Summary
Background
Activating mutations of EZH2, an epigenetic regulator, are present in approximately 20% of patients with follicular lymphoma. We investigated the activity and safety of tazemetostat, a first-in-class, oral EZH2 inhibitor, in patients with follicular lymphoma.
Methods
This study was an open-label, single-arm, phase 2 trial done at 38 clinics or hospitals in France, the UK, Australia, Canada, Poland, Italy, Ukraine, Germany, and the USA. Eligible patients were adults (≥18 years) with histologically confirmed follicular lymphoma (grade 1, 2, 3a, or 3b) that had relapsed or was refractory to two or more systemic therapies, had an Eastern Cooperative Oncology Group performance status of 0–2, and had sufficient tumour tissue for central testing of EZH2 mutation status. Patients were categorised by EZH2 status: mutant (EZH2mut) or wild-type (EZH2WT). Patients received 800 mg of tazemetostat orally twice per day in continuous 28-day cycles. The primary endpoint was objective response rate based on the 2007 International Working Group criteria for non-Hodgkin lymphoma, assessed by an independent radiology committee. Activity and safety analyses were done in patients who received one dose or more of tazemetostat. This study is registered with ClinicalTrials.gov, NCT01897571, and follow-up is ongoing.
Findings
Between July 9, 2015, and May 24, 2019, 99 patients (45 in the EZH2mut cohort and 54 in the EZH2WT cohort) were enrolled in the study. At data cutoff for the analysis (Aug 9, 2019), the median follow-up was 22·0 months (IQR 12·0–26·7) for the EZH2mut cohort and 35·9 months (24·9–40·5) for the EZH2WT cohort. The objective response rate was 69% (95% CI 53–82; 31 of 45 patients) in the EZH2mut cohort and 35% (23–49; 19 of 54 patients) in the EZH2WT cohort. Median duration of response was 10·9 months (95% CI 7·2–not estimable [NE]) in the EZH2mut cohort and 13·0 months (5·6–NE) in the EZH2WT cohort; median progression-free survival was 13·8 months (10·7–22·0) and 11·1 months (3·7–14·6). Among all 99 patients, treatment-related grade 3 or worse adverse events included thrombocytopenia (three [3%]), neutropenia (three [3%]), and anaemia (two [2%]). Serious treatment-related adverse events were reported in four (4%) of 99 patients. There were no treatment-related deaths.
Interpretation
Tazemetostat monotherapy showed clinically meaningful, durable responses and was generally well tolerated in heavily pretreated patients with relapsed or refractory follicular lymphoma. Tazemetostat is a novel treatment for patients with follicular lymphoma.
Funding
Epizyme.
Introduction
Follicular lymphoma is the most common indolent non-Hodgkin lymphoma, accounting for approximately 20% of all cases.1,2 Initial systemic treatment consists of anti-CD20-based chemoimmunotherapy,2–4 and the clinical course is remission followed by relapse in most patients.2,5 Despite the approval of new therapies for recurring follicular lymphoma, patients continue to relapse, indicating a continued need for novel therapeutic approaches.
Follicular lymphoma arises from germinal centre B cells. EZH2, a histone methyltransferase, is essential to the formation of the germinal centre, as the enzyme represses the transcription of genes that would otherwise limit B-cell proliferation and promote exit from the germinal centre.6 Gain-of-function mutations in the enzymatic domain of EZH2 are present in approximately 20% of patients with follicular lymphoma or diffuse large B-cell lymphoma derived from the germinal centre.7–9 Such activating mutations lead to the aberrant trimethylation of lysine 27 in histone 3 (H3K27me3),6,10,11 and the spread of H3K27me3 at target loci results in epigenetic silencing and maintenance of the germinal centre. This epigenetic silencing, in turn, allows B cells to proliferate and malignant clones to accumulate.10,12 EZH2 is also involved in tumour immune escape mechanisms, such as suppressing antigen presentation and preventing the trafficking of immune effector cells to the tumour microenvironment.13–15
By contrast with EZH2, which mediates transcriptional repression, CREB-binding protein (CREBBP) and KMT2D promote transcription. CREBBP and KMT2D are genetically altered in 60–75% of patients with follicular lymphoma.16–18 Somatic loss-of-function mutations in CREBBP or KMT2D result in a failure to activate the transcription of genes that promote B-cell exit from the germinal centre,17,19 enabling persistence of the germinal centre and accumulation of malignant germinal centre B cells. Given the crucial role of EZH2 in germinal centre formation, even patients with follicular lymphoma characterised by wild-type EZH2 (EZH2WT) are inherently reliant on this protein. Since follicular lymphomas with either EZH2WT or mutant EZH2 (EZH2mut) are dependent on EZH2, EZH2 represents a new therapeutic target in the treatment of follicular lymphoma.
