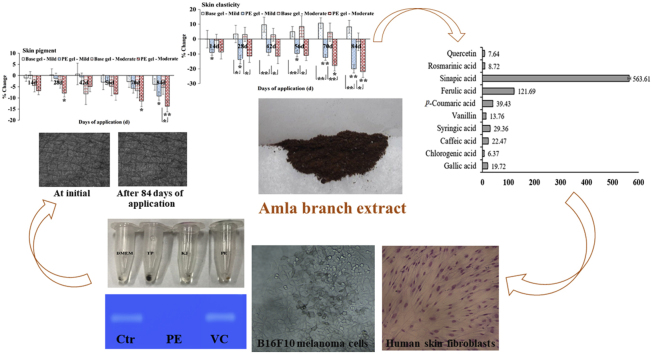
Abstract
Background and aim
Skin aging influences the changes in skin, including skin dryness, wrinkle, and irregular pigmentation. Amla (Phyllanthus emblica L.) branch has shown several benefits, but not the anti-skin aging. The study aimed to evaluate the anti-skin aging efficacy of amla branch.
Experimental procedure
Amla branches were standardized the phenolic acids. The extract was investigated anti-skin aging activities, including antioxidant, anti-tyrosinase, anti-melanogenesis, and matrix metalloproteinase-2 inhibitory assays. Topical gel containing extract was prepared and evaluated the skin irritation by a single closed patch test. Randomized, double-blind, placebo-control study was performed in 20 volunteers for 84 consecutive days. The tested skin was evaluated by Chromameter® CR 400, Dermalab® USB, Mexameter® MX 18, Corneometer® CM 825, and Visioscan® VC 98.
Results
Amla branch extract, a dark brown powder, consisted a variety of phenolic acids, mainly sinapic and ferulic acids. The extract exhibited the potent antioxidant and tyrosinase inhibitory activities in vitro assays and the melanin suppression through inhibition of tyrosinase and tyrosinase-related protein-2 activities, the strong antioxidant, and the potent matrix metalloproteinase-2 in cellular assays at 0.1 mg/mL. Topical gel containing 0.1% extract was a stable and safe formulation. Clinical study was proved the superior anti-skin aging efficacy, including the lightening skin color, the enhanced skin elasticity and hydration, and the skin wrinkle reduction.
Conclusion
The study results suggested that amla branch is a rich source of bioactive compounds and can be a potential ingredient for utilization in anti-skin aging products.
Keywords: Phenolic acids, Anti-Skin aging, In vitro assay, Cellular assay, Clinical trial
Graphical abstract
Highlights
-
•
Amla branches were standardized and investigated the anti-skin aging activities.
-
•
Sinapic and ferulic acids were major phenolics in extract.
-
•
In vitro and cellular assays exhibited the melanogenesis and matrix metalloproteinase-2 suppression, and antioxidant.
-
•
Clinical study proved the lightening skin color, the enhanced skin elasticity and hydration, and skin wrinkle reduction.
-
•
Study revealed that amla branch is a source of bioactive compounds for utilization as an anti-skin aging ingredient.
List of abbreviations
- AcOH
acetic acid
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- Cps
centipoise
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- GAE
gallic acid equivalent
- FeSO4
ferrous sulfate
- FRAP
ferric reducing ability of plasma
- MII
mean irritation index
- MMP
matrix metalloproteinase
- NO2CO3
sodium carbonate
- PE
Phyllanthus emblica L.
- SRB
sulforhodamine B
- TPC
total phenolic content
- TRP-2
tyrosinase-related protein-2
- UPLC
ultra-performance liquid chromatography
1. Introduction
Phyllanthus emblica L. (amla), a medicinal plant in family Phyllanthaceae, has generally been cultivated in tropical and subtropical countries, including China, India, Sri Lanka, and Thailand.1 It has been used for treatment of illnesses in traditional medicines, including Indian traditional healthcare system, Tibetan medicine, Sri Lankan medicine, Chinese herbal medicine, and Thai medicinal system.2 Amla demonstrates several biological activities, including antioxidant,3 anti-cancer activity,4,5 anti-diabetic activity,6 anti-inflammatory activity,7,8 anti-microbial activity,9 treatment of gastric ailments,10,11 adaptogenic activity,3 rejuvenating,1,12 and promoting health and longevity13,14 (details of each activity shown in Table A). Also, it is a rich source of nutrients, such as amino acids, vitamin C, carbohydrates, alkaloids, and phenolic acids. All parts of amla, particularly fruit, have been studied and reported to possess a variety of pharmacological activities in prevention and treatment of diseases.1,15, 16, 17, 18 Due to the fluctuations in fruit productivity and time of fruiting, other parts of amla have been investigated to use as the substituent for fruit.18,19 Amla branch has been studied and shown as a promising part for utilization in natural healthcare products, including food, health, and cosmetic products. The ethanolic extract of amla branch and its fractions exhibit the potent cytotoxicity against cancerous cells, including human leukemia (HL-60) and human hepatocellular carcinoma (SMMC-7721) cells.20 The branch extract also possesses the protective effect on testicular damage in valproic acid-induced rats,21 the antioxidant, the anti-melanogenesis, the anti-inflammatory, the anti-microbial, and the antimutagenic activities in vitro and cellular assays.19,22 According to literature, the free radicals and inflammation play the important role in skin aging via upregulation of matrix metalloproteinases (MMPs), thereby resulting in the degradation of connective tissue, tissue remodeling, and processes that orchestrate many of the degenerative processes associated with aging.23 However, the efficacy of amla branch extract against skin aging has not been reported so far.
Skin aging is a multifactorial process resulted from intrinsic and extrinsic factors. Intrinsic factors are associated with the influences of genetic, hormones, and metabolic slowdown, whereas extrinsic factors include the exposure to solar radiation, pollutants, and lifestyle behaviors.24 Both factors have influenced the changes in skin appearances, including skin dryness, laxity, dynamic and static wrinkles, and irregular pigmentation.25 As the current global human lifespan dramatically increases, people have paid attention to the aging-related issues, including anti-aging strategies, food supplement, and application of anti-aging products, for eradication of aging signs and living long with satisfactory health and well-being.26 The global anti-aging industry has gained the worth at 292 billion US dollars in 2015 and the market trend tends to be a robust growth.27 Currently, the natural origin ingredients and formulations have gained the increasing interest, because of consideration on health, environmental awareness, and safety of synthetic chemicals.28
In this study, amla branch was standardized and evaluated the anti-skin aging activities, including anti-melanogenesis, antioxidant, and matrix metalloproteinase (MMP)-2 inhibitory assays in vitro and cellular tests. Topical gel containing extract was also prepared and performed the safety test. The clinical efficacy study of gel containing extract was evaluated in 20 volunteers in a randomized, double-blind, placebo-control trial.
2. Materials and methods
2.1. Preparation and standardization of extract
Amla branches were harvested at Mae Fah Luang University, Chiang Rai, Thailand. The voucher specimen (PE_BR MFU18) was deposited for further reference at our laboratory herbarium at Mae Fah Luang University, Chiang Rai. The extraction was performed as previously described with some modification.11,22 The branches were cleansed and dried in hot air oven at 45 ± 2 °C for 24 h. The dried branches were ground and then macerated with 50% ethanol for 24 h. The proportion of branch powder and solvent was 1:5 (w/v). The extract was filtered and concentrated by using a rotary evaporator and a spray drier, respectively. The extraction was performed for 3 times and calculated for an extraction yield.
