Abstract
Background and aim
Postpartum depression (PPD) is a familiar problem which is associated with about 10–20% of women after child delivery. Fish oil (FO) has a therapeutic potentials to many diseases including mood disorders. However, there is paucity of data on the effects of FO supplementation on PPD rat model. Hence, this study aimed at investigating the potentials of FO in ameliorating depressive-like behaviors in PPD rat by evaluating the involvement of NLRP3-inflammasome.
Experimental procedure
Thirty six virgin adult female rats (n = 6) were randomly divided into six groups; Group 1–3 were normal control (NC), Sham (SHAM) and ovariectomized group (OVX) respectively whereas group 4–6 were PPD rats forced-fed once daily with distilled water (PPD), fish oil (PPD + FO; 9 g/kg) and Fluoxetine (PPD + FLX; 15 mg/kg) respectively from postpartum day 1 and continued for 10 consecutive days. Rats behaviors were evaluated on postpartum day 10 through open field test (OFT) and forced swimming test (FST), followed by biochemical analysis of NLRP3 inflammasome proteins pathway in their brain and determination of neutrophil to lymphocyte ratio (NLR).
Results
PPD-induced rats exhibited high immobility and low swimming time in FST with increased inflammatory status; NLR, IL-1β and NFкB/NLRP3/caspase-1 activity in their hippocampus. However, administration of FO or fluoxetine reversed the aforementioned abnormalities.
Conclusion
In conclusion, 10 days supplementation with FO ameliorated the depressive-like behaviors in PPD rats by targeting the NFкB/NLRP3/caspase-1/IL-1β activity. This has shed light on the potential of NLRP3 as a therapeutic target in treatment of PPD in rats.
Keywords: Menhaden fish oil, Postpartum depression, NLRP3-Inflammasome, Forced swimming test, Prefrontal cortex, Hippocampus
Abbreviations: FST, Forced swimming test; OFT, Open field test; IL-1β, Interleuki-1 beta; PPD, Postpartum depression; FO, Fish oil; MDD, Major depressive disorder; NLR, Neutrophil/lymphocyte ratio; NFкB, Nuclear factor kappa-light-chain-enhancer of activated B cells
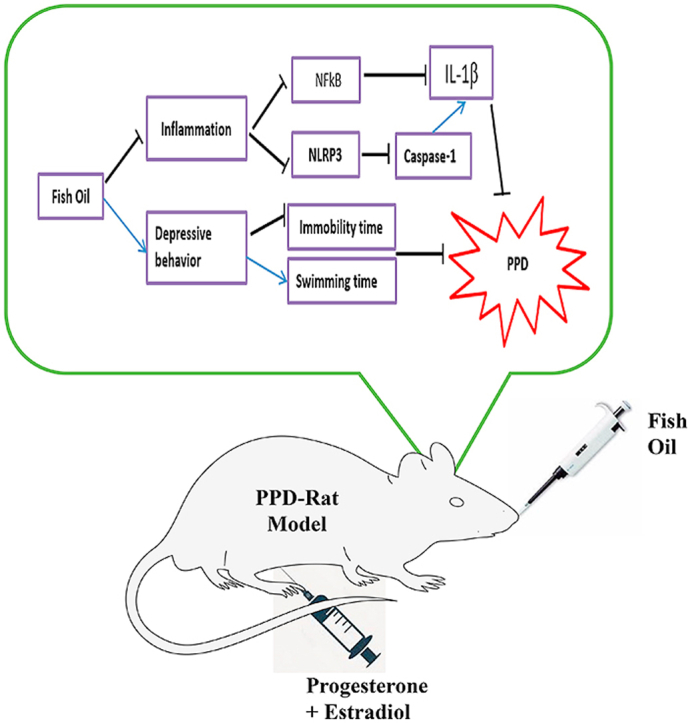
Graphical abstract
Highlights
-
•
Pioneer study on 10 days effect of Menhaden fish oil in post partum depression rat model.
-
•
Menhaden fish oil attenuates post partum depression in rat model.
-
•
Menhanden fish oil acted through NLRP3 inflammasome pathways.
1. Introduction
There is no clear understanding of the relationship between postpartum depression (PPD) and the resulting physiological changes, as results from studies on the aetiology of PPD are not consistent with each other.1, 2, 3, 4 The understanding of the pathophysiology of PPD is of critical importance. If not properly managed, the condition could affect the physiology and behaviour of newborn babies.5 PPD is associated with ovarian hormonal changes experienced after delivery and changes in some neurochemicals levels in the brain6, 7, 8. Animal model of PPD developed by modulation of hormones simulated pregnancy withdrawal (HSPW) exhibited long immobility time in forced swimming test (FST) (a test for screening of depression) and increased level of pro-inflammatory cytokines concentration such as interleukin one beta (IL-1β).9 The crucial role of IL-1β in PPD has been reviewed by Maes.10 Increased pro-inflammatory cytokine IL-1β in depressed animals was suggested to be linked with increased activation of an inflammatory transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NFкB) and nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome protein complex. Nevertheless, not many studies investigated the link between PPD and the modulation of the NLRP3 inflammasome complex. It was suggested that the NLRP3 inflammasome complex is associated with the pathophysiological changes of depression11 and NLRP3 could be a future potential therapeutic target in controlling depression.12, 13, 14
The multiprotein complex; NLRP3 inflammasome, consists of three proteins, which includes NLRP3, cysteinyl aspartate-specific proteinase-1 (caspase-1) and apoptosis-associated speck-like protein containing CARD (ASC).13 In normal condition, NLRP3 helps in the body’s self-defence by detecting stimuli that comes from bacteria, fungus and viral components. However, excessive activation of NLRP3 influences the onset of a wide spectrum of diseases such as type 2 diabetes, rheumatoid arthritis and depression. NLRP3 complex is associated with inflammation-induced depression,12,13 its up-regulation together with NFκB, caspase-1 and IL-1β pro-inflammatory cytokines lead to depression.15 The activation of NLRP3 influences maturation of caspase-1 from pro-caspase 1 that stimulated the activation of pro-IL-1β to matured IL-1β. Increased level of IL-1β showed a strong association with higher risk factors in depression. Thus, manipulation of NLRP3 functioning in PPD model could serve as a promising therapeutic strategy for PPD.
