Abstract
SDF-2 is a peptide released by prestalk cells during culmination that stimulates prespore cells to encapsulate. Genetic evidence indicates that the response is dependent on the dhkA gene. This gene encodes a member of the histidine kinase family of genes that functions in two-component signal transduction pathways. The sequence of the N-terminal half of DhkA predicts two hydrophobic domains separated by a 310-amino-acid loop that could bind a ligand. By inserting MYC6 epitopes into DhkA, we were able to show that the loop is extracellular while the catalytic domain is cytoplasmic. Cells expressing the MYC epitope in the extracellular domain of DhkA were found to respond only if induced with 100-fold-higher levels of SDF-2 than required to induce dhkA+ cells; however, they could be induced to sporulate by addition of antibodies specific to the MYC epitope. To examine the enzymatic activity of DhkA, we purified the catalytic domain following expression in bacteria and observed incorporation of labelled phosphate from ATP consistent with histidine autophosphorylation. Site-directed mutagenesis of histidine1395 to glutamine in the catalytic domain blocked autophosphorylation. Furthermore, genetic analyses showed that histidine1395 and the relay aspartate2075 of DhkA are both critical to its function but that another histidine kinase, DhkB, can partially compensate for the lack of DhkA activity. Sporulation is drastically reduced in double mutants lacking both DhkA and DhkB. Suppressor studies indicate that the cyclic AMP (cAMP) phosphodiesterase RegA and the cAMP-dependent protein kinase PKA act downstream of DhkA.
Amoebae of Dictyostelium discoideum aggregate into mounds containing up to 105 cells before forming fruiting bodies in which the spore mass is held up by a tapering stalk (9, 19, 20, 26). Although prespore and prestalk cells diverge and sort out soon after aggregation, they do not form spores and stalk cells until fruiting body formation is initiated. Since spores are unable to move once they have encapsulated, it is essential that their terminal differentiation be coordinated with their position on the elongating stalk; otherwise they would be left at the base. When the mass of prespore cells has been lifted off the substratum, a wave of expression of the spore-specific gene spiA can be seen to start in the prespore cells nearest the prestalk region that passes down through the mass of prespore cells in an hour or two (28). This temporal pattern suggests that prespore cells are responding to a signal secreted from prestalk cells as they undergo terminal differentiation.
The peptide signal SDF-2 is released from prestalk cells in a manner dependent on the ABC transporter TagC and induces rapid encapsulation of prespore cells (6, 31, 33). A candidate receptor for this signal is the putative histidine kinase DhkA, since strains carrying null mutations in dhkA form few spores even when they develop in chimeric mixtures with wild-type cells. When developed as pure populations, dhkA− cells proceed normally to culmination but then form fruiting bodies with long thin stalks that tend to topple over (42). Cells that are deficient in DhkA fail to respond to the sporulation-inducing factor SDF-2 (6), further implicating DhkA in the response to SDF-2. The predicted product of dhkA has two hydrophobic domains near the N terminus that could cross the membrane, leaving an extracellular loop of about 310 amino acids on the outside while keeping the putative catalytic domain and the aspartate relay site within the cytoplasm. This putative topology, together with the genetic evidence, suggests that DhkA may be the SDF-2 receptor.
DhkA is a member of the family of two-component signal transduction systems, in which autophosphorylation of a receptor kinase leads to modification of the activity of a response regulator (4, 38). These two-component systems are found in both bacteria and eukaryotes (3, 10, 11, 12, 14, 21, 23, 24, 29). Phosphorelay to an aspartate moiety on the response regulator either can be direct from the histidine phosphate of the sensor kinase or may go through various intermediates (13, 16, 25). DhkA is referred to as a “hybrid kinase” because it has both a catalytic domain and a response regulatory domain (42). Such hybrid kinases autophosphorylate the histidine in the sequence conserved just upstream of the catalytic domain and then pass the phosphate to the aspartate moiety in the response regulatory domain. In the yeast osmoregulatory signal transduction pathway, the phosphate linked to histidine on SLN1 is relayed to an aspartate near the carboxy end of SLN1 before being passed to a histidine carried by the small intermediate protein YPD1 and then on to its final destination on SSK1 (25). Phosphorylation of SSK1 keeps it from activating the mitogen-activated protein kinase kinase kinases SSK2 and SSK22 (22).
Dictyostelium cells that overexpress the catalytic subunit of PKA (40) as a result of carrying multiple copies of the pkaC gene can be induced to form spores rapidly after 24 h in monolayer cultures by addition of SDF-2 (6). While SDF-2 induces up to 50% of dhkA+ pkaC::pkaC cells to encapsulate within 30 min, it does not affect encapsulation in dhkA− pkaC::pkaC cells (6). These results demonstrate that DhkA plays an essential role in the response to SDF-2 and raises the possibility that this peptide is a ligand that activates DhkA and leads to rapid encapsulation. Since activation of PKA by addition of the membrane-permeable derivative of cyclic AMP (cAMP), 8-Br-cAMP, leads to rapid encapsulation even in dhkA− mutant cells (42), it is likely that PKA functions downstream of DhkA.
It has recently been shown that another histidine kinase, DhkB, functions in spores to ensure dormancy by maintaining high PKA activity (45). The germination inhibitor that accumulates during culmination, discadenine, was proposed as a candidate for the ligand that activates DhkB. Moreover, Zinda and Singleton (45) suggested that PKA activity is kept high in spores by inhibiting the cytoplasmic cAMP phosphodiesterase RegA (34, 41). Thus, both of the signal transduction pathways initiated by these histidine kinases may result in elevated levels of cAMP and PKA activity.
In the present study, we show that DhkA is a membrane-spanning histidine kinase and that modification of the extracellular domain by insertion of a MYC epitope reduces sensitivity to SDF-2. Moreover, the presence of the MYC epitope in the extracellular loop of DhkA makes it sensitive to activation by monoclonal antibody to MYC. We have found that inactivating either regA, the gene encoding the cytoplasmic cAMP phosphodiesterase, or pkaR, a gene encoding the regulatory subunit of PKA, partially suppresses the block to sporulation resulting from inactivation of dhkA. Previous studies have shown that activation of PKA in prespore cells leads to rapid encapsulation (15, 27). These findings are consistent with a signal transduction pathway in which the SDF-2 peptide activates DhkA on the surface of prespore cells, leading to inhibition of RegA and to subsequent activation of PKA.
