endocytosis. Some studies have suggested that titanium dioxide NPs (TiO2 NPs) can activate inflammatory signaling pathways including the c-Src, p38 MAP kinase, and NF-κB pathways [169].
7.3. Epigenetic modifications
In addition to inflammation and oxidative stress, NPs have been shown to induce epigenetic modifications. Recent in vitro studies demonstrated changes in DNA methylation, histone post-translational modifications, chromatin remodeling and RNA methylation following exposure to NPs [170–172]. However, it is not known if these epigenetic changes are true epigenetic modifications as we are still lacking data showing that these modifications are inheritable or that they mediate adverse health effects in humans and animals [170,173].
DNA methylation occurs at the C5 position of cytosines in the CpG islands. This chemical modification inhibits access of the transcriptional machinery to DNA and generally decreases gene expression. DNA methylation is catalyzed by DNA methyltransferases (DNMT3a/DNMT3b and DNMT1), and the expression of these enzymes have been shown to be altered following exposure to select NPs [174]. These include carbon-based NPs [175], titanium dioxide NPs [176] and selenium dioxide NPs [177]. These NPs have also been shown to cause changes in both local and global DNA methylation. Sharif et al. (2007) reported hypermethylation in the promoter region of poly (ADP-ribose) polymerase 1 (PARP-1) in human lung adenocarcinoma cells following their exposure to titanium dioxide NPs (TiO2 NPs) [178]. A more recent study demonstrated a 40% increase in DNA methylation at the promoter region of the alkaline phosphatase (ALPL) gene in mice after exposure to hydroxyapatite NPs [179]. A recent study used bisulfite pyrosequencing and reported a decrease in DNA methylation at the promoter region of interferon α (INF-α) and interferon-γ (INF-γ) in the lungs of mice exposed to carbon nanotubes via the pulmonary route [175].
Studies are still being conducted to assess whether NPs have any effects on the human methylome. One study published in 2013 demonstrated a decrease in global DNA methylation in human subjects after their exposure to micro- and nano-sized air pollutants including NO2 and O3 particles [180]. However, this finding has not been confirmed to date, and it’s difficult to separate out the effect of the NPs from that of toxicity.
The interaction of DNA with the cellular transcriptional machinery is dependent on how tightly DNA is wrapped around histones. DNA packaging depends on various chemical states of histones such as histone methylation, acetylation and phosphorylation [181]. NPs have been suggested to affect these chemical states by inducing histone post-translational modifications, mostly at their terminal amino tails, resulting in changes in DNA packaging and its accessibility to the transcriptional machinery [170]. Choi et al. (2008) demonstrated that exposure of human breast cancer cells to QD induced chromatin condensation and global histone hypo-acetylation and reduced gene transcription. In addition, exposure of these cells to QDs induced the expression of some apoptotic genes through activation of p53 [182]. AgNPs have also been suggested to induce histone post-translational changes by affecting various enzymes involved in chromatin remodeling including histone deacetylases [183]. The challenge in the future will be to separate these changes out from toxicity and to relate these epigenetic modifications to the human genetic variability and their effect on the susceptibility to specific NPs [170].
8. Factors that influence the toxicity of nanoparticles
Like all chemicals, the toxicity of NPs is dependent on their dose, structure and their physicochemical properties. For NPs these properties include size, surface area and surface charge [3]. Changes in the physico-chemical properties of NPs will affect their interactions with various proteins and other targets, which will ultimately affect their distribution and translocation to different organ systems [184]. Some studies suggested a direct correlation between the size distribution of NPs and the level of ROS generation in the kidneys [185,186]. This correlates with the observation that smaller size NPs usually exhibit higher tissue distribution and cause more serious side effects compared to larger sized NPs, even though these NPs are made of the same components. This size-dependent toxicity is demonstrated by the fact that AgNPs of 10 nm had higher liver distribution and more serious hepatobiliary toxicity compared to larger (40 and 100 nm) AgNPs with the same chemical composition [132,187].
The surface charge of some NPs, for example ZnO NPs, can affect their pharmacokinetics. Paek et al.(2013) suggested that the ionic forms of zinc oxide NPs, rather than the particulate form (uncharged), accumulated in organs such as the kidneys, liver and lungs following oral or intravenous administration. The authors investigated the effect of NPs size on their pharmacokinetics and concluded that the surface charge, rather than the size, is what determines the pharmacokinetic profile of ZnO NPs [122]. Shegokar et al. (2011) showed that the surface modifications of nevirapine nano-suspensions with serum albumin, polysaccharide and polyethylene glycol enhanced their accumulation in organs such as the brain, liver and spleen, which correlated to their toxicity in these organs [188].
The effect of surface properties of NPs can be modulated using different coating materials such as PEG. This fact can be utilized to alter the toxicity of NPs. For example, PEG has been shown to reduce the toxicity of NPs by affecting their interactions with proteins. However, it should be noted that too much PEG may also impair their cellular internalization and binding with target proteins and therefore their efficacy, as was shown for AuNPs [189]. Thus, any attempt to alter the toxicity of NPs by altering their biophysical properties must be balanced with the functionality of the NP. This is why more studies are needed on how the biophysical properties of NPs mediate the mechanisms of toxicity [190]. Such studies will facilitate the more “intelligent” design of NPs to benefit from their advantages while limiting their toxic health effects.
9. Evaluating and preventing the toxicity of nanoparticles
As mentioned above, the rapid growth of nanotechnology has created a gap-in-knowledge about their toxicity. Even though our current understanding of the toxicological status of NPs is not complete, we do know that many NPs may pose health concerns to humans and animals. To address these concerns a Research Team for Nano-Associated Safety Assessment (RT-NASA) was established by Kim and co-workers to investigate the toxicity of some NPs [158]. The RT-NASA studies were carried out in six steps: need assessment, physicochemical property, toxicity evaluation, toxicokinetics, peer review, and risk communication. The need assessment step involved responses from NPs consumers based on their age, sex, education and social status [158]. In the same spirit, the U.S. Environmental Protection Agency (EPA), the International Life Sciences Institute Research Foundation, and the Risk Science Institute developed working systems where experts in the field of nanotechnology from academia and government worked together to conduct toxicity screening and risk assessment associated with NPs [191]. Further, a global effort led by US and European experts has been focusing on mapping the potential risks of NPs [192–195]. Finally, it has been suggested that toxicity studies on NPs should be exchanged between toxicologists, chemists, material scientists, and medical doctors via effective networks that will allow for a more complete understanding of the area of nanotoxicology and its related risks in order to develop safe nanotechnology [90].
