Abstract
Background and aim
Multiple sclerosis (MS) is characterized by increasing symptom burden leading many people with MS to use complementary treatments. TRE (Tension and Trauma Releasing Exercises) is a mind-body therapeutic method aiming to release muscle tension and stress. People with MS (PwMS) have reported benefits from TRE, but no scientific studies have investigated the effects of TRE on PwMS. Aim: To test a TRE program for PwMS and thereby explore outcome measures to be applied in future randomized studies.
Experimental procedure
A nine-week TRE program was completed by nine participants: Five were women, age ranged from 44 to 66 years, and time since diagnosis ranged from 2 to 21 years. Outcome measures included self-reported day-to-day levels of nine different symptoms as well as sleep quality and stress level. Modified Fatigue Impact Scale (MFIS) fatigue score and spasticity level of the ankle plantar flexors, assessed using a Portable Spasticity Assessment Device (PSAD), were measured pre and post intervention.
Results
Decreases were seen in the mean scores of all nine self-reported day-to-day symptoms as well as stress level, while sleep quality mean score increased. LME analyses showed that all changes were statistically significant except one (bowel dysfunction). Mean MFIS-measured fatigue level decreased significantly from a score of 43.7 (SD = 13.6) to a score of 22.0 (SD = 12.3). No significant change was reported in PSAD-measured spasticity level.
Conclusion
The study indicates possible effects of TRE on PwMS on several self-reported outcome measures. Larger, randomized studies should be carried out to explore the findings further.
Keywords: Mind-body therapies, Outcome measures, Symptom management, Self-help therapy, Tension release
Graphical abstract
Highlights of the findings and novelties
-
•
This is the first original research study exploring trauma and tension releasing exercises (TRE) for persons with MS.
-
•
Improvements were seen from baseline to end-of study in eight out of nine self-reported symptoms as well as stress and sleep quality.
-
•
Spasticity measured objectively around the ankle flexors did not change significantly from baseline to end-of-study.
-
•
This pilot study can inform future research on the effects of TRE on persons with MS.
List of abbreviations
- LME
Linear Mixed-Effects
- MFIS
Modified Fatigue Impact Scale
- MS
Multiple Sclerosis
- PRO
Patient Reported Outcome
- PSAD
Portable Spasticity Assessment Device
- PwMS
People with Multiple Sclerosis
- TRE
Tension and Trauma Releasing Exercises
1. Introduction
Multiple sclerosis (MS) is a chronic, autoimmune, demyelinating disease.1 MS causes progressive neurodegeneration, often resulting in an increasing burden of symptoms and impairments over time, including fatigue, spasticity, walking difficulty, dizziness, and sensory disturbances.2 While disease modifying and symptomatic treatments exist, they are not always effective, and there is no curative treatment.3,4 Furthermore, the medical treatments are often associated with adverse effects.3 To supplement conventional treatment and rehabilitation, many people with MS (PwMS) turn to complementary treatments in the hopes of relieving symptoms and strengthening the body’s ability to handle the disease.5
Tension and Trauma Releasing Exercises (TRE) is a mind-body therapy that is designed to release deep muscle tension and reduce stress.6 The National Center for Biotechnology Information (NCBI) describes mind-body therapy as “Treatment methods or techniques which are based on the knowledge of mind and body interactions. These techniques can be used to reduce the feeling of tension and effect of stress, and to enhance the physiological and psychological well-being of an individual”.7
TRE is a self-help method which can be practiced at home once the method has been learned. TRE consists of seven stretching exercises designed to activate and exhaust the thigh flexor muscles, which can ultimately induce spontaneous neuromuscular tremors or shaking of the body. Six of the seven exercises are performed in a standing position while the final exercise is carried out lying flat on the back with the feet together, knees bent out and downward towards the floor. The tremors are induced while lying in this position.8 The exact mechanisms of action of TRE have not been scientifically demonstrated, but it is believed that the neuromuscular tremors release deep-rooted muscular tension and that it regulates the nervous system so that a state of relaxation and calm is obtained.6
Many PwMS experience tension of the muscles in the form of spasticity.9,10 According to Hugos and Cameron,11 spasticity can manifest in different ways, for example as spasms, resistance to passive stretch, pain, and tightness, and it can affect muscles throughout the body. At the same time, spasticity is linked to symptoms such as pain, sleep disturbance, fatigue, weakness, poor motor control, and bladder problems.11
We hypothesize that if TRE has the effect of releasing deep muscle tension and calming the nervous system, then it could relieve spasticity and affect a number of MS-related symptoms and conditions as mentioned above. For this reason, we chose to include various symptoms and conditions as outcome measures in this study.
