To the Editor:
Dermoscopy involves the use of a single handheld tool that incorporates illumination and magnification for improved visualization of skin structures. However, dermoscopy requires a knowledge base to be properly interpreted. In studies with new dermoscopists, dermoscopy was shown to provide no benefit to standard physical examinations,1 and might actually decrease sensitivity.2 In fact, one of the largest barriers to dermoscopy usage reported by dermatologists in the United States is the lack of training, which has resulted in only 48% of US dermatologists using dermoscopy.3
The triage amalgamated dermoscopic algorithm (TADA)4,5 differs from standard methods of dermoscopy education in that it starts by teaching the dermoscopic features of common benign lesions, so they can be excluded in further evaluation.6 The algorithm then teaches the identification of malignant skin lesions through abnormalities of the pigment network and vascular structure (Figs 1 and 2). This simplified algorithm has high sensitivity and specificity for both benign and malignant neoplasms and inherently caters to new dermoscopists.4
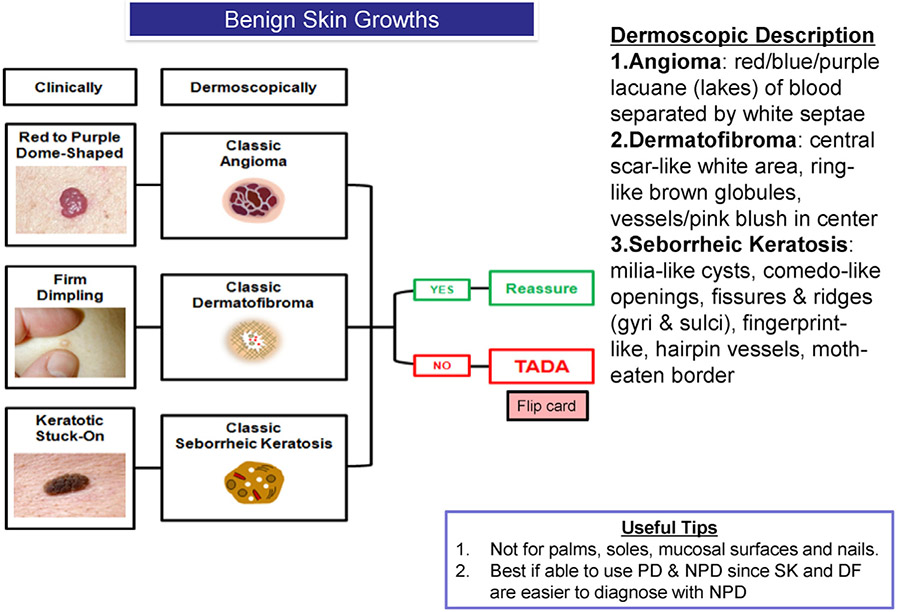
Fig 1.
Benign skin growths and description. DF, Dermatofibroma; NPD, nonpolarized dermoscopy; PD, polarized dermoscopy; SK, seborrheic keratosis; TADA, triage amalgamated dermoscopic algorithm.
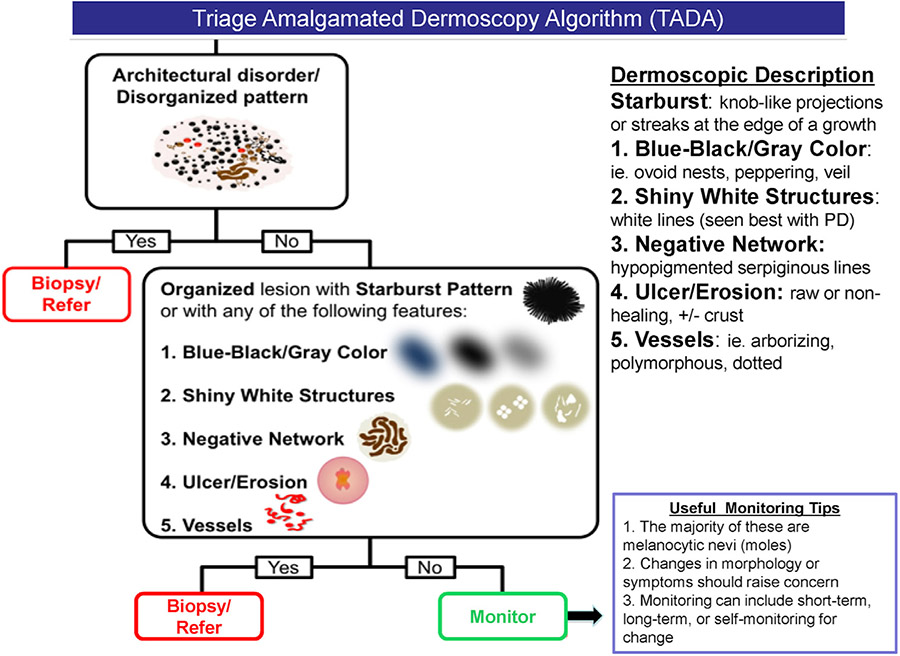
Fig 2.
