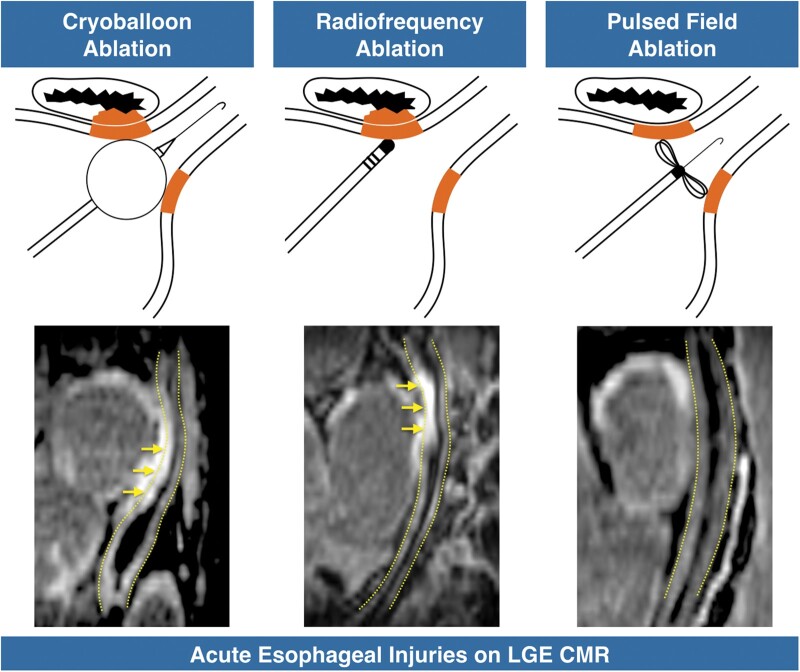
Abstract
Aims
Extra-atrial injury can cause complications after catheter ablation for atrial fibrillation (AF). Pulsed field ablation (PFA) has generated preclinical data suggesting that it selectively targets the myocardium. We sought to characterize extra-atrial injuries after pulmonary vein isolation (PVI) between PFA and thermal ablation methods.
Methods and results
Cardiac magnetic resonance (CMR) imaging was performed before, acutely (<3 h) and 3 months post-ablation in 41 paroxysmal AF patients undergoing PVI with PFA (N = 18, Farapulse) or thermal methods (N = 23, 16 radiofrequency, 7 cryoballoon). Oesophageal and aortic injuries were assessed by using late gadolinium-enhanced (LGE) imaging. Phrenic nerve injuries were assessed from diaphragmatic motion on intra-procedural fluoroscopy. Baseline CMR showed no abnormality on the oesophagus or aorta. During ablation procedures, no patient showed phrenic palsy. Acutely, thermal methods induced high rates of oesophageal lesions (43%), all observed in patients showing direct contact between the oesophagus and the ablation sites. In contrast, oesophageal lesions were observed in no patient ablated with PFA (0%, P < 0.001 vs. thermal methods), despite similar rates of direct contact between the oesophagus and the ablation sites (P = 0.41). Acute lesions were detected on CMR on the descending aorta in 10/23 (43%) after thermal ablation, and in 6/18 (33%) after PFA (P = 0.52). CMR at 3 months showed a complete resolution of oesophageal and aortic LGE in all patients. No patient showed clinical complications.
Conclusion
PFA does not induce any signs of oesophageal injury on CMR after PVI. Due to its tissue selectivity, PFA may improve safety for catheter ablation of AF.
Keywords: Pulsed field ablation, Atrial fibrillation, Catheter ablation, Oesophagus, Cardiac magnetic resonance
Graphical Abstract
What’s new?
Pulsed field ablation (PFA) selectively spares the oesophagus, with no acute oesophageal lesions detected on cardiac magnetic resonance (CMR) while these are common with thermal ablation methods, almost constantly observed on acute CMR when the oesophagus is in direct contact with a left atrial region targeted by ablation.
Transient aortic injuries are observed in a subset of patients after both PFA and thermal methods, with unclear pathological significance.