A systematic review of other types of mind-body therapies for MS found that interventions such as relaxation, mindfulness-based stress reduction, yoga and biofeedback could be helpful for symptoms such as depression, anxiety, fatigue, and bladder dysfunction, as well as for quality of life.12 However, no research has been carried out so far to explore the potential of TRE to relieve symptoms in MS. Indeed, there is a lack of published studies investigating TRE in general. One published pilot study explored effects of TRE on quality of life among non-professional caregivers in South Africa,13 and ongoing studies are examining TRE for stress release in military veterans,14,15 but further research is needed. People with MS have reported benefits from TRE,16 but the evidence so far is purely anecdotal.
Due to the current lack of evidence regarding the effects of TRE, this pilot study took an explorative approach in order to test a TRE program for PwMS and to investigate changes in various outcome measures over the course of a 9-week TRE program in order to identify possible outcome measures to apply in future randomized controlled trials.
2. Materials and methods
This was a single-group explorative intervention pilot study testing three types of outcome measures.
A 9-week TRE program was designed in collaboration between the researchers and a certified TRE instructor. The program consisted of weekly individual TRE training sessions with the instructor and, after the second session, daily practice at home. The home training sessions were expected to last between 30 and 45 min and included performing the seven stretching exercises, as well as remaining in the final tremor-state for as long as it felt comfortable, or for a maximum of 15 min.
2.1. Participants
As this was an exploratory study investigating a new intervention, sample size was not based on power calculations, but rather on convenience sampling. Eleven participants were recruited by the Danish MS Society via e-mail invitations sent to all members with MS who were registered with an e-mail address and who lived within a certain geographical area in and around Copenhagen.
Interested participants were included if they met the inclusion criteria which were; having been diagnosed with MS for more than one year, being 18 years or older, experiencing self-reported spasticity due to MS, having had no change in disease modifying treatment for the past 6 months, having had no change in spasticity medication for the past 3 months, and being able to carry out exercises in a standing position. Exclusion criteria were having previous experience with TRE, having had a relapse within the past 3 months, and having severe cognitive deficits or comorbidities such as severe depression or heart disease. Inclusion and exclusion criteria were assessed by the researchers based on self-report from the participants.
After reading the participant information, and with the opportunity to ask clarifying questions to the researchers, all participants provided written informed consent to partake in the study.
Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics at baseline.
| Participant no. | Sex | Age (years) | Years since diagnosis | MS subtype a | Walking ability b | Baseline MAS score for ankle plantar flexors (left/right) | Use of anti-spasticity medicine (Baclofen or Tizanidine) |
|---|---|---|---|---|---|---|---|
| 1 | Female | 56 | 19 | RRMS | >500 m | 0/0 | When needed |
| 2 | Male | 44 | 18 | SPMS | Walks with a cane | 1+/2 | Daily |
| 3 | Female | 48 | 21 | RRMS | >500 m | 0/0 | No |
| 4 | Female | 66 | 14 | PPMS | 500 m | 0/0 | No |
| 5 | Male | 50 | 8 | RRMS | >500 m | 3/3 | No |
| 6 | Male | 62 | 5 | PPMS | >500 m | 3/3 | Daily |
| 7 | Female | 51 | 10 | RRMS | >500 m | 1+/1 | Daily |
| 8 | Female | 43 | 2 | RRMS | 200–300 m | 2/2 | Daily |
| 9 | Male | 44 | 17 | RRMS | >500 m | 3/3 | No |
RRMS: Relapsing remitting multiple sclerosis. SPMS: Secondary progressive multiple sclerosis. PPMS: Primary progressive multiple sclerosis.
Maximum walking distance without using a walking aid or resting.
2.2. Ethics
The study was reported to the administration of the Danish National Committee on Health Research Ethics who decided that a formal review and approval was not necessary for this study as the intervention was mild and non-invasive. The study adhered to the EU General Data Protection Regulation.