10. Conclusion
Nanotechnology is one of the fastest growing fields of the last decade, with applications in disease diagnosis and treatment, biosensors, cosmetics, food industry and other technologies. However, the rapid growth in engineering and use of NPs has not been accompanied by a similar growth in our knowledge on their toxicity. As such, there is an urgent need for more pharmacokinetic and toxicological studies on NPs, especially before they reach the marketplace. Of particular importance is the need to understand the relationship between the physico-chemical and structural properties of NPs and their cellular reactivity and interaction within various organs and tissues. Such information will facilitate the intelligent design of NPs while decreasing the gap-in-knowledge regarding their toxicity.
Acknowledgements/Grant Support
This project was supported in part with funds from the National Institutes of Health; National Institute of Biomedical Imaging and Bioengineering [NIBIB (EB016100 to B.S.C. and R.D.A.)], a Department of Defense Prostate Cancer Research Program Idea Development Award (PC150431 GRANT11996600) to B.S.C, an Achievement Rewards for College Scientists Foundation Award and International Society of Pharmaceutical Engineering (ISPE), Women in Pharma Award to W.N.M.
Abbreviations:
- ADME
absorption distribution metabolism and excretion
- AgNPs
silver NPs
- ALP
alkaline phosphatase
- AuNPs
gold nanoparticles
- BBB
blood brain barrier
- CNPLex
carbon NP-DNA complex
- CNTs
carbon nanotubes
- DNMT
DNA methyltransferases
- EPA
Environmental Protection Agency
- EPR
enhanced permeability and retention effect
- GFP
green fluorescent protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GPT
glutamic pyruvic transaminase
- IL-6
interleukin-6
- LLC
Lewis lung carcinoma
- MAG
myelin-associated glycoprotein
- MARCO
macrophage receptors with collagenous structure
- MCP-1
monocyte chemoattractant protein-1
- MOG
myelin/oligodendrocyte glycoprotein
- MRI
magnetic resonance imaging
- NE
Nanoemulsion
- NP(s)
nanoparticle(s)
- NRK
normal rat kidney cells
- PEG
polyethylene glycol
- PLGA
poly-lactic glycolic acid
- PSA
prostate specific antigen
- PSMA
prostate specific membrane antigen
- PtNPs
platinum-based NPs
- QDs
quantum dots
- ROS
reactive oxygen species
- RT-NASA
Research Team for Nano-Associated Safety Assessment
- SiNPs-Cd
cadmium-containing silica NPs
- siRNA
small-interfering RNA
- SL
Stealth Liposomes
- TEM
transmission electron microscopy
- TNFα
tumor necrosis factor
References
- [1].Shapira P, Wang J, Follow the money, Nature 468 (2010) 627–628. [DOI] [PubMed] [Google Scholar]
- [2].Gwinn MR, Vallyathan V, Nanoparticles: health effects—pros and cons, Environ. Health Perspect 114 (2006) 1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu T, Tang M, Review of the effects of manufactured nanoparticles on mammalian target organs, J. Appl. Toxicol. : JAT (J. Appl. Toxicol.) (2017) 25–40. [DOI] [PubMed] [Google Scholar]
- [4].Wu W, Wu Z, Yu T, Jiang C, Kim WS, Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications, Sci. Technol. Adv. Mater 16 (2015) 023501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alwi R, Telenkov S, Mandelis A, Leshuk T, Gu F, Oladepo S, Michaelian K, Silica-coated super paramagnetic iron oxide nanoparticles (SPION) as biocompatible contrast agent in biomedical photoacoustics, Biomed. Optic Express 3 (2012) 2500–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lentschig MG, Reimer P, Rausch-Lentschig UL, Allkemper T, Oelerich M, Laub G, Breath-hold gadolinium-enhanced MR angiography of the major vessels at 1.0 T: dose-response findings and angiographic correlation, Radiology 208 (1998) 353–357. [DOI] [PubMed] [Google Scholar]
- [7].Taupin F, Flaender M, Delorme R, Brochard T, Mayol JF, Arnaud J, Perriat P, Sancey L, Lux F, Barth RF, Carriere M, Ravanat JL, Elleaume H, Gadolinium nanoparticles and contrast agent as radiation sensitizers, Phys. Med. Biol 60 (2015) 4449–4464. [DOI] [PubMed] [Google Scholar]
- [8].Lux F, Sancey L, Bianchi A, Cremillieux Y, Roux S, Tillement O, Gadolinium-based nanoparticles for theranostic MRI-radiosensitization, Nanomedicine 10 (2015) 1801–1815. [DOI] [PubMed] [Google Scholar]
- [9].Sancey L, Lux F, Kotb S, Roux S, Dufort S, Bianchi A, Cremillieux Y, Fries P, Coll JL, Rodriguez-Lafrasse C, Janier M, Dutreix M, Barberi-Heyob M, Boschetti F, Denat F, Louis C, Porcel E, Lacombe S, Le Duc G, Deutsch E, Perfettini JL, Detappe A, Verry C, Berbeco R, Butterworth KT, McMahon SJ, Prise KM, Perriat P, Tillement O, The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy, Br. J. Radiol 87 (2014) 20140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stuchinskaya T, Moreno M, Cook MJ, Edwards DR, Russell DA, Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates, Photochem. Photobiol. Sci 10 (2011) 822–831. [DOI] [PubMed] [Google Scholar]
- [11].Brown SD, Nativo P, Smith JA, Stirling D, Edwards PR, Venugopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ, Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin, J. Am. Chem. Soc 132 (2010) 4678–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD, Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity, Small 1 (2005) 325–327. [DOI] [PubMed] [Google Scholar]
- [13].Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM, Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles, ACS Nano 2 (2008) 2213–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Au L, Zheng D, Zhou F, Li ZY, Li X, Xia Y, A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells, ACS Nano 2 (2008) 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ashraf S, Pelaz B, del Pino P, Carril M, Escudero A, Parak WJ, Soliman MG, Zhang Q, Carrillo-Carrion C, Gold-based nanomaterials for applications in nanomedicine, Top. Curr. Chem 370 (2016) 169–202. [DOI] [PubMed] [Google Scholar]
- [16].Han G, Ghosh P, Rotello VM, Multi-functional gold nanoparticles for drug delivery, Adv. Exp. Med. Biol 620 (2007) 48–56. [DOI] [PubMed] [Google Scholar]
- [17].Wang H, Zhang Y, Yu H, Wu D, Ma H, Li H, Du B, Wei Q, Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles, Anal. Biochem 434 (2013) 123–127. [DOI] [PubMed] [Google Scholar]
