Abstract
P-glycoprotein (P-gp) acts as a pump to transport cytotoxic drugs out of cells and is upregulated in cancer cells. Suppressing the expression of P-gp is an effective strategy to overcome multidrug resistance in cancer chemotherapy. Temozolomide (TMZ) is the recommended drug for the standard treatment of patients with glioblastoma, but its clinical application is restricted due to drug resistance. Transient receptor potential channel-5 (TRPC5), a Ca2+-permeable channel, has been attributed to a different drug resistance mechanism except DNA repair system; therefore, we aimed to elucidate the mechanism regarding the role of TRPC5 in TMZ resistance. TRPC5 and P-glycoprotein (P-gp) are upregulated in TMZ-resistant glioblastoma cell lines. The downregulation of TRPC5 inhibited P-gp expression and led to a significant reversal of TMZ resistance in TMZ-resistant cell lines. TRPC5-siRNA restricted the growth of tumour xenografts in an athymic nude mouse model of TMZ-resistant cells. In specimens from patients with recurrent glioblastoma, TRPC5 was found to be highly expressed, accompanied by the upregulation of P-gp expression. The nuclear factor of activated T cell isoform c3 (NFATc3), which acts as a transcriptional factor, bridges TRPC5 activity to P-gp induction. In conclusion, these results demonstrate the functional role of the TRPC5-NFATc3-P-gp signalling pathway in TMZ resistance in glioblastoma cells.
Keywords: TRPC5, P-gp, Drug resistance, Glioblastoma, Temozolomide
Introduction
Glioblastoma is the most common, malignant primary brain tumour in adults, accounting for approximately 65% of adult central nervous system (CNS) tumours. The current standard of care for this malignancy is a combination of surgical resection, radiotherapy, and chemotherapy. However, patients with glioblastoma have a poor prognosis and their median overall survival time is less than 15 months [4,14]. These data highlight the urgent need for innovative and effective therapeutic strategies against this disease.
Temozolomide (TMZ), a lipophilic second-generation imidazotetrazine prodrug, has improved the prognosis of patients with glioblastoma either treated directly or followed after surgical resection because it can cross the blood brain barrier (BBB) [10], spontaneously undergo hydrolysis, and generate the active metabolite 5-(3-dimethyl-1-triazenyl)-imidazole-4-carboxamide at physiological pH [17], forming O6-methylguanine adducts, which mispairs with thymine. Mispaired thymines cannot be repaired; hence, they induce the breakage of single- and double-strand DNA and trigger senescence and apoptotic activation in glial cells [9,15]. However, the clinical application of TMZ is limited because of drug resistance in some patients. Several drug-resistance mechanisms may be responsible for the therapeutic failure of TMZ in glioblastoma.
The chemotherapeutic effect in cancer is usually restricted by drug resistance, either because tumour cells are intrinsically resistant to drug action or because tumour cells are initially positive to therapy, but after a period of time, they become capable of circumventing drug action and are therefore selected within the cell population [12]. There is a large body of knowledge describing acquired drug resistance from studies on cellular models. These mechanisms include increased drug efflux, decreased drug uptake into cells, the activation of the DNA repair system, the activation of detoxifying enzymes (such as cytochrome P450), and the inhibition of apoptotic signalling pathways [7]. The mechanism of TMZ resistance has been associated with the activation of DNA repair systems. However, the downregulation of the enzyme cannot fully induce TMZ sensitivity in TMZ-resistant glioblastoma cells. Therefore, other mechanisms are involved in TMZ resistance. The overexpression of ATP-binding cassette (ABC) transporters to increase drug efflux is a common mechanism for inducing cellular resistance to doxorubicin (DOX) and other anticancer agents, such as paclitaxel and vinblastine [3,7]. ABCB1 is a member of the ABC transporter family and encodes the membrane-bound P-glycoprotein (P-gp; also called ATP-binding cassette sub-family B member 1[ABCB1] or multidrug resistance protein 1 [MDR1]), which participates in an efflux pump responsible for multiple drug resistance (MDR) [6]. Multiple resistance proteins are highly expressed in more than 70% of the tumour specimens from the CNS, and P-gp expression has been detected in up to 18% of high-grade glioblastomas [1]. P-gp and other transporters positively transport substrates out of the brain, thereby restricting their effects on the CNS [5,24,26]. However, the precise mechanism by which P-gp removes TMZ in glioblastoma cells is still under debate.
An important indicator of the transcriptional regulation of P-gp is cytosolic Ca2+ level([Ca2+]i) [16,23,25]. Studies have shown that the chelation of Ca2+ by 1, 2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraaceticacid abolishes the P-gp expression induced by many drugs, whereas thapsigargin, which inhibits sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, increases [Ca2+]i, and therefore enhances P-gp production [23,25]. Moreover, several different Ca2+ channel antagonists have been shown to inhibit P-gp expression [16]. TRP channels, a group of cation channels, consist of several isoforms such as TRPM and TRPC. TRPC5, a TRPC channel, is a canonical Ca2+-permeable, receptor-operated channel that is widely expressed in the brain, testis, kidney, adrenal gland, ovary, uterus, and endothelial cells. In breast cancer, TRPC5 participates in the production of P-gp by changes in [Ca2+] [18]. However, the precise function of TRPC5 in glioblastoma remains unknown. Hence, we hypothesised that P-gp is upregulated in TMZ-resistant cells, and TRPC5 upregulation mediates P-gp expression and contributes to TMZ resistance.
Materials and methods
Cells and reagents
U87 wild-type (U87/WT) and U251 wild-type (U251/WT) cells were obtained from the Chinese Academy of Sciences (Shanghai, China), and HEK-293 cells were obtained from the Institute of Neuroscience, Soochow University. All cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM)/F12 culture medium containing 10% foetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA). TMZ-resistant human glioblastoma cells (U87/TMZ) were derived by exposing U87/WT cells to TMZ at a high dose of 400 nM for 6 months. TMZ was reconstituted in dimethyl sulphoxide (DMSO) prior to use, resulting in an effective TMZ concentration of 25 µM. When the cells were in the logarithmic growth phase, TMZ was combined with DMEM to a final concentration of 400 nM. Subsequently, at every 24 h incubation interval, the spent medium was discarded and replaced with the fresh medium with identical TMZ concentration. Dead cells were discarded by washing with PBS after 3 days, and the remaining cells were diluted at a density of 2 × 105 cells/ml in DMEM containing 10% FBS and cultured in a 6 cm cell culture dish; this procedure was repeated for 6 months. Finally, a cell line resistant to 400 nM TMZ (termed U87/TMZ) was derived from the U87/WT cells after 6 months of culture. All cells were incubated at 37˚C in a humidified air chamber containing 5% CO2.