2.2. Analysis of total phenolic contents (TPC)
A serial dilution of standard gallic acid and extract were mixed with water, Folin-Ciocalteu reagent, and Na2CO3, respectively. The mixture was mixed and incubated for 1 h. The absorbance was measured. TPC of extract was calculated and expressed as mg of gallic acid equivalent per g of extract (mg GAE/g extract).29 The measurement was performed in triplicate.
2.3. Analysis of phenolic profile by ultra-performance liquid chromatography (UPLC)
UPLC analysis was performed on an ACQUITY H-Class system equipped with an ACQUITY UPLC PDA eλ detector using a BEH C18 1.7 μm column (2.1✕100 mm). Gallic, protocatechuic, chlorogenic, caffeic, syringic, p-coumaric, ferulic, sinapic, and rosmarinic acids, vanillin, and quercetin at various concentrations in acetonitrile were used to prepare the calibration curve. The sample was separated by a gradient mobile phase consisting of acetonitrile (A) and 3% acetic acid (AcOH) (B). The eluent was set as follows: 0 min 100% B, 1.5 min 95% B, 3 min 85% B, 5 min 80% B, and 8 min 70% B at a flow rate of 0.6 mL/min. The characterization of phenolic acids in extract (1 mg/mL) was performed in triplicate.29
2.4. In vitro assays
2.4.1. Antioxidant activity
The antioxidant activity was performed by DPPH scavenging assay, ABTS scavenging assay, and ferric reducing ability of plasma (FRAP), and used vitamin C as a standard. The antioxidant value obtained from DPPH and ABTS scavenging assays was presented as the concentration required to scavenge 50% of free radicals (IC50), whereas that from FRAP was expressed as mg FeSO4/mg extract. Each assay was performed in triplicate.30
2.4.2. Anti-tyrosinase activity
Anti-tyrosinase activity was evaluated by dopachrome formation method with l-dopa as the substrate. A serial dilution of extract and kojic acid, a standard, was prepared and mixed with a mixture of phosphate buffer and mushroom tyrosinase. l-dopa was added into mixture and incubated for 20 min. The absorbance was measured. The concentration that was able to deactivate enzyme at 50% (IC50) was calculated. The assay was performed in triplicate.30
2.5. Cellular assays
2.5.1. Cell culture
B16F10 melanoma cells (ATCC® CRL-6475™) and human skin fibroblasts (PromoCell® at the 15th-18th passages) were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution in a humidified incubator at 37 °C with 5% carbon dioxide. Cells were harvested to perform each experiment in triplicate.
2.5.2. Cytotoxicity assay
The assay was evaluated by sulforhodamine B (SRB) method.28 Briefly, cells were seeded and incubated for 24 h. Cells were then treated with different concentrations of samples. After 72 h, cells were fixed and stained with SRB. The excess dye was washed away, and the bound dye was solubilized in tris buffer to measure absorbance. The percent of cell viability was calculated in compared to solvent control.
2.5.3. Analysis of anti-skin aging activities
2.5.3.1. Melanogenesis assay
The assay was consisted of melanin content measurement and activities of tyrosinase and tyrosinase-related protein (TRP)-2.28 Briefly, B16F10 melanoma cells were plated and treated with noncytotoxic concentrations of samples for 72 h. Cells were then detached to determine melanin content and enzyme activities. The total protein content was performed by the Bradford protein assay for calculation of actual melanin content and enzyme activities. The percent of relative ratio of melanin content and enzyme activities was calculated in compared to solvent control.
2.5.3.2. Antioxidant assay
The cellular antioxidant was evaluated as previous method.28 Briefly, human skin fibroblasts were seeded and treated with noncytotoxic concentrations of samples. After 24 h incubation, all samples were replaced with fresh medium containing 150 μM hydrogen peroxide and further incubated for 4 h. The cells were then fixed and evaluated the cell viability by SRB assay.
2.5.3.3. MMP-2 inhibitory assay
Human skin fibroblasts were seeded and incubated overnight. The culture medium was replaced with fresh medium without serum supplement. The greatest noncytotoxic concentration of samples was added and incubated for 72 h. The culture medium was collected to analyze the enzyme activity by SDS-PAGE zymography with gelatin as the substrate. The zymographic bands were determined with the Bio-Rad Gel Doc Imaging System. The percent of MMP-2 inhibitory activity was calculated in compared to solvent control.31
2.6. Topical gel formulations
Base gel composing of water, propylene glycol, glycerin, hydroxyethyl cellulose and liquid germall™ plus and PE gel consisting of 0.1% extract incorporated into base gel (data shown in Table B) were prepared. Both base and PE gels were performed the physicochemical properties, including pH, viscosity, and physical appearance, at initial and after stability test by 7 cycles of heating-cooling cycle.29
2.7. Clinical assays
2.7.1. Ethical conduction
The study protocol in healthy volunteers was followed the guidelines of Declaration of Helsinki and was approved by The Mae Fah Luang University Ethics Committee with registered approval no. REH-62117.
2.7.2. Volunteer recruitment
Twenty healthy Thai volunteers aged 25–50 years old showing mild to moderate clinical signs of skin aging with no existing skin conditions and history of allergic reactions were screened to enroll according to the inclusion and exclusion criteria. All procedures were described to all volunteers, and the informed consent statements were signed prior to conducting the test.
2.7.3. Safety test
The safety test was performed by a single closed patch application.29 The samples, including base gel, PE gel, and deionized water, were applied to the volar forearm of volunteers for 24 h. After patch removal, the skin reaction, which was assessed at 30 min and 24 h, was focused on degree of skin erythema and edema, and graded as following score: 0 = no reaction, 0.5 = very slight reaction (barely visible), 1.0 = slight reaction, 2.0 = obvious reaction, and 3.0 = significant reaction. The mean irritation index (MII) of each sample was calculated and degree of irritation was categorized based on the MII value as nonirritating (MII<0.2), slightly irritating (0.2≤MII<0.5), moderately irritating (0.5≤MII<1), and strongly irritating (MII≥1).
2.7.4. Analysis of clinical anti-skin aging efficacy
Twenty healthy volunteers who had not shown skin reactions in safety test were included in a randomized, double-blind, placebo-control study. Regarding the inter-individual variability, all volunteers were randomly assigned to apply two formulations, base and PE gel, to each half of their faces twice daily after face cleansing in morning and evening for 84 consecutive days. During study, any skin treatment on treated areas was prohibited as well as smoking and liquor drinking. The tested skin was also protected against strong sun radiation and any skin insulting. All volunteers washed their faces and then rested for 30 min in a waiting room. The measurement was performed in the climate-controlled room at 20 ± 2 °C and 40–60% relative humidity. The tested skin was evaluated the CIE L∗a∗b∗ color system by Chromameter® CR 400 (Konica Minolta, Japan), the melanin index by Mexameter® MX 18, the skin elasticity by Dermalab® USB elasticity probe (Cortex Technology, Denmark), the skin hydration by Corneometer® CM 825, and the skin wrinkles (SEw) by Visioscan® VC 98 (Courage and Khazaka, Germany) at baseline and after 14, 28, 42, 56, 70, and 84 days of application.29 The comparison of clinical assessment was performed between baseline and the post-application evaluation and presented as the percent change. Upon the final assessment, all volunteers were requested to complete the questionnaires for subjective satisfaction assessment.