- [18].Swanner J, Mims J, Carroll DL, Akman SA, Furdui CM, Torti SV, Singh RN, Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells, Int. J. Nanomed 10 (2015) 3937–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chaudhari AA, Ashmore D, Nath SD, Kate K, Dennis V, Singh SR, Owen DR, Palazzo C, Arnold RD, Miller ME, Pillai SR, A novel covalent approach to bioconjugate silver coated single walled carbon nanotubes with antimicrobial peptide, J. Nanobiotechnol 14 (2016) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spain E, Gilgunn S, Sharma S, Adamson K, Carthy E, O’Kennedy R, Forster RJ, Detection of prostate specific antigen based on electrocatalytic platinum nanoparticles conjugated to a recombinant scFv antibody, Biosens. Bioelectron 77 (2016) 759–766. [DOI] [PubMed] [Google Scholar]
- [21].Chuang CH, Brown PR, Bulovic V, Bawendi MG, Improved performance and stability in quantum dot solar cells through band alignment engineering, Nat. Mater 13 (2014) 796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun Q, Subramanyam G, Dai L, Check M, Campbell A, Naik R, Grote J, Wang Y, Highly efficient quantum-dot light-emitting diodes with DNA-CTMA as a combined hole-transporting and electron-blocking layer, ACS Nano 3 (2009) 737–743. [DOI] [PubMed] [Google Scholar]
- [23].Yang DZ, Xu SK, Chen QF, Applications of quantum dots to biological probes, Guang pu xue yu guang pu fen xi = Guang pu 27 (2007) 1807–1810. [PubMed] [Google Scholar]
- [24].Fang M, Peng CW, Pang DW, Li Y, Quantum dots for cancer research: current status, remaining issues, and future perspectives, Canc. Biol. Med 9 (2012) 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi HS, Frangioni JV, Nanoparticles for biomedical imaging: fundamentals of clinical translation, Mol. Imag 9 (2010) 291–310. [PMC free article] [PubMed] [Google Scholar]
- [26].Matea CT, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, Iancu C, Mocan L, Quantum dots in imaging, drug delivery and sensor applications, Int. J. Nanomed 12 (2017) 5421–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ow H, Larson DR, Srivastava M, Baird BA, Webb WW, Wiesner U, Bright and stable core-shell fluorescent silica nanoparticles, Nano Lett. 5 (2005) 113–117. [DOI] [PubMed] [Google Scholar]
- [28].Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M, Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine, Nano Lett. 9 (2009) 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Serpell CJ, Kostarelos K, Davis BG, Can carbon nanotubes deliver on their promise in Biology? Harnessing unique properties for unparalleled applications, ACS Central Science 2 (2016) 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klumpp C, Kostarelos K, Prato M, Bianco A, Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics, Biochim. Biophys. Acta 1758 (2006) 404–412. [DOI] [PubMed] [Google Scholar]
- [31].Zhang Y, Petibone D, Xu Y, Mahmood M, Karmakar A, Casciano D, Ali S, Biris AS, Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine, Drug Metabolism Reviews 46 (2014) 232–246. [DOI] [PubMed] [Google Scholar]
- [32].Kolosnjaj J, Szwarc H, Moussa F, Toxicity studies of carbon nanotubes, Adv. Exp. Med. Biol 620 (2007) 181–204. [DOI] [PubMed] [Google Scholar]
- [33].Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL, A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks, Crit. Rev. Toxicol 36 (2006) 189–217. [DOI] [PubMed] [Google Scholar]
- [34].Bangham AD, Standish MM, Watkins JC, Diffusion of univalent ions across the lamellae of swollen phospholipids, J. Mol. Biol 13 (1965) 238–252. [DOI] [PubMed] [Google Scholar]
- [35].Bozzuto G, Molinari A, Liposomes as nanomedical devices, Int. J. Nanomed 10 (2015) 975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C, Nanoliposomes and their applications in food nanotechnology, J. Liposome Res 18 (2008) 309–327. [DOI] [PubMed] [Google Scholar]
- [37].Mozafari MR, Liposomes: an overview of manufacturing techniques, Cell. Mol. Biol. Lett 10 (2005) 711–719. [PubMed] [Google Scholar]
- [38].Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H, Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation, J. Liposome Res 20 (2010) 228–243. [DOI] [PubMed] [Google Scholar]
- [39].Woodbury DJ, Richardson ES, Grigg AW, Welling RD, Knudson BH, Reducing liposome size with ultrasound: bimodal size distributions, J. Liposome Res 16 (2006) 57–80. [DOI] [PubMed] [Google Scholar]
- [40].Hope MJ, Bally MB, Webb G, Cullis PR, Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential, Biochim. Biophys. Acta 812 (1985) 55–65. [DOI] [PubMed] [Google Scholar]
- [41].Ghanbari Safari M, Hosseinkhani S, Lipid composition of cationic nanoliposomes implicate on transfection efficiency, J. Liposome Res 23 (2013) 174–186. [DOI] [PubMed] [Google Scholar]
- [42].Abou DS, Thorek DL, Ramos NN, Pinkse MW, Wolterbeek HT, Carlin SD, Beattie BJ, Lewis JS, 89)Zr-labeled paramagnetic octreotide-liposomes for PET-MR imaging of cancer, Pharm. Res. (N. Y.) 30 (2013) 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Laverman P, Brouwers AH, Dams ET, Oyen WJ, Storm G, van Rooijen N, Corstens FH, Boerman OC, Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose, J. Pharmacol. Exp. Therapeut 293 (2000) 996–1001. [PubMed] [Google Scholar]
- [44].Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D, Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times, Biochim. Biophys. Acta 1070 (1991) 187–192. [DOI] [PubMed] [Google Scholar]
- [45].Sharma US, Sharma A, Chau RI, Straubinger RM, Liposome-mediated therapy of intracranial brain tumors in a rat model, Pharm. Res. (N. Y.) 14 (1997) 992–998. [DOI] [PubMed] [Google Scholar]
- [46].Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK, Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft, Canc. Res 54 (1994) 3352–3356. [PubMed] [Google Scholar]
- [47].Mock JN, Costyn LJ, Wilding SL, Arnold RD, Cummings BS, Evidence for distinct mechanisms of uptake and antitumor activity of secretory phospholipase A2 responsive liposome in prostate cancer, Integr.Biol. : Quantitative Biosciences from Nano to Macro 5 (2013) 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Al-Azayzih A, Missaoui WN, Cummings BS, Somanath PR, Liposome-mediated delivery of the p21 activated kinase-1 (PAK-1) inhibitor IPA-3 limits prostate tumor growth in vivo, Nanomedicine 12 (2016) 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rai VK, Mishra N, Yadav KS, Yadav NP, Nanoemulsion as pharmaceutical Carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications, J. Contr. Release 270 (2017) 203–225. [DOI] [PubMed] [Google Scholar]