The following antibodies were used: anti-β-actin (cat. no. sc-47778; 1:200) and anti-NFATc3 (cat. no. sc-8405; 1:500) from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), anti-TRPC5 (cat. no. ACC-020; 1:200) from Alomone Labs (Jerusalem, Israel); and anti-MDR1 (cat. no. 13978S; 1:200) from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The plasmids pcDNA3.1-TRPC5, pcDNA3.1-GFP-NFATc3, and a control plasmid were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). The TRPC5-siRNA (sense strand, 5’-CCA AUG GAC UGA ACC AGC UUU ACU U-3’; antisense strand, 5’-AAG UAA AGC UGG UUC AGU CCA UUG G-3’) was purchased from Shanghai Genechem Co., Ltd. (Shanghai, China). TRPC5 shRNA(h) lentiviral particles (sc-42670-V) were purchased from Santa Cruz Biotechnology(Dallas, TX, USA). TRPC5 shRNA(h) lentiviral particles is a pool of three different shRNA plasmids:
A(5’-GATCCGAACCAGCTTTACTTCTATTTCAAGAGAATAGAAGTAAAGCTGGTTCTTTTT-3’);
B(5’-GATCCCTACCATGTTTGGAACATATTCAAGAGATATGTTCCAAACATGGTAGTTTTT-3’);
C(5’-GATCCCCATCTTTGTTGCCATTCATTCAAGAGATGAATGGCAACAAAGATGGTTTTT-3’);
A scramble sequence
(5’-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG) was used as a control.
TMZ chemosensitivity assay
The cytotoxicity of TMZ was tested in vitro using the Cell Counting Kit-8(CCK8) assay. Briefly, 5 × 103 cells per well were plated in 96-well plates. After 24 h, the spent medium was aspirated, replaced with TMZ, and the cells were culture for another 24 h. The concentrations of TMZ used in each group were 25, 50, 100, 200, 400, 800, 1600, 3200, and 6400 μM. After the treatment, the TMZ-containing medium was replaced with 10μL of CCK-8 and 100 μL of complete culture medium for 4h. The absorbance of the cells at 590 nm was measured using a microplate reader (Thermo, Synergie HT, USA). Five wells were used for each concentration, and the entire experiment was repeated at least three times. The IC50 (concentration resulting in 50% inhibition of cell growth) value for TMZ was calculated using SPSS16 software.
Intracellular calcium level measurement
U87/WT cells were added to 96-well plates (5000 cells/well) and cultured overnight at 37 ˚C. The cells were transfected with TRPC5 plasmid or TRPC5-siRNA for 24 h. Subsequently, Fluo-4 (2 mM/l; cat. no. F14201; Thermo Fisher Scientific, Inc.) was added for 30 min at 37 ˚C, and fluorescence was measured at 485 nm using a spectrophotometer.
Immunofluorescence staining
U87/WT and HEK-293 cells were plated at a density of 8 × 103 cells/well. The cells were then transfected with GFP-tagged NFATc3 and TRPC5 plasmid or TRPC5-siRNA for 24 h. Afterwards, the cells were fixed in 4% paraformaldehyde for 15 min and permeabilised with 0.3% Triton-X 100 for 10 min at room temperature. The samples were then blocked with 1% bovine serum albumin (Bytotime Biotechnology, Beijing, China) in PBS for 1 h at room temperature, incubated overnight with a primary antibody at 4 °C, and then incubated with a fluorescently labelled secondary antibody for 2 h at room temperature. Images were captured using a confocal laser scanning microscope with a 63 × oil-immersion objective lens.
Western blot
Cells or tissues were collected and lysed in RIPA lysis buffer containing 1% PMSF protease and phosphatase inhibitors. BCA protein assay kit from Biotime (Shanghai, China) was used to achieve equal protein loading of the lysates. Samples were loaded onto a Tris-glycine SDS-PAGE and transferred to nitrocellulose membranes. The immunoblots were probed overnight at 4 °C with the appropriate primary antibody after blocking with 5% skim milk in TBST. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h. ECL kits (Bytotime Biotechnology, Beijing, China) were used to visualise the protein bands.
Reverse transcriptase (RT)-PCR
Total RNA was extracted using TRIzol reagent (Sigma-Aldrich), and complementary DNA was synthesised using a SuperScript First-strand cDNA synthesis kit (Invitrogen, Carlsbad, USA) according to the manufacturer's protocol. Quantitative PCR was conducted with 40 cycles of 95 °C for 10 s, and 60 °C for 40 s. Data were analysed using Graphpad Prism 8. GAPDH was used as an endogenous control. The primer sequences used were:
GAPDH forward 5′-AAGGTCGGAGTCAACGGATTTGGT-3′, reverse 5′-AGTGATGGCATGGACTGTGGTCAT-3′;
TRPC5 forward 5′-TGAACTCCCTCTACCTGGCAAC-3′; reverse 5′-CGAAGAGTGCTTCCGCAATCAGT-3′; and
mdr forward 5′-CTGTTTGACTGCAGCATTGCTGAGAACAT-3′, reverse 5′-CTGGCGCTTTGTTCCAGCCTGGACACTGAC-3′
Mouse xenograft models
All mice were housed in an air-filtered pathogen-free environment. To generate subcutaneous tumours, U87/WT or U87/TMZ cells were first treated with or without TRPC5-shRNA lentivirus particles for 72 h, then 4 × 106 cells were injected subcutaneously into the nude mice after an intraperitoneal injection of 5% chloral hydrate (300 mg/kg). Each group contained 6 mice, and the mice with tumours derived from U87/WT and U87/TMZ cells were checked and administered with 75 mg/m2 TMZ every 3 days. The humane endpoint was determined to be the weight of mice decreased by 20%. After 21 days, the mice were enthanized using spinal cord dislocation (none of them died), and all of them had a single subcutaneous tumour. The longest diameter of a single subcutaneous tumour was 1.905cm in the TRPC5/TMZ group and tumour volumes were measured using the formula: volume(cm3) = (length) × (width)2/2.