2.8. Statistical analysis
Data were presented as means ± standard error of mean (S.E.) of three independent experiments. In vitro and cellular data were compared using one-way analysis of variance (ANOVA) and the least significant difference test (LSD), whereas clinical efficacy data were analyzed by one-way repeated measures ANOVA and Independent t-test. A p-value <0.05 was considered statistically significant.
3. Results and discussion
3.1. Preparation and standardization of extract
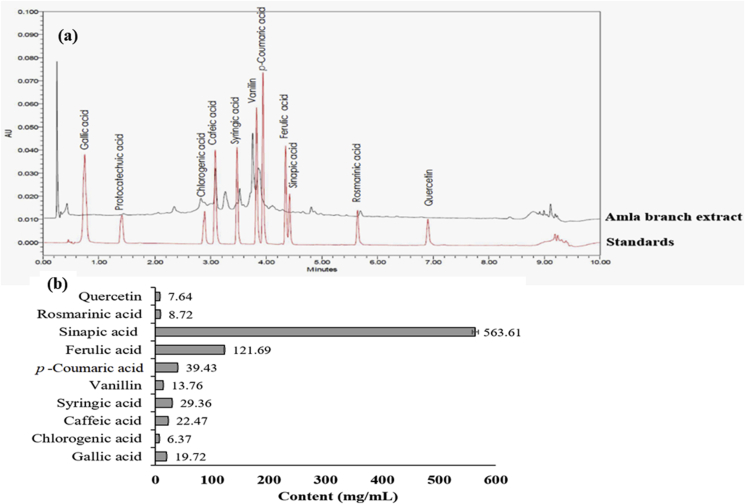
Amla branch extract was dark brown powder with an extraction yield of 10.40 ± 0.20% (w/w). TPC and analysis of phen olic profile presents in Table 1 and Fig. 1, respectively. Sinapic and ferulic acids were the major bioactive components in extract, along with the presence of eight minor phenolics. Since phenolic compounds play the main role in plant biological activities, the determination of phenolic content is an important criterion for plant quality.32 The study reported that TPC of different amla fruit cultivars are in the range of 239.6–274.3 mg GAE/100 g dry matter33 and the major phenolics in fruit extract are gallic and tannic acids.16 Interestingly, TPC and the major phenolics of branch extract were different from those of fruit extract. The variation in content and phenolic acids in plant may be influenced by the difference in genetic, maturity, stages and agronomic conditions, and activity of polyphenoloxidase enzyme among the plant cultivars.33
Table 1.
TPC and in vitro assays.
| Parameter | PE extract | Standard |
|---|---|---|
| Total phenolic content (mg GAE/g extract) | 673.96 ± 3.68 | – |
| Antioxidant activity | ||
| DPPH assay (IC50; μg/mL) | 3.65 ± 0.27 | Vitamin C: 2.98 ± 0.08∗ |
| ABTS assay (IC50; μg/mL) | 2.83 ± 0.12∗ | Vitamin C: 3.15 ± 0.05 |
| FRAP (mg FeSO4/mg extract) | 9.08 ± 0.05 | – |
| Anti-tyrosinase activity (IC50; μg/mL) | 310.34 ± 3.84 | Kojic acid: 30.82 ± 1.99∗∗ |
∗ and ∗∗ indicate the significant difference at p-value<0.05 and 0.001, respectively.
Fig. 1.
Standardization of phenolic profile: (a) ULPC chromatogram of standards and amla branch extract, and (b) content of phenolic acids in extract.
Sinapic acid, the major constituent, along with other phenolics in extract have reported the benefit effects on human health, including anti-carcinogen and anti-inflammation.34 Accordingly, the biological activities of branch extract against skin aging were performed.
3.2. In vitro assays
Table 1 presents TPC and in vitro assays. Amla branch extract exhibited the potent free radical scavenging and the tyrosinase inhibitory activities. The ability of extract for combating radicals and inhibiting tyrosinase may be attributed to the phenolics. The study reported that plant antioxidant activity depends on TPC, and position and availability of free hydroxyl groups to scavenge radicals.35,36 Additionally, the high phenolic content is associated with strong antioxidant by electron transfer mechanism17 and tyrosinase inhibitory activity. Ferulic and p-coumaric acids have demonstrated to act as the competitive tyrosinase inhibitors.29 To investigate the factors affecting skin aging, including antioxidant, anti-melanogenesis, and MMP inhibition, the amla branch extract was evaluated the anti-skin aging activity in the cellular assays compared with sinapic acid, a major phenolic constituent.
3.3. Cellular assays
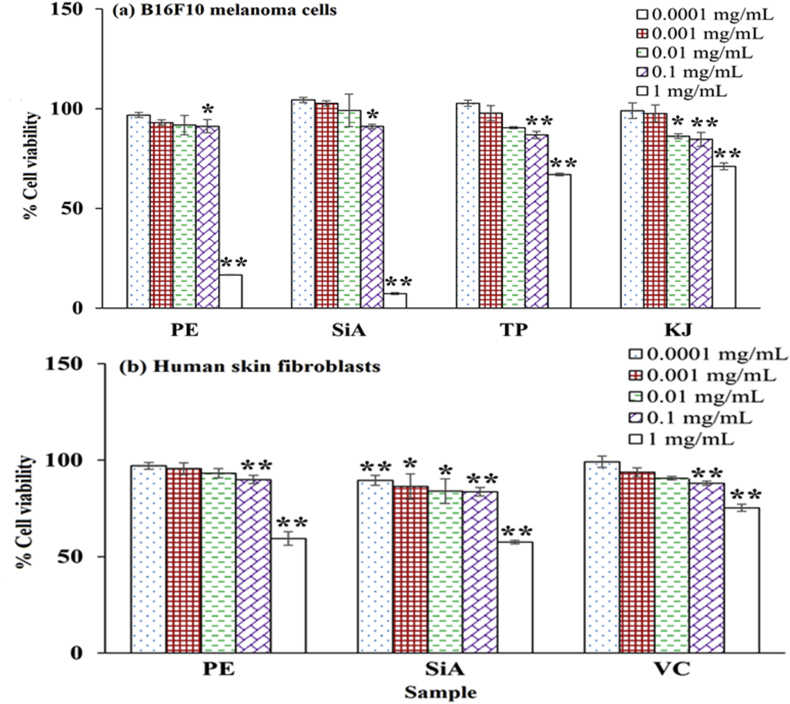
3.3.1. Cytotoxicity assay
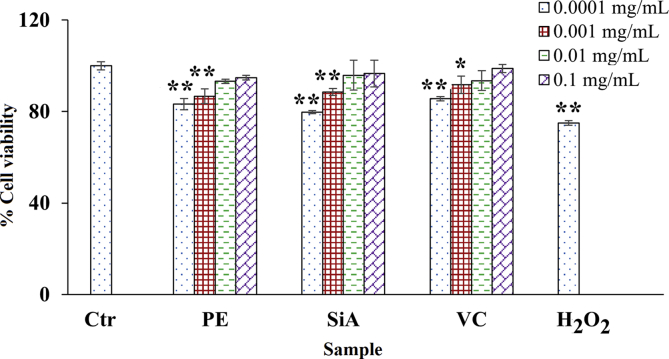
Since the cytotoxic concentration of treated samples influenced the anti-skin aging activity, the cytotoxicity assay was performed to obtain the noncytotoxic concentrations. Fig. 2 presents the viability of cells treated with extract, sinapic acid, theophylline, kojic acid, and vitamin C. The similar cell viability results were observed in B16F10 melanoma cells and human skin fibroblasts. The noncytotoxic concentrations of extract, sinapic acid, theophylline, kojic acid, and vitamin C, which demonstrated cell viability greater than 80%, were found in the range of 0.0001–0.1 mg/mL. The decreased cell viability was observed when treated with the increased concentration. The cytotoxic concentration of 1 mg/mL extract, sinapic acid, theophylline, and kojic acid resulted in the decreased cell viability to 16.67 ± 0.20, 7.24 ± 0.51, 67.04 ± 0.69, and 71.02 ± 1.69%, respectively, in B16F10 melanoma cells. For human skin fibroblasts, the cytotoxic concentration of 1 mg/mL extract, sinapic acid, and vitamin C treatment reduced the cell viability to 59.35 ± 3.48, 57.49 ± 0.93, and 75.23 ± 1.87%, respectively.