Recent studies have shown an association between fish oil (FO) and decreased risk of developing depression. A cross-sectional study supported the benefits of FO towards PPD in women,16 whereas some studies showed no association between FO intake and the onset of PPD.17 FO which is a rich source of omega-3 fatty acids has been used to manage and control maternal behaviours.18 Studies have shown that 9 g/kg of Menhaden FO supplementation for fifteen days reduced the risk of PPD in rats, as the rats exhibited decreased immobility time in forced swimming test (FST) as well as decreased plasma levels of IL-1β.8 Therefore, the current study is aimed at further evaluation of the potential mechanism of actions of Menhaden FO (rich in omega-3) supplementation on PPD rat’s model. The PPD rats’ model was supplemented with 9 g/kg of FO once daily for 10 days. Further, antidepressant-like effects of the FO was evaluated through FST on days 2 and 10 PPD, while the levels of IL-1β, NLRP3, caspase-1 and NFкB were measured both in brain prefrontal cortex (PFC) and hippocampus at the end of menhaden FO supplementation through enzyme-linked immunosorbent assay (ELISA).
2. Materials and methods
2.1. Animals
Female albino Wistar rats with an average weight of 180–200 g were used in this study. The rats were supplied by Takrif Bistari Enterprise, Taman Kembangsari, Seri Kembangan, Selangor, Malaysia and were kept at Animal Behaviour Room, Faculty of Medicine & Health Sciences, Universiti Putra Malaysia. The rats were housed individually per cage under 12 h/12 h day/light cycle with free access to water and standard rat chow, and they acclimatized for 2 weeks before the experiment began. All the experimental procedures were conducted following Animal handling and ethical guidelines approved by the Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (UPM/IACUC/AUP-R097/2015).
2.2. Experimental design
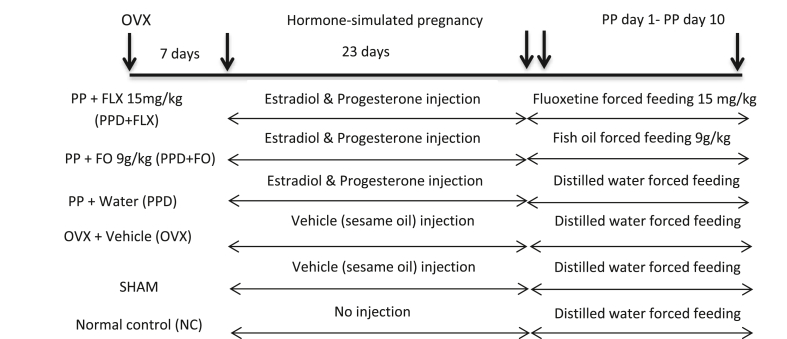
The rats were randomly allocated into 6 groups (Fig. 1) containing six animals per group. Normal control (NC), Sham-operated (SHAM), Ovariectomized (OVX), PPD rats fed with distilled water (PPD), PPD rats fed with fish oil (PPD + FO) 9 g/kg and PPD rats fed with antidepressant fluoxetine (PPD + FLX) 15 mg/kg. The doses of FO and fluoxetine selected for this study were based on previous research, where PPD rats were supplemented with FO for 15 days.8 The ovariectomized rats were intraperitoneally injected with hormones, estradiol benzoate and progesterone for 23 days; mimicking the gestation period of rodents, thus inducing postpartum state (Fig. 1)19 The sudden decrease of oestrogen and progesterone hormones leads to mood changes known as postpartum blues that will eventually end up as a PPD.20 All treatments commenced on PPD day 1 and ended onPPD day 10. Forced swimming test and open field test (OFT) were both carried-out on PPD days 2 and 10 respectively to screen for depressive-like behaviours in the rats during the 10 days fish oil supplementation.19,21 All the rats were euthanized on PPD day 10 through decapitation and their prefrontal cortex (PFC) and hippocampus were harvested for evaluation of NFкB, NLRP3, caspase-1 and IL-1β concentrations.
Fig. 1.
Timeline and treatment of experimental rats groups, OVX = ovariectomy, FO = fish oil, FLX = fluoxetine, PP = Postpartum.
2.3. Postpartum depression model induction
The induction of an animal model of PPD was done using HSPW protocol.8,18,19 It began with ovariectomy followed by 23 days of ovarian hormones injection and subsequent withdrawal of the hormonal injections to mimic the postpartum condition in the rats. At the beginning of the experiment, rats were ovariectomized bilaterally using an aseptic technique. The ovariectomy was conducted under anaesthesia (ketamine 80 mg/kg and xylazine 10 mg/kg; im). The ovariectomized rats were observed daily for seven days post-surgery to full recovery. Iodine was applied unto the wounds, while their cages were cleaned daily to prevent infections and contamination. Each rat was maintained in a separate cage during the observation period. Sham-operated rats were just sutured back as their ovaries were left intact.8,18 One week after the ovariectomy, hormone regimens were started. The rats were injected with ovarian hormones estradiol benzoate and progesterone daily for 23 days (Table 1). From day 1–16, 2.5 μg/rat of Estradiol benzoate and 4 mg/rat of Progesterone were administered. However, from day 17–23 only 50μg/rat Estradiol benzoate was injected to mimic the normal gestation period of rats. The doses used were enough to induce postpartum behaviour as earlier reported.8,18 Maternal nesting test behaviour was conducted on day 22 of the hormone injection to ascertain a pregnancy-like state of the PPD rats’ model. After the last injection of hormones on day 22, paper towels were provided to each cage as nesting material for the rats. The rats were expected to make a nest with the paper towels as preparation for having their offspring as if they were pregnant. 24 h later, the cages were examined for the presence of nest-like shape which indicates a positive result for maternal nesting behaviour test. Rats with positive results for maternal nesting test behaviour were considered a success in the induction of hormone-simulated pregnancy-like state and were withdrawn from the hormones injection to finalise PDD induction. The postpartum depressed rats were allocated into PPD experimental groups administered with distilled water (PPD), fish oil (PPD + FO) and fluoxetine (PPD + FLX) respectively.