2.3. Outcome measures
As TRE has the potential to affect a range of symptoms and aspects of wellbeing, an exploratory approach was chosen in which a range of outcome measures were tested to see whether a potential effect could be indicated. The outcome measures were mainly self-reported, but also included an objective measure of spasticity. Three different measurement approaches were taken:
Fatigue level was measured using a standard pre- and post-intervention self-rated measure of fatigue level using the Modified Fatigue Impact Scale (MFIS).17 The MFIS questionnaire was filled out by participants in the week prior to the TRE intervention start (week 1) and in the week following the final TRE session (week 11).
Day-to-day self-rated reports of symptom severity levels were registered using a newly developed patient reported outcome (PRO) tool18 that was made approachable via a smartphone app. The tool is currently being validated and includes nine items that score individual MS symptoms on 11-point numeric rating scales scoring from 0 to 10, with 0 being that the symptom is non-existent and 10 being that the symptom is the worst possible. The symptoms are fatigue, walking difficulty, sensory disturbances, dizziness, spasticity, muscle weakness, pain, bladder dysfunction and bowel dysfunction. In addition, the tool includes items scoring overall stress level on an 11-point numeric rating scale and sleep quality during the previous night on a 5-point numeric rating scale. Participants were asked to complete the questionnaire every day, or as often as possible, beginning the week prior to the first TRE session (week 1), and ending with the completion of the TRE program (in week 10).
Finally, to include an objective measure of spasticity, we used the Portable Spasticity Assessment Device (PSAD). The PSAD contains two accelerometers, a gyroscope, two electromyography (EMG) channels and a dynamometer and allow for quantification of reflex mediated torque, a measure of spasticity. The PSAD is described in detail in Yamaguchi et al. 2018.19 In this study, we used the PSAD device to measure the reflex torque of the ankle plantar flexors. Reflex torque was measured at baseline (week 1) and at the end of the study (week 11). Co-author C Svane, who has experience with the PSAD method, carried out the measurements. The Modified Ashworth Scale (MAS) was used to determine participants’ baseline spasticity level of the ankle plantar flexors.
2.4. Statistical analysis
Mean scores and standard deviations were calculated for all outcome measures. For day-to-day symptom-, stress-, and sleep quality levels, as well as total symptom burden levels (all nine symptom scores added together), weekly mean scores for the individual participants as well as for the whole group were calculated and presented as timeline graphs.
Total MFIS score was calculated by summation of all 21 items in the scale. Subscale scores were calculated by summation of the specific sets of items pertaining to the subscale. Differences in mean MFIS scores were assessed using paired sample, two-tailed t-tests.
To examine potential effects of TRE on day-to-day symptom-, stress-, and sleep quality levels, as well as total symptom burden level, linear mixed-effects regression (LME) was performed with each outcome as the dependent continuous variable, and time as the independent variable. A random intercept, random slope model was used to account for individual levels at baseline as well as individual slopes of progression. This model also handles the floor effect which can arise due to the lower limit of the scale. LME is a common approach when analyzing longitudinal data because it focuses on individual change over time, while taking missing or unequal data and variation in timing of repeated measures into account.20 Model assumptions of LME regarding linearity and normal distribution were tested. PSAD spasticity scores were likewise analyzed using LME regression.
All statistical analyses were carried out using Stata 16 software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.)
3. Results
Eleven participants were recruited, and nine completed the program. Two participants left the study prematurely due to health issues and their data was excluded from the study. These were both women, 38 and 51 years old, and had had the MS diagnosis for 2 and 4 years, respectively.
Out of the nine participants who completed the TRE intervention, five (56%) were women. Age of participants ranged from 44 to 66 years (mean = 51.6). Time since diagnosis ranged between 2 and 21 years (mean = 12.7). Different MS sub-diagnoses were represented in the sample, but most participants had relapsing-remitting MS. Most participants reported being able to walk 500 m or more without a walking aid or rest. One was able to walk up to 300 m without aid or rest, and one needed a cane to walk. This indicates that most participants would score 4 or lower on the MS-specific disability scale EDSS (Expanded Disability Status Scale)21 which ranges from 0 to 10, while two would score between 4.5 and 6.0. However, EDSS scores were not clinically evaluated for this study. All participants reported that they experienced spasticity to some degree. However, only four participants took anti-spasticity medication daily at baseline, and only six participants had spasticity in the ankle plantar flexors at baseline according to the MAS score. Baseline data are presented in Table 1.