Fig. 2.
Cytotoxicity assay in (a) B16F10 melanoma cells and (b) human skin fibroblasts treated with amla branch extract (PE), sinapic acid (SiA), theophylline (TP), kojic acid (KJ), and vitamin C (VC) at 0.0001–1 mg/mL ∗ indicates the significant difference from the control (∗p < 0.05, ∗∗p < 0.001).
The cytotoxicity of extract was in agreement with that of sinapic acid, a main component in extract. Sinapic acid and its derivatives have demonstrated the anticancer effect,37 while other phenolics in extract, including ferulic, p-coumaric, and gallic acids, have also reported to inhibit cell proliferation, particularly cancerous cells.38,39 The cytotoxicity of theophylline, kojic acid, and vitamin C, which were used as the controls in the further assays, was in agreement with previous studies.28,40 The extract, sinapic acid, and controls at noncytotoxic concentrations (0.0001–0.1 mg/mL), that demonstrated the cell viability greater than 80%, were further tested the anti-skin aging activities.
3.3.2. Analysis of anti-skin aging activities
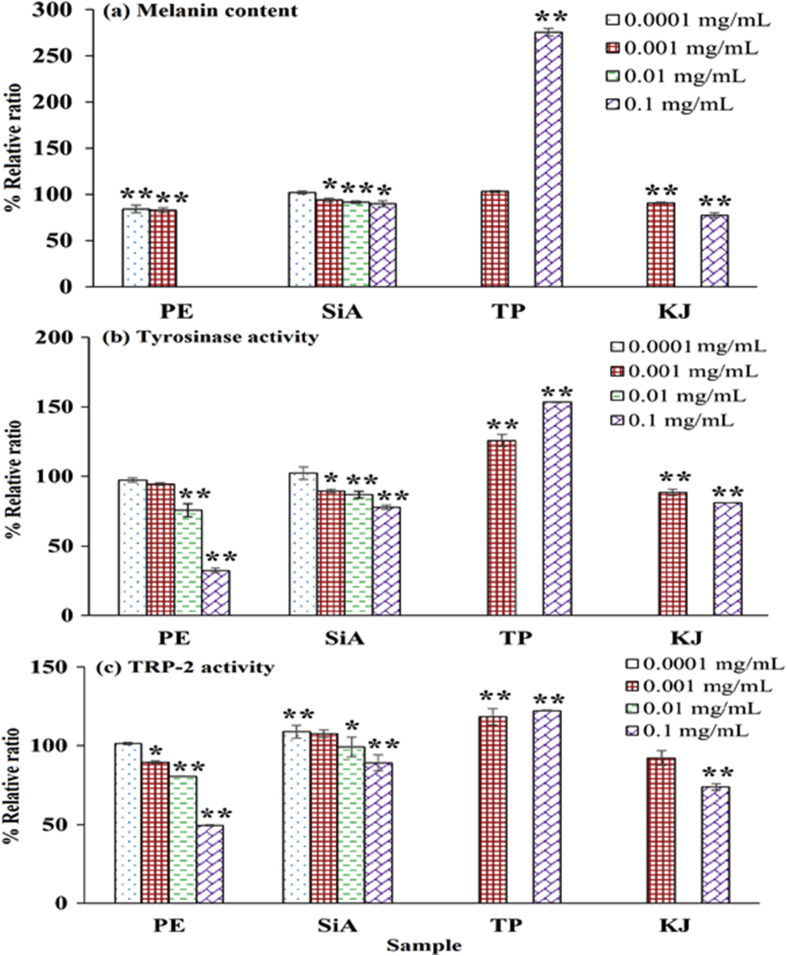
3.3.2.1. Melanogenesis assay
Fig. 3 presents the melanogenesis assay of extract and sinapic acid compared with theophylline and kojic acid. Due to the interference color of the brownish-red extract, at 0.01–0.1 mg/mL, in the end point measurement of melanin content analysis, so, only 0.0001 and 0.001 mg/mL amla branch extract were analyzed the effect on melanin content. The remarkable decline in melanin content and melanogenic enzyme activities was observed in a concentration-dependent manner. Theophylline stimulated the melanin content and enzyme activities, whereas kojic acid suppressed the pigment and enzyme activities. Regarding melanogenesis suppression, the activity of amla branch extract, sinapic acid and kojic acid was mediated through suppression of tyrosinase and TRP-2 activities. In addition, extract at the greatest tested concentration significantly suppressed the melanin content and the melanogenic enzyme activities greater than sinapic acid and kojic acid.
Fig. 3.
Melanogenesis assay in B16F10 melanoma cells treated with amla branch extract (PE), sinapic acid (SiA), theophylline (TP), and kojic acid (KJ) at 0.0001–0.1 mg/mL: (a) melanin content, (b) tyrosinase activity, and (c) TRP-2 activity. ∗ indicates the significant difference from the control (∗p < 0.05, ∗∗p < 0.001).