MATERIALS AND METHODS
Chemicals.
Proteinase K was purchased from Boehringer-Mannheim, Indianapolis, Ind. Monoclonal antibody 9E10 against c-Myc was purchased from Santa Cruz Biotechnology, Santa Cruz, Calif. Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) and goat anti-rabbit IgG were purchased from Sigma, St. Louis, Mo. Custom-synthesized oligonucleotides were from Operon Technologies Inc., Alameda, Calif.
Plasmids.
The knockout vector for disruption of pkaR was a gift from A. Kuspa. The expression vector pkaC::pkaC (A-7 Neo) has been previously described (5). pBluescript-MYC6 was a gift from M. Yaffe. Knockout vectors for disruption of regA (30) and expression of dhkA under its own promoter (42) were as described previously. Site-directed mutagenesis, insertion of DNA sequences encoding six consecutive c-Myc epitope tags, and in-frame deletion in dhkA were performed by standard molecular biology techniques, and the mutated alleles were cloned into the dhkA expression vector (42). All of the new constructs were verified by sequencing with an ABI Prism 377 DNA sequencer. The dhkA mutant alleles generated were dhkAH1395Q, which carries a T-to-A substitution at bp 4185 such that histidine 1395 is changed into glutamine; dhkAD2075N, which carries a G-to-A substitution at bp 5223 such that aspartate 2075 is changed into asparagine; dhkA900MYC6, in which a 302-bp PstI DNA fragment was generated by PCR from pBluescript-MYC6 and introduced into the PstI site at bp 2701 in dhkA; and dhkA2025MYC6, in which a 291-bp HindIII DNA fragment was generated by PCR from pBluescript-MYC6 and introduced into the HindIII site at bp 6076 in dhkA.
Plasmids pdhkAcat and pdhkAcatH1395Q encode His6-DhkA fusion proteins that can be expressed in bacteria. They were constructed by ligating PCR-amplified DNA encoding amino acids 1275 to 1884 of dhkA (42) between the SalI and BamHI sites of pQE-9 (Qiagen, Palo Alto, Calif.). The template used to generate the insert in pdhkAcatH1395Q was full-length dhkA DNA with the site-directed mutation H1395Q. The single T-to-A substitution in the insert of pdhkAcatH1395Q was verified by sequencing. All other bases were identical to those in pdhkAcat. These plasmids were expressed in Escherichia coli M15 (pREP4) following induction with IPTG (isopropyl-β-d-thiogalactopyranoside).
Cells, growth, transformation, and development.
Strains used in this work were wild-type AX4 cells (17), dhkA null mutants (42), and regA null mutants (30). All strains were grown in HL5 medium and maintained on SM nutrient agar in association with bacteria (39). Synchronous development of cells on nitrocellulose filters and sporulation assays were performed as described previously (31, 32).
pkaR and regA were disrupted in a dhkA− background by homologous recombination, as described previously (34). Transformation with expression vectors for dhkA alleles and pkaC::pkaC, selection for G418 resistance, and maintenance of transformed cell lines were as described previously (32). All genetic modifications were confirmed by Southern blot analyses, as described previously (32). In the double transformant carrying dhkAH1395Q and dhkAD2075N, the transforming plasmids were identical except for the respective point mutations. A 2.6-kb fragment spanning both mutation sites was amplified by PCR from genomic DNA of transformed cells, digested with EcoRI, and analyzed by agarose gel electrophoresis to confirm the presence of the mutation in dhkAD2075N. The respective digested DNA fragments were purified from the gel and sequenced to confirm the presence of the mutation in dhkAH1395Q.
dhkB was disrupted in strains AX4 (dhkA+) and AK299 (dhkA−) (42). The knockout plasmid p2C3/BSR, in which the histidine kinase domain of dhkB is replaced with a blasticidin S resistance cassette (45), was digested with PvuII and EcoRI to isolate the BSR cassette flanked by dhkB sequences. Cells of strains AX4 and AK299 were transformed with this linearized fragment by electroporation, followed by selection for blasticidin resistance, as described by Kuspa and Loomis (18). The disruption of dhkB was confirmed by Southern blot analysis.
Subcellular fractionation of DhkA.
Cells (108) expressing the 900MYC6 epitope-tagged DhkA protein were developed on nitrocellulose filters for 18 h. The cells were collected into buffer (20 mM Tris [pH 8.3], 150 mM NaCl, 1 mM EDTA) and frozen at −80°C. Cells were thawed on ice, the sample was split in two, and 0.5% Nonidet P-40 (NP-40) was added to one of the aliquots. After centrifugation at 1,250 × g for 5 min at 4°C, the supernatant was centrifuged at 40,000 × g for 30 min at 4°C. The resulting supernatant and pellet were saved. One-fifth of each fraction or one-fifth of a sample containing an equal number of unfractionated cells was mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and the proteins were resolved by electrophoresis on a 5.5% polyacrylamide-SDS gel. Proteins were subjected to Western blot analysis with the 9E10 monoclonal antibody against c-Myc (Santa Cruz Biotechnology), as described previously (37).
Sensitivity of DhkA to proteinase K treatment of intact cells.