Table 1.
Hormone regime for induction of hormone-simulated pregnancy model (postpartum depression model induction).8
| Hormones | Days 1–16 | Days 17–23 |
|---|---|---|
| Estradiol benzoate | 2.5 μg/rat | 50 μg/rat |
| Progesterone | 4 mg/rat | – |
2.4. Supplementation protocol
The FO supplementation protocol was conducted according to previous reports from the same laboratory with slight modifications.8 Briefly, the FO was administered to the rats through oral gavage, the feeding regimen lasted for 10 days postpartum (Fig. 1). The dose of the FO (Menhaden fish oil, Sigma F8020) administered was calculated daily using the formula (body weight (g) x 0.009)/density (g/ml) for dose 9 g/kg. While its composition includes, 30% omega-3 fatty acids with 10–15% Eicosapentaenoic acid and 8–15% Docosahexaenoic acid which has been proven to be safe in rats.8,22,23
2.5. Neutrophil-lymphocyte ratio (NLR) analysis
Tail vein blood was taken from the rats for evaluation of neutrophil/lymphocyte ratio (NLR) on day 23 of hormone-simulated pregnancy. A thin blood smear was prepared and stained with Leishman’s stain. Observations were made using a light microscope at 40x magnification to calculate the number of neutrophils and lymphocytes. The NLR is a good indicator of distress condition in rats.24 Reports revealed that there would be an increased level of neutrophils and decreased level of lymphocytes in depressed subjects.25
2.6. Behavioral tests
2.6.1. Forced swimming test
To evaluate the antidepressant-like effects of 10 days FO supplementation on PPD rats’ model, FST was conducted. The protocol for FST was previously described,8 the rats were forced to swim and both their passive and active behaviours were recorded using a video camera for later evaluation. In passive behaviour, their time of immobility was recorded, while in active behaviour both swimming and climbing times were recorded and subsequently calculated.
2.6.2. Open field test
The OFT was conducted to evaluate the locomotor activities of the rats following treatments given. The behaviour of rats crossing the squares on the floor of a square box measuring 75 cm length x 75 cm breadth and 42 cm high were observed. The floor of the box was further subdivided by black lines into 25 smaller squares of equal dimensions, 15 cm square each. Each rat was allowed an hour for habituation in the behavioural room, prior to the test. The test session for each rat lasted for 5 min and was recorded using a video for later use8,18
2.7. Brain tissues collection and ELISA
On PPD day 10, rats were euthanized using a guillotine, their brains were collected as described previously.8,18 Briefly, the brain was dissected out carefully and washed with cold 1x phosphate-buffered saline (PBS) (BR0014G, Oxoid Ltd, UK), 1 tablet dissolved in 100 mL deionized water. The rats prefrontal cortex and hippocampus were carefully harvested, weighted and homogenized using Polytron PT-MR 1600 E (Kinematica AG, Switzerland)with cold PBS (100 mg wet tissue in 1 mL of 1 x PBS) for 3 min. The homogenates were freeze-thawed for 2 cycles to further break down the cells and were centrifuged for 5 min at 5000×g, 4 °C. The supernatants were pipetted and used for the analysis of NFкB, NLRP3, caspase-1 and IL-1β levels, all the biomarkers were measured using their respective ELISA kits according to the user manuals; pro-inflammatory cytokines interleukin-1 Beta (IL-1β) (Rat Il-1β/IL-IF2, E-EL-0012, Elabscience, USA), inflammatory transcription factor, Nuclear Factor Kappa B (NF-kB) (Rat NF-кB, E-EL-0673, Elabscience, USA), inflammasome complex (caspase-1 (Rat CASP1, E-EL-0371, Elabscience, USA) and NACHT, LRR and PYD Domains- Containing Protein 3 (Rat NLRP3, E-EL-1463, Elabscience, USA) which is associated with the production of IL-1β.
2.8. Statistical analysis
Data were analysed by one-way analysis of variance (ANOVA) followed by Tukey’s posthoc multiple comparisons using statistical software SPSS IBM version 23. All the results were expressed as mean ± SEM. Significance difference was accepted when the p-value is equal or less than 0.05 (p < 0.05).
3. Results
3.1. Effects of 10 days FO supplementation on the bodyweight of the PPD rat model
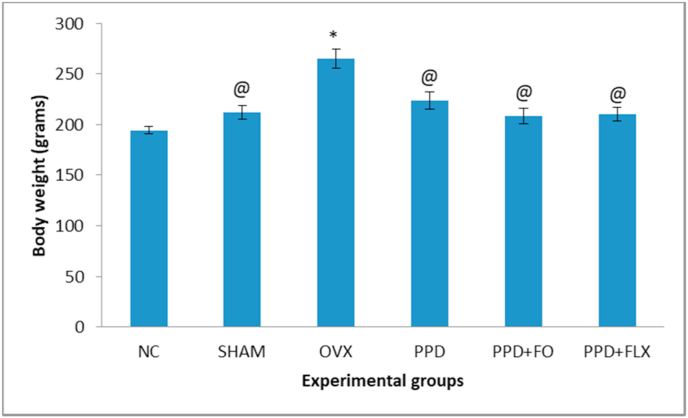
The result showed that there was a statistically significant decrease in body weight of all experimental groups when compared to the OVX group (p < 0.05). However, administration of FO or FLX to the PPD-induced rats did not affect their weights, as no significant differences were observed, when compared to the NC group (Fig. 2).