3.1. Fatigue
All nine participants completed the MFIS questionnaire at baseline and at end of study. The total average MFIS score decreased significantly from 43.7 (SD = 13.6) at baseline to 22.0 (SD = 12.3) at end of study (p = 0.0014), that is, an almost 50% decrease. The average MFIS cognitive subscale score decreased from 17.3 (SD = 7.8) to 9.1 (SD = 4.2) (p = 0.0048), the physical subscale score decreased from 21.8 (SD = 6.2) to 10.9 (SD = 7.5) (p = 0.0012) and the psychosocial subscale score decreased from 4.6 (SD = 1.8) to 2.0 (SD = 2.1) (p = 0.0051).
3.2. Day-to-day symptom, stress, and sleep quality levels
One participant (male, 50 years old) only completed the day-to-day questionnaire for the first two weeks of the intervention. This participant’s day-to-day data were excluded from the analysis. The remaining 8 participants had an overall completion rate (proportion of days out of the total 10 weeks) of 73%, ranging between 59% and 93% between participants and 63%–84% between weeks.
The total mean symptom burden score (on a scale from 0 to 90) for the nine recorded symptoms was reduced from 23.9 in week 1 to 11.9 in week 10. LME analysis showed that the change in total mean symptom score was significant with a coefficient of −0.22 (p < 0.001). This signifies that there was a daily mean reduction of 0.22 points on the total symptom burden scale.
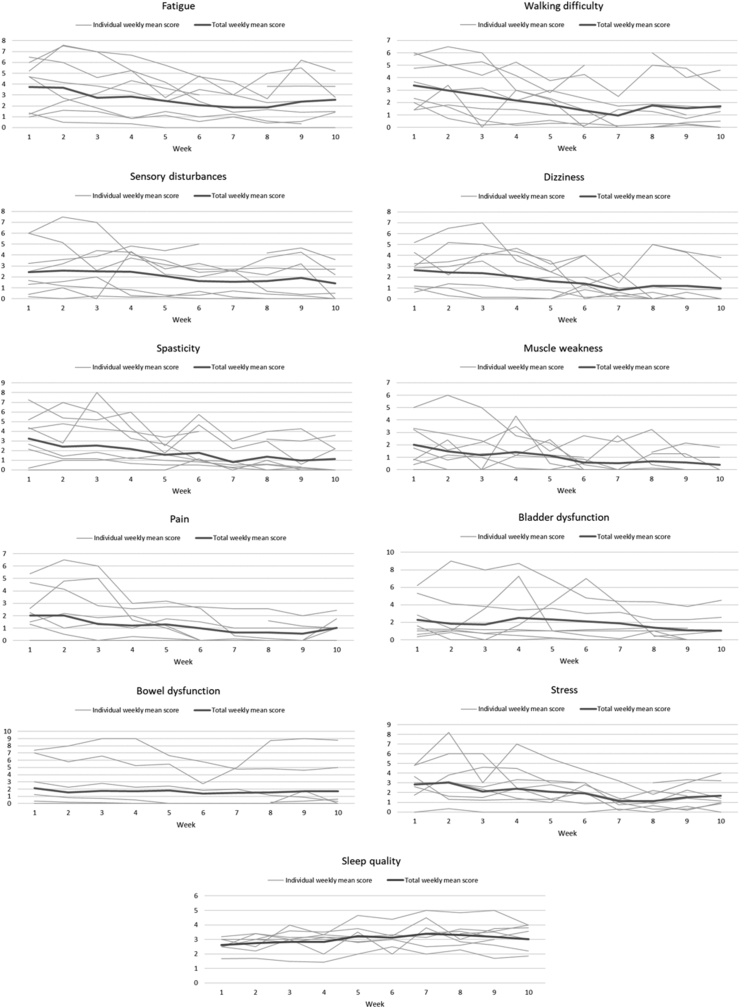
Fig. 1 illustrates weekly mean scores in day-to-day symptoms, as well as stress and sleep quality, over the 10 study weeks. Looking at change on the 11-point scale between week 1 and week 10, symptoms were reduced by between 0.4 points (bowel dysfunction) and 2.1 points (spasticity), and stress was reduced by 1.2 points. Sleep quality increased by 0.4 points on the 5-point scale.
Fig. 1.
Changes in mean weekly individual and total scores for symptoms, stress and sleep quality.