Patients
Human glioblastoma samples (n = 20) were obtained from the Affiliated Wuxi No. 2 Hospital of Nanjing Medical University.
Inclusion and exclusion criteria
Patients who met all of the following criteria at the start of the treatment are eligible for the study:
-
1
At least 18 years of age and provided a signed written informed consent.
-
2
Patients undergoing open skull surgery.
-
3
The pathology results showing WHO Ⅱ to Ⅳglioblastoma.
-
4
Patients who were receiving standard TMZ treatment after surgery.
Patients who met any of the following criteria at the start of treatment were not eligible for the study:
-
1
Patients undergoing radiotherapy.
-
2
Patients with other malignant tumours.
The patients were separated into two groups: the first group included 10 patients (mean age: 40.3; age range: 30 to 59 years; six females and four males) with primary glioblastoma, and the other group included the same patients who received TMZ treatment and had recurrent glioblastoma. Informed consent was obtained for the anonymous specimens provided by all human participants in this study. Patients were recruited between January 2013 and December 2018. All patients received the standard STUPP protocol and the clinical outcomes were assessed using MRI.
Ethics statement
All experiments involving patients and animals were conducted in accordance with the Guide for Animal Care and Use of Laboratory Animals published by the National Institutes of Health, USA. All experiments were approved by the Animal Experimentation Ethics Committee of Nanjing Medical University. The use of clinical samples was approved by the review board and ethics committee of the Affiliated Wuxi No.2 Hospital of Nanjing Medical University (Reference Number: 20121104).
Statistics
All experiments were repeated at least three times. Quantitative data were statistically analysed as the average value of replicates with the t-test and presented as the representative experimental means± SD (standard deviation). P <0.05 was considered statistically significant difference. *: P <0.05; **: P <0.01; ***: P <0.001.
Results
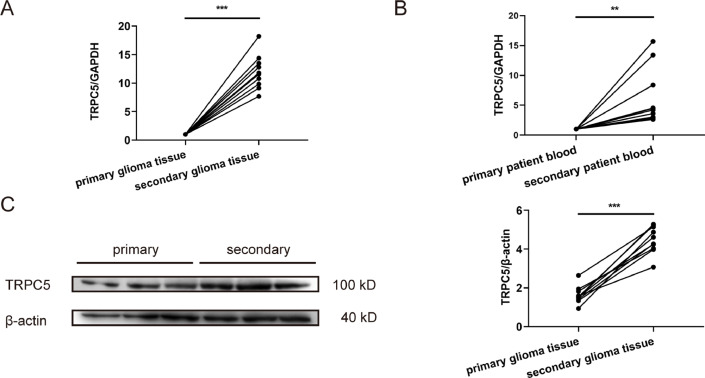
Increased TRPC5 expression in human secondary glioblastoma tissue
To investigate the clinical expression of TPRC5 in patients with glioblastoma, we analysed the specimens of 10 patients whose glioblastoma was primary and those that were recurrent after TMZ treatment. RT-PCR analysis indicated that TRPC5 mRNA expression in the recurrent group was higher than that in the primary group(Fig. 1A). To investigate the TRPC5 mRNA expression in peripheral blood, we also assessed TRPC5 mRNA expression in peripheral blood collected from the same 10 patients using RT-PCR. The results indicated that TRPC5 mRNA levels in the peripheral blood after chemotherapy were significantly higher than those in the control group(Fig. 1B). TRPC5 protein levels in glioblastoma tissues were also measured and it was observed that TRPC5 expression was upregulated in the recurrent group compared to that in the primary group (Fig. 1C). Thus, TRPC5 mRNA and protein expression increased in patients with recurrent glioblastoma after TMZ treatment.
Fig. 1.
TRPC5 expression is increased in secondary glioblastoma tissue.
(A) TRPC5 mRNA expression in primary or secondary glioblastoma tissues. TRPC5 mRNA levels increased in secondary patients after TMZ treatment (n =10 each group). Data were analysed using Student's t-test. (B) TRPC5 mRNA expression in the peripheral blood of primary or secondary patients. TRPC5 mRNA expression increased in the peripheral blood of secondary patients (n =10 each group). Data were analysed using Student's t-test. (C) TRPC5 protein levels in primary or secondary glioblastoma tissue. TRPC5 protein expression was increased in patients postchemotherapy (n =10 each group). Data were analysed using Student's t-test.
Expression of TRPC5 mRNA and protein in U87/TMZ and U251/TMZ cells
The TMZ-resistant U87 and U251 cell lines were established via high-dose treatment with TMZ as described and were named U87/TMZ and U251/TMZ, respectively. We conducted the CCK-8 assay to confirm the resistance of U87/TMZ and U251/TMZ cells to TMZ. The IC50 values for U87/WT and U87/TMZ cells were 162.45 μM and 3134.15 μM (P<0.01), and the IC50 values for U251/WT and U251/TMZ cells were 96.66 μM and 1633.62 μM (P < 0.01), respectively (Supplementary.1A). The resistance index (RI), which is defined as the ratio between the IC50 of drug-resistant and drug-sensitive cell lines, of the U87/TMZ and U251/TMZ cell lines were 19.29 and 16.90, respectively, indicating the successful establishment of TMZ-resistant cell lines.
TRPC5 mRNA and protein expression were examined in both U87/TMZ and U251/TMZ cells. RT-PCR results indicated that TRPC5 mRNA levels increased in U87/TMZ or U251/TMZ cells compared to their respective wild-type counterparts by almost 60-fold, and the difference was statistically significant (P < 0.05)(Supplementary. 1B). TRPC5 (110-120 kDa) protein levels were also considerably elevated in U87/TMZ and U251/TMZ cells than those in their corresponding wild-type counterparts (P < 0.05)(Supplementary. 1C). β-Actin was used as an internal control.