Fig. 2.
The effects of FO supplementation on the bodyweights of the PPD-induced rat model. Data are presented as mean ± SEM (n = 6). @p < 0.05 vs OVX group, ∗p < 0.05 vs NC group.
3.2. Effects of 10 days FO supplementation on NLR of PPD rat model
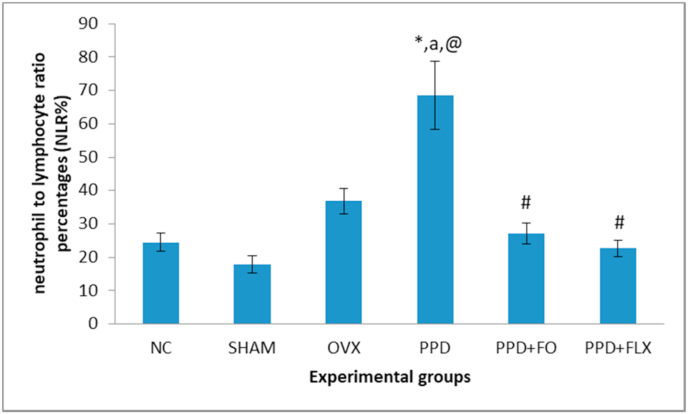
The results showed a significant (p < 0.05) increase in NLR of ovariectomized rats relative to the control group. Whereas, supplementation with FO (9 mg/kg) for 10 days or fluoxetine have significantly reduced the NLR when compared to PPD group (Fig. 3).
Fig. 3.
Antidepressant-like effect of FO on NLR of PPD rat model. Data represented as mean ± SEM (n = 6). ∗p < 0.05 vsNC, ap< 0.05vs SHAM, @P < 0.05 vs OVX, #p < 0.05 vs PPD.
3.3. Effects of 10 days FO supplementation on FSTbehavior of PPD rat model
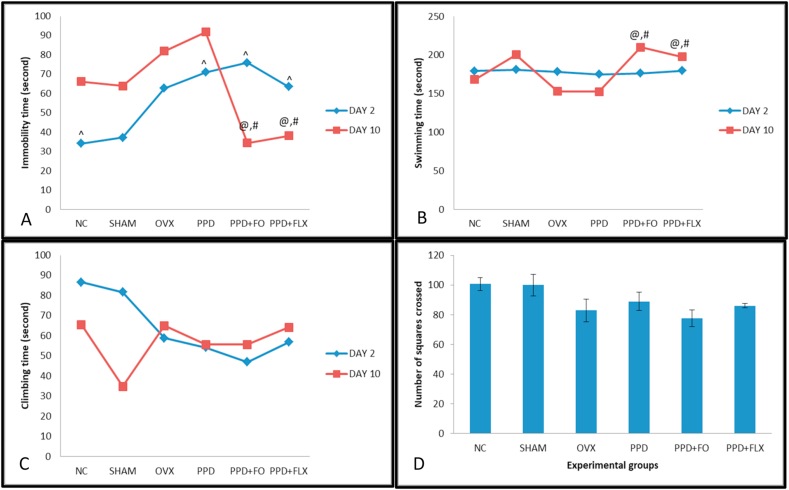
The supplementation of PPD rat model with FO has significantly reduced their FST immobility time (p ˂ 0.05) on day 10 postpartum when compared to the control. However, no changes were observed in the immobility time on day 2 postpartum among all the rat groups. Furthermore, reduction in immobility time was also observed in fluoxetine treated group of rats on day 10 postpartum. In addition, 10 days FO supplementation increased swimming time significantly in PPD rats (p < 0.05) but not in climbing time (Fig. 4A, B, C).
Fig. 4.
The antidepressant-like effects of 10 days FO supplementation on FST and OFT behaviours of PPD rat model. (A) Immobility time, (B) Swimming time, (C) Climbing time, (D) Number of squares crossed. Data are presented as mean ± SEM (n = 6). (^) significant difference at p < 0.05 of each experimental group when comparing between day 2 PPD and day 10 PPD. @p < 0.05 vs OVX, #p < 0.05 vs PPD.
3.4. Effects of 10 days FO supplementation on locomotor activity of PPD rat model
The OFT results revealed that 10 days FO supplementation on PPD rats does not affect the number of squares crossed by the rat groups (p ˃ 0.05) (Fig. 4), as no statistically significant differences were observed among them.
3.5. Effects of 10 days FO supplementation on the levels of IL-1β, NLRP3 inflammasome, NFκB and caspase-1in the hippocampus and PFC of PPD-induced animals
The results (Table 2) showed the changes in levels of hippocampal and PFC Il-1β, caspase-1, NLRP3 and NfкB. The hippocampal levels of IL-1β, NLRP3 inflammasome, NFκB and caspase-1 in PPD rats that were supplemented with FO (9 g/kg) for 10 days decreased significantly (p < 0.05), relative to PPD rats group (negative control). Consequently, the negative control group had higher levels of IL-1β, NLRP3 inflammasome, NFκB and caspase-1 when compared to the normal control group (p < 0.05). Moreover, the levels of IL-1β, NLRP3 inflammasome, NFκB and caspase-1 observed in the group supplemented for 10 days with 9 g/kg of FO were comparable to the levels seen in fluoxetine treated group. However, 10 day of FO 9 g/kg has little effect on the rats PFC, as no significant changes were observed in the levels of IL-1β, NLRP3 inflammasome, NFκB and caspase-1 in their PFC.
Table 2.