LME analyses showed that eight out of the nine symptoms as well as stress were significantly reduced, and sleep quality significantly increased (p < 0.05). Change in bowel dysfunction was found to be borderline significant (p = 0.050). The results of the LME analyses are presented in Table 2.
Table 2.
Linear mixed effects regressions results for day-to-day symptoms, stress level and sleep quality.a
| Coef. | Random effect parameters |
p-value | 95% CI | ||
|---|---|---|---|---|---|
| SD intercept | SD slope | ||||
| Fatigue | −0.029 | 1.7 | 2.17 e−08 | <0.001 | −0.036; −0.021 |
| Walking difficulty | −0.028 | 0.001 | 0.00007 | <0.001 | −0.035; −0.021 |
| Sensory disturbance | −0.022 | 1.4 | 9,4 e−07 | <0.001 | −0.030; −0.014 |
| Dizziness | −0.030 | 1.3 | 6.0 e−07 | <0.001 | −0.038; −0.022 |
| Spasticity | −0.034 | 1.5 | 1.9 e−09 | <0.001 | −0.042; −0.026 |
| Reduced muscle strength | −0.024 | 0.6 | 2.6 e−08 | <0.001 | −0.032; 0.016 |
| Pain | −0.026 | 1.0 | 1.90 e−10 | <0.001 | −0.032; −0.020 |
| Bladder dysfunction | −0.023 | 1.7 | 7.8 e−06 | <0.001 | −0.030; −0.015 |
| Bowel dysfunction | −0.007 | 2.6 | 2.89 e−07 | 0.050 | −0,014; 7,41 e−06 |
| Stress | −0.029 | 1.2 | 3.6 e−12 | <0.001 | −0.036; −0.021 |
| Sleep quality | 0,010 | 0.002 | 0.00003 | <0.001 | 0.006; 0.014 |
Based on data from 8 participants.
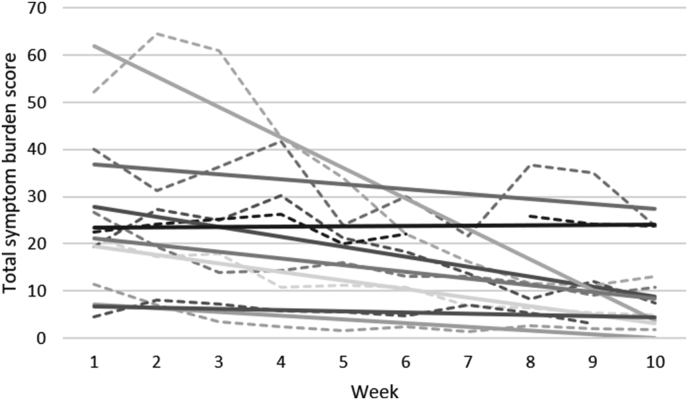
As this study had a very limited number of subjects but still showed significant results in many of the day-to-day measured endpoints, we chose to examine the data further to explore how individual outcomes may have affected the overall scores. Individual mean weekly total symptom scores with trend lines for each of the 8 participants who had day-to-day data are shown in Fig. 2. All participants showed declining trend-lines. However, one participant stood out as his/her trend line had a much steeper slope compared to the other participants. To check whether this participant’s data alone could explain the large number of significant changes found in the day-to-day data, the analyses were run again with exclusion of the data from this participant. Even with the exclusion of this participant’s data, the analysis still showed significant changes on all day-to-day measures that were found significant in the original model (data not shown). However, bowel dysfunction became clearly insignificant (p = 0.653). Hence, it seems that the trend indicated in the original analysis for bowel dysfunction was explained by this participant’s data.
Fig. 2.
Individual changes in weekly total symptom burden scores, with trend-lines, for the 8 participants who have day-to-day data.
3.3. Spasticity
Due to technical difficulties and because one of the subjects suffered from a sprained ankle, baseline and end of study PSAD data from the left ankle was only obtained from six of the nine participants. Right ankle data was obtained in all nine participants.
LME analysis showed no statistically significant difference in reflex torque measured with the PSAD method for neither left nor right plantar flexors between baseline and end of study (Table 3).
Table 3.
Linear mixed effects regressions results for changes in mean reflex torque between week 1 (baseline) and week 11 (end of study)a.
| Coef. | Random effect parameters |
p-value | 95% CI | ||
|---|---|---|---|---|---|
| SD intercept | SD slope | ||||
| Right ankle | 1.140 | 0.94 | 1.54 e−10 | 0.057 | −0.033; 2.313 |
| Left ankle | 0.492 | 0.68 | 0.65 | 0.406 | −0.667; 1.651 |
Based on data from 9 participants for right ankle, and 6 participants for left ankle (3 missing).