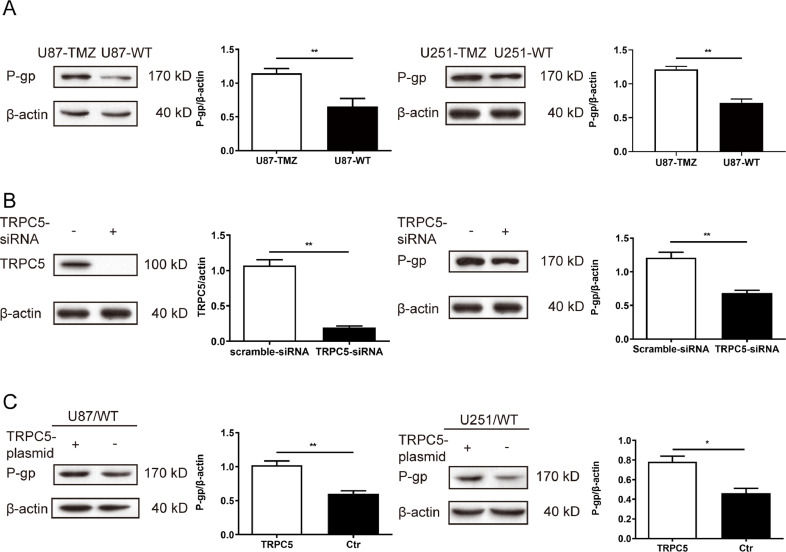
Regulation of P-gp expression and TMZ resistance via TRPC5 upregulation or downregulation
To further determine the mechanism of TMZ resistance in the cell lines used, we focused on a common target of drug resistance—P-glycoprotein (P-gp), an efflux pump protein that acts as a multidrug binding site by moving drugs from the endoplasm to the extracellular matrix. Although P-gp has been found in many other tumour cells, its exact role in glioblastoma remains unknown.
First, P-gp protein expression was determined in different cell lines via western blot analysis. The protein (170 kDa) was highly expressed in U87/TMZ or U251/TMZ cells, whereas only a low expression was detected in its parental line U87/WT (P < 0.01)(Fig. 2A). TRPC5 was inhibited by TRPC5-siRNA, which also inhibited P-gp expression in U87/TMZ cells (Fig. 2B). In contrast, the knockdown of P-gp had no effect on TRPC5 protein levels (data not shown), suggesting that TRPC5 could regulate P-gp expression. The TRPC5 plasmid was transfected into U87/WT and U251/WT cells, and it was observed that TRPC5 enhanced the expression of P-gp in both U87/WT and U251/WT cells (Fig. 2C). Furthermore, mock transfection with an empty plasmid did not affect P-gp expression.
Fig. 2.
TRPC5 activated P-gp induction in glioblastoma cells.
(A) Protein levels of P-gp detected via western blot showed that the protein expression of P-gp increased in U87/TMZ or U251/TMZ cells compared with the corresponding control cells. These changes were statistically significant by Student's t-test (P < 0.05). (B) TRPC5 was inhibited by treatment with TRPC5-siRNA (5 nM, 24 h) in U87/TMZ cells, and western blot analysis of TRPC5 and P-gp expression indicated that the levels of these proteins were lower than those in the control. (C) Overexpression of TRPC5 using a TRPC5 expression-plasmid led to an enhanced expression of TRPC5 and P-gp. This difference was statistically significant by Student's t-test.
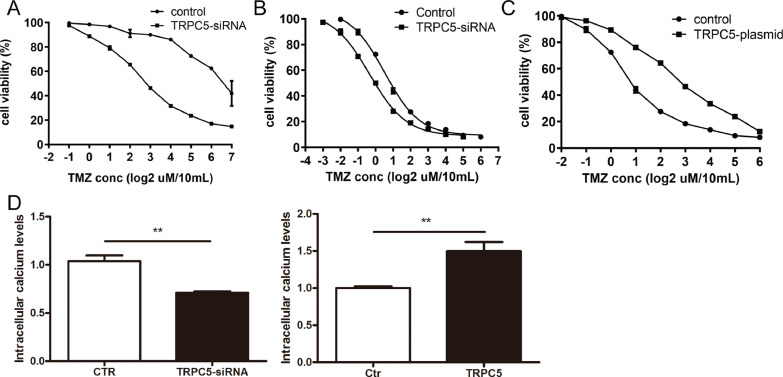
We also investigated the possibility of inhibiting TRPC5 to reverse TMZ resistance in U87/TMZ cells. CCK8 assays indicated that compared to U87/WT cells, U87/TMZ cells were much more resistant (19.29-fold) to TMZ-induced cell death (Supplementary. 1A). Many other chemotherapeutic drugs have also been used to evaluate the drug-resistance of U87/TMZ cells. U87/TMZ cells also displayed resistance to these drugs, including paclitaxel and vincristine (data not shown). Treatment with TRPC5-siRNA led to a reversal of TMZ resistance in U87/TMZ cells, with TMZ IC50 values reduced from 3134.15 μM to 594.22 μM (P < 0.001)(Fig. 3A). Furthermore, we observed enhanced sensitivity to TMZ in TRPC5-knockdown U87/WT cells. The TMZ IC50 values for U87/WT and TRPC5-knockdown U87/WT cells were 162.45 μM and 93.78 μM, respectively (P < 0.05) (Fig. 3B). To further confirm the effect of TRPC5 on drug resistance, the cells were transfected with a TRPC5-overexpression plasmid, and the TMZ IC50 value for the transfected U87/WT cells was 703.72 μM (P < 0.01)(Fig. 3C). Previous research has pointed out that TRPC5 accelerates Ca2+ influx to activate P-gp expression. Thus, we measured Ca2+ influx in U87/WT cells after transfection with TRPC5-plasmid or TRPC5-siRNA for 24 h. Our results indicated that TRPC5 overexpression upregulated Ca2+ influx in U87/WT cells, whereas TRPC5-siRNA inhibited Ca2+ influx (Fig. 3D). Therefore, the results showed that TRPC5 accelerated Ca2+ influx to activate P-gp expression and mediate TMZ resistance.
Fig. 3.