Effects of 10 days FO supplementation on the levels of IL-1β, NLRP3 inflammasome, NFκB and caspase-1 of the hippocampus and PFC of PPD rat model. Data represent as mean ± SEM (n = 3), #p < 0.05.
| Experimental groups on day 10 PPD | Biochemical changes in the hippocampus |
|||
|---|---|---|---|---|
| IL-1β | Caspase-1 | NLRP3 | NFκB | |
| NC | 126.157 ± 12.34# | 157.067 ± 14.90# | 3.967 ± 0.32# | 6.767 ± 0.25# |
| SHAM | 158.667 ± 13.56# | 173.267 ± 17.04# | 4.233 ± 0.21 | 7.900 ± 0.47# |
| OVX | 280.050 ± 19.57 | 314.300 ± 33.02 | 5.467 ± 0.31 | 11.483 ± 0.36 |
| PPD | 335.850 ± 14.43 | 382.33 ± 25.65 | 6.067 ± 0.36 | 13.467 ± 0.29 |
| PPD + FO | 262.000 ± 15.60# | 228.300 ± 11.50# | 4.017 ± 0.27# | 11.033 ± 0.84# |
| PPD + FLX | 245.583 ± 15.23# |
193.417 ± 13.64# |
4.150 ± 0.32# |
10.267 ± 0.43# |
|
Biochemicals changes in the PFC |
||||
| IL-1β | Caspase-1 | NLRP3 | NFκB | |
| NC | 199.017 ± 5.53 | 154.917 ± 4.22# | 3.633 ± 0.25 | 9.600 ± 0.77# |
| SHAM | 216.933 ± 42.00 | 169.433 ± 16.61# | 4.317 ± 0.49 | 9.033 ± 0.96# |
| OVX | 270.417 ± 35.76 | 236.667 ± 20.23 | 4.967 ± 0.21 | 12.950 ± 0.26 |
| PPD | 280.583 ± 19.20 | 266.400 ± 23.37 | 5.683 ± 0.22 | 13.167 ± 0.51 |
| PPD + FO | 242.083 ± 13.39 | 202.33 ± 7.94 | 4.567 ± 0.24 | 11.50 ± 0.76 |
| PPD + FLX | 215.033 ± 7.66 | 198.867 ± 14.21 | 4.117 ± 0.26# | 10.333 ± 1.15 |
4. Discussion
The present study has shown the antidepressive-like effects of 10 days FO supplementation of PPD rats model involves NLRP3 inflammasome cascade. The PPD state of the rats used in the study was induced using the HSPW protocol, the rats exhibited high levels of pro-inflammatory cytokines in their hippocampus and high NLR paralleled with nesting behaviour and high immobility time in FST. The study investigated the pro-inflammatory cytokine IL-1β production cascade (NFкB/NLRP3/caspase-1/IL-1β) in the brain tissues; prefrontal cortex and hippocampus of PPD rats after supplementation with FO. The antidepressant-like effects of 10 days FO 9 g/kg supplementation in PPD induced rat model was exerted by decreasing the levels of pro-inflammatory cytokine in the hippocampus, which resulted in decreased immobility time during FST. FO supplementation of PPD rats has managed to influence the inflammasome cascade NLRP3 which is responsible for IL-1β pro-inflammatory cytokine production as early as 10 days postpartum. The effects of FO in this study is comparable to that of fluoxetine, which is an approved anti-depressant available in the market.
The present study revealed no statistically significant differences in the body weight changes among the PPD groups. However, in ovariectomized control rats, the body weight was significantly higher when compared to the normal control group. It was observed previously that ovariectomy influences body weight changes because low estrogen levels in ovariectomized animals lead to increased body abdominal fats.26 Furthermore, ovariectomy reportedly increased body weight by regulating the energy expenditure mechanism.27 Additionally, the effect of ovariectomy in increasing body weight has been postulated to be similar to the intake of high fats diet.28 This current study induced PPD in rats using hormone-simulated pregnancy regime in ovariectomized rats. The withdrawal of these hormones after 23 days in ovariectomized rats has induced PPD condition. In postpartum human, 14–25% of women showed increased body weight within 1 year of its onset. Similarly, rats in the present study showed increased body weight due to PPD induction.The administration of the FO or fluoxetine in this study has no statistically significant effect on the reduction of the body weights of PPD induced rats.
FST is an established test for checking depressive-like behaviours among rats.29,30 The present study evaluated the antidepressive-like effects of FO in PPD rats and its possible mechanism of action involving inflammation. This study revealed that FO supplementation for 10 days in PPD induced rats have reduced their immobility time and further increased their swimming but not climbing times significantly during FST. An increased in active behaviours of rats were indicators of antidepressive-like effects of the compound being tested.31 Any tested compound with potential antidepressive-like effects may decrease the immobility time observed in FST and increased the swimming and climbing times.32, 33, 34 Thus, FO showed its antidepressive-like effects in this study after 10 days supplementation to PPD rats. This study agrees with a previous study that showed the antidepressive-like effects of FO in PPD rats after 15 days supplementation postpartum.8Similar findings were also observed in PPD rats supplemented with fish flesh extract for 15 days.18 There was a linear dose-response relationship between FO intake and risk of depression.35 Studies have shown that certain class of drugs such as convulsants and stimulants which has potentials of increasing locomotor activity can as well decrease the immobility time in FST, thereby producing false-positive result.21 Hence, OFT was carried out in the present study to rule-out any probable confounding effect of FO on locomotor activity of the rats which in turn may cause bias on FST results. However, no statistically significant differences were observed among the rat groups after OFT, as the numbers of boxes they crossed were approximately the same. This is suggestive that the 10 days FO supplementation does not have any effect on the immobility time of FST.