Tazemetostat is a first-in-class, selective, oral inhibitor of mutant and wild-type EZH2 that is under clinical investigation for the treatment of non-Hodgkin lymphoma. In a phase 1 trial, monotherapy with tazemetostat showed anticancer activity and a favourable safety profile in patients with relapsed or refractory non-Hodgkin lymphoma.20 We aimed to investigate the activity and safety of tazemetostat in patients with either EZH2WT or EZH2mut follicular lymphoma.
Methods
Study design and participants
This study was an open-label, single-arm, phase 2 trial done at 38 clinics or hospitals in France, the UK, Australia, Canada, Poland, Italy, Ukraine, Germany, and the USA (appendix p 3). Patients with either EZH2mut or EZH2WT follicular lymphoma were enrolled into one of two follicular lymphoma cohorts (out of a total of six cohorts in the study; appendix p 10); these data are presented herein. Results from the other cohorts will be reported separately.
Patients were eligible if they were aged 18 years or older and had histologically confirmed follicular lymphoma (grade 1, 2, 3a, or 3b) that had relapsed or was refractory to two or more standard systemic therapies, measurable disease per the International Working Group criteria for non-Hodgkin lymphoma (IWG-NHL),21 and sufficient tissue available for central testing of EZH2 mutation status. To be eligible, patients had to have histological confirmation based on the local pathology report confirmed at initial diagnosis or in one previous line of therapy, and biopsy was not mandatory at baseline. Patients could have transformed follicular lymphoma (appendix p 1). Inclusion criteria also required an Eastern Cooperative Oncology Group performance status of 0–2, a life expectancy of 3 months or more, and adequate renal (calculated creatinine clearance of ≥40 mL/min by use of the Cockcroft-Gault formula), bone marrow (absolute neutrophil count ≥750 cells per µL, platelet count ≥75 000 platelets per µL, and a blood concentration of haemoglobin ≥90 g/L), and liver (total blood bilirubin concentration ≤1·5 × the upper limit of normal [ULN] and blood concentrations of alkaline phosphatase and alanine aminotransferase ≤3 × ULN [≤5 × ULN if the patient had liver metastases]) function. To receive the first dose of tazemetostat, patients must not have had cytotoxic chemotherapy in the preceding 21 days, non-cytotoxic chemotherapy or local site radiotherapy in the preceding 14 days, or monoclonal antibodies in the preceding 28 days. Key exclusion criteria included the presence of non-cutaneous malignancies other than B-cell lymphoma, leptomeningeal or brain metastases, or thrombocytopenia, neutropenia, or anaemia of grade 3 or more.
This study, which was initiated by Eisai and completed by Epizyme, was done in compliance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable national and local regulatory requirements. Each study site obtained ethical approval from institutional review boards or independent ethics commitees; the protocol can be found in the appendix. All patients provided written informed consent.
Procedures
Eligible patients received 800 mg of tazemetostat orally twice per day in continuous 28-day cycles until confirmed disease progression, unacceptable toxicity, withdrawal of consent, or for up to 2 years of treatment. Patients who received 2 years of therapy were eligible to continue treatment in a roll-over study (TRuST; NCT02875548). Dose reductions and interruptions of tazemetostat were permitted (appendix p 4).
Central EZH2 testing was done by use of the cobas EZH2 Mutation Test (Roche Molecular Systems, Pleasanton, CA, USA). The specific tissue type or source was not protocol-mandated; however, most EZH2 testing was done on formalin-fixed paraffin-embedded tissue from a resection or excisional lymph node biopsy. Additional details regarding EZH2 testing are described in the appendix (p 1). Patients underwent CT (or MRI) scans before the administration of the first dose of tazemetostat, then every 8 weeks up to month 6, and every 12 weeks thereafter. A whole body ¹⁸F-fluorodeoxyglucose (FDG) PET scan was recommended at first indication of a possible complete or partial response. If a bone marrow biopsy was done at baseline, the biopsy was repeated upon suspicion of disease progression or relapse or at first indication of a complete response if bone marrow involvement was present at screening. Tumour responses were assessed by an independent radiology committee and investigators per the IWG-NHL response criteria.21 Laboratory tests, including analyses of haematology, electrolyte chemistry, liver function, renal function, and urine, were completed before tazemetostat administration on days 1 and 15 of each cycle. Treatment-emergent adverse events were graded per the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03 and were evaluated throughout the study.