TRPC5 increased TMZ resistance in glioblastoma cells
(A) U87/TMZ cells were first treated with TRPC5-siRNA (5 nM) for 24 h, and then with increasing concentrations of TMZ for another 24 h. The IC50 values of the U87/TMZ and U87/TMZ-TRPC5-siRNA cells to TMZ were 3134.15 μM and 538.52 μM, respectively. (B) U87/WT cells were first treated with TRPC5-siRNA (5 nM) for 24 h, and then with increasing concentrations of TMZ for another 24 h. The IC50 value of the U87/WT cell line was 93.78 μM. (C) U87/WT cells were first treated with a TRPC5-overexpression plasmid (1 μM) for 24 h, and then with increasing concentrations of TMZ for another 24 h. The IC50 value for the U87/WT cell line was 703.72 μM. (D) The U87/WT cell line was treated with either a TRPC5-plasmid or TRPC5-siRNA for 24 h, and then Ca2+ influx was measured. TRPC5-plasmid transfection upregulated Ca2+ influx in U87/WT cells, while TRPC5-siRNA inhibited it.
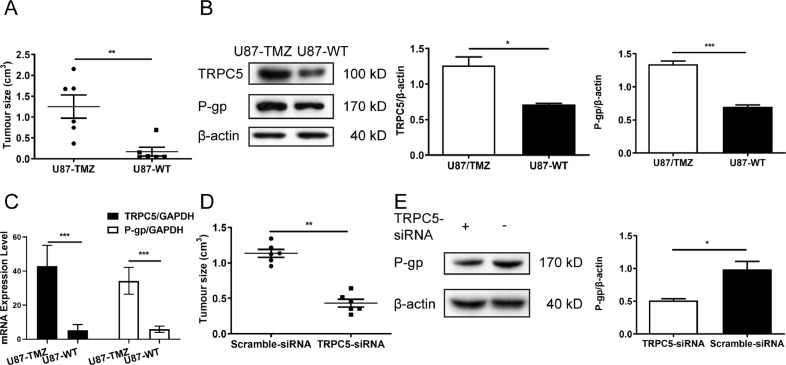
TRPC5-siRNA restricted the growth of U87/TMZ tumour xenografts
An animal model of human glioblastoma was established by injecting U87/TMZ or U87/WT cells into athymic nude mice (BALB/cAnNCr-nu/nu). After TMZ treatment, the tumour continued to grow in size, indicating that cells were resistant to TMZ (Fig. 4A). Immunoblotting analysis indicated that TRPC5 and P-gp levels increased in U87/TMZ-injected tumours compared to those in U87/WT-injected tumours (Fig. 4B). TRPC5 and P-gp mRNA expression was then examined, and it was found that both TRPC5 and P-gp mRNA expression upregulated in U87/TMZ-injected tumours (Fig. 4C). U87/TMZ cells pre-treated with TRPC5-siRNA lentivirus particles showed restricted tumour growth (Fig. 4D), whereas P-gp expression decreased in TRPC5-siRNA lentivirus-treated tumours compared to those in scramble lentivirus-treated tumours (Fig. 4E).
Fig. 4.
Inhibition of TRPC5 restricted the growth of human glial tumour xenografts in athymic nude mice. (A) Athymic nude mice were injected subcutaneously with U87/TMZ and U87/WT cells, then treated with TMZ for 21 d. After harvest, the tumour sizes were measured. Each group contained six mice. (B) Western blot analysis of TRPC5 and P-gp expression showed that protein levels of TRPC5 and P-gp in tumours were significantly higher in U87/TMZ cells than in U87/WT cells. (Student's t-test) (C) U87/TMZ cells were first treated with TRPC5-lentiviral particles for 72 h, and then athymic nude mice were injected subcutaneously with TRPC5-lentivirus-treated U87/TMZ cells. After harvest, the tumour sizes were measured. Each group contained six mice. (D) Western blot analysis of P-gp expression showed that P-gp levels were significantly decreased in U87/TMZ tumours treated with TRPC5-lentiviral particles compared with untreated U87/TMZ tumours. (Student's t-test) Values are means ± SD of three to six experiments.
Involvement of NFATc3 in TRPC5-regulated P-gp induction
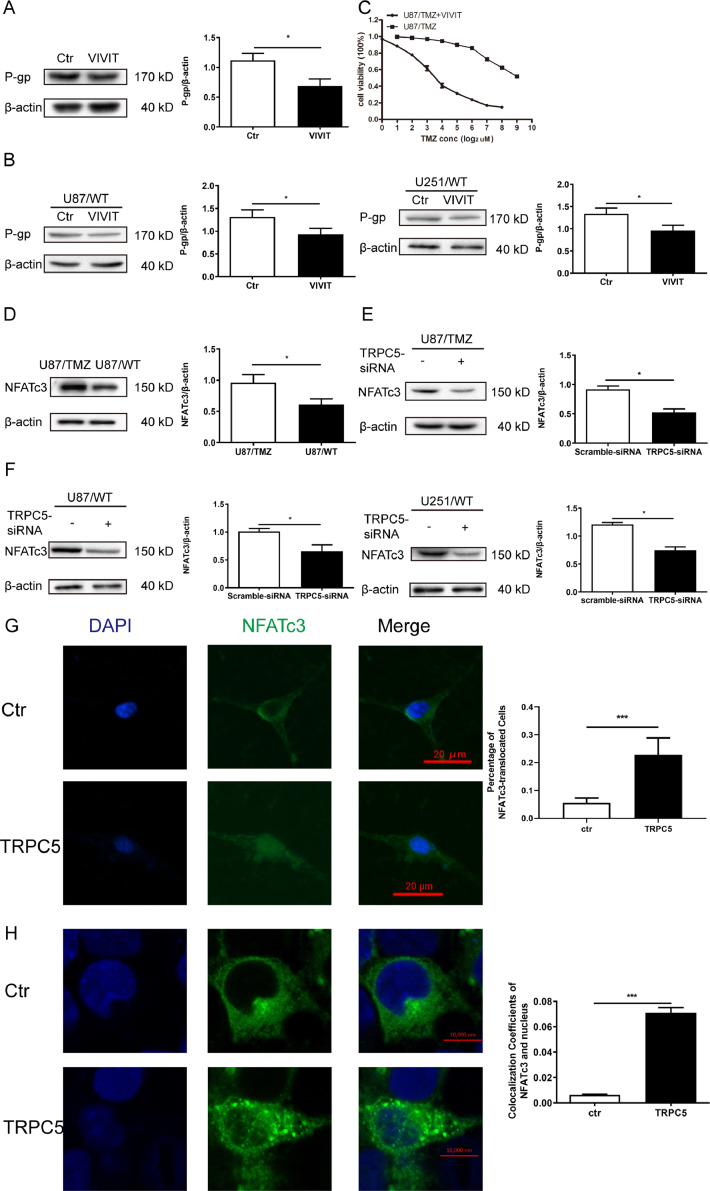
According to our previous results, P-gp mRNA expression was much higher in U87/TMZ cells than in U87/WT cells (data not shown). According to previous research [18], it was reported that TRPC5 mediates P-gp expression via a Ca2+-dependent transcription factor, a nuclear factor of activated T cells (NFAT). Thus, we attempted to inhibit NFAT activity via VIVIT, a cell permeable peptide inhibitor of NFAT that selectively inhibits calcineurin-mediated NFAT dephosphorylation. The results showed that VIVIT reduced P-gp expression in U87/TMZ cells (Fig. 5A). Next, we treated U87/WT and U251/WT cells with VIVIT, and this inhibitor also reduced P-gp expression in both cell lines (Fig. 5B). In CCK8 assays, VIVIT caused a remarkable reversal of TMZ resistance, upgrading the sensitivity of U87/TMZ cells to TMZ (P <0.01)(Fig. 5C).