Cells expressing the 900MYC6 epitope-tagged DhkA protein or the 2025MYC6 epitope-tagged DhkA protein were developed on nitrocellulose filters for 18 h. Multicellular structures were collected into isotonic buffer (30 mM HEPES [pH 7.5], 10 mM magnesium acetate, 10 mM NaCl, and 10% sucrose) and triturated by passage through a 22-gauge needle. Samples of 2.5 × 107 cells per ml were treated with freshly prepared proteinase K solution at 0°C as indicated. After an hour, 1 mM phenylmethylsulfonyl fluoride (PMSF) was added to inhibit the protease, and the cells were washed three times by centrifugation and resuspended in 1 ml of isotonic buffer containing 1 mM PMSF. The cells were then lysed in SDS-PAGE sample buffer, and one-fifth of each sample was resolved by electrophoresis on a 7.5% polyacrylamide-SDS gel. Proteins were subjected to Western blot analysis with the 9E10 monoclonal antibody against c-Myc (Santa Cruz Biotechnology) or with a polyclonal antibody against the intracellular TipA protein (37), as described previously (31).
Encapsulation assay.
Cells were dissociated from early to mid-culminants and deposited at 2 × 104/cm2 in 24-well Falcon plastic plates with 0.5 ml of buffer containing 10 mM MES [2-(N-morpholino)ethanesulfonic acid] (pH 6.5), 20 mM NaCl, 20 mM KCl, 1 mM CaCl2, and 1 mM MgSO4. Purified SDF-2 or anti-MYC monoclonal antibody 9E10 was added at various concentrations, and spore-like cells were scored by phase-contrast microscopy (7). Each assay was repeated three to six times, and at least 200 cells were scored. One unit of SDF-2 activity is defined as the amount necessary to induce 50% of K-P cells to form spores.
Protein kinase assay.
Extracts were prepared from E. coli M15(pREP4) carrying pdhkAcat, pdhkAcatH1395Q, or the vector pQE-9 (Qiagen) with no insert. When the cultures reached an optical density at 600 nm of 0.7, they were induced with 1 mM IPTG for 1 h and 1 ml was harvested in a solution of 10 mM Tris-HCl (pH 8.0), 50 mM sodium phosphate (pH 8.0), 100 mM NaCl, and 0.1 mM PMSF before being broken by sonication. After centrifugation at 16,000 × g for 5 min, the supernatants were mixed with 50 μl of Talon Metal Affinity Resin (Clontech Laboratories, Inc., Palo Alto, Calif.) for 20 min at 4°C. Talon beads were washed with a solution of 50 mM Tris-HCl (pH 7.5), 50 mM KCl, 5 mM MgCl2, and 0.1 mM PMSF; collected by centrifugation; and washed a second time with the same buffer supplemented with 2 mM 2-mercaptoethanol.
Talon beads carrying His6 anchored proteins were resuspended in wash buffer with 2-mercaptoethanol, incubated at 22°C for 45 min with 50 μM [γ-32P]ATP (6,000 Ci/mmol), washed three times with buffer, and then eluted with 50 μl of 100 mM EDTA. Samples were electrophoretically separated on 10% polyacrylamide-SDS gels at 4°C and subsequently exposed to REFLECTION X-ray film (NEN) at −70°C with an intensifying screen. The proteins were transferred to a PROTRAN nitrocellulose membrane (Schleicher & Schuell) by electroblotting, exposed, and then treated with 1 M HCl for 2 h to hydrolyze histidine phosphates (25) before reexposure. This experiment was repeated three times with essentially the same results.
To determine the levels of expression of the wild-type and mutated forms of the DhkA catalytic domain, equal numbers of bacteria were lysed in a solution of 8 M urea, 0.1 mM PMSF, 20 mM Tris-HCl (pH 8.0), and 100 mM NaCl at 1 h after induction with 1 mM IPTG. The extracts were centrifuged and the supernatants were mixed with Talon beads. After two washes with lysis buffer, bound proteins were eluted from the beads with 75 mM imidazole in lysis buffer and electrophoretically resolved on SDS gels. A major silver-stained band of the size expected for the catalytic domain of DhkA (70 kDa) was present at equal levels on the gels of material from bacteria carrying either pdhkAcat or pdhkAcatH1395Q. This band was absent on gels of material from bacteria carrying only the vector plasmid. The 70-kDa band was recognized by antibodies specific to the RGS-His epitope (Qiagen) when proteins in the gel were transferred to nitrocellulose, indicating that this band contained the DhkA catalytic domain.
RESULTS
DhkA spans the plasma membrane.
The primary sequence of dhkA suggested that the encoded protein might be membrane associated, due to the presence of two hydrophobic stretches of about 20 amino acids each between amino acids 770 and 790 and between amino acids 1100 and 1120 (42). To directly determine the membrane topology of DhkA, we generated two independent epitope-tagged alleles of dhkA by introducing a DNA fragment encoding six successive MYC epitopes into different regions in the gene. One of the MYC6 epitopes was introduced into the predicted extracellular region of DhkA between amino acids 900 and 901 (dhkA900MYC6), and the other was introduced into the carboxy terminus between amino acids 2025 and 2026 (dhkA2025MYC6). The tagged genes were transformed into dhkA null mutants, and the localization of the tagged protein was tested.
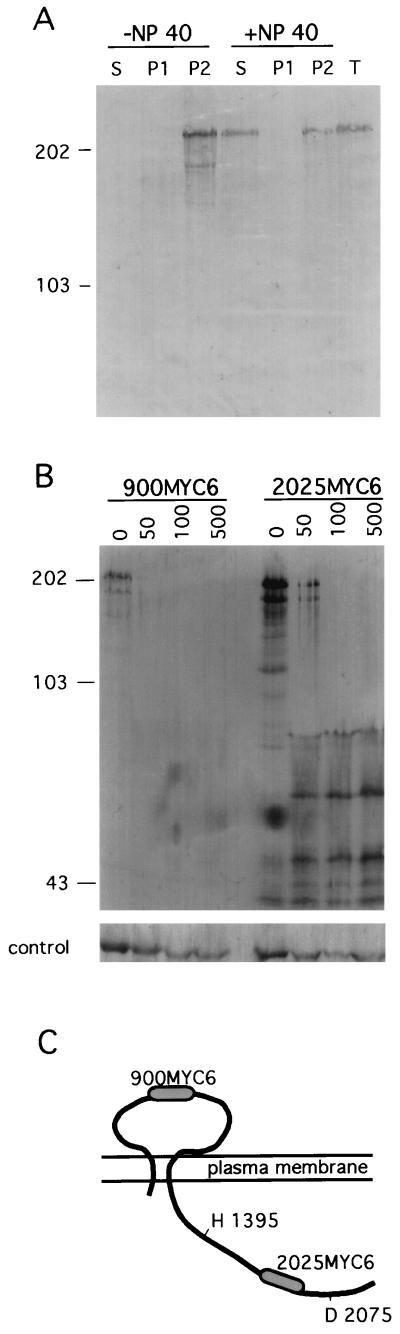
Extracts were prepared from cells expressing dhkA900MYC6 that were disrupted by freezing at −80°C and thawing on ice and fractionated by centrifugation at 40,000 × g for 30 min. The supernatant and pelleted material were subjected to SDS-PAGE and analyzed by Western blotting with an anti-MYC monoclonal antibody. The results presented in Fig. 1A show that the epitope-tagged DhkA protein was found exclusively in the pellet unless the cells were treated with detergent (0.5% NP-40) which partially solubilized the protein. These results support the prediction that DhkA is a membrane-associated protein.