Introduction
Pulmonary vein isolation (PVI) is a recommended therapeutic option in patients with symptomatic drug-refractory atrial fibrillation (AF).1 To this day, PVI has mostly been achieved with thermal methods, in which tissue necrosis is obtained through resistive heating2 or freezing.3 However, as thermal approaches do not specifically target the myocardium, anatomical structures surrounding the left atrium are at risk of collateral damage. Extra-atrial damage includes oesophageal,4 right phrenic nerve,5 and aortic injuries.6 Although oesophageal lesions after AF ablation have been assessed with oesophagoscopy, this method is invasive and shows suboptimal sensitivity to detect non-transmural and transient oesophageal lesions.7,8 Late gadolinium-enhanced cardiac magnetic resonance (LGE-CMR) can depict oesophageal injuries after catheter ablation.9 A recent CMR study reported high rates of transient oesophageal lesions (40%) after AF ablation using thermal methods.10 Likewise, CMR studies have shown that injuries on the descending aorta were also common after PVI,6 although the pathological significance remains ambiguous. Pulsed field ablation (PFA) is a non-thermal ablative approach in which cell death is obtained by applying high voltage ultra-short pulses to induce pores in cell membranes.11 Using this technology, initial clinical studies have reported high durability of PVI and procedural safety.12 Indeed, with the threshold for irreversible injury after PFA being dependent on cell size, shape and orientation, the method can be tissue-specific, and preclinical data suggests that PFA may selectively target cardiomyocytes while sparing the oesophagus, nerves and blood vessels.13–16 The aim of this study was to assess the rate of extra-atrial injury on CMR following PVI using PFA in patients with paroxysmal AF, as compared to thermal ablation methods.
Methods
Population and study design
From November 2018 to November 2019, we prospectively considered for inclusion all patients with paroxysmal AF referred for a first catheter ablation procedure at Bordeaux University Hospital, and without contra-indication to contrast-enhanced CMR. Included subjects were not consecutive patients since the inclusion depended on MR systems’ availability. All patients underwent CMR at baseline <4 days prior to ablation as part of the routine pre-operative work-up, as well as CMR at the acute stage <3 h post-ablation, and at 3-month follow-up. The study population comprised 18 patients treated with PFA (Farapulse, formerly IOWA APPROACH), and 23 patients treated with thermal methods, i.e. radiofrequency (RF) in 16 (Thermocool Smarttouch, Biosense Webster) and cryo-ablation in 7 (Arctic Front Advance, Medtronic). Patients treated with PFA were part of IMPULSE (A Safety and Feasibility Study of the IOWA Approach Endocardial Ablation System to Treat Atrial Fibrillation) (NCT03700385) and PEFCAT (A Safety and Feasibility Study of the FARAPULSE Endocardial Ablation System to Treat Paroxysmal Atrial Fibrillation) (NCT03714178), two single-arm feasibility trials with nearly identical structure. Baseline, acute and follow-up CMR studies were reviewed to assess injury on the oesophagus and descending aorta. Phrenic damage was assessed on intra-procedural fluoroscopy. The rates of these extra-atrial injuries were compared between PFA and thermal methods. The study was approved by our institutional ethics committee, and all patients provided informed consent.
Catheter ablation
Left atrial (LA) thrombus was ruled out via pre-operative computed tomography scan. All procedures were performed under conscious sedation and uninterrupted oral anticoagulation. Intravenous heparin was administered after transseptal puncture. In all patients, the procedural endpoint was PVI. In the PFA group, a 12 F over-the-wire PFA ablation catheter (Farawave, Farapulse) with five splines, each containing four electrodes, was deployed in either a flower petal or basket configuration, depending on pulmonary vein (PV) anatomy. The catheter was advanced over a guidewire such that the splines achieved circumferential contact/proximity with the PV antra. The right ventricle was paced to synchronize PFA delivery to just after QRS onset in patients of IMPULSE (N = 8), while no such synchronization was used in patients of PEFCAT (N = 10). The ablative energy consisted of microsecond-scale biphasic pulses delivered in bipolar fashion with output ranging from 1800 to 2000 V. With the latest waveform version used in PEFCAT, each application comprised five delivery packets. Applications were repeated eight times per vein, with repositioning and/or rotation of the catheter every two applications to ensure circumferential PV ostial and antral coverage. In the thermal group, patients were treated with either a contact-force irrigated RF ablation catheter (Thermocool Smarttouch, Biosense Webster) or a cryoballoon (Arctic Front Advance, Medtronic). When using RF, we applied 0.9% saline irrigation, and delivered RF during 30–60 s applications, with a temperature limited to 52°C, a minimum contact force of 20 g on the anterior wall and 10 g on the posterior wall, and a maximum power of 30 W (25 W on the posterior wall). PVI was performed under 3D electroanatomical mapping guidance, using a point-by-point and/or a dragging technique. In the PFA and RF groups, PVI was confirmed using 3D maps (Carto, Biosense Webster or Rhythmia, Boston Scientific), or a circular mapping catheter (Lasso, Biosense Webster, Irvine, CA, USA). When using cryo-ablation, PVI was performed using a 28‐mm cryoballoon catheter (Arctic Front Advance; Medtronic), under fluoroscopic guidance. PV occlusion was tested with the retention/leakage of contrast agent after injection at the distal tip of the balloon. A minimum of two freezes were delivered to each PV with a targeted duration of 180 s. The octapolar mapping catheter incorporated in the ablation device (Achieve; Medtronic) was used to confirm electrical PVI. Neither oesophageal temperature monitoring nor mechanical oesophageal deviation was used in any of the groups. In all patients, phrenic injury was assessed by analysing diaphragmatic motion on per-procedural fluoroscopic images.