Melanogenesis, a pigment biosynthesis in melanocytes, involves at least 3 melanogenic enzymes, including tyrosinase, TRP-1 and TRP-2. Tyrosinase is known as a key enzyme in the pathway.28 With aging, the irregular pigmentation is associated with the uneven distribution of pigment cells, greater α-dihydroxyphenylalanine (dopa) positivity in sun-exposed skin, a local loss of melanocytes, and a modification in interaction between melanocytes and keratinocytes.41 According to study results, melanogenesis suppression of extract may be attributed to the synergistic effect of phenolic acids. Sinapic acid slightly inhibits the enzymatic oxidation of l-dopa,42 whereas other phenolics, including ferulic, p-coumaric, and gallic acids, are reported to inhibit the melanogenic enzyme activities.29,39 Theophylline, a pigment stimulating agent, induces pigment by activation of cyclic adenosine monophosphate and increasing of dopa- and gamma-glutamyl transpeptidase-reactive cells,43 whereas kojic acid, a skin lightening agent, reduces pigment through complex formation of enzyme and inhibitor, resulting in enzyme malfunction.44
3.3.2.2. Antioxidant assay
To evaluate the protective effect of antioxidant against free radical-induced cytotoxicity, cells were treated with extract, sinapic acid, and vitamin C prior to induction of cell damage. Hydrogen peroxide is used to cause damage by cell membrane leakage and DNA damage.45 Fig. 4 demonstrates the antioxidant activity of extract, sinapic acid, and vitamin C compared with solvent control and oxidative control (H2O2). Cells treated with hydrogen peroxide (oxidative control) significantly decreased cell viability to 74.98 ± 0.99%, indicating cytotoxicity. In contrast, cells treated with antioxidants exhibited the greater cell viabilities than oxidative control. The cell viabilities of 0.01 mg/mL extract, sinapic acid, and vitamin C were 93.24 ± 0.88, 95.89 ± 6.52, and 93.48 ± 4.33%, respectively, which were comparable to solvent control.
Fig. 4.
Antioxidant activity assay in human skin fibroblasts treated with solvent control (ctr), H2O2 (oxidative control), amla branch extract (PE), sinapic acid (SiA), and vitamin C (VC) at 0.0001–0.1 mg/mL ∗ indicates the significant difference from the control (∗p < 0.05, ∗∗p < 0.001).
Currently, free radicals are associated with the causes of several disorders, including hyperpigmentation and skin aging. Free radicals, that are produced by endogenous metabolic processes and exogenous factors, such as sun irradiation and pollution, are largely attributed to a complex cascade of cellular oxidative damage. Strategies for manipulating the generated free radicals have been extensively studied. Antioxidant, one of several strategies, can deactivate and slow down the damaging reactions induced by free radicals.46 The ability of extract for radical scavenging, which was in agreement with in vitro assays, may be attributed to phenolic acids.29,37,47 Vitamin C, a known antioxidant, is mediated activity by reacting with aqueous peroxyl radicals and restoring the antioxidant properties of vitamin E.48
3.3.2.3. MMP-2 inhibitory assay
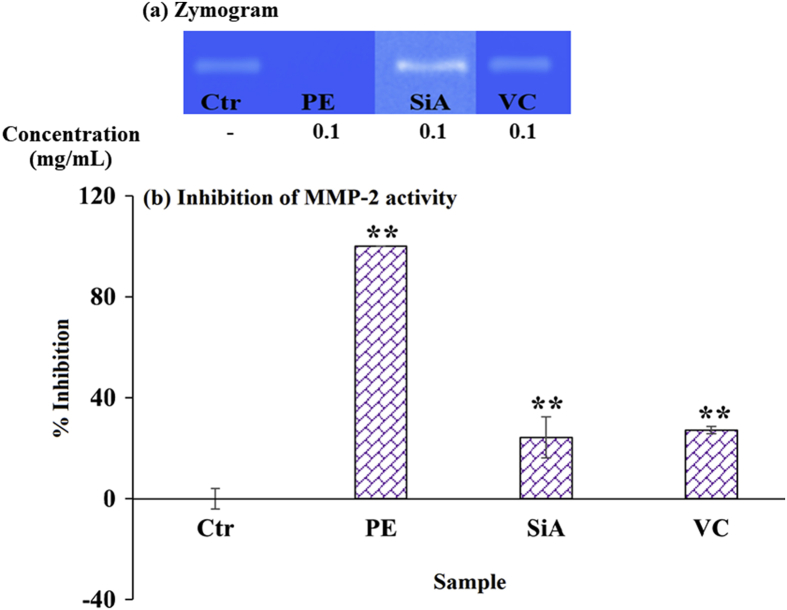
MMPs, the zinc-dependent endopeptidases, can degrade extracellular matrices and lead to the turnover and remodeling of tissue. In aging skin, the increased expression of MMPs results in the breakdown of fibrils, including collagen, gelatin, and elastin, and subsequently causes skin laxity and wrinkles. Among several classes of MMPs, MMP-2 takes its role in cleavage of dermal extracellular matrices, including basement membrane glycoproteins and proteoglycans.23 As shown in Fig. 5, amla branch extract exhibited the greatest MMP-2 inhibition compared to sinapic acid, and vitamin C.
Fig. 5.
MMP-2 inhibitory activity in human skin fibroblasts treated with solvent control (ctr), amla branch extract (PE), sinapic acid (SiA), and vitamin C (VC) at 0.1 mg/mL: (a) zymogram, and (b) inhibition of MMP-2 activity. ∗ indicates the significant difference from the control (∗p < 0.05, ∗∗p < 0.001).
The MMP deactivation is associated with specific zinc chelating agent at enzyme active site as well as non-specific inhibiting by natural substances, including plant polyphenols and terpenoids.23 The enzyme suppression of extract may be obtained from high phenolic content. The anti-inflammation and antioxidant of sinapic acid together with other phenolics, including quercetin, ferulic, p-coumaric, and gallic acids, are associated with the inhibition of MMP gene expression, resulting in prevention of enzyme substrate.17,23,29,47,49
3.4. Topical gel formulations
As amla branch extract exhibited several in vitro and cellular anti-skin aging effects, the topical gels, including base and PE gels, were prepared. For concentration of extract incorporated into topical formulation, it was the extrapolated value from the greatest noncytotoxic concentration. In cytotoxicity assay, the cells were directly exposed to the tested substance, but the percutaneous absorption would influence the topically applied formulation. Many factors affecting percutaneous absorption of substances, including base formulation, physicochemical properties, skin application sites, population variability, and skin surface conditions, are taken into consideration, thus, the extrapolated concentration of amla branch extract would be in the range of 0.05–0.1%.28,50 The previous study reported the extrapolated in vitro noncytotoxic concentration (0.1 mg/mL) to the relevant concentration (0.1 and 0.2%) added into cosmetic product is the safe concentration without induction of skin irritation in the clinical study.29 So, 0.1% was selected to add in the gel preparation.
Base gel was a colorless and viscous formulation, whereas PE gel was a slightly turbid, light brown, and more viscous formulation. The pH of PE gel (5.10 ± 0.10) was slightly less than base formulation (6.40 ± 0.10), owing to acidic characteristics of amla branch extract. After the completion of heating-cooling cycle, both formulations were observed the slight changes in physicochemical properties but were stable without phase separation (data shown in Table C). The chemical stability of extract was performed in the aqueous solution by measurement of TPC at initial and after 7 cycles of heating-cooling cycle. The decreased TPC of extract was observed but appeared to be the non-significant change (p-value = 0.33) compared to the initial content (data not shown). This result indicated the stable phenolic contents in extract over time.29
3.5. Clinical assays
3.5.1. Safety test
The safety test was performed in 20 female volunteers by a single closed patch test. During 24 h of patch application and after patch removal, all volunteers did not report and show any sign accompanied by skin erythema and edema on all treated areas. The results indicated that base and PE gels were nonirritating products with MII value of 0.