4. Discussion
The purpose of the study was to test a TRE program for PwMS and to point towards outcome measures for future randomized, controlled studies. Due to the small sample size and the non-controlled, non-randomized design of this study, the results should not be interpreted as confirming or disproving effects of TRE on PwMS, but they may be used to indicate possible benefits as well as the type of outcome measures to be used in future trials.
For explorative purposes, a range of outcome measures were used in this study. The results do not point towards specific outcome measures to be used in future studies. Rather, they indicate that TRE may affect several symptoms and conditions. TRE has the stated aim of reducing deep muscle tension. MS is associated with involuntary muscle activity such as spasticity, stiffness, and cramps. It is reasonable to assume that a reduction in involuntary muscle activity/tension could affect other symptoms such as fatigue, pain, walking difficulty, poor bladder control etc., which would explain why a reduction in all these symptoms was seen in this study.11 If future studies wish to select one primary outcome measure, they may consider using a measure of fatigue, as this study found significant reduction in fatigue when measured on the MFIS scale as well as on the day-to-day Likert scale. Another relevant focus in future trials could be sleep quality, as poor sleep is associated with a number of MS-related symptoms.22 Sleep quality may therefore be indicative of the impact of symptoms in PwMS. In addition, sleep quality may be measured more objectively, using for example actigraphy,23 if the study wishes to use other measures than self-reported. Even though most of the self-reported outcome measures showed statistically significant improvements, the results should be interpreted with care due to the limitations of a non-randomized, small study such as this. The lack of a control group means that we cannot assess whether factors other than the intervention have influenced the measured outcomes. Selection bias24 may have been present as participants who volunteered may differ from the MS population as a whole in various aspects, such as age and gender composition, level of education and income, stage of the disease, etc. Related to this, volunteer bias25 and compliance bias26 may have been present. Participants may have been highly motivated to try TRE and thus have had a higher adherence to the intervention. They also may have had a tendency to respond more positively to the intervention than other PwMS would have. Two participants left the study early due to health issues, which may have caused attrition bias. With attrition bias, the characteristics of the group changes if the participants who leave the study early differ from the rest of the group.27 For example, the two participants left the study prematurely due to health problems which may suggest that they have poorer overall health than the rest of the group. Overall health may have an impact on the ability to practice TRE as well as the possible effects of TRE. Thus, the results may have been skewed due to this loss to follow-up.
The small sample size of the study also increases the risk that the observed changes are due to chance rather than being indicative of a true effect.28 This is why larger, controlled trials, which can better eliminate these biases, are needed to confirm the findings of this pilot study.
Except for self-reported spasticity, it was not a requirement for inclusion that the participants experienced the symptoms used as outcome measures. This implied that some participants had baseline levels of some symptoms which were zero or close to zero. Also, most participants were not severely affected by MS in terms of walking ability. This may have implications for the study’s ability to measure improvements. For future studies using several endpoints, it could be considered to use stricter inclusion criteria when it comes to baseline scoring. For example a criteria could be that the participant has to score higher than a certain level on at least three of the endpoints.
It has been shown that self-reported symptoms in PwMS, such as pain and fatigue, vary substantially within the same person over even short periods of time.29 Using a day-to-day measurement tool for self-reported outcomes in this study allowed for a detailed picture of changes occurring over the entire intervention period to be obtained.
The day-to-day measurement tool may increase data validity as compared to more traditional, single point instruments. As Kratz et al.30 point out, when self-reported outcomes are assessed at a single time point by asking about participants’ experience of a certain symptom or function within a retrospective time period (e.g. fatigue during the past two weeks), there is great risk of recall bias affecting the data quality. This problem may be exacerbated by the cognitive decline often experienced by PwMS.30
The day-to-day symptom scale was developed recently, and while it has partly undergone validity testing, a peer-reviewed paper has not yet been published on the validity and reliability of the scale. Neither have clinically relevant changes on the scale been determined yet.