Cardiac magnetic resonance
CMR studies were performed at baseline within 4 days prior to each ablation procedure, as well as acutely, i.e. less than 3 h post-ablation, and at 3-month follow-up. Studies were conducted on a 1.5-Tesla system (MAGNETOM AERA®, Siemens Medical Systems, Erlangen, Germany), equipped with a 32-channel cardiac coil. Atrial LGE imaging was initiated 20 min after the intravenous injection of 0.2 mmol/kg gadoterate meglumine (Guerbet, Aulnay-sous-bois, France). Imaging was acquired in trans-axial orientation at a mid-diastolic phase using a three-dimensional, inversion-recovery-prepared, electrocardiogram-gated, respiration-navigated gradient-echo pulse sequence with fat-saturation.17 Typical imaging parameters were: voxel size 1.25 × 1.25 × 2.5 mm, flip angle 22°, TR/TE 6.1/2.4 ms, inversion time 260–320 ms depending on the results of a TI scout scan performed immediately before acquisition, parallel imaging with GRAPPA technique with R = 2, 42 reference lines, acquisition time 5–10 min depending on patient’s heart and breath rate. Images were reviewed in trans-axial, four-chamber and sagittal oblique views parallel to the oesophagus using a multi-planar three-dimensional viewer (Horos open source software, Horosproject.org). A reader with 15 years of experience in CMR analysed all images, blinded from procedural and patient characteristics. On baseline CMR, the LA surface area was measured on a four-chamber view to look for differences in atrial size between groups. However, these areas being measured at mid-diastole, they could not be compared to normal reference values. At each time point, images were reviewed to qualitatively assess atrial, oesophageal, and aortic LGE. In addition, the proximity between the oesophagus/descending aorta and the LA wall was analysed. On baseline CMR, it was categorized as direct contact if the oesophagus or the aorta showed less than 1 mm distance with the LA wall on at least one location. On acute post-ablation CMR, it was categorized as in direct contact with an LA region targeted by ablation if the oesophagus or the aorta showed less than 1 mm distance from an LA wall region exhibiting acute LGE.
Statistical analysis
The Shapiro–Wilk test of normality was used to assess whether quantitative data conformed to the normal distribution. Continuous data are expressed as mean ± standard deviation when following a normal distribution, and as median (interquartile range Q1–Q3) otherwise. Categorical data are expressed as a proportion (%). Independent continuous variables were compared using independent-sample parametric (unpaired Student’s t-test) or non-parametric tests (Mann–Whitney U test) depending on data normality. Dependent continuous variables were compared using paired-sample parametric or non-parametric tests (paired Student’s t-test, Wilcoxon signed-rank test) depending on data normality. Independent categorical variables were compared using χ2 test when expected frequencies were ≥5, and Fisher’s exact test when they were <5. Dependent categorical variables were compared using the paired-sample McNemar’s test. All statistical tests were two-tailed. A P-value <0.05 was considered to indicate statistical significance. Analyses were performed using NCSS 8 (NCSS Statistical Software, Kaysville, UT, USA).
Results
Population baseline characteristics
Patient baseline characteristics are shown in Table 1. No significant differences were found between PFA (N = 18, age 58 ± 9 years, 83% males) and thermal groups (N = 23, age 59 ± 9 years, 74% males) in terms of demographics, left ventricular ejection fraction, cardiovascular risk factors, medical history, or drugs. On baseline CMR, the LA area did not differ between groups (19.6 ± 2.6 and 19.7 ± 2.8 cm2 in PFA and thermal groups, respectively, P = 0.85). The oesophagus and descending aorta were found to be in direct contact with the left atrium in 31/41 (76%) and 28/41 (68%) of the total population, respectively, with no significant differences between groups (P = 0.78 and P = 0.64 for the oesophagus and aorta, respectively). None of the patients from any of the two groups showed oesophageal or aortic LGE at baseline (0/41).
Table 1.