FIG. 1.
Membrane association and orientation of the DhkA protein. (A) Cells expressing the 900MYC6 epitope-tagged DhkA protein were disrupted in the presence or absence of 0.5% NP-40, as indicated. The supernatant (S) and pelleted material (P) following high-speed centrifugation, as well as unfractionated extract (T), were resolved by gel electrophoresis and analyzed by Western blotting with a monoclonal antibody against the MYC epitope. Each sample contains material from 2 × 107 cells. The sizes and positions of protein markers are indicated on the left, in kilodaltons. (B) Intact cells expressing the 900MYC6 epitope or the 2025MYC6 epitope were disaggregated by trituration in isotonic buffer and treated with 0 to 500 μg of proteinase K per ml, as indicated above the respective lanes. Cells were washed free of the protease and resuspended in sample buffer. Samples representing 2 × 107 cells each were resolved by gel electrophoresis and analyzed by Western blotting with a monoclonal antibody against the MYC epitope. Numbers on the left indicate sizes, in kilodaltons. As a control, aliquots from the same samples were analyzed with antibodies against the intracellular protein TipA. (C) Schematic representation of the DhkA protein (2150 amino acids) relative to the plasma membrane. The MYC6 epitopes are represented by shaded areas. H 1393 and D 2075 represent the conserved histidine residue in the H motif and the conserved aspartic acid residue in the D motif, respectively. The drawing is not to scale.
In order to determine whether or not DhkA has an extracellular domain, we subjected intact cells to a protease protection assay. Cells expressing dhkA900MYC6 were treated with various concentrations of proteinase K for 1 h on ice. At the end of the reaction, the cells were washed and lysed. Tagged DhkA was electrophoretically separated on polyacrylamide-SDS gels and analyzed on Western blots with an anti-MYC monoclonal antibody. The results in Fig. 1B show that the 900MYC6 epitope tag was sensitive to treatment of intact cells with protease, consistent with the predicted extracellular localization of the epitope. As a control, we show that the intracellular protein TipA (37) was not affected by treating whole cells with proteinase K.
To determine the localization of the putative catalytic domain of DhkA, we performed a similar assay on cells carrying the 2025MYC6 epitope tag. The size of that epitope-tagged protein was reduced following treatment of intact cells with protease (Fig. 1B). Note that while the 900MYC6 epitope was completely degraded by protease treatment, the DhkA protein carrying the 2025MYC6 epitope was trimmed to fragments ranging in size between 45 and 70 kDa, apparently by intracellular proteases following release of the extracellular domain, but not completely degraded. These observations are consistent with extracellular localization of the 900MYC epitope and intracellular localization of the 2025MYC6 epitope. The simplest interpretation of the results presented in Fig. 1A and B is that DhkA is a plasma membrane protein in which the region surrounding amino acid 900 is extracellular and the region including amino acid 2025 is intracellular (Fig. 1C).
Modification of the extracellular loop reduces sensitivity to SDF-2.
Insertion of the MYC6 epitope tag either into the extracellular loop or near the C terminus was found to affect the function of dhkA, since neither the dhkA900MYC6 allele nor the dhkA2025MYC6 allele was able to fully complement the sporulation defect in dhkA null cells (Table 1). If DhkA is a receptor kinase, then the epitope tag in amino acid 900 could interfere with ligand binding. The epitope tag in amino acid 2025 is adjacent to the predicted D motif around the aspartic acid residue at amino acid 2075 and could possibly interfere with phosphorylation of that residue.
TABLE 1.
Complementation of dhkA null mutants by dhkA alleles
|
dhkA allele in complementation vector |
% Viable sporesa |
|---|---|
| None | 4 ± 3 |
| Wild-type dhkA | 100 ± 8 |
| dhkA900MYC6 | 24 ± 5 |
| dhkA2025MYC6 | 9 ± 3 |
| dhkAH1395Q | 15 ± 7 |
| dhkAD2075N | 35 ± 8 |
| dhkAH1395Q and dhkAD2075N | 100 ± 7 |
The number of detergent-resistant viable spores after 36 h of development on filters is presented as a percentage of the original number of cells developed (5 × 107). The number of viable spores was determined in at least three separate experiments with each strain.
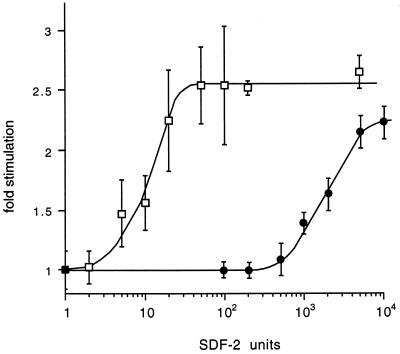
To determine whether the sensitivity for SDF-2 was reduced in dhkA− cells expressing dhkA900MYC6, we dissociated them from mid-culminants and dispersed them as a monolayer under buffer. Purified SDF-2 was added at various concentrations to induce sporulation. As can be seen in Fig. 2, 100-fold-higher concentrations of SDF-2 were required for the maximal response in dhkA900MYC6 cells than were needed with wild-type cells. On the other hand, even 5,000 units of SDF-2 failed to increase the level of sporulation in the host dhkA− cells (data not shown). It appears that the MYC epitope in the extracellular loop significantly reduces the sensitivity of DhkA for SDF-2 but does not abolish it.