- [50].Jaiswal M, Dudhe R, Sharma PK, Nanoemulsion: an advanced mode of drug delivery system, 3 Biotech 5 (2015) 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, Chourasia MK, Nanoemulsion: concepts, development and applications in drug delivery, J. Contr. Release 252 (2017) 28–49. [DOI] [PubMed] [Google Scholar]
- [52].Mundada V, Patel M, Sawant K, Submicron emulsions and their applications in oral delivery, Crit. Rev. Ther. Drug Carrier Syst 33 (2016) 265–308. [DOI] [PubMed] [Google Scholar]
- [53].Desai N, Challenges in development of nanoparticle-based therapeutics, AAPS J. 14 (2012) 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang Y, Li P, Truong-Dinh Tran T, Zhang J, Kong L, Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer, vol. 6, Nanomaterials, Basel, Switzerland), 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE, Biodegradable polymeric nanoparticles as drug delivery devices, J. Contr. Release 70 (2001) 1–20. [DOI] [PubMed] [Google Scholar]
- [56].Salmaso S, Caliceti P, Stealth properties to improve therapeutic efficacy of drug nanocarriers, J. Drug Deliv 2013 (2013) 374252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ghanbarzadeh B, Oleyaei SA, Almasi H, Nanostructured materials utilized in biopolymer-based plastics for food packaging applications, Crit. Rev. Food Sci. Nutr 55 (2015) 1699–1723. [DOI] [PubMed] [Google Scholar]
- [58].Rohilla S, Dureja H, Recent patents, formulation and characterization of nanoliposomes, Recent Patents on Drug Delivery & Formulation 9 (2015) 213–224. [DOI] [PubMed] [Google Scholar]
- [59].Mousavi SZ, Nafisi S, Maibach HI, Fullerene nanoparticle in dermatological and cosmetic applications, Nanomedicine 13 (2017) 1071–1087. [DOI] [PubMed] [Google Scholar]
- [60].Dellinger A, Zhou Z, Connor J, Madhankumar AB, Pamujula S, Sayes CM, Kepley CL, Application of fullerenes in nanomedicine: an update, Nanomedicine 8 (2013) 1191–1208. [DOI] [PubMed] [Google Scholar]
- [61].Dellinger A, Olson J, Link K, Vance S, Sandros MG, Yang J, Zhou Z, Kepley CL, Functionalization of gadolinium metallofullerenes for detecting atherosclerotic plaque lesions by cardiovascular magnetic resonance, J. Cardiovasc. Magn. Reson. : Offic. J. Soc. Cardiovasc. Magn. Reson 15 (2013) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Moghimi SM, Hunter AC, Murray JC, Nanomedicine: current status and future prospects, Faseb. J.: Offic. Pub. Fed. Am. Soc. Exp. Biol 19 (2005) 311–330. [DOI] [PubMed] [Google Scholar]
- [63].Sabnis S, Sabnis NA, Raut S, Lacko AG, Superparamagnetic reconstituted highdensity lipoprotein nanocarriers for magnetically guided drug delivery, Int. J. Nanomed 12 (2017) 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nagesh PKB, Johnson NR, Boya VKN, Chowdhury P, Othman SF, Khalilzad-Sharghi V, Hafeez BB, Ganju A, Khan S, Behrman SW, Zafar N, Chauhan SC, Jaggi M, Yallapu MM, PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer, Colloids Surfaces B Biointerfaces 144 (2016) 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bao W, Liu R, Wang Y, Wang F, Xia G, Zhang H, Li X, Yin H, Chen B, PLGA-PLL-PEG-Tf-based targeted nanoparticles drug delivery system enhance antitumor efficacy via intrinsic apoptosis pathway, Int. J. Nanomed 10 (2015) 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Broz P, Ben-Haim N, Grzelakowski M, Marsch S, Meier W, Hunziker P, Inhibition of macrophage phagocytotic activity by a receptor-targeted polymer vesicle-based drug delivery formulation of pravastatin, J. Cardiovasc. Pharmacol 51 (2008) 246–252. [DOI] [PubMed] [Google Scholar]
- [67].Botella P, Abasolo I, Fernandez Y, Muniesa C, Miranda S, Quesada M, Ruiz J, Schwartz S Jr., Corma A, Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation, J. Contr. Release 156 (2011) 246–257. [DOI] [PubMed] [Google Scholar]
- [68].Allen TM, Cullis PR, Drug delivery systems: entering the mainstream, Science 303 (2004) 1818–1822. [DOI] [PubMed] [Google Scholar]
- [69].Gabizon A, Shmeeda H, Barenholz Y, Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies, Clin. Pharmacokinet 42 (2003) 419–436. [DOI] [PubMed] [Google Scholar]
- [70].Reddy JA, Allagadda VM, Leamon CP, Targeting therapeutic and imaging agents to folate receptor positive tumors, Curr. Pharmaceut. Biotechnol 6 (2005) 131–150. [DOI] [PubMed] [Google Scholar]
- [71].Bwatanglang IB, Mohammad F, Yusof NA, Abdullah J, Hussein MZ, Alitheen NB, Abu N, Folic acid targeted Mn:ZnS quantum dots for theranostic applications of cancer cell imaging and therapy, Int. J. Nanomed 11 (2016) 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mkandawire MM, Lakatos M, Springer A, Clemens A, Appelhans D, Krause-Buchholz U, Pompe W, Rodel G, Mkandawire M, Induction of apoptosis in human cancer cells by targeting mitochondria with gold nanoparticles, Nanoscale 7 (2015) 10634–10640. [DOI] [PubMed] [Google Scholar]
- [73].Gopalan B, Ito I, Branch CD, Stephens C, Roth JA, Ramesh R, Nanoparticle based systemic gene therapy for lung cancer: molecular mechanisms and strategies to suppress nanoparticle-mediated inflammatory response, Technol. Cane. Res. Treat 3 (2004) 647–657. [DOI] [PubMed] [Google Scholar]
- [74].Prabha S, Labhasetwar V, Nanoparticle-mediated wild-type p53 gene delivery results in sustained antiproliferative activity in breast cancer cells, Mol. Pharm 1 (2004) 211–219. [DOI] [PubMed] [Google Scholar]
- [75].Kaul G, Amiji M, Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies, Pharm. Res. (N. Y.) 22 (2005) 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Guo J, O’Driscoll CM, Holmes JD, Rahme K, Bioconjugated gold nanoparticles enhance cellular uptake: a proof of concept study for siRNA delivery in prostate cancer cells, Int. J. Pharm 509 (2016) 16–27. [DOI] [PubMed] [Google Scholar]
- [77].Niemeyer CM, Adler M, Lenhert S, Gao S, Fuchs H, Chi L, Nucleic acid supercoiling as a means for ionic switching of DNA–nanoparticle networks, Chembiochem. : A Eur. J. Chem. Biol 2 (2001) 260–264. [DOI] [PubMed] [Google Scholar]
- [78].Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP, Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots, Nat. Biotechnol 21 (2003) 41–46. [DOI] [PubMed] [Google Scholar]