Patient baseline characteristics
| Group | Total | Thermal (N = 23) | PFA (N = 18) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 58 ± 9 | 59 ± 9 | 58 ± 9 | 0.87 |
| Male gender | 32 (78%) | 17 (74%) | 15 (83%) | 0.78 |
| Clinical status and history | ||||
| LVEF (%) | 61 ± 7 | 61 ± 8 | 62 ± 6 | 0.44 |
| Hypertension | 8 (20%) | 4 (17%) | 4 (22%) | 0.71 |
| Diabetes | 1 (2%) | 0 (0%) | 1 (6%) | 0.26 |
| Smoking | 17 (41%) | 9 (39%) | 8 (44%) | 0.74 |
| Dyslipidaemia | 7 (17%) | 4 (17%) | 3 (17%) | 0.95 |
| Obesity | 5 (12%) | 1 (4%) | 4 (22%) | 0.09 |
| Stroke or TIA | 3 (7%) | 1 (4%) | 2 (11%) | 0.54 |
| CAD | 3 (7%) | 2 (9%) | 1 (6%) | 0.71 |
| Medication | ||||
| Warfarin | 2 (5%) | 2 (9%) | 0 (0%) | 0.21 |
| NOAC | 39 (95%) | 21 (91%) | 18 (100%) | 0.21 |
| AAD class I | 16 (39%) | 7 (30%) | 9 (50%) | 0.21 |
| AAD class II | 17 (41%) | 9 (39%) | 8 (44%) | 0.74 |
| AAD class III | 15 (36%) | 10 (43%) | 5 (28%) | 0.31 |
| No AAD | 10 (24%) | 6 (26%) | 4 (22%) | 0.78 |
AAD, anti-arrhythmic drug; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; NOAC, novel oral anticoagulant; PFA, pulsed field ablation; TIA, transient ischaemic attack.
Catheter ablation
Procedural characteristics are shown in Table 2. PVI was successfully obtained in all 41 patients. In the PFA group, the mean fluoroscopy time was 24 ± 9 min. The mean total skin-to-skin procedure time was 126 ± 37 min. Of note, this procedure time included the acquisition of baseline and post-ablation voltage maps, as part of the PEFCAT and IMPULSE research protocols. The total PFA energy delivery time was less than 1 min/patient. In the thermal group, the mean fluoroscopy time was 25 ± 14 min (27 ± 16 min with RF and 21 ± 4 min with cryoballoon). The mean total skin-to-skin procedure time was 142 ± 51 min (156 ± 53 min with RF and 110 ± 28 min with cryoballoon). The mean total RF duration was 45 ± 23 min, and the typical total freezing duration was 17 ± 3 min. Fluoroscopy time and total procedure time did not differ between groups (P = 0.78 and P = 0.27, respectively). Phrenic palsy was observed in none of the patients (0/41). There were no device-related complications in any of the groups. Groin haematoma was observed in two patients from the thermal group and one patient from the PFA group (P = 0.71), without further complication. There were no severe complications in any of the groups. Mild chest discomfort was reported after the procedure by 3/23 (13%) patients from the thermal group and 2/18 (11%) patients from the PFA group (P = 0.86). These symptoms did not motivate additional explorations and spontaneously resolved in all patients over a few days.
Table 2.
Procedural characteristics
| Group | Total | Thermal (N = 23) | PFA (N = 18) | P-value |
|---|---|---|---|---|
| Total procedure time (min) | 141 ± 50 | 142 ± 51 | 126 ± 37 | 0.27 |
| Fluoro time (min) | 24 ± 11 | 25 ± 14 | 24 ± 9 | 0.78 |
| Energy delivery duration (min) | NA | RF: 45 ± 23 CRYO: 17 ± 3 | 0.57 ± 0.08 | <0.001a |
| Succesfull PVI | 41 (100%) | 23 (100%) | 18 (100%) | NA |
| Phrenic palsy on fluoro | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Procedure-related complications | 2 (5%) | 2 (9%) | 1 (6%) | 0.71 |
CRYO, cryoballoon; PFA, pulsed field ablation; PVI, pulmonary vein isolation; RF, radiofrequency; NA, not assessable.
Significant against both RF and CRYO values.
Oesophageal findings on acute cardiac magnetic resonance
CMR performed less than 3 h post-ablation showed atrial LGE encircling all PVs at either the antral or the ostial level in all patients. Acute CMR showed that the oesophagus was in direct contact with an LA region targeted by ablation in 11/23 (48%) patients in the thermal group, and in 11/18 (61%) patients in the PFA group, with no significant difference between groups (P = 0.41). In the thermal group, oesophageal injuries were found on LGE images in 10/23 (43%) patients (6/16 ablated with RF and 4/7 with cryoballoon). All injuries involved the anterior wall of the oesophagus, which was found to be facing the antrum or ostium of the left inferior PV in 7/10, the antrum or ostium of the right inferior PV in 2/10, and the posterior LA wall in 1/10. LGE was transmural throughout the oesophageal wall in 5/10 (50%) cases, and associated with a thickening of the oesophageal wall as compared to baseline in 4/10 (40%). All oesophageal lesions were found in patients showing a direct contact between the oesophagus and an LA region targeted by ablation, and among the 11 patients showing such direct contact, 10 (91%) exhibited oesophageal injury. In contrast, oesophageal lesions were observed in none of the 18 patients ablated with PFA (0%, P < 0.001 vs. thermal methods), including in the 11 patients with the oesophagus in direct contact with an LA region targeted by ablation. Examples of oesophageal injuries following thermal ablation are shown in Figure 1. The sparing of the oesophagus after PFA is illustrated in Figure 2.