3.5.2. Analysis of clinical anti-skin aging efficacy
To investigate clinical efficacy, 20 female volunteers aged 25–50 years were enrolled in the study. According to the different ages of included volunteers, the analysis of clinical data was classified into 2 groups, including mild aging and moderate aging. The mild aging group was recruited 10 volunteers aged 25–29 years (average age 25.60 ± 0.64 years), whereas the moderate aging group was included 10 volunteers aged 30–50 years (average age 40.20 ± 2.34 years). The non-invasive instruments were employed to determine effects of gel application at baseline, and post-application at 14, 28, 42, 56, 70, and 84 days. Throughout the study, all volunteers did not report any adverse events, confirming the safety of base and PE gel. Clinical efficacy and subjective satisfaction assessment after base and PE gel treatment in the mild and moderate aging groups are shown as percent change in Fig. 6. The skin measurement in clinical anti-skin aging efficacy presents in Table D.
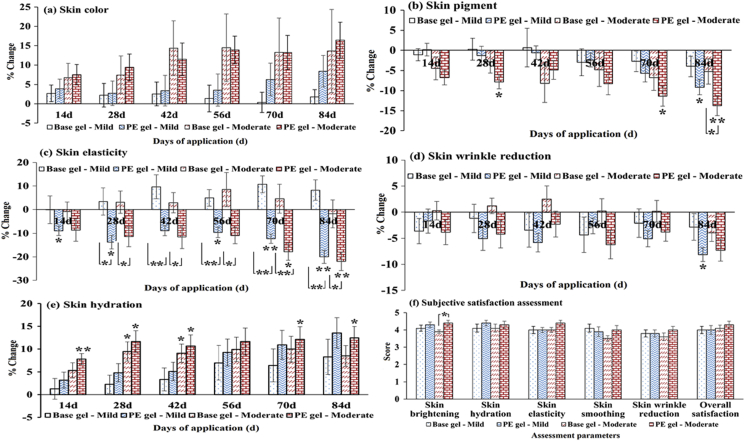
Fig. 6.
Clinical anti-skin aging efficacy of base and PE gels: (a) skin color, (b) skin pigment, (c) skin elasticity, (d) skin wrinkle reduction, (e) skin hydration, and (f) subjective assessment at the completion of study period. ∗ indicates the significant difference from the baseline (∗p < 0.05, ∗∗p < 0.001).
3.5.2.1. Skin color
Chromameter® CR 400 was used for skin color assessment. The measured L∗a∗b∗ values, which indicated the skin lightness, red/green component, and blue/yellow component, respectively, were converted into the individual typology angle (ITA°), an index for determining overall skin color degree of a subject. The ITA° values in both mild and moderate aging groups at baseline were not different. The ITA° values indicated the tan to intermediate skin color in the included volunteers.51 The greater ITA° value, showing the lighter skin color, was observed after treatment with base and PE gels for 14 days in both mild and moderate aging groups. At the completion of study, the increased ITA° was greater when treated with PE gel in both aging groups, but there was no statistical difference.
3.5.2.2. Skin pigment
The baseline melanin indexes of volunteers included in the mild and moderate aging groups were not different. According to Fitzpatrick skin type, the skin color of volunteers was classified as skin type III.52 After application of base and PE gels, a decrease in melanin indexes was observed in the mild and moderate aging groups. Compared to baseline, PE gel significantly reduced the skin pigment in both aging groups, but the effect was found in the moderate aging group in the shorter time of application (28 days, p = 0.031) compared with the mild aging group (84 days, p = 0.018). At the completion of study, PE gel significantly decreased skin pigment greater than base gel (p = 0.048).
3.5.2.3. Skin elasticity
The skin elasticity analyzed by Dermalab® USB elasticity probe measures the retraction time after skin stretched by pressure within the vacuum chamber in the device.53 The improved skin elasticity corresponds to the shorter retraction time. Among mild and moderate aging groups, the retraction times at baseline were significantly different, owing the aging characteristics of volunteers. Compared to baseline, PE gel significantly shortened the retraction times, resulting in the enhanced skin elasticity after 14 (p = 0.036) and 70 (p = 0.019) days of application in the mild and moderate aging groups, respectively. Base gel demonstrated the change in retraction times, but there was no significant difference. The significant improvement in skin elasticity after PE gel treatment was found after 28 days of application in both mild (p = 0.016) and moderate (p = 0.035) aging groups.
3.5.2.4. Skin wrinkle reduction
Skin wrinkle (SEw) values determined by Visioscan® VC 98 in mild and moderate aging groups at baseline were not different. After treatment with base and PE gels, the decrease in skin wrinkles was observed. The significant skin wrinkle reduction was found after 84 days of PE gel treatment in the mild aging group. At the completion of study, the skin wrinkle reduction in PE gel were greater than base gel, but there was no significant difference.
3.5.2.5. Skin hydration
Skin hydration values measured by Corneometer® CM 825 in the mild and moderate aging groups at baseline were not different. The improved skin hydration in both mild and moderate aging groups were observed when treated with base and PE gels. The significantly increased skin hydration was found only in the moderate aging group after treatment with base and PE gels for 28 (p = 0.032) and 14 (p = 0.002) days, respectively. There was no significant difference in skin hydration among two formulations.
3.5.2.6. Subjective satisfaction assessment
After completion of study, the 5-point scale questionnaires of subjective assessment were completed by volunteers to compare the satisfaction on base and PE gels. The results showed that the overall satisfaction scores for PE gel were 4.00 ± 0.26 and 4.30 ± 0.21 in the mild and moderate aging groups, respectively. The satisfaction scores for base and PE gels were comparable in the mild aging group, whereas those were different in the moderate aging group. The skin brightening score for PE gel in this group was significantly greater than base gel (p = 0.026). The satisfaction scores for other parameters after PE gel treatment were greater than that of base gel treatment, but there was no significant difference. However, the slight difference in the overall satisfaction score among base and PE gels may be due to the slight changes in skin conditions that were unnoticeable by volunteers’ perception.
According to clinical assessment, several non-invasive instruments were employed to evaluate the anti-skin aging efficacy of prepared formulations. Skin color was determined by 2 devices, including Chromameter® CR 400 measured by the CIE L∗a∗b∗ color system and Mexameter® MX 18 measured by the narrow-band simple reflectance meter. The strong correlation between ITA° and melanin indexes have been reported to assess the skin pigmentation.52 The greater ITA° and less melanin indexes than baseline after application of PE gel in both mild and moderate aging groups indicated the lightening skin color. Additionally, volunteers in the moderate aging group significantly noted the change in skin color after PE gel application, thereby leading to the greater skin brightening score than base gel in the subjective assessment. The skin lightening effect in clinical study was in accordance with in vitro anti-tyrosinase and cellular anti-melanogenesis effects. According to literature, the aging skin is associated with the loss of skin hydration and elasticity as well as the appearance of skin wrinkles and deep furrows.54 The determination of skin elasticity, wrinkles and hydration in clinical study was performed to investigate the anti-skin aging efficacy of PE gel compared to base gel. The improvement in skin elasticity, wrinkles and hydration after PE gel treatment was observed, indicating the younger skin properties. The clinically relevant improvement of skin elasticity and wrinkles were associated with the strong antioxidant and the MMP inhibitory activities of extract in vitro and cellular assays. Sinapic acid, a major component, together with other phenolic acids in extract may synergistically act on the anti-skin aging effects.1,29,47 The significantly increased skin hydration after treatment with base and PE gels was found only in the moderate aging group. Since dry skin is a prominent clinical manifestation of skin aging, the application of moisturizing product would make an effort to moisturize the skin.55 The similar skin hydration effect after treatment with base and PE gels may be attributed to the incorporation of humectants, including propylene glycol and glycerin, in the formulation.