- [79].Gao X, Cui Y, Levenson RM, Chung LW, Nie S, In vivo cancer targeting and imaging with semiconductor quantum dots, Nat. Biotechnol 22 (2004) 969–976. [DOI] [PubMed] [Google Scholar]
- [80].Zhu Y, Sun Y, Chen Y, Liu W, Jiang J, Guan W, Zhang Z, Duan Y, In vivo molecular MRI imaging of prostate cancer by targeting PSMA with polypeptide-labeled superparamagnetic iron oxide nanoparticles, Int. J. Mol. Sci 16 (2015) 9573–9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhao Y, Shaffer TM, Das S, Perez-Medina C, Mulder WJ, Grimm J, Near-infrared quantum dot and 89Zr dual-labeled nanoparticles for in vivo cerenkov imaging, Bioconjugate Chem. 28 (2017) 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pan LH, Kuo SH, Lin TY, Lin CW, Fang PY, Yang HW, An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles, Biosens. Bioelectron 89 (2017) 598–605. [DOI] [PubMed] [Google Scholar]
- [83].Misra SK, Ohoka A, Kolmodin NJ, Pan D, Next generation carbon nanoparticles for efficient gene therapy, Mol. Pharm 12 (2015) 375–385. [DOI] [PubMed] [Google Scholar]
- [84].Yeh CY, Hsiao JK, Wang YP, Lan CH, Wu HC, Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer, Biomaterials 99 (2016) 1–15. [DOI] [PubMed] [Google Scholar]
- [85].Rizzitelli S, Giustetto P, Faletto D, Delli Castelli D, Aime S, Terreno E, The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model, J. Contr. Release 230 (2016) 57–63. [DOI] [PubMed] [Google Scholar]
- [86].Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW, Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination, J. Contr. Release 240 (2016) 332–348. [DOI] [PubMed] [Google Scholar]
- [87].McConnell KI, Shamsudeen S, Meraz IM, Mahadevan TS, Ziemys A, Rees P, Summers HD, Serda RE, Reduced cationic nanoparticle cytotoxicity based on serum masking of surface potential, J. Biomed. Nanotechnol 12 (2016) 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Akhtar S, Cationic nanosystems for the delivery of small interfering ribonucleic acid therapeutics: a focus on toxicogenomics, Expet Opin. Drug Metabol. Toxicol 6 (2010) 1347–1362. [DOI] [PubMed] [Google Scholar]
- [89].Dick CA, Brown DM, Donaldson K, Stone V, The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types, Inhal. Toxicol 15 (2003) 39–52. [DOI] [PubMed] [Google Scholar]
- [90].Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ, Nanotoxicology Occup. Environ. Med 61 (2004) 727–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Isama K, [In vitro safety evaluation of nanomaterials–cellular response to metal oxide nanoparticles], Yakugaku zasshi, J. Pharm. Soc. Jpn 134 (2014) 731–735. [DOI] [PubMed] [Google Scholar]
- [92].Almeida JP, Chen AL, Foster A, Drezek R, In vivo biodistribution of nanoparticles, Nanomedicine 6 (2011) 815–835. [DOI] [PubMed] [Google Scholar]
- [93].Shi H, Magaye R, Castranova V, Zhao J, Titanium dioxide nanoparticles: a review of current toxicological data, Part. Fibre Toxicol 10 (2013) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Inoue K, Takano H, Aggravating impact of nanoparticles on immune-mediated pulmonary inflammation, Sci. Wor. J 11 (2011) 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tang L, Zhang C, Song G, Jin X, Xu Z, In vivo skin penetration and metabolic path of quantum dots, Science China, Life Sciences 56 (2013) 181–188. [DOI] [PubMed] [Google Scholar]
- [96].Nangia S, Sureshkumar R, Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes, Langmuir 28 (2012) 17666–17671. [DOI] [PubMed] [Google Scholar]
- [97].Tak YK, Pal S, Naoghare PK, Rangasamy S, Song JM, Shape-dependent skin penetration of silver nanoparticles: does it really matter? Sci. Rep 5 (2015) 16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M, Understanding biophysicochemical interactions at the nano-bio interface, Nat. Mater 8 (2009) 543–557. [DOI] [PubMed] [Google Scholar]
- [99].Nel A, Xia T, Madler L, Li N, Toxic potential of materials at the nanolevel, Science 311 (2006) 622–627. [DOI] [PubMed] [Google Scholar]
- [100].Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV, Nanodrug applications in photodynamic therapy, Photodiagn. Photodyn. Ther 8 (2011) 14–29. [DOI] [PubMed] [Google Scholar]
- [101].Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW, Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis, Toxicol. Lett 201 (2011) 92–100. [DOI] [PubMed] [Google Scholar]
- [102].Pope CA 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD, Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution, Jama 287 (2002) 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Raj S, Jose S, Sumod US, Sabitha M, Nanotechnology in cosmetics: opportunities and challenges, J. Pharm. BioAllied Sci 4 (2012) 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nazarenko Y, Han TW, Lioy PJ, Mainelis G, Potential for exposure to engineered nanoparticles from nanotechnology-based consumer spray products, J. Expo. Sci. Environ. Epidemiol 21 (2011) 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Risom L, Moller P, Loft S, Oxidative stress-induced DNA damage by particulate air pollution, Mutat. Res 592 (2005) 119–137. [DOI] [PubMed] [Google Scholar]
- [106].Mehta M, Chen LC, Gordon T, Rom W, Tang MS, Particulate matter inhibits DNA repair and enhances mutagenesis, Mutat. Res 657 (2008) 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rollerova E, Tulinska J, Liskova A, Kuricova M, Kovriznych J, Mlynarcikova A, Kiss A, Scsukova S, Titanium dioxide nanoparticles: some aspects of toxicity/focus on the development, Endocr. Regul 49 (2015) 97–112. [DOI] [PubMed] [Google Scholar]
- [108].Wan R, Mo Y, Zhang Z, Jiang M, Tang S, Zhang Q, Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice, Part. Fibre Toxicol 14 (2017) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sarfraz M, Roa W, Bou-Chacra N, Lobenberg R, Inflammation caused by nanosized delivery systems: is there a benefit? Mol. Pharm 13 (2016) 3270–3278. [DOI] [PubMed] [Google Scholar]
- [110].Silva RM, Teesy C, Franzi L, Weir A, Westerhoff P, Evans JE, Pinkerton KE, Biological response to nano-scale titanium dioxide (TiO2): role of particle dose, shape, and retention, J. Toxicol. Environ. Health Part a 76 (2013) 953–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Schulte P, Geraci C, Zumwalde R, Hoover M, Kuempel E, Occupational risk management of engineered nanoparticles, J. Occup. Environ. Hyg 5 (2008) 239–249. [DOI] [PubMed] [Google Scholar]