FIG. 2.
Reduced sensitivity to SDF-2 in dhkA900MYC6 cells. Wild-type cells of strain AX4 (□) and dhkA− cells carrying the DhkA 900MYC6 construct (●) were developed on filters until early culmination, dissociated, and deposited as a monolayer under buffer. Purified SDF-2 was added at various concentrations, and spores were counted after 6 h. Fold stimulation was calculated relative to the number of spores seen in cultures incubated in the absence of added SDF-2. As a control, we added 50 units of SDF-1 and found that sporulation was stimulated 2.3 ± 0.2-fold for both wild-type dhkA+ cells and dhkA900MYC6 cells.
The presence of the MYC epitope in DhkA allowed us to test whether specific antibodies to it would activate the cells in a manner similar to activation of mammalian lymphocytes by cross-linking of surface antigens with antibodies (8, 36, 43, 44). Monoclonal antibody to MYC was added at various dilutions to monolayers of dhkA900MYC6 cells dissociated from early culminants. Sporulation was induced in dhkA900MYC6 cells even when the monoclonal antibody was diluted 20,000-fold, while the antibody even at 40-times-higher concentrations had no significant effect on wild-type cells that do not carry the MYC epitope (Table 2). There was no response of cells of either strain to 1:500 dilutions of a monoclonal antibody (MLJ11) to an unrelated surface protein (Table 2) or nonspecific mouse IgG (data not shown). The response of dhkA900MYC6 cells to anti-MYC antibodies further confirms that the epitope is extracellular and indicates that the loop is directly involved in activation of DhkA.
TABLE 2.
Stimulation of sporulation by MYC antibodies
| Monoclonal antibody (dilution) | Fold stimulationa
|
|
|---|---|---|
| AX4 | DhkA 900MYC6 | |
| Anti-MYC (1:500) | 1.02 ± 0.03 | 2.5 ± 0.3 |
| Anti-MYC (1:10,000) | 2.1 ± 0.2 | |
| Anti-MYC (1:20,000) | 1.6 ± 0.2 | |
| Anti-MYC (1:50,000) | 1.2 ± 0.1 | |
| Anti-MLJ11 (1:500) | 0.9 ± 0.1 | 1.1 ± 0.1 |
Wild-type cells of strain AX4 and dhkA− cells carrying the DhkA 900MYC6 construct were developed on filters until early to mid-culmination, dissociated, and deposited as a monolayer under buffer. Various dilutions of monoclonal antibody 9E10, specific to the MYC epitope, or monoclonal antibody to a surface protein, MLJ11, were added, and spores were counted after 3 h. Fold induction by antibody was calculated relative to the number of spores seen in cultures incubated in the absence of antibody.
Site-directed mutations.
DhkA is similar to other hybrid kinases in that it carries both an H motif in the predicted catalytic histidine kinase domain and a D motif in the carboxy terminus. The importance of these two domains for the activity of DhkA is demonstrated by the results shown in Table 1. The dhkA gene was modified by site-directed mutagenesis to change either the conserved histidine residue in the H motif into glutamine (dhkAH1395Q) or to change the conserved aspartic acid residue in the D motif into asparagine (dhkAD2075N). The mutant alleles were cloned into separate dhkA expression vectors and transformed individually or in combination into dhkA null mutant cells. Sporulation of the resulting transformed cell lines was measured. As shown in Table 1, the wild-type dhkA expression vector was able to fully complement the dhkA− mutation, and site-directed mutations in either the H or D motif reduced that ability, demonstrating the importance of each of these residues for the activity of DhkA. When both the H1395Q and D2075N constructs were cotransformed into the same dhkA null host cell line, they complemented each other and were able to fully rescue the sporulation defect of the dhkA null host (Table 1). This result indicates that, as in the yeast osmoregulatory sensor kinase SLN1 (25), phosphorylation of the aspartic acid in the D motif is not necessarily a monomolecular event. In addition, this finding indicates that these single amino acid substitutions did not have a general effect on the structure of the protein but rather a specific effect on the predicted functional domains.
Partial redundancy of DhkA and DhkB.
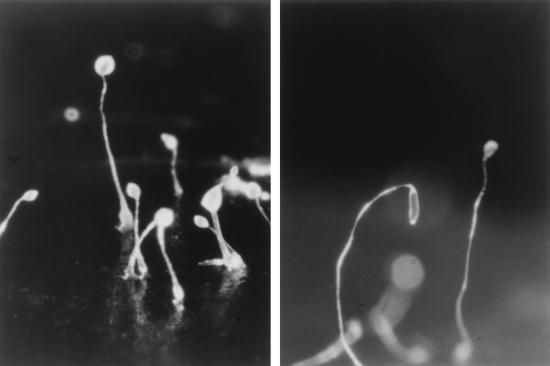
Although sporulation is severely reduced and delayed in dhkA− strains, it is not totally blocked (Table 1). Residual sporulation may result from partial overlap of other pathways functioning during culmination. The histidine kinase DhkB has been shown to play an essential role in maintaining dormancy once spores have encapsulated (45). It has been proposed that DhkB responds to the adenine derivative discadenine, which accumulates during culmination and is essential for maintaining dormancy (1). Since it appears that activation of either DhkA or DhkB can result in accumulation of cAMP to levels that activate PKA, DhkB may be responsible for the residual sporulation seen in the absence of DhkA. Therefore, we knocked out dhkB in a dhkA− null mutant by using homologous recombination and determined the sporulation efficiency of the double mutant. Cells lacking both histidine kinases form fruiting bodies with long thin stalks resembling those of dhkA− mutants and make very few spores (Fig. 3; Table 3). Since inactivation of dhkB alone does not significantly affect encapsulation (45), we would have expected the double mutants to form at least a few percent spores, but less than 1 in 104 of the cells encapsulated. The dhkA− dhkB− double mutant could be induced to sporulate efficiently by incubating cells dissociated from early culminants in the presence of 20 mM 8-Br-cAMP to activate PKA (data not shown). These results indicate that PKA activation by DhkB may account for the low level of sporulation that is seen in dhkA− strains.