Outcomes
The primary endpoint was objective response rate based on the IWG-NHL 2007, defined as the proportion of patients with a best overall response of a complete or partial response.21 Determinations of the independent radiology committee were used for the primary evaluation of the primary endpoint, with investigator assessments considered supportive. Secondary endpoints were the duration of response (defined as the time from first complete or partial response to recurrence, objectively documented disease progression, or death) and progression-free survival (defined as the time from the first study dose to the first documented relapse, disease progression, or death). Safety and tolerability were also evaluated as secondary endpoints. The prespecified, exploratory endpoints were overall survival (defined as the time from the date of the first dose of study drug until the date of death from any cause), disease control rate (defined as the proportion of patients who had either a confirmed complete response or partial response of any duration or who had stable disease lasting at least 12, 18, or 24 months from the start of the study drug treatment), and time to first response (defined as the time from the date of the first dose of study drug until the date of first response). Exploratory pharmacokinetic, pharmacodynamic, and biomarker analyses will be reported separately. Pharmacokinetic data from the phase 1 study have been reported.20
Statistical analysis
We calculated the final sample size for each cohort of patients with follicular lymphoma using the modified two-stage Green-Dahlberg design, which allows for early cohort termination for futility. The first ten patients of each cohort were enrolled in stage one; if only one or no patients responded (complete or partial response) among these ten patients, cohort enrolment would be terminated for futility. If the futility boundary was surpassed, 35 additional patients were enrolled in stage two. The sample size, which was based on the primary endpoint (objective response rate), increased from an initially planned 30 patients to 45 patients when the protocol was amended on April 15, 2016, to increase the power of the study by use of the two-stage Green-Dahlberg design. A target enrolment of 45 patients per cohort provided a power of approximately 85% to test the alternative hypothesis that objective response rate would be 40% or more against the null hypothesis that objective response rate would be 20% or less, at a one-sided significance level of 0·025.
Activity and safety analyses were done in a modified intention-to-treat population, which comprised patients who received one dose or more of tazemetostat. Patients with confirmed transformed follicular lymphoma (appendix p 1) were included in the intention-to-treat population. Objective response rate was analysed per the intention-to-treat principle and summarised by use of the number and proportion of patients, and the Clopper-Pearson exact 95% binomial CI. The concordance between the independent radiology committee and investigator assessments of objective response rate and best overall response was calculated. We estimated duration of response, progression-free survival, and overall survival using the Kaplan–Meier method and report the median with associated two-sided 95% CIs. Overall disease control rate was calculated as the proportion of patients with a complete response, partial response, or stable disease. For each patient with a response, the time to first response (in months) was defined as the time from the date of the first dose of the study drug until the date of first response (either a complete response or a partial response, whichever came first). Objective response rate, duration of response, and progression-free survival were examined in subgroups based on baseline demographic and disease characteristics, including patients treated with immunochemotherapy who had had disease progression within 24 months of disease diagnosis (the POD24 subgroup), those who were double refractory (no objective response to any rituximab-containing therapy and relapsed within 6 months or refractory to any alkylator-based chemotherapy) or refractory to rituximab (no objective response to rituximab-containing therapy, or progressive disease within 6 months of completion of rituximab-containing therapy), and by number of lines of previous treatment, refractoriness, previous radiotherapy, tumour burden, sex, age, time since last treatment, region, and Groupe d’Etude des Lymphomes Folliculaires status (meeting at least one of the following criteria: target lesion >7 cm in diameter, three nodal target lesions >3 cm in diameter each, B symptoms at baseline, serum concentration of lactate dehydrogenase higher than the upper limit of normal, haemoglobin ≤10 g/dL, neutrophil count ≤1500 cells per μL, or platelet count ≤100 000 platelets per mL). In a post-hoc exploratory analysis, we evaluated the number of patients with a deepening response, defined as the number of patients who had a partial response before having a complete response. Analysis of objective response rate in the subgroup of patients who received previous treatment with a PI3K inhibitor or immunomodulatory drugs was not prespecified.
We summarised the incidence of treatment-emergent adverse events and treatment-related adverse events using descriptive statistics for all patients (irrespective of EZH2 mutational status). We used SAS (version 9.4) for all statistical analyses. Additional information is provided in the statistical analysis plan and protocol (appendix). This study is registered with ClinicalTrials. gov, NCT01897571. A data monitoring committee oversaw this study.
Role of the funding source
The sponsor (Epizyme) designed the study and collected the data. Epizyme was involved in data analysis and interpretation and employed a professional medical writer to assist in the writing of this report. All authors had access to the raw data, and the corresponding author had final responsibility for the decision to submit for publication.
Results
Between July 9, 2015, and May 24, 2019, 45 patients with EZH2mut follicular lymphoma and 54 patients with EZH2WT follicular lymphoma were enrolled, administered tazemetostat, and were included in the modified intention-to-treat population (appendix p 11). The data cutoff date for the primary analysis was Aug 9, 2019, and the data cutoff date for the safety analysis was May 24, 2019, with a median follow-up of 22·0 months (IQR 12·0–26·7) in the EZH2mut cohort and 35·9 months (24·9–40·5) in the EZH2WT cohort. Baseline patient characteristics are shown in table 1. 11 (24%) patients in the EZH2mut cohort and 21 (39%) patients in the EZH2WT cohort had relapsed after receiving a PI3K inhibitor or an immunomodulatory drug. Three patients had transformed follicular lymphoma, all of whom were in the EZH2WT cohort.