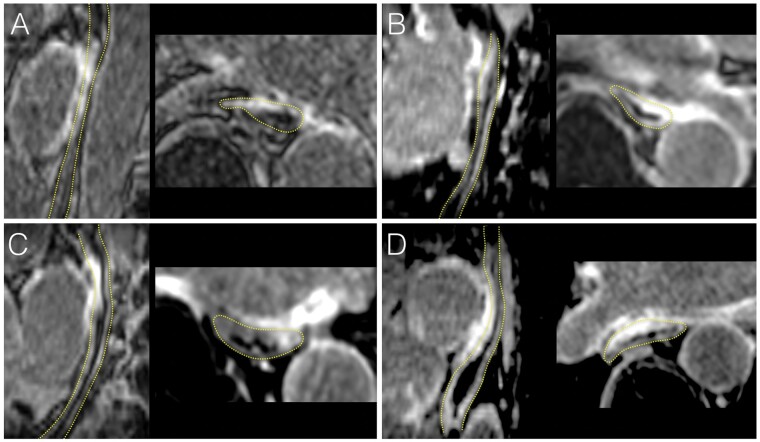
Figure 1.
Examples of acute oesophageal injuries on CMR following thermal ablation. LGE CMR images acquired less than 3 h post-ablation are shown in four patients treated with RF (A, C) or cryoballoon (B, D). In each, the oesophagus is shown in a sagittal oblique view parallel to the oesophagus (left image), and in a transaxial view (right image). The dotted yellow lines indicate oesophagus location. All patients show intense and transmural oesophageal LGE in direct contact with atrial areas targeted by ablation, also exhibiting LGE. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; RF, radiofrequency.
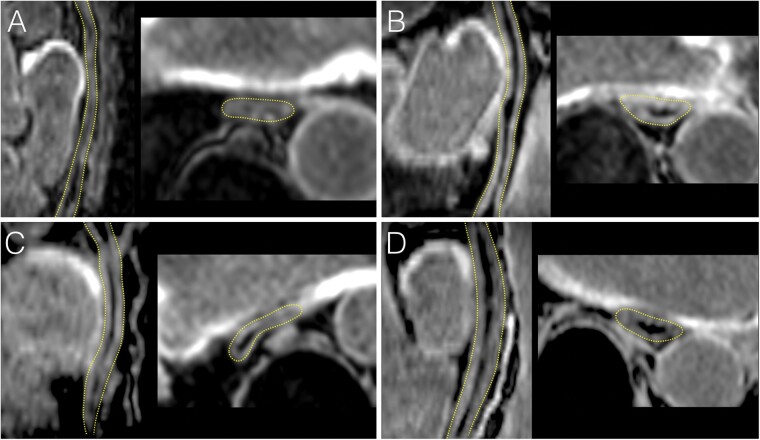
Figure 2.
Absence of acute oesophageal injury on CMR after PVI using PFA. LGE CMR images acquired less than 3 h post-ablation are shown in four patients treated with PFA (A–D). In each, the oesophagus is shown in a sagittal oblique view parallel to the oesophagus (left image), and in a trans axial view (right image). The dotted yellow lines indicate oesophagus location. No oesophageal LGE is seen in any of the patients despite direct contact with LA wall regions targeted by ablation, and showing intense LGE. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; PFA, pulsed field ablation; PVI, pulmonary vein isolation.
Aortic findings on acute cardiac magnetic resonance
On acute CMR, the descending aorta showed a direct contact with a LA region targeted by ablation in 13/23 (56%) patients in the thermal group, and in 12/18 (67%) patients in the PFA group, with no significant difference between groups (P = 0.52). Aortic lesions were found on acute LGE images in 10/23 (43%) patients in the thermal group, and in 6/18 (33%) patients in the PFA group, with no significant difference between groups (P = 0.52). These consisted of focal LGE on the anterior aortic wall, all being observed in patients with direct contact between the aorta and a left inferior PV antral or ostial region targeted by ablation. As compared to baseline images, there was no modification of aortic shape suggestive of aneurysm in any of the studied patients. Examples of aortic injuries following thermal ablation or PFA are shown in Figure 3. The rates of acute extra-atrial damage after thermal ablation and PFA are summarized in Figure 4.