It is interesting that the self-reported level of spasticity was reduced significantly during the study while no significant change was observed for the PSAD-measured spasticity levels of the ankle plantar flexors. Discrepancy between subjectively and objectively measured spasticity outcomes has also been shown in other trials among MS patients, for example in studies of Sativex.31 The discrepancy in this study could partly be due to lack of sensitivity of the PSAD method and the missing data in the PSAD analysis. Another explanation may lie in how spasticity is understood and reported. The PSAD method measures spasticity according to the formal, clinical definition “velocity dependent increase in muscle tone with exaggeration of stretch reflex circuitry”.19 However, there is a general lack of consensus regarding the definition of spasticity, and other researchers, as well as clinicians and PwMS themselves, may have a broader understanding of what spasticity is.11,19,32 Thus, the participants may have experienced bodily discomfort which they interpreted as spasticity, but which would not have been classified as spasticity according to the clinical definition. This is also illustrated by the fact that three out of the nine participants who reported experiencing spasticity at baseline, had no measurable spasticity in the ankle plantar flexor at baseline assessed on the MAS scale.
Another explanation for the discrepancy between PSAD-measured and self-reported spasticity level changes could be that participants have experienced spasticity in parts of the body other than around the ankle, or they may have experienced spasticity at other times of the day than around midday when the PSAD measurements were carried out.
The findings of this study suggest that future studies on PwMS that wish to evaluate the effects of TRE or other interventions on spasticity levels should consider that spasticity is perhaps not well defined and that self-reported spasticity may cover a broader sense of bodily discomfort than spasticity measured for example via the PSAD method.
5. Conclusions
The study indicates that TRE may affect a number of MS-related symptoms and conditions. MS is a disease that manifests in many ways and is characterized by fluctuating and individually determined symptom patterns. Therefore, future studies investigating TRE in PwMS should consider using more than one outcome measure and should also consider using tools that allow for measurement over time, such as the day-to-day symptom scale used in this pilot study. Fatigue and sleep quality measures may be of particular interest.
TRE shows potential as a tool for PwMS to alleviate symptoms and thus to improve quality of life. Such tools are in high demand among PwMS. However, randomized studies investigating TRE among PwMS are needed.
Funding
The study was funded by the Danish MS Society.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors thank certified TRE instructor Michael Nissen, MSc Psychology, who carried out the TRE intervention.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Nylander A., Hafler D.A. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barin L., Salmen A., Disanto G. The disease burden of Multiple Sclerosis from the individual and population perspective: which symptoms matter most? Mult Scler Relat Disord. 2018;25:112–121. doi: 10.1016/j.msard.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Carrithers M.D. Update on disease-modifying treatments for multiple sclerosis. Clin Therapeut. 2014;36(12):1938–1945. doi: 10.1016/j.clinthera.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kesselring J., Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol. 2005;4(10):643–652. doi: 10.1016/S1474-4422(05)70193-9. [DOI] [PubMed] [Google Scholar]
- 5.Skovgaard L., Nicolajsen P.H., Pedersen E. Use of complementary and alternative medicine among people with multiple sclerosis in the nordic countries. Autoimmune Dis. 2012;2012:1–13. doi: 10.1155/2012/841085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berceli D. Shake it off naturally: reduce stress, anxiety and tension with (TRE) CreateSpace. 2015;IX - XII [Google Scholar]
- 7.Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894 National center for Biotechnology information. https://www.ncbi.nlm.nih.gov/
- 8.Berceli D. Namaste Pub.; 2008. The Revolutionary Trauma Release Process. [Google Scholar]
- 9.Rizzo M.A., Hadjimichael O.C., Preiningerova J., Vollmer T.L. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler Houndmills Basingstoke Engl. 2004;10(5):589–595. doi: 10.1191/1352458504ms1085oa. [DOI] [PubMed] [Google Scholar]