In addition to FST, to evaluate more on the status of stress and depression of the PPD induced rats model, NLR was also evaluated. The NLR in the PPD group of rats was increased significantly when compared to the normal control group. This is a pioneer report linking increased NLR in PPD rats to inflammation. The measurements of NLR is an easy procedure besides being cheap, which can be used for the detection of inflammation in depression. It was earlier documented that, NLR was high in depressed and mood disorder subjects.36 The link between inflammation and depression with regards to NLR was previously reported in a major depressive disorder (MDD) patients.20 The finding was supported by the work of Mazza,36 which reported the roles of NLR in detecting inflammatory changes during depression. The analyses, which considered the first one to be claimed were done based on heterogeneity sensitivity and meta-regression analyses that included the bipolar disorder and MDD patients compared to healthy subjects.36 Administration of FO for 10 days reduced the NLR in PPD rats. This indicated the possibility of FO’santidepressive-like effects on PPD rats by modulating the NLR thus linking it with inflammation. Fish oil was earlier reported as a good anti-inflammatory agent which might be of benefit to many inflammatory diseases.37 Therefore, its benefits towards depression were suggested to be influenced by its ability to abolish NLRP3inflammasome activation and secretion of pro-inflammatory cytokines IL-1β.38 It was reported previously that high expression of NLRP3 influenced the production of IL-1β.36 High expression of NLRP3 activates procaspase-1 to caspase-1 which in-turn activates the precursor of IL-1β into matured IL-1β. Thus, the high levels of NLRP3 and caspase-1 helped in increasing IL-1β production which subsequently increased the risk of early-onset of depression. Notwithstanding, the association of inflammation with the pathophysiology of PPD is not fully understood39,40 and the findings on PPD pathophysiology were not consistent with each other.40, 41, 42 Thus, further studies need to be carried out to get a clearer picture of pathophysiological changes associated with PPD.
A preliminary study had indicated the increased risk of PPD in postpartum women who have a higher level of IL-1β in early postpartum.43 While in PPD rats, significant reduction of IL-1β concentration was recorded after 15 days of FO supplementation.8 However, there is a paucity of information linking the production of IL-1β in PPD induced rats’ model to the NLRP3 inflammasome complex. Although a previous study has highlighted the association of FO antidepressive-like effects mechanism with NLRP3 neuromodulation,37 this study has investigated the involvement of NLRP3 inflammasome in IL-1β production. Pro-inflammatory cytokine IL-1β increased significantly in hippocampus but not in PFC of PPD rats. The significant increase of IL-1β seen in the hippocampus of PPD rats relates directly with the increases in inflammasome NLRP3 complex and caspase-1 concentration as well. Besides, PPD model applied the estrogen deficiency mechanism to induce the state of PPD in rats. In line with a previous study, low estrogen help to increase NLRP3 and IL-1β pro-inflammatory cytokines which resulted in depression as a result of an increase in the level of neuroinflammation in the hippocampus.44 Furthermore, this study has recorded a high concentration of inflammatory transcription factors, NFκB in the hippocampus of PPD induced rats which is statistically significant. The findings from this study conformed with a previous report which showed that NF-κB has been linked with the NLRP3 with regards to depression onset.45 This was earlier asserted by previous studies which showed high levels of NFкB and IL-1β in the serum of PPD induced rat models.8,18
In this study, menhaden FO used consisted of 30% omega-3 fatty acid of which the ratio is low in the formula, and many previous studies and meta-analyses documented that using low concentration of omega-3 regimen (EPA + DHA lower than 70%) mostly resulted in to negative outcome. However, the present study used 9 g/kg of the FO consecutively for 10 days, this dose is believed to be high enough and effective as reported by Arbabi et al., 2014. Additionally, some studies have shown that lower dose of FO 1.5 g/kg, was high enough to show antidepressant effect without causing stomach regurgitation or diarrhoea.46, 47, 48 Further, supplementation with a dosage of 3 g/kg of FO containing 12% of EPA and 18% of DHA for approximately 2 months in rats has demonstrated its antidepressant-like effects.49 Further epidemiological studies reported that supplementation with high ω-3 PUFAs is linked with reduced occurrence and severity of depressive condition in patients.50 Finally, it is noteworthy that the present study used oral gavage for direct administration of the FO in to the stomach of the rats, as mode of administration might influenced the outcome of the results. Gastric gavage (deliver directly into the stomach) has more accurate and precise dosing for animals in administering substances into the gastro-intestinal tract as it eliminates risks of variability in intake between individual animals that may arise when substances are administered through delivery in food and/or water.51 Therefore, is it suggestive that the antidepressant effect seen in this study might be attributed to the high dose of FO 9 g/kg used as well as the effectiveness of its delivery through oral gavage. Taken together, the current results suggest that FO may exerts it antidepressant-like effects in various dosages.
This study found that 10 days menhaden FO supplementation to PPD induced rats have reduced the levels of NFκB, NLRP3, caspase-1 and IL-1β in their hippocampus. The reduction was significant in hippocampal tissues when compared to PFC. It was reported previously that FO administration was able to decrease the production of IL-1β by interfering on the inflammasome activation processes.37 These biochemical findings were in parallel with its behaviour test findings and NLR ratio which indicated the potentials of FO as anti-depressant in PPD rats. Taken together, the current data showed that the antidepressive-like effects of menhaden FO in PPD rats were influenced by the inactivation of NLRP3 inflammasome complex cascade and IL-1β concentration modulation. However, there are some limitations in this study as it failed to measure the concentration of the various fatty acids in the brain or serum of the PPD rats. Chen and Su measured the concentration of fatty acid in the brains (hypothalamus, hippocampus, frontal cortex, cerebellum and olfactory bulb) of rats pups in which their parents were fed with FO during their pregnancy and lactation. The study recommended the use of FO to reduce anxiety in postpartum rats.52 Even though the present study did not measure the fatty acid in either brain or serum of the rats, but it agrees with Chen and Su and concluded that this could be the way FO exerts it’s antidepressant-like effect on the PPD rats. Thus, in this context, additional research need to be done to investigate which nutritional ingredients play a major role in the antidepressant-like effects of FO in PPD rat model.