Printable triage amalgamated dermoscopic algorithm for quick reference. NPD, Nonpolarized dermoscopy; PD, polarized dermoscopy.
Approval was obtained from the institutional review boards of Pennsylvania State University (CR9551) and the US Air Force 59 Medical Wing (FWH20180132H). Voluntary, fully informed consent of the participants used in this research was obtained as required by 32 CFR 219 and DODI 3216.02_AFI40-402. We administered a 1-hour live seminar to the 59 physicians in Pennsylvania and a recorded e-learning version of the same training to the 43 physicians in Florida who consented to the study. Both groups had limited previous exposure to dermoscopy and completed a test of 30 benign and malignant dermoscopy images before training and a separate test after training.
All participants had significant improvement (P < .001) in sensitivity for detecting malignant skin lesions with good specificity (Table I). The live lecture yielded an increase in sensitivity from 62% to 88%, and the e-learning method yielded an increase from 70% to 92%. Although the participants in the e-learning method had a higher baseline score than the live lecture (P ≤ .01), the resultant final sensitivities after education were not significantly different (P = .13; noninferiority t test with 10% margin, P < .01). We conclude that the e-learning method is at least noninferior to a live lecture setting for teaching dermoscopy and has many inherent benefits. E-learning enables training of larger audiences using the internet on individual monitors with perhaps better color and detail than a projector at a pace that is individualized to the learner. Indeed, e-learning was the preferred method of the participants in this arm of the study.
Table I.
Physician performance with different delivery methods of the triage amalgamated dermoscopic algorithm used to teach dermoscopy
| Category | Mean (%) score | Median (range) score | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| Live lecture, n = 59 | ||||
| Before training | 17.9 (60) | 19 (5-26) | 62.1 | 90.3 |
| After training | 23.5 (80) | 23 (17-26) | 88.0 | 87.8 |
| P value | ≤.001 | ≤.001 | ||
| E-learning, n = 43 | ||||
| Before training | 20.3 (68) | 20 (12-27) | 70.4 | 89.8 |
| After training | 24.7 (82) | 25 (17-29) | 91.5 | 87.6 |
| P value | ≤.001 | ≤.001 |
Dermatoscopes have been described as stethoscopes for the skin because of their utility in cutaneous diagnosis. However, a dermatoscope, like a stethoscope, is only 1 tool used in the full physical examination of a patient, not the single element used in physical diagnosis. With training, the ability to utilize dermoscopy increases. The e-learning video used in our study is now available for free use as part of the American Academy of Dermatology basic dermatology curriculum (see https://data.mendeley.com/datasets/jgdt3nxm8d/1). The authors hope that this study and the resources it provides will serve as an initial step for new users increasing their use of dermoscopy within our community and starting a journey toward broader use of this powerful tool in the clinical examination of patients.
Acknowledgments
We wish to thank Jisuk Park, PhD, for assistance with the statistical analysis of the data.
Footnotes
Conflicts of interest: Dr Marghoob received honorarium for speaking on dermoscopy for 3GEN. Dr Susong, Dr Ahrns, Dr Daugherty, and Dr Seiverling have no conflicts of interest to disclose.
Previously presented preliminary work in a poster presentation at the North American Primary Care Research Group annual meeting in Chicago, Illinois, November 9-13, 2018.
Disclaimer: The views expressed are those of the authors and do not reflect the official views or policy of the Department of Defense or its components.
IRB review: USAF: FWH20180132H Penn State: CR9551.
REFERENCES
- 1.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–165. [DOI] [PubMed] [Google Scholar]
- 2.Binder M, Schwarz M, Winkler A, et al. Epiluminescence microscopy. A useful tool for the diagnosis of pigmented skin lesions for formally trained dermatologists. Arch Dermatol. 1995;131(3):286–291. [DOI] [PubMed] [Google Scholar]
- 3.Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63(3):412–419, 419.e1-2. [DOI] [PubMed] [Google Scholar]
- 4.Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the triage amalgamated dermoscopic algorithm (TADA). J Am Board Fam Med. 2016;29(6):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers T, Marino M, Dusza SW, Bajaj S, Marchetti MA, Marghoob A. Triage amalgamated dermoscopic algorithm (TADA) for skin cancer screening. Dermatol Pract Concept. 2017;7(2):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiverling EV, Ahrns HT, Greene A, et al. Teaching benign skin lesions as a strategy to improve the triage amalgamated dermoscopic algorithm (TADA). J Am Board Fam Med. 2019;32(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]