4. Conclusions
Amla branch extract consisted of a variety of phenolics, mainly sinapic and ferulic acids, demonstrated the biological activities in vitro, cellular, and clinical assays against skin aging. The viabilities of cells treated with 0.1 mg/mL extract, the greatest noncytotoxic concentration, were 91.21 ± 3.33 and 89.98 ± 2.11% in B16F10 melanoma cells and human skin fibroblasts, respectively. The in vitro and cellular anti-skin aging results, including antioxidant, anti-melanogenesis, and MMP-2 inhibitory activities, were strongly associated with the improved skin conditions in clinical trial. The lightening skin color, the enhanced skin elasticity and hydration, and the reduced skin wrinkles after PE gel application were correlated to younger skin properties. The study results suggested that amla branch can be a potential ingredient for utilization in anti-skin aging products.
CRedit authorship contribution statement
Puxvadee Chaikul: Methodology, Software, Investigation, Writing-original draft, review, and editing, Funding acquisition.
Jariya Somkumnerd: Methodology, Software, Investigation.
Mayuree Kanlayavattanakul: Methodology, Investigation, Writing-review and editing.
Nattaya Lourith: Methodology, Investigation, Writing-review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Mae Fah Luang University, Thailand [grant numbers 621B02014, 2019].
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.02.004.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Fujii T., Wakaizumi M., Ikami T., Saito M. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J Ethnopharmacol. 2008;119:53–57. doi: 10.1016/j.jep.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Ekanayake E., Feng M., Murindahabi T., Nissanka A., Patrick G. Contribution of Indian gooseberry (Phyllanthus emblica) to household economy in Sri Lanka: a case study from Udadumbara Divisional Secretariat. Small-Scale For. 2018;17:277–292. [Google Scholar]
- 3.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaveni M., Mirunalini S. Chemopreventive efficacy of Phyllanthus emblica L.(amla) fruit extract on 7, 12-dimethylbenz (a) anthracene induced oral carcinogenesis–A dose–response study. Environ Toxicol Pharmacol. 2012;34:801–810. doi: 10.1016/j.etap.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Vadde R., Radhakrishnan S., Eranda Karunathilake Kurundu H., Reddivari L., Vanamala J.K.P. Indian gooseberry (Emblica officinalis Gaertn.) suppresses cell proliferation and induces apoptosis in human colon cancer stem cells independent of p53 status via suppression of c-Myc and cyclin D1. J Funct Foods. 2016;25:267–278. [Google Scholar]
- 6.Kasabri V., Flatt P., Abdel-Wahab Y. Emblica officinalis stimulates the secretion and action of insulin and inhibits starch digestion and protein glycation in vitro. Eur J Med Plants. 2014;4:753–770. doi: 10.1017/S0007114509991577. [DOI] [PubMed] [Google Scholar]
- 7.Rao T.P., Okamoto T., Akita N., Hayashi T., Kato-Yasuda N., Suzuki K. Amla (Emblica officinalis Gaertn.) extract inhibits lipopolysaccharide-induced procoagulant and pro-inflammatory factors in cultured vascular endothelial cells. Br J Nutr. 2013;110:2201–2206. doi: 10.1017/S0007114513001669. [DOI] [PubMed] [Google Scholar]
- 8.Middha S.K., Goyal A.K., Lokesh P. Toxicological evaluation of Emblica officinalis fruit extract and its anti-inflammatory and free radical scavenging properties. Phcog Mag. 2015;11:S427. doi: 10.4103/0973-1296.168982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrotra S., Srivastava A.K. Comparative antimicrobial activities of neem, amla, aloe, Assam tea and clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. J Med Plants Res. 2010;4:2393–2398. [Google Scholar]
- 10.Al-Rehaily A.J., Al-Howiriny T.A., Al-Sohaibani M.O., Rafatullah S. Gastroprotective effects of “Amla” Emblica officinalis on in vivo test models in rats. Phytomedicine. 2002;9:515–522. doi: 10.1078/09447110260573146. [DOI] [PubMed] [Google Scholar]
- 11.Mehmood M.H., Rehman A., Nu Rehman, Gilani A.H. Studies on prokinetic, laxative and spasmodic activities of Phyllanthus emblica in experimental animals. Phytother Res. 2013;27:1054–1060. doi: 10.1002/ptr.4821. [DOI] [PubMed] [Google Scholar]
- 12.Yokozawa T., Kim H.Y., Kim H.J., Okubo T., Chu D.-C., Juneja L.R. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br J Nutr. 2007;97:1187–1195. doi: 10.1017/S0007114507691971. [DOI] [PubMed] [Google Scholar]
- 13.Pathak P., Prasad B.R.G., Murthy N.A., Hegde S.N. The effect of Emblica officinalis diet on lifespan, sexual behavior, and fitness characters in Drosophila melanogaster. Ayu. 2011;32:279–284. doi: 10.4103/0974-8520.92544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawal S., Singh P., Gupta A., Mohanty S. Dietary intake of Curcuma longa and Emblica officinalis increases life span in Drosophila melanogaster. BioMed Res Int. 2014;2014:910290. doi: 10.1155/2014/910290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokozawa T., Kim H.Y., Kim H.J. Amla (Emblica officinalis Gaertn.) attenuates age-related renal dysfunction by oxidative stress. J Agric Food Chem. 2007;55:7744–7752. doi: 10.1021/jf072105s. [DOI] [PubMed] [Google Scholar]
- 16.Kumar G.S., Nayaka H., Dharmesh S.M., Salimath P.V. Free and bound phenolic antioxidants in amla (Emblica officinalis) and turmeric (Curcuma longa) J Food Compos Anal. 2006;19:446–452. [Google Scholar]
- 17.Pientaweeratch S., Panapisal V., Tansirikongkol A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: an in vitro comparative study for anti-aging applications. Pharm Biol. 2016;54:1865–1872. doi: 10.3109/13880209.2015.1133658. [DOI] [PubMed] [Google Scholar]
- 18.Variya B.C., Bakrania A.K., Patel S.S. Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res. 2016;111:180–200. doi: 10.1016/j.phrs.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Sripanidkulchai B., Junlatat J. Bioactivities of alcohol based extracts of Phyllanthus emblica branches: antioxidation, antimelanogenesis and anti-inflammation. J Nat Med. 2014;68:615–622. doi: 10.1007/s11418-014-0824-1. [DOI] [PubMed] [Google Scholar]
- 20.Qi W.-Y., Li Y., Hua L., Wang K., Gao K. Cytotoxicity and structure activity relationships of phytosterol from Phyllanthus emblica. Fitoterapia. 2013;84:252–256. doi: 10.1016/j.fitote.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Iamsaard S., Arun S., Burawat J. Phyllanthus emblica L. branch extract ameliorates testicular damage in valproic acid-induced rats. Int J Morphol. 2015;33:1016–1022. [Google Scholar]
- 22.Sripanidkulchai B., Fangkrathok N. Antioxidant, antimutagenic and antibacterial activities of extracts from Phyllanthus emblica branches. Songklanakarin J Sci Technol. 2014;36:669–674. [Google Scholar]