- 10.Barnes M.P., Kent R.M., Semlyen J.K., McMullen K.M. Spasticity in multiple sclerosis. Neurorehabilitation Neural Repair. 2003;17(1):66–70. doi: 10.1177/0888439002250449. [DOI] [PubMed] [Google Scholar]
- 11.Hugos C.L., Cameron M.H. Assessment and measurement of spasticity in MS: state of the evidence. Curr Neurol Neurosci Rep. 2019;19(10):79. doi: 10.1007/s11910-019-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senders A., Wahbeh H., Spain R., Shinto L. Mind-body medicine for multiple sclerosis: a systematic review. Autoimmune Dis. 2012;2012:1–12. doi: 10.1155/2012/567324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berceli D., Salmon M., Bonifas R., Ndefo N. Effects of self-induced unclassified therapeutic tremors on quality of life among non-professional caregivers: a pilot study. Glob Adv Health Med. 2014;3(5):45–48. doi: 10.7453/gahmj.2014.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austrian-Military-Research-Project-Outline.pdf. http://traumaprevention.com/wp-content/uploads/2016/04/Austrian-Military-Research-Project-Outline.pdf
- 15.VA-Research-Announcement.pdf. http://traumaprevention.com/wp-content/uploads/2016/04/VA-Research-Announcement.pdf
- 16.Nissen M. Shake it off Naturally: Reduce Stress, Anxiety and Tension with (TRE) CreateSpace; 2015. Using TRE with people with multiple sclerosis (MS) in the Danish multiple sclerosis society. [Google Scholar]
- 17.Larson R.D. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013;15(1):15–20. doi: 10.7224/1537-2073.2012-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karnoe A. vol. 25. 2019. Development and feasibility test of a scale for patient-reported outcome measures in multpile sclerosis; pp. 1031–1072. (Multiple Sclerosis Journal). [DOI] [Google Scholar]
- 19.Yamaguchi T., Hvass Petersen T., Kirk H. Spasticity in adults with cerebral palsy and multiple sclerosis measured by objective clinically applicable technique. Clin Neurophysiol. 2018;129(9):2010–2021. doi: 10.1016/j.clinph.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Caruana E.J., Roman M., Hernández-Sánchez J., Solli P. Longitudinal studies. J Thorac Dis. 2015;7(11):E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11) doi: 10.1212/WNL.33.11.1444. 1444-1444. [DOI] [PubMed] [Google Scholar]
- 22.Bøe Lunde H.M., Aae T.F., Indrevåg W. Poor sleep in patients with multiple sclerosis. Paul F., editor. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandner M.A., Rosenberger M.E. Sleep and Health. Elsevier; 2019. Actigraphic sleep tracking and wearables: historical context, scientific applications and guidelines, limitations, and considerations for commercial sleep devices; pp. 147–157. [DOI] [Google Scholar]
- 24.Nunan D., Bankhead C., Aronson J. Selection bias. Catalog of bias. 2017. https://catalogofbias.org/biases/selection-bias/ Published March 28.
- 25.Brassey J., Mahtani K., Spencer E., Heneghan C. Volunteer bias. Catalog of bias. 2017. https://catalogofbias.org/biases/volunteer-bias/ Published November 17.
- 26.Spencer E., Heneghan C. Compliance bias. Catalog of bias. 2018. https://catalogofbias.org/biases/compliance-bias/ Published May 24.
- 27.Bankhead C., Aronson J., Nunan D. Attrition bias. Catalog of bias. 2017. https://catalogofbias.org/biases/attrition-bias/ Published July 20. [DOI] [PubMed]
- 28.Spencer E., Brassey J., Mahtani K., Heneghan C. Wrong sample size bias. Catalog of Bias. 2017. https://catalogofbias.org/biases/wrong-sample-size-bias/ Published July 20.
- 29.Kratz A.L., Murphy S.L., Braley T.J. Ecological momentary assessment of pain, fatigue, depressive, and cognitive symptoms reveals significant daily variability in multiple sclerosis. Arch Phys Med Rehabil. 2017;98(11):2142–2150. doi: 10.1016/j.apmr.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kratz A.L., Alschuler K.N., Ehde D.M. A randomized pragmatic trial of telephone-delivered cognitive behavioral-therapy, modafinil, and combination therapy of both for fatigue in multiple sclerosis: the design of the “COMBO-MS” trial. Contemp Clin Trials. 2019;84:105821. doi: 10.1016/j.cct.2019.105821. [DOI] [PubMed] [Google Scholar]
- 31.Giacoppo S., Bramanti P., Mazzon E. Sativex in the management of multiple sclerosis-related spasticity: an overview of the last decade of clinical evaluation. Mult Scler Relat Disord. 2017;17:22–31. doi: 10.1016/j.msard.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra S., Pandyan A., Day C., Jones P., Hermens H. Spasticity, an impairment that is poorly defined and poorly measured. Clin Rehabil. 2009;23(7):651–658. doi: 10.1177/0269215508101747. [DOI] [PubMed] [Google Scholar]