FIG. 3.
Terminal differentiation of the dhkA− dhkB− double mutant. Wild-type (AX4) cells (left) and a dhkB− derivative of strain AK299 (dhkA−) (right) were developed for 36 h on filter supports. The fruiting bodies of the double-mutant (dhkA− dhkB−) strain had long weak stalks that often toppled over before they could be photographed. Prespore cells of the double mutant ascended the stalks but never encapsulated to form spores.
TABLE 3.
Sporulation of dhkA− dhkB− double mutants
| Genotype | % Viable sporesa
|
|
|---|---|---|
| 24 h | 36 h | |
| dhkA− | 2 ± 2 | 4 ± 3 |
| dhkA− dhkB− | 0 | 0 |
| dhkA− dhkB−/dhkA+ | 15 ± 6 | 5 ± 4 |
| dhkA− dhkB−/dhkAH1395Q | 0 | 0 |
| dhkA− dhkB−/dhkAD2075N | 0 | 0 |
The number of detergent-resistant viable spores is presented as a percentage of the total number of cells (5 × 107). The number of viable spores was determined in at least three separate experiments with each strain. Less than 1 in 103 cells formed spores in the double-mutant strains except for that transformed with wild-type dhkA.
DhkB may also account for the higher levels of spores seen in dhkA− strains expressing mutant forms of DhkA than in the parental dhkA− strain that completely lacks DhkA (Table 1). To test for cross talk among the histidine kinases, we transformed the dhkA− dhkB− double mutant with constructs for the expression of wild-type dhkA or one of the point mutations, dhkAH1395Q or dhkAD2075N. While expression of the wild-type dhkA construct led to the formation of fairly normal fruiting bodies resembling those seen in dhkB− strains and resulted in a high level of sporulation, neither of the constructs expressing mutant forms of DhkA had any effect on fruiting body morphology, and they failed to rescue sporulation (Table 3). The residual sporulation seen in dhkA− cells transformed with these mutant constructs appears to depend on the function of DhkB.
Autophosphorylation of the catalytic domain of DhkA.
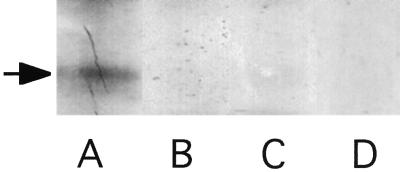
To test whether the putative catalytic domain of DhkA is able to catalyze its own phosphorylation, we expressed the region encoding amino acids 1275 to 1884 of DhkA (42), fused to a His6 tag, in E. coli. This DhkA derivative lacks the transmembrane domains and intervening loop as well as the carboxy-terminal portion where the relay aspartate is found. We also prepared a variant of this protein in which the essential histidine codon was modified to encode glutamine, as in the dhkAH1395Q mutation. Both of these proteins were expressed at high levels in E. coli (see Materials and Methods). The His6 fusion proteins were bound to metal affinity beads and incubated with radiolabelled ATP while still on the beads. The proteins were then eluted, electrophoretically separated on gels, and autoradiographed. Material prepared from bacteria transformed with the construct expressing the wild-type catalytic domain incorporated label into a protein of the expected size (Fig. 4A). A protein of the same size (70 kDa) was recognized by antibodies to the His6 epitope (data not shown). Extracts from bacteria expressing the construct modified by site-directed mutagenesis did not incorporate label into a protein of this size (Fig. 4B), although a protein with the His6 epitope was present at the same level (data not shown). To test whether the label in the 70-kDa material had the properties of histidine-phosphate, blots of the gels were exposed before and after being treated with 1 M HCl for 2 h (25). As expected, all of the radioactivity in the 70-kDa band was removed by mild acid hydrolysis (Fig. 4D). Together with the fact that replacement of histidine1395 by glutamine precluded phosphorylation, these results show that DhkA is an autophosphorylating histidine kinase.
FIG. 4.
Autophosphorylation of DhkA. Proteins from an equal number of bacteria carrying pdhkAcat (A), pdhkAcatH1395Q (B), or vector alone (C) were bound to metal affinity beads and incubated with [32P]ATP. Proteins were eluted, resolved by gel electrophoresis, and exposed to X-ray film. (D) Proteins were then transferred to a nitrocellulose filter that was treated with 1 M HCl for 2 h, and the portion shown in lane A was exposed for the same period of time to X-ray film. The arrow indicates the position of the 70-kDa protein.
Genetic analysis.
Null mutations in dhkA result in reduced sporulation and enhanced prestalk differentiation (42), a phenotype opposite that observed with either regA null mutants or pkaR null mutants where prestalk and stalk differentiation are compromised and prespore differentiation is accelerated (2, 30, 35). We therefore generated double null mutants in which either regA or pkaR were disrupted in a dhkA null background. Both the dhkA− regA− double null mutants and the dhkA− pkaR− double null mutants sporulated well in comparison to the parental dhkA− strain, as did a dhkA null mutant that was transformed with a vector leading to overexpression of the catalytic subunit of PKA encoded by the pkaC gene (Table 4). Thus, RegA and PKA appear to function downstream of DhkA. Previous results have shown that all of these genes function downstream of the ABC transporter TagC and that the effects of RegA are mediated by PKA (30, 34).
TABLE 4.