- 23.Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 24.Verschoore M., Nielson M. The Rationale of anti-aging cosmetic ingredients. J Drugs Dermatol. 2017;16:s94–s97. [PubMed] [Google Scholar]
- 25.Gao L., Kang H., Li Y. Clinical efficacy and safety of 3DEEP multisource radiofrequency therapy combined with fractional skin resurfacing for periocular skin aging. J Clin Aesthet Dermatol. 2020;13:41–44. [PMC free article] [PubMed] [Google Scholar]
- 26.Vaiserman A.M., Lushchak V., Koliada A.K. Anti-aging pharmacology: promises and pitfalls. Ageing Res Rev. 2016;31:9–35. doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Calasanti T., King N., Pietilä I., Ojala H. Rationales for anti-aging activities in middle age: aging, health, or appearance? Gerontol. 2018;58:233–241. doi: 10.1093/geront/gnw111. [DOI] [PubMed] [Google Scholar]
- 28.Chaikul P., Lourith N., Kanlayavattanakul M. Antimelanogenesis and cellular antioxidant activities of rubber (Hevea brasiliensis) seed oil for cosmetics. Ind Crop Prod. 2017;108:56–62. [Google Scholar]
- 29.Kanlayavattanakul M., Lourith N., Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607–616. doi: 10.1016/j.jep.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Kanlayavattanakul M., Lourith N., Tadtong S., Jongrungruangchok S. Rice panicles: new promising unconventional cereal product for health benefits. J Cereal Sci. 2015;66:10–17. [Google Scholar]
- 31.Lourith N., Kanlayavattanakul M., Chaikul P., Chansriniyom C., Bunwatcharaphansakun P. In vitro and cellular activities of the selected fruits residues for skin aging treatment. An Acad Bras Cienc. 2017;89:577–589. doi: 10.1590/0001-3765201720160849. [DOI] [PubMed] [Google Scholar]
- 32.Miliauskas G., Venskutonis P.R., van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. [Google Scholar]
- 33.Sonkar N., Rajoriya D., Chetana R., Murthy K.V. Effect of cultivars, pretreatment and drying on physicochemical properties of Amla (Emblica officinalis) gratings. J Food Sci Technol. 2020;57:980–992. doi: 10.1007/s13197-019-04131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilar-Hernández I., Afseth N.K., López-Luke T., Contreras-Torres F.F., Wold J.P., Ornelas-Soto N. Surface enhanced Raman spectroscopy of phenolic antioxidants: a systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vib Spectrosc. 2017;89:113–122. [Google Scholar]
- 35.Mishra P., Dutta N., Mahanta C.L. Partial extraction and identification of phenolics in Amla (Emblica officinalis) seed coat powder. J Food Sci Technol. 2015;52:6990–7001. [Google Scholar]
- 36.Ismail H.F., Hashim Z., Soon W.T., Rahman N.S.A., Zainudin A.N., Majid F.A.A. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J Tradit Complement Med. 2017;7:452–465. doi: 10.1016/j.jtcme.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nićiforović N., Abramovič H. Sinapic acid and its derivatives: natural sources and bioactivity. Compr Rev Food Sci F. 2014;13:34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 38.Panwar R., Sharma A.K., Kaloti M., Dutt D., Pruthi V. Characterization and anticancer potential of ferulic acid-loaded chitosan nanoparticles against ME-180 human cervical cancer cell lines. Appl Nanosci. 2016;6:803–813. [Google Scholar]
- 39.Chaikul P., Khat-udomkiri N., Iangthanarat K., Manosroi J., Manosroi A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur J Pharmaceut Sci. 2019;131:39–49. doi: 10.1016/j.ejps.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Slotkin T.A., Seidler F.J. Antimitotic and cytotoxic effects of theophylline in MDA-MB-231 human breast cancer cells. Breast Canc Res Treat. 2000;64:259–267. doi: 10.1023/a:1026508605951. [DOI] [PubMed] [Google Scholar]
- 41.Haddad M.M., Xu W., Medrano E.E. Aging in epidermal melanocytes: cell cycle genes and melanins. J Invest Dermatol Symp Proc. 1998;3:36–40. [PubMed] [Google Scholar]
- 42.Choi S.W., GM Sapers. Purpling reaction of sinapic acid model systems containing L-DOPA and mushroom tyrosinase. J Agric Food Chem. 1994;42:1183–1189. [Google Scholar]
- 43.Hu F. Theophylline and melanocyte-stimulating hormone effects on gamma-glutamyl transpeptidase and DOPA reactions in cultured melanoma cells. J Invest Dermatol. 1982;79:57–62. doi: 10.1111/1523-1747.ep12510659. [DOI] [PubMed] [Google Scholar]
- 44.Cabanes J., Chazarra S., Garcia-Carmona F. KojicAcid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J Pharm Pharmacol. 1994;46:982–985. doi: 10.1111/j.2042-7158.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 45.Whittemore E.R., Loo D.T., Watt J.A., Cotmans C.W. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience. 1995;67:921–932. doi: 10.1016/0306-4522(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 46.McDaniel D., Farris P., Valacchi G. Atmospheric skin aging—contributors and inhibitors. J Cosmet Dermatol. 2018;17:124–137. doi: 10.1111/jocd.12518. [DOI] [PubMed] [Google Scholar]
- 47.Chen C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid Med Cell Longev. 2016;2016:3571614. doi: 10.1155/2016/3571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bendich A., Machlin L.J., Scandurra O., Burton G.W., Wayner D.D.M. The antioxidant role of vitamin C. Adv Free Radical Biol. 1986;2:419–444. [Google Scholar]
- 49.Pei K., Ou J., Huang J., Ou S. p-Coumaric acid and its conjugates: dietarysources, pharmacokinetic propertiesand biological activities. J Sci Food Agric. 2016;96:2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 50.Law R.M., Ngo M.A., Maibach H.I. Twenty clinically pertinent factors/observations for percutaneous absorption in humans. Am J Clin Dermatol. 2020;21:85–95. doi: 10.1007/s40257-019-00480-4. [DOI] [PubMed] [Google Scholar]
- 51.Del Bino S., Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 2013;169:33–40. doi: 10.1111/bjd.12529. [DOI] [PubMed] [Google Scholar]
- 52.Wilkes M., Wright C.Y., du Plessis J.L., Reeder A. Fitzpatrick skin type, individual typology angle, and melanin index in an African population: steps toward universally applicable skin photosensitivity assessments. JAMA Dermatol. 2015;151:902–903. doi: 10.1001/jamadermatol.2015.0351. [DOI] [PubMed] [Google Scholar]
- 53.Willey A., Kilmer S., Newman J. Elastometry and clinical results after bipolar radiofrequency treatment of skin. Dermatol Surg. 2010;36:877–884. doi: 10.1111/j.1524-4725.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 54.Choi J.W., Kwon S.H., Huh C.H., Park K.C., Youn S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19:e349–e355. doi: 10.1111/j.1600-0846.2012.00650.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee D.E., Huh C.-S., Ra J. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: a randomized, double blind, placebo-controlled study. J Microbiol Biotechnol. 2015;25:2160–2168. doi: 10.4014/jmb.1509.09021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.