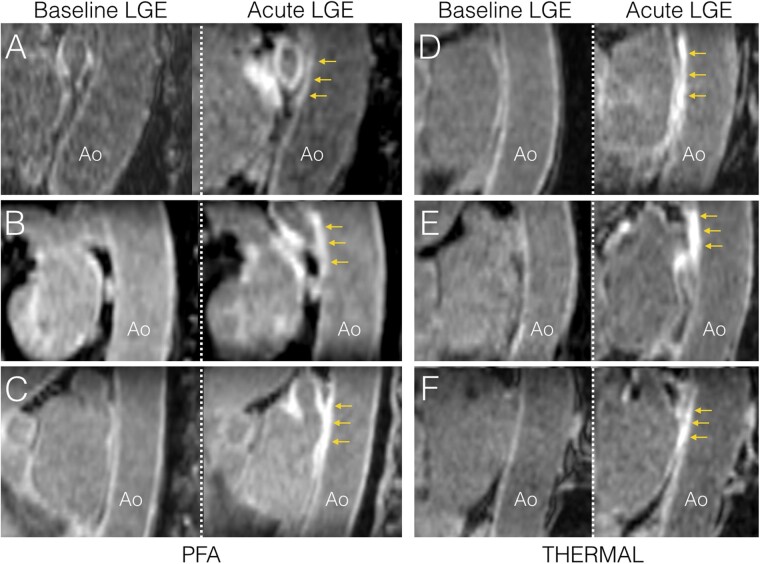
Figure 3.
Examples of acute aortic injuries on CMR following thermal ablation or PFA. Sagittal oblique images parallel to the descending aorta are shown in three patients treated with PFA (A–C), and three patients treated with thermal methods (D: RF, E and F: cryoballoon). In each, baseline imaging is shown in the left column, and acute imaging acquired less than 3 h post-ablation is shown in the right column. All patients show LGE on the anterior wall of the descending aorta (yellow arrows), in direct contact with atrial areas targeted by ablation, also exhibiting LGE. Ao, aorta; CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; PFA, pulsed field ablation; RF, radiofrequency.
Figure 4.
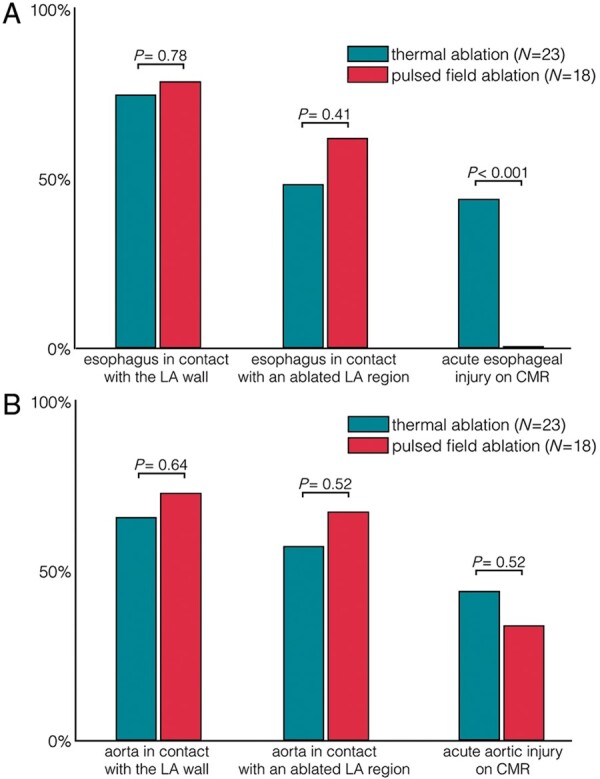
Acute extra-atrial damage on CMR after PVI with thermal vs. pulsed field ablation. (A) oesophageal injuries and (B) aortic injuries. CMR, cardiac magnetic resonance; LA, left atrium; PVI, pulmonary vein isolation.
Patient outcomes and cardiac magnetic resonance results at 3-month follow-up
Due to insufficient follow-up duration, arrhythmia recurrence was not assessed at 3 months. In both groups, none of the patients had shown any procedure-related complication during follow-up. Particularly, none of the patients had experienced clinical signs suggestive of atrio-oesophageal fistula or aortic complication. Follow-up CMR showed a complete resolution of both oesophageal and aortic LGE in all patients. As compared to baseline images, there was no modification of aortic shape suggestive of aneurysm in any of the patients studied.
Discussion
This study is the first to compare extra-atrial injury following PFA, RF and cryo-ablation for AF in humans. It confirms that PFA selectively spares the oesophagus, with no acute oesophageal lesions detected on CMR in any of the patients treated with PFA, while these are common with thermal ablation methods, almost constantly observed on acute CMR when the oesophagus is in direct contact with an LA region targeted by ablation. Transient aortic injuries are observed in a subset of patients after both PFA and thermal methods, with unclear pathological significance. These findings illustrate the tissue specificity of PFA.