Epistasis relationships in the sporulation pathway
| Genotype | % Viable sporesa |
|---|---|
| Wild type | 100 ± 7 |
| regA− | 50 ± 5 |
| pkaR− | 52 ± 5 |
| pkaC::pkaC | 59 ± 6 |
| dhkA− | 4 ± 3 |
| dhkA− regA− | 34 ± 8 |
| dhkA− pkaR− | 15 ± 7 |
| dhkA− pkaC::pkaC | 60 ± 6 |
The number of detergent-resistant viable spores after 36 h of development on filters is presented as a percentage of the original number of cells developed (5 × 107). The number of viable spores was determined in at least three separate experiments with each strain.
DISCUSSION
SDF-2 is released from prestalk cells during culmination and appears to diffuse throughout the sorus (6, 7). Prestalk cells respond by releasing a burst of SDF-2 within a few minutes while prespore cells respond by encapsulating within an hour (6, 7). The responses of both cell types are dependent on the histidine kinase encoded by dhkA (6). Histidine kinases are widespread in two-component signal transduction mechanisms of bacteria and have been found to mediate a variety of responses in plants, fungi, and Dictyostelium (4, 21). They all share a conserved motif surrounding the histidine that is autophosphorylated and show sequence similarity in the catalytic domain. Likewise, there is a telltale sequence surrounding the aspartate to which the phosphate is relayed.
The primary sequence of DhkA shows that, in addition to the conserved motifs, there are two potential transmembrane domains near the N terminus separated by a 310-amino-acid loop (42). When a MYC6 epitope tag was positioned at amino acid 900 in the loop, it was rapidly degraded when proteinase K was added to the extracellular medium. Protease treatment was carried out at 0°C to minimize internalization of the enzyme by endocytosis. The lack of significant internalization was verified by showing that a cytoplasmic protein, TipA, was not degraded under these conditions. When the MYC6 epitope was positioned at amino acid 2025 of DhkA near the receptor aspartate, it was protected from protease degradation although DhkA was trimmed. The topology of DhkA was confirmed by the demonstration that addition of antibody to the MYC6 epitope induced sporulation in cells expressing dhkA900MYC6. Insertion of the MYC6 epitope at amino acid 900 of DhkA appears to reduce its sensitivity to SDF-2 by about 100-fold. Thus, the 310-amino-acid loop between the transmembrane domains of DhkA is exposed to the intercellular medium and appears to be critical for ligand binding and activation of DhkA. The carboxy-terminal portion of DhkA that carries the conserved aspartate to which the phosphate is relayed appears to be internal, where it can affect its response regulator.
Using a His6 derivatized version of the central portion of dhkA, we showed that DhkA is a protein kinase able to autophosphorylate on a histidine residue (Fig. 4). Site-directed mutagenesis of histidine1395 to glutamine in the catalytic domain identified that residue as essential for autophosphorylation (Fig. 4). The physiological significance of the conserved histidine in DhkA was further demonstrated in vivo. We found that a full length dhkA construct carrying this site-directed mutation failed to fully rescue sporulation in dhkA− mutants (Table 1). Likewise, the importance of the conserved aspartate near the C terminus of DhkA was demonstrated by site-directed mutagenesis to asparagine to preclude phosphorylation. The dhkAD2075N mutation also compromised the ability of the gene to complement the dhkA− null mutation (Table 1). Whereas each of the alleles was unable to fully complement the dhkA null mutation, cotransformation of dhkA− cells with both of the mutant constructs gave strains that sporulated normally, indicating an interaction between the modified proteins.
Phosphotransfer from endogenous DhkB to the aspartate in the H1395Q version of DhkA may account for the increased sporulation seen in dhkA− cells expressing dhkAH1395Q relative to untransformed dhkA− cells, while phosphotransfer from the histidine in the D2075N version of DhkA to the aspartate in DhkB may account for the fact that dhkA− cells expressing dhkAD2075N make about a third as many spores as wild-type cells (Table 1). Double mutants lacking both histidine kinases, DhkA and DhkB, make almost no spores even when transformed with constructs expressing either dhkAH1395Q or dhkAD2075N (Table 3). Intermolecular cooperation has also been observed in the hybrid kinase SLN1p, which is responsible for osmoregulation in yeast (25). The results presented here show that DhkA is a membrane-spanning histidine kinase and is likely to be a receptor which mediates the cellular response to SDF-2 in the genetic pathway that eventually leads to PKA-dependent differentiation.
ACKNOWLEDGMENTS
We thank Adam Kuspa for insightful suggestions, Allyson Andrews for technical assistance, and Negin Iranfar for sequencing analyses. The dhkB knockout plasmid was a kind gift of Charles Singleton.
Fredrik Söderbom is a Fellow of the Swedish Foundation for International Cooperation in Research and Higher Education. Christophe Anjard benefited from an EMBO fellowship (ALTF 560-1996). This work was supported by the National Science Foundation (grant 9728463).