- [112].Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S, Lead JR, Stone V, Fernandes TF, Jepson M, van Aerle R, Tyler CR, Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout, Toxicol. Sci. : An Official Journal of the Society of Toxicology 115 (2010) 521–534. [DOI] [PubMed] [Google Scholar]
- [113].Debia M, Bakhiyi B, Ostiguy C, Verbeek JH, Brouwer DH, Murashov V, A systematic review of reported exposure to engineered nanomaterials, Ann. Occup. Hyg 60 (2016) 916–935. [DOI] [PubMed] [Google Scholar]
- [114].Pietroiusti A, Magrini A, Engineered nanoparticles at the workplace: current knowledge about workers’ risk, Occup. Med. (Oxf.) 64 (2014) 319–330. [DOI] [PubMed] [Google Scholar]
- [115].Shang L, Nienhaus K, Nienhaus GU, Engineered nanoparticles interacting with cells: size matters, J. Nanobiotechnol 12 (2014) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Iavicoli I, Leso V, Beezhold DH, Shvedova AA, Nanotechnology in agriculture: opportunities, toxicological implications, and occupational risks, Toxicol. Appl. Pharmacol 329 (2017) 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Frohlich E, Roblegg E, Oral uptake of nanoparticles: human relevance and the role of in vitro systems, Arch. Toxicol 90 (2016) 2297–2314. [DOI] [PubMed] [Google Scholar]
- [118].Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE, Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress, Toxicol. Appl. Pharmacol 261 (2012) 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].He X, Zhang H, Ma Y, Bai W, Zhang Z, Lu K, Ding Y, Zhao Y, Chai Z, Lung deposition and extrapulmonary translocation of nano-ceria after intratracheal instillation, Nanotechnology 21 (2010) 285103. [DOI] [PubMed] [Google Scholar]
- [120].Bakalova R, Zhelev Z, Nikolova B, Murayama S, Lazarova D, Tsoneva I, Aoki I, Lymph node mapping using quantum dot-labeled polymersomes, Gen. Physiol. Biophys 34 (2015) 393–398. [DOI] [PubMed] [Google Scholar]
- [121].Ballou B, Andreko SK, Osuna-Highley E, McRaven M, Catalone T, Bruchez MP, Hope TJ, Labib ME, Nanoparticle transport from mouse vagina to adjacent lymph nodes, PLoS One 7 (2012) e51995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Paek HJ, Lee YJ, Chung HE, Yoo NH, Lee JA, Kim MK, Lee JK, Jeong J, Choi SJ, Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo, Nanoscale 5 (2013) 11416–11427. [DOI] [PubMed] [Google Scholar]
- [123].Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, Van Eijkeren JC, Geertsma RE, De Jong WH, The kinetics of the tissue distribution of silver nanoparticles of different sizes, Biomaterials 31 (2010) 8350–8361. [DOI] [PubMed] [Google Scholar]
- [124].Balasubramanian SK, Jittiwat J, Manikandan J, Ong CN, Yu LE, Ong WY, Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats, Biomaterials 31 (2010) 2034–2042. [DOI] [PubMed] [Google Scholar]
- [125].Lee JA, Kim MK, Paek HJ, Kim YR, Kim MK, Lee JK, Jeong J, Choi SJ, Tissue distribution and excretion kinetics of orally administered silica nanoparticles in rats, Int. J. Nanomed 9 (2) (2014) 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tang H, Feng X, Zhang T, Dai Y, Zhou Z, Chen H, Liu L, Li X, Zhuang T, Liu X, Zhang Q, Stability, pharmacokinetics, biodistribution and safety assessment of folate-conjugated pullulan acetate nanoparticles as cervical cancer targeted drug carriers, J. Nanosci. Nanotechnol 15 (2015) 6405–6412. [DOI] [PubMed] [Google Scholar]
- [127].Sutunkova MP, Katsnelson BA, Privalova LI, Gurvich VB, Konysheva LK, Shur VY, Shishkina EV, Minigalieva IA, Solovjeva SN, Grebenkina SV, Zubarev IV, On the contribution of the phagocytosis and the solubilization to the iron oxide nanoparticles retention in and elimination from lungs under long-term inhalation exposure, Toxicology 363–364 (2016) 19–28. [DOI] [PubMed] [Google Scholar]
- [128].Onoue S, Yamada S, Chan HK, Nanodrugs: pharmacokinetics and safety, Int. J. Nanomed 9 (2014) 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K, Histological analysis of 70-nm silica particles-induced chronic toxicity in mice, Eur. J. Pharm. Biopharm.: Offic. J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 72 (2009) 626–629. [DOI] [PubMed] [Google Scholar]
- [130].Sung JH, Ji JH, Park JD, Yoon JU, Kim DS, Jeon KS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Chang HK, Lee JH, Cho MH, Kelman BJ, Yu IJ, Subchronic inhalation toxicity of silver nanoparticles, Toxicol. Sci. : Offic. J. Soc. Toxicol 108 (2009) 452–461. [DOI] [PubMed] [Google Scholar]
- [131].Warheit DB, Sayes CM, Frame SR, Reed KL, Pulmonary exposures to Sepiolite nanoclay particulates in rats: resolution following multinucleate giant cell formation, Toxicol. Lett 192 (2010) 286–293. [DOI] [PubMed] [Google Scholar]
- [132].Wu T, Tang M, Toxicity of quantum dots on respiratory system, Inhal. Toxicol 26 (2014) 128–139. [DOI] [PubMed] [Google Scholar]
- [133].Ma-Hock L, Brill S, Wohlleben W, Farias PM, Chaves CR, Tenorio DP, Fontes A, Santos BS, Landsiedel R, Strauss V, Treumann S, Ravenzwaay B, Short term inhalation toxicity of a liquid aerosol of CdS/Cd(OH)(2) core shell quantum dots in male Wistar rats, Toxicol. Lett 208 (2012) 115–124. [DOI] [PubMed] [Google Scholar]
- [134].Gosens I, Cassee FR, Zanella M, Manodori L, Brunelli A, Costa AL, Bokkers BG, de Jong WH, Brown D, Hristozov D, Stone V, Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure, Nanotoxicology 10 (2016) 1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bhirde AA, Patel S, Sousa AA, Patel V, Molinolo AA, Ji Y, Leapman RD, Gutkind JS, Rusling JF, Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice, Nanomedicine 5 (2010) 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Tseng WK, Chieh JJ, Yang YF, Chiang CK, Chen YL, Yang SY, Horng HE, Yang HC, Wu CC, A noninvasive method to determine the fate of Fe(3)O(4) nanoparticles following intravenous injection using scanning SQUID biosusceptometry, PLoS One 7 (2012) e48510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kim YS, Song MY, Park JD, Song KS, Ryu HR, Chung YH, Chang HK, Lee JH, Oh KH, Kelman BJ, Hwang IK, Yu IJ, Subchronic oral toxicity of silver nanoparticles, Part. Fibre Toxicol 7 (20) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wang C, Cheng K, Zhou L, He J, Zheng X, Zhang L, Zhong X, Wang T, Evaluation of long-term toxicity of oral zinc oxide nanoparticles and zinc sulfate in mice, Biol. Trace Elem. Res 178 (2017) 276–282. [DOI] [PubMed] [Google Scholar]