Fig. 5.
NFATc3 mediated P-gp induction and affected TMZ resistance in U87/TMZ cells. (A) U87/TMZ cells were treated with VIVIT overnight and P-gp protein expression was measured via western blot. VIVIT treatment significantly reduced P-gp level in U87/TMZ cells.(Student's t-test) (B) U87/WT and U251/WT cells were treated with VIVIT overnight at the indicated concentrations. P-gp expression level significantly decreased in U87/WT or U251/WT cells after VIVIT treatment.(Student's t-test) (C) U87/TMZ cells were treated overnight with VIVIT, then TMZ was incubated at different concentrations for 24 h. Each point represents the mean ± SD of three measurements. (D) NFATc3 protein levels increased in U87/TMZ cells compared with U87/WT cells. (E) The protein expression of NFATc3 decreased in U87/TMZ cells after treatment with TRPC5-siRNA for 24 h. (F) Knockdown of TRPC5 downregulated NFATc3 expression in U87/WT and U251/WT cells. (G) U87/WT cells were co-transfected TRPC5 and GFP-tagged NFATc3 plasmids for 24 h and a challenge with 100 μM carbachol was performed at the indicated time. Representative images of the migration of GFP-tagged NFATc3 from the cytosol to the nucleus are shown. (H) HEK-293 cells were co-transfected with TRPC5 and NFATc3 plasmid for 24 h and a challenge with 100 μM carbachol was performed at the indicated time. Representative images of the migration of GFP-tagged NFATc3 from the cytosol to the nucleus are shown. Values were means ± SD of the three experiments. 50 cells were measured in one experiment and the procedures were repeated at least three times.
There are four isoforms of NFAT, namely NFATc1-4. Previous research has pointed out that in drug-resistant breast cancerous cells, NFATc3 activates P-gp expression [18]. Hence, we determined the expression levels of NFATc3 and found that NFATc3 expression in U87/TMZ cells was higher than in U87/WT cells (Fig. 5D). NFAT is a Ca2+-dependent transcription factor that is translocated from the cytosol to the nucleus by a rise in [Ca2+]i, which stimulates gene transcription [11]. Treatment with TRPC5-siRNA decreased NFATc3 expression in U87/TMZ cells (Fig. 5E). NFATc3 expression was also downregulated in TRPC5-knockdown U87/WT and U251/WT cells (Fig. 5F). Rescue experiments were performed and the result indicated that VIVIT inhibited NFATc3 expression (Supplementary 2A). Indeed, the co-transfection of TRPC5 and GFP-tagged NFATc3 plasmid in U87/WT cells, along with the stimulation of TRPC5 expression using carbachol, induced NFATc3 translocation from the cytoplasm to the nucleus (Fig. 5G). Next, we transfected TRPC5 and GFP-tagged NFATc3 plasmids in HEK-293 cells. The results indicated NFATc3 translocation from the cytoplasm to the nucleus (Fig. 5H and Supplementary 2B). Taken together, these results revealed a signalling pathway involving the overexpression of TRPC5, NFATc3 nuclear translocation, and P-gp induction.
High expression of TRPC5 and P-gp in human glioblastoma samples
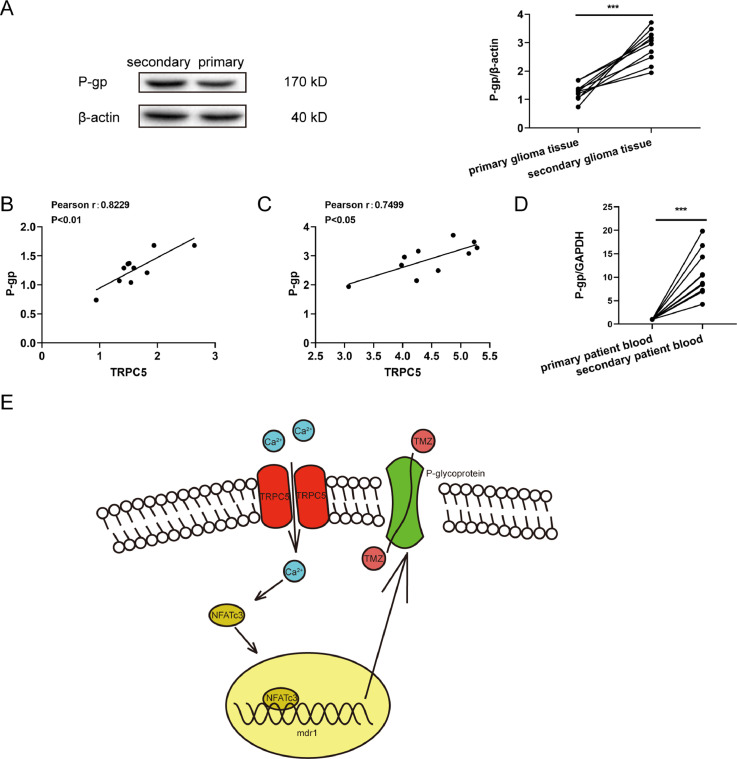
To investigate the clinical potential of detecting P-gp expression in glioblastoma tissue, we re-analyzed the specimens of 10 patients whose glioblastomas were primary and recurrent after TMZ treatment. Immunoblots indicated that P-gp expression was increased in the chemotherapy group (Fig. 6A). Meanwhile, P-gp levels in cancer tissues were positively correlated with TRPC5 expression in primary (Fig. 6.B) and secondary glioblastoma tissues (Fig. 6C). We also assessed P-gp mRNA expression in peripheral blood and the results indicated that the level of P-gp in peripheral blood after chemotherapy was significantly higher than that in the control group (Fig. 6D).