Oesophageal injury after thermal ablation vs. pulsed field ablation
Several prior studies have assessed the prevalence of oesophageal lesion following AF ablation. Clinically, up to 20% of patients may experience symptoms suggestive of oesophageal injury, including odynophagia, dysphagia, chest discomfort, gastric reflux, gastroparesis, or dysmotility.7 Knopp et al.4 reported thermal oesophageal injuries in 11% of patients after ablation with RF, and similar rates have been reported after cryoballoon ablation.18 The present study used CMR to detect such lesions, and we found oesophageal LGE to be extremely frequent after either RF or cryoballoon ablation (43%), and particularly almost constant when the oesophagus is in contact with a LA region targeted by ablation (91%). This rate of oesophageal LGE is consistent with a prior CMR study.10 It suggests that CMR is more sensitive than oesophagoscopy for the assessment of sub-clinical oesophageal injuries as it can detect non-transmural lesions. In contrast, we observed no oesophageal injury in any of patients treated with PFA, including those exhibiting a direct contact between the oesophagus and a LA region targeted by ablation. This confirms the tissue specificity of PFA, which selectively spares the oesophageal tissue. The electrical impulses delivered during PFA create pores in cells by polarizing its bi-layered lipidic membrane.11 The threshold to induce irreversible damage with PFA depends on the pulse design, but more importantly on cell size, cell shape, and orientation of the cells within the tissue.19 Fortunately, cardiomyocytes have a low threshold for irreversible electroporation, owing to their specific characteristics and to the cardiac myofibrillar architecture. Several pre-clinical reports had suggested a selective sparing of the oesophageal tissue, as well as nerves and blood vessels.13–16 Our study is the first to document this tissue selectivity in a clinical setting, with major implications for the safety of catheter ablation.
Other extra-cardiac damage
In the present study, the risk of phrenic palsy could not be analysed since it was observed in none of the studied patients. To document the impact of PFA on large vessels, the descending aorta was analysed on CMR. Aortic LGE has already been reported as quite common after PVI using thermal methods.6 Our results are in line with this prior study, with acute LGE being found in 43% of the patients immediately after thermal ablation. Interestingly, a substantial number of patients treated with PFA also exhibited transient LGE lesions on the descending aorta. This suggests that the aortic wall may also have a rather low threshold to PFA, although the clinical significance of these sub-clinical findings remain equivocal. To our knowledge, the descending aorta has not been associated with specific complications after catheter ablation with the modalities used in this study, including PFA.12 Nonetheless, with the descending aorta and the oesophagus being immediately adjacent to each other and in the vicinity of atrial ablation targets, the presence of acute aortic lesions following PFA demonstrates that the electric field indeed extended beyond the atrial wall. The lack of LGE on the oesophageal wall despite aortic LGE substantiates PFA’s tissue-selective mechanism.
Clinical implications
Atrio-oesophageal fistula is the most dramatic complication following catheter ablation. Although mitigation measures have been proposed, such as oesophageal temperature monitoring,20 oesophageal cooling systems21 or mechanical oesophageal displacement,22 they did not fully alleviate the risk of oesophageal injury. As a consequence, the energy delivered during catheter ablation is always a compromise between safety and efficacy, resulting in a substantial occurrence of non-durable PVI.23 The present study shows that PFA selectively spares the oesophagus, and may thus dramatically improve the safety of AF ablation. Besides alleviating safety concerns, this may also impact ablation effectiveness, because for the first time one can deliberately overpower therapy without compromising safety.
Study limitations
The effects of PFA are highly parameter-dependent and the observations reported herein can only be associated with the system used in this study. Oesophagoscopy was not performed to confirm CMR findings. Prior studies have related oesophageal LGE on acute CMR after thermal ablation to abnormalities on oesophagoscopy,9–10 but we acknowledge that most of the CMR findings reported here are subclinical and not linked to clinical manifestations, and that beyond research it is still unclear how CMR could play a clinical role to detect and manage clinically relevant oesophageal injuries. Larger studies should be conducted to document an impact on patient outcomes. Our study did not employ specific methods to mitigate the risk of oesophageal injury such as oesophageal temperature monitoring or oesophageal deviation. Thus, the incremental value of PFA over these methods cannot be assessed. Last, our study has a limited sample size which particularly prevented us from analysing potential differences between thermal methods (RF vs. cryoballoon ablation), as well as phrenic injuries. This was due to the limited availability of CMR to perform acute studies immediately post-ablation, and to the COVID pandemic preventing us from recruiting enough patients treated with thermal methods to eventually compare three groups of comparable size. Still, this does not prevent us from drawing clinically and statistically relevant conclusions on PFA.