5. Conclusion
In conclusion, the results obtained from the present study revealed that 10 days of menhaden FO 9 g/kg (rich in omega-3 fatty acid, Eicosapentaenoic acid and Docosahexaenoic acid) supplementation to PPD induced rat model can produce antidepressive-like effects on the rats. The rats exhibited a significant reduction of immobility time in FST which is independent of the effect of their locomotor activity. The FO supplementation attenuated the depressive-like behaviour through the reduction of the levels of NFκB, NLRP3, caspase-1 and IL-1β of their hippocampus, which levels were observed to be high in PPD induced rat model. These findings suggest that the antidepressant-like effects of FO influence the NLRP3 inflammasome protein complex that controls the production of IL-1β proinflammatory cytokines in PPD induced rats. However, this study is limited by not measuring the concentrations of the individual FO fatty acids from the hippocampus or serum of the rats.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgement
The authors would like to express gratitude to the Ministry of Higher Education Malaysia for the financial support provided under the Fundamental Research Grant Scheme (FRGS) Grant. Cost center FRGS/1/2015/SKK08/UPM/02/10.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.02.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hamazaki K., Takamori A., Tsuchida A. Dietary intake of fish and n-3 polyunsaturated fatty acids and risks of perinatal depression: the Japan Environment and Children’s Study (JECS) J Psychiatr Res. 2018;98:9–16. doi: 10.1016/j.jpsychires.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Silverman M.E., Reichenberg A., Savitz D.A. The risk factors for postpartum depression: a population-based study. Depress Anxiety. 2017;34:178–187. doi: 10.1002/da.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buglione-Corbett R., Deligiannidis K.M., Leung K. Archives of women’s mental health; 2018. Expression of Inflammatory Markers in Women with Perinatal Depressive Symptoms; pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Wosu A.C., Gelaye B., Williams M.A. History of childhood sexual abuse and risk of prenatal and postpartum depression or depressive symptoms: an epidemiologic review. Arch Wom Ment Health. 2015;18:659–671. doi: 10.1007/s00737-015-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skalkidou A., Hellgren C., Comasco E., Sylvén S., Poromaa I.S. Biological aspects of postpartum depression. Women’s health. 2012;8:659–672. doi: 10.2217/whe.12.55. [DOI] [PubMed] [Google Scholar]
- 6.Kim S., Soeken T.A., Cromer S.J., Martinez S.R., Hardy L.R., Strathearn L. Oxytocin and postpartum depression: delivering on what’s known and what’s not. Brain Res. 2014;1580:219–232. doi: 10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiller C.E., Meltzer-Brody S., Rubinow D.R. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20:48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbabi L., Baharuldin M.T.H., Moklas M.A.M., Fakurazi S., Muhammad S.I. Antidepressant-like effects of omega-3 fatty acids in postpartum model of depression in rats. Behavioral brain research. 2014;271:65–71. doi: 10.1016/j.bbr.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Yim I.S., Stapleton L.R.T., Guardino C.M., Hahn-Holbrook J., Schetter C.D. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11 doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maes M., Song C., Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets. 2012;16:1097–1112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- 11.Su W.J., Zhang Y., Chen Y. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res. 2017;322:1–8. doi: 10.1016/j.bbr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Liu L., Liu Y.Z. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int J Neuropsychopharmacol. 2015;18:6. doi: 10.1093/ijnp/pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du R.H., Tan J., Sun X.Y., Lu M., Ding J.H., Hu G. Fluoxetine inhibits NLRP3 inflammasome activation: implication in depression. Int J Neuropsychopharmacol. 2016;19:37. doi: 10.1093/ijnp/pyw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue N., Huang H., Zhu X. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14:102. doi: 10.1186/s12974-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia K.K., Ding H., Yu H.W., Dong T.J., Pan Y., Kong L.D. Mediators of inflammation; 2018. Huanglian-Wendan Decoction Inhibits NF-Κb/nlrp3 Inflammasome Activation in Liver and Brain of Rats Exposed to Chronic Unpredictable Mild Stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EdalatiFard F., Mirghafourvand M., Mohammad-Alizadeh-Charandabi S., Farshbaf-Khalili A., AsghariJafaraabadi M. The relationship between diet and postpartum depression in postpartum women in Tabriz. The Iranian Journal of Obstetrics, Gynecology and Infertility. 2016;18:1–10. [Google Scholar]
- 17.Kobayashi M., Ogawa K., Morisaki N., Tani Y., Horikawa R., Fujiwara T. Dietary n-3 polyunsaturated fatty acids in late pregnancy and postpartum depressive symptom among Japanese women. Front Psychiatr. 2017;8:241. doi: 10.3389/fpsyt.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukkoor A., Saleem M., Baharuldin M.T.H.B. Antidepressant-like effect of lipid extract of channastriatus in postpartum model of depression in rats. Evid base Compl Alternative Med. 2017;2017 doi: 10.1155/2017/1469209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoffel E.C., Craft R.M. Ovarian hormone withdrawal-induced ‘depression’ in female rats. Physiol Behav. 2004;83(3):505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Schiller C.E., O’Hara M.W., Rubinow D.R., Johnson A.K. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav. 2013;119:137–144. doi: 10.1016/j.physbeh.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galea L.A., Wide J.K., Barr A.M. Estradiol elleviates depression-like symptoms in a novel animal model of post-partum depression. Behavior Brain Research Journal. 2001;122(1):1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 22.Hélène Plamondon, Marie-Claude Roberge. Dietary PUFA supplements reducememory deficits but not CA1 ischemic injury in rats. Physiol Behav. 2008;95(3):492–500. doi: 10.1016/j.physbeh.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Su Kuan-Pin. Omega-3 fatty acids in major depressive disorder: a preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(4):267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 24.Swan M.P., Hickman D.L. Evaluation of the neutrophil-lymphocyte ratio as a measure of distress in rats. Lab Anim. 2014;43:276. doi: 10.1038/laban.529. [DOI] [PubMed] [Google Scholar]