Fig. 6.
Clinical utility of measuring TRPC5 and P-gp expression in patients with glioblastoma. (A) The P-gp expression level in glioblastoma tissue specimens was measured via western blot and a representative image is shown. P-gp protein level increased in postchemotherapy patients (n =10 each group) compared to the same patients before surgery. Data were analysed using Student's t-test. (B and C) The Spearman's rank test was used to analyse the relationship between TRPC5 and P-gp expression levels between primary and secondary glioblastoma tissues. (D) RT-PCR analysis of P-gp in the peripheral blood of patients with glioblastoma showed that the mRNA level of P-gp increased in postchemotherapy patients (n =10 each group). Data were analysed via Student t-test. (E) The signalling pathway involved in TRPC5-activated P-gp expression in glioma cells under exposure to TMZ is shown.
Discussion
The efficacy of TMZ as the first-choice treatment for glioblastoma is limited by a high rate of drug resistance. Considerable research has focused on the enzyme—O6-methylguanine-DNA methyltransferase (MGMT), which is the effector site of TMZ binding in glioma cells [2]. However, MGMT inhibition failed to fully inhibit TMZ resistance. For this reason, this study focused on P-gp, which is the most important factor in many drug-resistant cancer cells. A previous study has pointed out that increased transcription of MDR1 led to an autonomous induction of TMZ resistance and increasing concentrations of TMZ competed with calcein for P-gp [19]. Our results indicated that P-gp expression was elevated in U87/TMZ and U251/TMZ cells. Previous research has identified that an increase in [Ca2+]i stimulates P-gp induction in cancer cells [16,23]. TRPC5 is a Ca2+-permeable non-selective cation channel that increases Ca2+ influx and plays an important role in neuronal growth cone extension [8,13], vascular smooth muscle migration [13], and animal fear behaviour [22]. Ma et al. determined that TRPC5 upregulation in breast cancer leads to adriamycin resistance by increasing P-gp expression [18].
TRPC5 is abundantly expressed in the brain tissues; however, the function of TRPC5 in TMZ resistance in glioblastoma is not well understood. In the present study, we first evaluated TRPC5 mRNA and protein levels in both patient specimens and glioblastoma cell lines. Our results confirmed that in patients with recurrent glioblastoma, both TRPC5 mRNA and protein expression was upregulated. Meanwhile, TRPC5 mRNA and protein levels in U87/TMZ and U251/TMZ cells were significantly different, and the difference between TRPC5 mRNA levels was more obvious than the protein expression levels. This may be affected by translation being monitored via other mechanisms, which requires further investigation. Next, we determined the function of TRPC5 in the stimulation of P-gp induction and TMZ resistance in glioblastoma cells. To monitor TMZ resistance, we determined two different indices, P-gp expression and TMZ-induced cancer cell death. In TMZ-resistant U87 and U251 cells, P-gp expression increased, and both cell lines were more resistant to TMZ-induced cell death compared to wild-type cell lines. Inhibition of TRPC5 by TRPC5-siRNA decreased P-gp expression and reversed TMZ resistance. To further confirm the effect of TRPC5 on P-gp expression and TMZ resistance, the cells were transfected with a TRPC5 expression plasmid. We observed an increase in P-gp expression and drug resistance to TMZ in these cells. We also confirmed that TRPC5 regulated P-gp expression and TMZ resistance in vivo. Meanwhile, P-gp mRNA and protein expression increased in patients with recurrent glioblastoma and was positively correlated with TRPC5 expression. These data demonstrate that TRPC5 is a prominent protein that regulates P-gp expression and mediates TMZ resistance in vivo and in vitro.
NFAT is a transcription factor activated by Ca2+, which is mediated through the Ca2+-binding proteins calmodulin and calcineurin [11], where TRPC channels could mediate Ca2+ entry [20]. Previous research has documented that Ca2+ entry through other TRPC channels, including TRPC1, TRPC3, and TRPC6, could accelerate NFAT-dependent gene transcription [20,21]. A putative NFAT binding site can be found in the 5’-flanking sequence of MDR1. In breast cancer cells, TRPC5 stimulates the expression of NFAT, especially NFATc3 [18]. Thus, we explored the possible mechanism of action of NFAT in P-gp expression mediated by TRPC5. The NFAT inhibitor, VIVIT, substantially reduced P-gp expression in U87/WT or U251/WT cells. It also increased cell death and upregulated the efficacy of TMZ. In our study, we found that TRPC5 upregulation or downregulation could accelerate or inhibit Ca2+ influx and intracellular Ca2+ concentration, which could activate or inhibit the nuclear translocation of NFATc3, respectively. Meanwhile, the stimulation of TRPC5 by carbachol also induced the nuclear translocation of NFATc3. These data suggest that overexpression of TRPC5 stimulates the nuclear translocation of NFATc3 to enhance P-gp induction.