Conclusions
Pulsed electrical fields used to isolate the PVs in patients with paroxysmal AF create transmural myocardial lesions and, in some cases, extend beyond the posterior atrial wall as demonstrated by the presence on CMR of acute lesions on the descending aorta. Crucially, however, PFA does not induce any signs of oesophageal injury while these are commonly observed after thermal ablation. This first clinical demonstration of the tissue specificity of PFA has major implications for the safety of catheter ablation in patients with AF.
Funding
This work was supported by the l’Agence Nationale de la Recherche (ANR) under Grant Agreements (Equipex MUSIC ANR-11-EQPX-0030, LIRYC ANR-10-IAHU-04); and the European Research Council (ERC n 715093 to H.C.).
Conflict of interest: H.C. has served as a consultant for Farapulse. V.Y.R. owns stock in Farapulse, and has served as a consultant for Farapulse. P.J. owns stock in Farapulse, and has received honoraria from Farapulse. C.E., C.S. and R.V. are Farapulse employees. The remaining authors have nothing to disclose.
Data availability
We hereby confirm that the data analysed in the present study may be made available to third party upon reasonable request.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr. et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 2.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A. et al. ; ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 3.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG. et al. ; STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713–23. [DOI] [PubMed] [Google Scholar]
- 4.Knopp H, Halm U, Lamberts R, Knigge I, Zachäus M, Sommer P. et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm 2014;11:574–8. [DOI] [PubMed] [Google Scholar]
- 5.Sacher F, Monahan KH, Thomas SP, Davidson N, Adragao P, Sanders P. et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol 2006;47:2498–503. [DOI] [PubMed] [Google Scholar]
- 6.Tung P, Hong SN, Chan RH, Peters DC, Hauser TH, Manning WJ. et al. Aortic injury is common following pulmonary vein isolation. Heart Rhythm 2013;10:653–8. [DOI] [PubMed] [Google Scholar]
- 7.Kapur S, Barbhaiya C, Deneke T, Michaud GF.. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–55. [DOI] [PubMed] [Google Scholar]
- 8.Gorman DR, Peterson KA, Fang J, Olpin J, Sommers DO, McFadden M. et al. Cross-sectional imaging obtained immediately following radiofrequency atrial fibrillation ablation does not predict endoscopic evidence of esophageal injury. Dig Dis Sci 2011;56:3453–8. [DOI] [PubMed] [Google Scholar]
- 9.Badger TJ, Adjei-Poku YA, Burgon NS, Kalvaitis S, Shaaban A, Sommers DN. et al. Initial experience of assessing esophageal tissue injury and recovery using delayed-enhancement MRI after atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2009;2:620–5. [DOI] [PubMed] [Google Scholar]
- 10.Baher A, Kheirkhahan M, Rechenmacher SJ, Marashly Q, Kholmovski EG, Siebermair J. et al. High-power radiofrequency catheter ablation of atrial fibrillation: using late gadolinium enhancement magnetic resonance imaging as a novel index of esophageal injury. JACC Clin Electrophysiol 2018;4:1583–94. [DOI] [PubMed] [Google Scholar]
- 11.Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M.. Cell membrane electroporation—part 1: the phenomenon. IEEE Electr Insul Mag 2012;28:14–23. [Google Scholar]
- 12.Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H. et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 13.Neven K, van Es R, van Driel V, van Wessel H, Fidder H, Vink A. et al. Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ Arrhythm Electrophysiol 2017;10:e004672. [DOI] [PubMed] [Google Scholar]
- 14.van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH.. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm 2015;12:1838–44. [DOI] [PubMed] [Google Scholar]
- 15.Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R. et al. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol 2019;12:e007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck ED. et al. Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 2020;13:e008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN. et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed H, Neuzil P, d'Avila A, Cha Y-M, Laragy M, Mares K. et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm 2009;6:962–9. [DOI] [PubMed] [Google Scholar]
- 19.Ben-David E, Ahmed M, Faroja M, Moussa M, Wandel A, Sosna J. et al. Irreversible electroporation: treatment effect is susceptible to local environment and tissue properties. Radiology 2013;269:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fürnkranz A, Bordignon S, Böhmig M, Konstantinou A, Dugo D, Perrotta L. et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 2015;12:268–74. [DOI] [PubMed] [Google Scholar]
- 21.Arruda MS, Armaganijan L, Di Biase L, Rashidi R, Natale A.. Feasibility and safety of using an esophageal protective system to eliminate esophageal thermal injury: implications on atrial-esophageal fistula following AF ablation. J Cardiovasc Electrophysiol 2009;20:1272–8. [DOI] [PubMed] [Google Scholar]
- 22.Koruth JS, Reddy VY, Miller MA, Patel KK, Coffey JO, Fischer A. et al. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:147–54. [DOI] [PubMed] [Google Scholar]
- 23.Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN.. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation 2004;109:1226–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We hereby confirm that the data analysed in the present study may be made available to third party upon reasonable request.