REFERENCES
- 1.Abe H, Uchiyama M, Tanaka Y, Saito H. Structure of discadenine, a spore germination inhibitor from the cellular slime mold Dictyostelium discoideum. Tetrahedron Lett. 1976;42:3807–3810. [Google Scholar]
- 2.Abe K, Yanagisawa K. A new class of rapid developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev Biol. 1983;95:200–210. doi: 10.1016/0012-1606(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 3.Alex L, Borkovich K, Simon M I. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc Natl Acad Sci USA. 1996;93:3416–3421. doi: 10.1073/pnas.93.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alex L, Simon M. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. doi: 10.1016/0168-9525(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 5.Anjard C, Pinaud S, Kay R R, Reymond C D. Overexpression of DdPK2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development. 1992;115:785–790. doi: 10.1242/dev.115.3.785. [DOI] [PubMed] [Google Scholar]
- 6.Anjard C, Zeng C, Loomis W F, Nellen W. Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev Biol. 1998;193:146–155. doi: 10.1006/dbio.1997.8804. [DOI] [PubMed] [Google Scholar]
- 7.Anjard C, Chang W, Gross J, Nellen W. Production and activity of spore differentiation factors (SDFs) in Dictyostelium. Development. 1998;125:4067–4075. doi: 10.1242/dev.125.20.4067. [DOI] [PubMed] [Google Scholar]
- 8.Berberich I, Shu G, Clark E. Cross-linking CD40 on B cells rapidly activates nuclear factor NF-κB. J Immunol. 1994;153:4357–4366. [PubMed] [Google Scholar]
- 9.Bonner J T. The cellular slime molds. 2nd ed. Princeton, N.J: Princeton University Press; 1967. [Google Scholar]
- 10.Burret R B, Borkovich K A, Simon M I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Kwok S F, Bleecker A B, Meyerowitz E M. Arabidopsis ethylene-response gene ETR1—similarity of product to 2-component regulators. Science. 1993;262:245–249. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 12.Hess J F, Bourret R, Simon M. Histidine phosphorylation and phosphoryl group transfer in bacterial chemotaxis. Nature. 1988;336:139–143. doi: 10.1038/336139a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoch J. The phosphorelay signal transduction pathway in the initiation of Bacillus subtilis sporulation. J Cell Biochem. 1993;51:55–61. doi: 10.1002/jcb.240510111. [DOI] [PubMed] [Google Scholar]
- 14.Kakimoto T. CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science. 1996;274:982–985. doi: 10.1126/science.274.5289.982. [DOI] [PubMed] [Google Scholar]
- 15.Kay R R. Evidence that elevated intracellular cyclic AMP triggers spore maturation in Dictyostelium. Development. 1989;105:753–759. [Google Scholar]
- 16.Keener J, Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci USA. 1988;80:3599–3603. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knecht D A, Cohen S M, Loomis W F, Lodish H F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986;6:3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuspa A, Loomis W F. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomis W F. Dictyostelium discoideum. A developmental system. New York, N.Y: Academic Press; 1975. [Google Scholar]
- 20.Loomis W F. Genetic networks that regulate development in Dictyostelium cells. Microbiol Rev. 1996;60:135–150. doi: 10.1128/mr.60.1.135-150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomis W F, Shaulsky G, Wang N. Histidine kinases in signal transduction pathways of eukaryotes. J Cell Sci. 1997;110:1141–1145. doi: 10.1242/jcs.110.10.1141. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Takekawa M, Saito H. Activation of the yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 23.Maeda T, Wurgler-Murphy S, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 24.Ninfa A J, Magasanik B. Covalent modifications of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci USA. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 26.Raper K B. Pseudoplasmodium formation and organization in Dictyostelium discoideum. J Elisha Mitchell Sci Soc. 1940;56:241–282. [Google Scholar]
- 27.Richardson D L, Hong C B, Loomis W F. A prespore gene, Dd31, expressed during culmination of Dictyostelium discoideum. Dev Biol. 1991;144:269–280. doi: 10.1016/0012-1606(91)90421-x. [DOI] [PubMed] [Google Scholar]
- 28.Richardson D L, Loomis W F, Kimmel A R. Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development. 1994;120:2891–2900. doi: 10.1242/dev.120.10.2891. [DOI] [PubMed] [Google Scholar]
- 29.Schuster S C, Noegel A A, Oehme F, Gerisch G, Simon M I. The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 1996;15:3880–3889. [PMC free article] [PubMed] [Google Scholar]
- 30.Shaulsky G, Escalante R, Loomis W F. Developmental signal transduction pathways uncovered by genetic suppressors. Proc Natl Acad Sci USA. 1996;93:15260–15265. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaulsky G, Kuspa A, Loomis W F. A multidrug resistance transporter serine protease gene is required for prestalk specialization in Dictyostelium. Genes Dev. 1995;9:1111–1122. doi: 10.1101/gad.9.9.1111. [DOI] [PubMed] [Google Scholar]
- 32.Shaulsky G, Loomis W F. Cell type regulation in response to expression of ricin-A in Dictyostelium. Dev Biol. 1993;160:85–98. doi: 10.1006/dbio.1993.1288. [DOI] [PubMed] [Google Scholar]
- 33.Shaulsky G, Loomis W F. Initial cell type divergence in Dictyostelium is independent of DIF-1. Dev Biol. 1996;174:214–220. doi: 10.1006/dbio.1996.0067. [DOI] [PubMed] [Google Scholar]
- 34.Shaulsky G, Fuller D, Loomis W F. A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development. 1998;125:691–699. doi: 10.1242/dev.125.4.691. [DOI] [PubMed] [Google Scholar]
- 35.Simon M N, Pelegrini O, Veron M, Kay R R. Mutation of protein kinase-A causes heterochronic development of Dictyostelium. Nature. 1992;356:171–172. doi: 10.1038/356171a0. [DOI] [PubMed] [Google Scholar]
- 36.Skov S. Intracellular signal transduction mediated by ligation of MHC class I molecules. Tissue Antigens. 1998;51:215–223. [PubMed] [Google Scholar]
- 37.Stege J T, Shaulsky G, Loomis W F. Sorting of the initial cell types in Dictyostelium is dependent on the tipA gene. Dev Biol. 1997;185:34–41. doi: 10.1006/dbio.1997.8538. [DOI] [PubMed] [Google Scholar]
- 38.Stout V, Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990;172:659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- 40.Taylor S S, Buechler J A, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;9:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 41.Thomason P, Traynor D, Cavet G, Chang W-T, Harwood A, Kay R R. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998;17:2823–2845. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang N, Shaulsky G, Escalante R, Loomis W F. A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J. 1996;15:3890–3898. [PMC free article] [PubMed] [Google Scholar]
- 43.Worm M, Tsytsykova A, Geha R. CD40 ligation and IL-4 use different mechanisms of transcriptional activation of the human lymphotoxin a promoter in B cells. Eur J Immunol. 1998;28:901–906. doi: 10.1002/(SICI)1521-4141(199803)28:03<901::AID-IMMU901>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 44.Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- 45.Zinda M J, Singleton C K. The hybrid histidine kinase dhkB regulates spore germination in Dictyostelium discoideum. Dev Biol. 1998;196:171–183. doi: 10.1006/dbio.1998.8854. [DOI] [PubMed] [Google Scholar]