- 25.Demir S., Atli A., Bulut M. Neutrophil–lymphocyte ratio in patients with major depressive disorder undergoing no pharmacological therapy. Neuropsychiatric Dis Treat. 2015;11:2253. doi: 10.2147/NDT.S89470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley E.M., Pourafshar S., Navaei N., Akhavan N.S., George K.S., Arjmandi B.H. The effects of ipriflavone, isoflavone, and 17b-estradiol on body composition in hamster models of ovariectomy. Faseb J. 2017;31:645. [Google Scholar]
- 27.Ding L.C., Gong Q.Q., Li S.W., Fu X.L., Jin Y.C., Zhang J., Sun X.Y. Rcan2 and estradiol independently regulate body weight in female mice. Oncotarget. 2017;8 doi: 10.18632/oncotarget.18259. 48098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasa T., Matsuzaki T., Yano K., Irahara M. The effects of ovariectomy and lifelong high-fat diet consumption on body weight, appetite, and lifespan in female rats. Horm Behav. 2018;97:25–30. doi: 10.1016/j.yhbeh.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Porsolt R.D., Le Pichon M., Jalfre M.L. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 30.Detke M.J., Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behavioral Brain Research. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 31.Detke M.J., Rickels M., Lucki L. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121(1):66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 32.Vines A., Delattre A.M., Lima M.M. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology. 2012;62:184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Reyes-Mendez M.E., Castro-Sánchez L.A., Dagnino-Acosta A. Capsaicin produces antidepressant-like effects in the forced swimming test and enhances the response of a sub-effective dose of amitriptyline in rats. Physiol Behav. 2018;195:158–166. doi: 10.1016/j.physbeh.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Galea L.A., Wide J.K., Barr A.M. Estradiol elleviates depressive-like symptoms in a novel animal model of post-partum depression. Behavior Brain Research Journal. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 35.Grosso G. Dietary n-3 PUFA, fish consumption and depression: a systematicreview and meta-analysis of observational studies. J Affect Disord. 2016;205:269–281. doi: 10.1016/j.jad.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Mazza M.G., Lucchi S., Tringali A.G.M., Rossetti A., Botti E.R., Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog Neuro Psychopharmacol Biol Psychiatr. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Dang R., Zhou X., Tang M., Xu P., Gong X., Liu Y., Jiang P. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr. 2018;57:893–906. doi: 10.1007/s00394-016-1373-z. [DOI] [PubMed] [Google Scholar]
- 38.Tang M., Dang R., Liu S. Ω-3 fatty acids supplementing in gestation alleviates neuroinflammation and modulates neurochemistry in rats. Lipids Health Dis. 2018;17(1):1–11. doi: 10.1186/s12944-018-0894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackmore E.R., Moynihan J.A., Rubinow D.R., Pressman E.K., Gilchrist M., O’Connor T.G. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom Med. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackmore E.R., Groth S.W., Chen D.-G., Gilchrist M.A., O’Connor T.G., Moynihan J.A. Depressive symptoms and proinflammatory cytokines across the perinatal period in African American women. J Psychosom Obstet Gynecol. 2014;35:8–15. doi: 10.3109/0167482X.2013.868879. [DOI] [PubMed] [Google Scholar]
- 41.Roomruangwong C., Anderson G., Berk M., Stoyanov D., Carvalho A.F., Maes M. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog Neuro Psychopharmacol Biol Psychiatr. 2018;81:262–274. doi: 10.1016/j.pnpbp.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Shorey S., Ing C.C.Y., Ng E.D., Huak C.Y., San W.T.W., Seng C.Y. Prevalence and incidence of postpartum depression among healthy mothers: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:235–248. doi: 10.1016/j.jpsychires.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Corwin E.J., Johnston N., Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs. 2008;10:128–133. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y., Sheng H., Bao Q., Wang Y., Lu J., Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression-and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Su K.P. Omega-3 polyunsaturated fatty acids in prevention of mood andanxiety disorders. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2015;13:129–137. doi: 10.9758/cpn.2015.13.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamaziere A., Richard D., Barbe U. Differential distribution of DHA-phospholipids in rat brain after feeding: a lipidomic approach. Prostagl Leukot Essent Fat Acids. 2011;84(1-2):7–11. doi: 10.1016/j.plefa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y.Q., Dang R.L., Tang M.M. Long chain omega-3 polyunsaturated fatty acid supplementation alleviates doxorubicin-induced depressive-like behaviors and neurotoxicity in rats: involvement of oxidative stress and neuroinflammation. Nutrients. 2016;8(4):243. doi: 10.3390/nu8040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dang R., Zhou X., Tang M., Xu P., Gong X., Liu Y., Jiang P. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr. 2018;57(3):893–906. doi: 10.1007/s00394-016-1373-z. [DOI] [PubMed] [Google Scholar]
- 49.Carabelli B., Delattre A.M., Pudell C. The antidepressant-like effect of fish oil: possible role of ventral hippocampal 5-HT 1A post-synaptic receptor. Mol Neurobiol. 2015;52(1):206–215. doi: 10.1007/s12035-014-8849-8. [DOI] [PubMed] [Google Scholar]
- 50.Grosso G., Micek A., Marventano S. Dietary n-3 PUFA, fish consumptionand depression: a systematic review and meta-analysis of observational studies. J Affect Disord. 2016;205:269–281. doi: 10.1016/j.jad.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: routes of administration and factors to consider. JAALAS. 2011;50(5):600–613. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H.F., Su H.M. Fish oil supplementation of maternal rats on an n-3 fatty acid-deficient diet prevents depletion of maternal brain regional docosahexaenoic acid levels and has a postpartum anxiolytic effect. J Nutr Biochem. 2012;23(3):299–305. doi: 10.1016/j.jnutbio.2010.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.