- [139].Bartneck M, Ritz T, Keul HA, Wambach M, Bornemann J, Gbureck U, Ehling J, Lammers T, Heymann F, Gassler N, Ludde T, Trautwein C, Groll J, Tacke F, Peptide-functionalized gold nanorods increase liver injury in hepatitis, ACS Nano 6 (2012) 8767–8777. [DOI] [PubMed] [Google Scholar]
- [140].Dumkova J, Smutna T, Vrlikova L, Le Coustumer P, Vecera Z, Docekal B, Mikuska P, Capka L, Fictum P, Hampl A, Buchtova M, Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs, Part. Fibre Toxicol 14 (2017) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wiemann M, Vennemann A, Blaske F, Sperling M, Karst U, Silver Nanoparticles in the Lung: Toxic Effects and Focal Accumulation of Silver in Remote Organs vol. 7, Nanomaterials, Basel, Switzerland), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gustafsson A, Jonasson S, Sandstrom T, Lorentzen JC, Bucht A, Genetic variation influences immune responses in sensitive rats following exposure to TiO2 nanoparticles, Toxicology 326 (2014) 74–85. [DOI] [PubMed] [Google Scholar]
- [143].Couto D, Freitas M, Costa VM, Chiste RC, Almeida A, Lopez-Quintela MA, Rivas J, Freitas P, Silva P, Carvalho F, Fernandes E, Biodistribution of polyacrylic acid-coated iron oxide nanoparticles is associated with proinflammatory activation and liver toxicity, J. Appl. Toxicol. : JAT (J. Appl. Toxicol.) 36 (2016) 1321–1331. [DOI] [PubMed] [Google Scholar]
- [144].Nahar M, Jain NK, Preparation, characterization and evaluation of targeting potential of amphotericin B-loaded engineered PLGA nanoparticles, Pharm. Res. (N. Y.) 26 (2009) 2588–2598. [DOI] [PubMed] [Google Scholar]
- [145].Park EJ, Kim SW, Yoon C, Kim Y, Kim JS, Disturbance of ion environment and immune regulation following biodistribution of magnetic iron oxide nanoparticles injected intravenously, Toxicol. Lett. 243 (2016) 67–77. [DOI] [PubMed] [Google Scholar]
- [146].Xu J, Peng C, Yu M, Zheng J, Renal clearable noble metal nanoparticles: photoluminescence, elimination, and biomedical applications, Wiley interdisciplinary reviews, Nanomed.Nanobiotechnol 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Riviere JE, Pharmacokinetics of nanomaterials: an overview of carbon nanotubes, fullerenes and quantum dots, Wiley interdisciplinary reviews, Nanomed.Nanobiotechnol 1 (2009) 26–34. [DOI] [PubMed] [Google Scholar]
- [148].Coccini T, Barni S, Mustarelli P, Locatelli C, Roda E, One-month persistence of inflammation and alteration of fibrotic marker and cytoskeletal proteins in rat kidney after Cd-doped silica nanoparticle instillation, Toxicol. Lett 232 (2015) 449–457. [DOI] [PubMed] [Google Scholar]
- [149].Chen X, Zhouhua W, Jie Z, Xinlu F, Jinqiang L, Yuwen Q, Zhiying H, Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-kappaB signaling pathway, Int. J. Nanomed 10 (2015) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Hoet PH, Bruske-Hohlfeld I, Salata OV, Nanoparticles - known and unknown health risks, J. Nanobiotechnol 2 (2004) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Oberdorster E, Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass, Environ. Health Perspect 112 (2004) 1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Sharma HS, Sharma A, Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology, Prog. Brain Res 162 (2007) 245–273. [DOI] [PubMed] [Google Scholar]
- [153].Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad Z, Ren G, A review of nanoparticle functionality and toxicity on the central nervous system, J. R. Soc. Interface 7 (4) (2010) S411–S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ze Y, Zheng L, Zhao X, Gui S, Sang X, Su J, Guan N, Zhu L, Sheng L, Hu R, Cheng J, Cheng Z, Sun Q, Wang L, Hong F, Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice, Chemosphere 92 (2013) 1183–1189. [DOI] [PubMed] [Google Scholar]
- [155].Kolter M, Ott M, Hauer C, Reimold I, Fricker G, Nanotoxicity of poly(n-butylcyano-acrylate) nanoparticles at the blood-brain barrier, in human whole blood and in vivo, J. Contr. Release 197 (2015) 165–179. [DOI] [PubMed] [Google Scholar]
- [156].Dabrowska-Bouta B, Zieba M, Orzelska-Gorka J, Skalska J, Sulkowski G, Frontczak-Baniewicz M, Talarek S, Listos J, Struzynska L, Influence of a low dose of silver nanoparticles on cerebral myelin and behavior of adult rats, Toxicology 363–364 (2016) 29–36. [DOI] [PubMed] [Google Scholar]
- [157].Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJ, Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain, Drug Chem. Toxicol 37 (2014) 336–347. [DOI] [PubMed] [Google Scholar]
- [158].Kim YR, Park SH, Lee JK, Jeong J, Kim JH, Meang EH, Yoon TH, Lim ST, Oh JM, An SS, Kim MK, Organization of research team for nano-associated safety assessment in effort to study nanotoxicology of zinc oxide and silica nanoparticles, Int. J. Nanomed 9 (2) (2014) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Korani M, Rezayat SM, Gilani K, Arbabi Bidgoli S, Adeli S, Acute and subchronic dermal toxicity of nanosilver in Guinea pig, Int. J. Nanomed 6 (2011) 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ryu HJ, Seo MY, Jung SK, Maeng EH, Lee SY, Jang DH, Lee TJ, Jo KY, Kim YR, Cho KB, Kim MK, Lee BJ, Son SW, Zinc oxide nanoparticles: a 90-day repeated-dose dermal toxicity study in rats, Int. J. Nanomed 9 (2) (2014) 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Nemmar A, Yuvaraju P, Beegam S, Yasin J, Kazzam EE, Ali BH, Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles, Int. J. Nanomed 11 (2016) 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K, Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance, Inhal. Toxicol 12 (2000) 1113–1126. [DOI] [PubMed] [Google Scholar]
- [163].Teodoro JS, Silva R, Varela AT, Duarte FV, Rolo AP, Hussain S, Palmeira CM, Low-dose, subchronic exposure to silver nanoparticles causes mitochondrial alterations in Sprague-Dawley rats, Nanomedicine 11 (2016) 1359–1375. [DOI] [PubMed] [Google Scholar]
- [164].Khanna P, Ong C, Bay BH, Baeg GH, Nanotoxicity: an Interplay of Oxidative Stress, vol. 5, Inflammation and Cell Death, Nanomaterials, Basel, Switzerland, 2015, pp. 1163–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, Li Y, Li B, Ge C, Zhou G, Gao Y, Zhao Y, Chai Z, Potential neurological lesion after nasal instillation of TiO(2) nanoparticles in the anatase and rutile crystal phases, Toxicol. Lett 183 (2008) 72–80. [DOI] [PubMed] [Google Scholar]