There were some limitations to our study. The inhibition of TRPC5 did not fully restrict P-gp expression. P-gp acts as a drug transporter in the cell membrane to receive numerous signals to mediate drug transport; hence, other mechanisms underlying P-gp regulation may exist without changing TRPC5 expression. In vivo, we generated subcutaneous glioblastoma tumours to determine the effect of TRPC5-shRNA lentiviral particles on P-gp and TMZ resistance. There may exist several differences between subcutaneous glioblastoma tumours and glioblastoma tumours in the brain; therefore, we need to orthotopically implant U87 cells in the brain to further confirm our results. In addition, there is still doubt regarding the origin of the U87 cell line. Although we have conducted our experiments both in U87 and U251 cell lines, more research should be conducted on other glioblastoma cell lines.
In conclusion, our results demonstrate that the overexpression of TRPC5 stimulates NFATc3 translocation and increases P-gp expression and TMZ resistance both in vivo and in vitro. The inhibition/suppression of TRPC5 can inhibit P-gp expression and reverse TMZ resistance in glioblastoma cells and enhance the therapeutic effects of TMZ on glioblastoma both in cell lines and animal models.
CRediT authorship contribution statement
Yan Zou: Conceptualization, Methodology, Software, Investigation. Zi'xiang Liu: Investigation, Data curation. Yi'nan Zhou: Investigation. Jing Wang: Formal analysis. Qin'yi Xu: Software, Validation, Visualization. Xu'dong Zhao: Writing – original draft, Supervision. Zeng'li Miao: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Compliance with Ethical Standards
Ethical approval for this investigation was obtained from the Research Ethics Committee of the Affiliated Wuxi No.2 Hospital of Nanjing Medical University.
Funding
This work was supported by Major project of Wuxi Health Commission[Grant No. Z201809].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101214.
Appendix. Supplementary materials
References
- 1.Abe T., Mori T., Wakabayashi Y., Nakagawa M., Cole S.P., Koike K., ..., Hori S. Expression of multidrug resistance protein gene in patients with glioma after chemotherapy. J. Neurooncol. 1998;40(1):11–18. doi: 10.1023/a:1005954406809. [DOI] [PubMed] [Google Scholar]
- 2.Alonso M.M., Gomez-Manzano C., Bekele B.N., Yung W.K., Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67(24):11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar S.V., Dey S., Hrycyna C.A., Ramachandra M., Pastan I., Gottesman M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 4.Brandes A.A., Tosoni A., Franceschi E., Reni M., Gatta G., Vecht C. Glioblastoma in adults. Crit. Rev. Oncol. Hematol. 2008;67(2):139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen C., Hanson E., Watson J.W., Lee J.S. P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab. Dispos. 2003;31(3):312–318. doi: 10.1124/dmd.31.3.312. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.J., Chin J.E., Ueda K., Clark D.P., Pastan I., Gottesman M.M., Roninson I.B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Greka A., Navarro B., Oancea E., Duggan A., Clapham D.E. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat. Neurosci. 2003;6(8):837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 9.Gunther W., Pawlak E., Damasceno R., Arnold H., Terzis A.J. Temozolomide induces apoptosis and senescence in glioma cells cultured as multicellular spheroids. Br. J. Cancer. 2003;88(3):463–469. doi: 10.1038/sj.bjc.6600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegi M.E., Diserens A.C., Gorlia T., Hamou M.F., de Tribolet N., Weller M., ..., Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 11.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(18):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 12.Hutter G., Sinha P. Proteomics for studying cancer cells and the development of chemoresistance. Proteomics. 2001;1(10):1233–1248. doi: 10.1002/1615-9861(200110)1:10<1233::AID-PROT1233>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L.H., Gamper N., Beech D.J. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr. Drug Targets. 2011;12(5):724–736. doi: 10.2174/138945011795378568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson D.R., O'Neill B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 2012;107(2):359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 15.Kheirelseid E.A., Miller N., Chang K.H., Curran C., Hennessey E., Sheehan M., Kerin M.J. Mismatch repair protein expression in colorectal cancer. J. Gastrointest. Oncol. 2013;4(4):397–408. doi: 10.3978/j.issn.2078-6891.2013.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komoto C., Nakamura T., Yamamori M., Ohmoto N., Kobayashi H., Kuwahara A., ..., Sakaeda T. Reversal effects of Ca2+ antagonists on multidrug resistance via down-regulation of MDR1 mRNA. Kobe J. Med. Sci. 2008;53(6):355–363. [PubMed] [Google Scholar]
- 17.Koukourakis G.V., Kouloulias V., Zacharias G., Papadimitriou C., Pantelakos P., Maravelis G., ..., Kouvaris J. Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article. Molecules. 2009;14(4):1561–1577. doi: 10.3390/molecules14041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X., Cai Y., He D., Zou C., Zhang P., Lo C.Y., ..., Yao X. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109(40):16282–16287. doi: 10.1073/pnas.1202989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz J.L., Walker N.D., Scotto K.W., Rameshwar P. Temozolomide competes for P-glycoprotein and contributes to chemoresistance in glioblastoma cells. Cancer Lett. 2015;367(1):69–75. doi: 10.1016/j.canlet.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Ohba T., Watanabe H., Murakami M., Takahashi Y., Iino K., Kuromitsu S., ..., Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J. Mol. Cell Cardiol. 2007;42(3):498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Poteser M., Schleifer H., Lichtenegger M., Schernthaner M., Stockner T., Kappe C.O., ..., Groschner K. PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proc. Natl. Acad. Sci. U. S. A. 2011;108(26):10556–10561. doi: 10.1073/pnas.1106183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riccio A., Li Y., Moon J., Kim K.S., Smith K.S., Rudolph U., ..., Clapham D.E. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137(4):761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riganti C., Campia I., Polimeni M., Pescarmona G., Ghigo D., Bosia A. Digoxin and ouabain induce P-glycoprotein by activating calmodulin kinase II and hypoxia-inducible factor-1alpha in human colon cancer cells. Toxicol. Appl. Pharmacol. 2009;240(3):385–392. doi: 10.1016/j.taap.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Schinkel A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999;36(2-3):179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 25.Shtil A.A., Azare J. Redundancy of biological regulation as the basis of emergence of multidrug resistance. Int. Rev. Cytol. 2005;246:1–29. doi: 10.1016/S0074-7696(05)46001-5. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Dai H., Shaik N., Elmquist W.F. Drug efflux transporters in the CNS. Adv. Drug Deliv. Rev. 2003;55(1):83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.