- [166].Manshian BB, Poelmans J, Saini S, Pokhrel S, Grez JJ, Himmelreich U, Mädler L, Soenen SJ, Nanoparticle-induced inflammation can increase tumor malignancy, Acta Biomater. 68 (2018. March 1) 99–112, 10.1016/j.actbio.2017.12.020 [Epub 2017 Dec 20]. [DOI] [PubMed] [Google Scholar]
- [167].Pandey RK, Prajapati VK, Molecular and immunological toxic effects of nanoparticles, Int. J. Biol. Macromol. 107 (Pt A) (2018. February) 1278–1293, 10.1016/j.ijbiomac.2017.09.110 Epub 2017 Oct 7. Review. [DOI] [PubMed] [Google Scholar]
- [168].Roy R, Kumar S, Tripathi A, Das M, Dwivedi PD, Interactive threats of nanoparticles to the biological system, Immunol. Lett 158 (2014) 79–87. [DOI] [PubMed] [Google Scholar]
- [169].Moon C, Park HJ, Choi YH, Park EM, Castranova V, Kang JL, Pulmonary inflammation after intraperitoneal administration of ultrafine titanium dioxide (TiO2) at rest or in lungs primed with lipopolysaccharide, J. Toxicol. Environ. Health Part a 73 (2010) 396–409. [DOI] [PubMed] [Google Scholar]
- [170].Sierra MI, Valdes A, Fernandez AF, Torrecillas R, Fraga MF, The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome, Int. J. Nanomed 11 (2016) 6297–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Shyamasundar S, Ng CT, Yung LY, Dheen ST, Bay BH, Epigenetic mechanisms in nanomaterial-induced toxicity, Epigenomics 7 (2015) 395–411. [DOI] [PubMed] [Google Scholar]
- [172].Smolkova B, El Yamani N, Collins AR, Gutleb AC, Dusinska M, Nanoparticles in food. Epigenetic changes induced by nanomaterials and possible impact on health, Food Chem. Toxicol.: Int. J. Pub. Br. Ind. Biol. Res. Assoc 77 (2015) 64–73. [DOI] [PubMed] [Google Scholar]
- [173].Stoccoro A, Karlsson HL, Coppede F, Migliore L, Epigenetic effects of nanosized materials, Toxicology 313 (2013) 3–14. [DOI] [PubMed] [Google Scholar]
- [174].Chen ZX, Riggs AD, DNA methylation and demethylation in mammals, J. Biol. Chem 286 (2011) 18347–18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Brown TA, Lee JW, Holian A, Porter V, Fredriksen H, Kim M, Cho YH, Alterations in DNA methylation corresponding with lung inflammation and as a biomarker for disease development after MWCNT exposure, Nanotoxicology 10 (2016) 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Lu X, Miousse IR, Pirela SV, Melnyk S, Koturbash I, Demokritou P, Short-term exposure to engineered nanomaterials affects cellular epigenome, Nanotoxicology 10 (2016) 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Gong C, Tao G, Yang L, Liu J, Liu Q, Zhuang Z, SiO(2) nanoparticles induce global genomic hypomethylation in HaCaT cells, Biochem. Biophys. Res. Commun 397 (2010) 397–400. [DOI] [PubMed] [Google Scholar]
- [178].Bai W, Chen Y, Gao A, Cross talk between poly(ADP-ribose) polymerase 1 methylation and oxidative stress involved in the toxic effect of anatase titanium dioxide nanoparticles, Int. J. Nanomed 10 (2015) 5561–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Ha SW, Jang HL, Nam KT, Beck GR Jr., Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression, Biomaterials 65 (2015) 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].De Prins S, Koppen G, Jacobs G, Dons E, Van de Mieroop E, Nelen V, Fierens F, Int Panis L, De Boever P, Cox B, Nawrot TS, Schoeters G, Influence of ambient air pollution on global DNA methylation in healthy adults: a seasonal follow-up, Environ. Int 59 (2013) 418–424. [DOI] [PubMed] [Google Scholar]
- [181].Paluch BE, Naqash AR, Brumberger Z, Nemeth MJ, Griffiths EA, Epigenetics: a primer for clinicians, Blood Reviews 30 (2016) 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Choi AO, Brown SE, Szyf M, Maysinger D, Quantum dot-induced epigenetic and genotoxic changes in human breast cancer cells, J. Mol. Med. (Berl.) 86 (2008) 291–302. [DOI] [PubMed] [Google Scholar]
- [183].Dubey P, Matai I, Kumar SU, Sachdev A, Bhushan B, Gopinath P, Perturbation of cellular mechanistic system by silver nanoparticle toxicity: cytotoxic, genotoxic and epigenetic potentials, Adv. Colloid Interface Sci 221 (2015) 4–21. [DOI] [PubMed] [Google Scholar]
- [184].Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G, Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs, Inhal. Toxicol 21 (1) (2009) 55–60. [DOI] [PubMed] [Google Scholar]
- [185].Elsaesser A, Howard CV, Toxicology of nanoparticles, Adv. Drug Deliv. Rev 64 (2012) 129–137. [DOI] [PubMed] [Google Scholar]
- [186].Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M, Toxicity of nanomaterials, Chem. Soc. Rev 41 (2012) 2323–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, Mattiello S, Cubadda F, Aureli F, D’Amato M, Raggi A, Lenardi C, Milani P, Scanziani E, Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects, Part. Fibre Toxicol 13 (2016) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Shegokar R, Singh KK, Surface modified nevirapine nanosuspensions for viral reservoir targeting: in vitro and in vivo evaluation, Int. J. Pharm 421 (2011) 341–352. [DOI] [PubMed] [Google Scholar]
- [189].Simpson CA, Salleng KJ, Cliffel DE, Feldheim DL, In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles, Nanomedicine 9 (2013) 257–263. [DOI] [PubMed] [Google Scholar]
- [190].Buzea C, Pacheco II, Robbie K, Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2 (2007) Mr17–71. [DOI] [PubMed] [Google Scholar]
- [191].Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H, Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy, Part. Fibre Toxicol 2 (8) (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Colvin VL, The potential environmental impact of engineered nanomaterials, Nat. Biotechnol 21 (2003) 1166–1170. [DOI] [PubMed] [Google Scholar]
- [193].Brumfiel G, Nanotechnology: a little knowledge, Nature 424 (2003) 246–248. [DOI] [PubMed] [Google Scholar]
- [194].Mogharabi M, Abdollahi M, Faramarzi MA, Toxicity of nanomaterials; an undermined issue, Daru : Journal of Faculty of Pharmacy, Tehran University of Medical Sciences 22 (59) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Mukherjee B, Maji R, Roychowdhury S, Ghosh S, Toxicological concerns of engineered nanosize drug delivery systems, Am. J. Therapeut 23 (2016) e139–150. [DOI] [PubMed] [Google Scholar]