Table 1:
Baseline patient and disease characteristics
| EZH2mut (n=45) | EZH2WT (n=54) | |
|---|---|---|
| Age, years | 62 (57–68) | 61 (53–67) |
| Sex | ||
| Male | 19 (42%) | 34 (63%) |
| Female | 26 (58%) | 20 (37%) |
| ECOG performance status | ||
| 0 | 21 (47%) | 26 (48%) |
| 1 | 24 (53%) | 23 (43%) |
| 2 | 0 | 4 (7%) |
| Missing | 0 | 1 (2%) |
| Satisfied GELF criteria* | ||
| Yes | 31 (69%) | 40 (74%) |
| No | 14 (31%) | 14 (26%) |
| Time from initial diagnosis, years | 4·7 (1·7–6·4) | 6·3 (3·4–9·0) |
| Histology | ||
| Grade 1, 2, or 3a | 42 (93%) | 51 (94%)† |
| Grade 3b or transformed follicular lymphoma‡ | 3 (7%) | 6 (11%)† |
| Previous lines of anticancer therapy§ | ||
| One | 2 (4%) | 1 (2%) |
| Two | 22 (49%) | 16 (30%) |
| Three | 10 (22%) | 11 (20%) |
| Four | 4 (9%) | 10 (19%) |
| Five or more | 7 (16%) | 16 (30%) |
| Median | 2 (2–43) | 3 (2–5) |
| Refractory to last regimen¶ | 22 (49%) | 22 (41%) |
| Poor risk features | ||
| Refractory to a rituximab-containing regimen|| | 22 (49%) | 32 (59%) |
| Double refractory** | 9 (20%) | 15 (28%) |
| Previous haematopoietic stem-cell transplant | 4 (9%) | 21 (39%) |
| Disease progression within 24 months of disease diagnosis in patients treated with first-line immunochemotherapy (POD24) | 19 (42%) | 32 (59%) |
Data are median (IQR) or n (%). ECOG=Eastern Cooperative Oncology Group. GELF=Groupe d’Etude des Lymphomes Folliculaires.
Defined as a target lesion of more than 7 cm in diameter, three nodal target lesions of more than 3 cm in diameter each, the presence of B symptoms at baseline, a concentration of serum lactate dehydrogenase higher than the upper limit of normal, a serum haemoglobin concentration of 100 g/L or less, a neutrophil count of 1500 cells per µL or less, or a platelet count of 100 000 platelets per mL or less.
Some patients were counted in more than one category.
Patients with confirmed grade 3b or transformed follicular lymphoma following a central pathology review.
Excludes maintenance, consolidation, adjuvant, and neoadjuvant therapies when counted as their own line.
Patients with stable disease or progressive disease to the most recent previous anticancer therapy.
Refractory to either rituximab monotherapy or rituximab-containing therapy or progressive disease within 6 months of completion of rituximab-containing therapy.
Refractory to rituximab (as a monotherapy or as part of a combination therapy) and a chemotherapy induction regimen containing one or more alkylating agent or purine nucleoside antagonist and have relapsed within 6 months.
As of the final assessment of responses by the independent data monitoring committee for the interim futility analysis (September, 2016), four of five evaluable patients in the EZH2mut cohort had a response and two of ten evaluable patients in the EZH2WT cohort had a response. At data cutoff (Aug 9, 2019), the objective response rate assessed by the independent radiology committee was 69% (95% CI 53–82; 31 of 45 patients) in the EZH2mut cohort and 35% (23–49; 19 of 54 patients) in the EZH2WT cohort and included complete responses in six (13%) patients in the EZH2mut cohort and two (4%) patients in the EZH2WT cohort (table 2). Of the 42 patients who had a partial response assessed by the independent radiology committee, only eight (19%; four in the EZH2mut cohort and four in the EZH2WT cohort) had ¹⁸F-FDG PET scans; all eight remained in the partial response classification per the ¹⁸F-FDG PET scan assessment. Of the 50 patients who had a response in either cohort, three in the EZH2mut cohort and seven in the EZH2WT cohort subsequently had an autologous or allogenic haematopoietic stem-cell transplant. Concordance between the assessment of responses by the independent radiology committee and investigators was high (86 [87%] of 99 patients; table 2). 44 (98%) of 45 patients in the EZH2mut cohort and 35 (65%) of 54 patients in the EZH2WT cohort showed evidence of a reduction in tumour volume (figure 1). Five patients in the EZH2WT cohort were excluded from this analysis because tumour responses were not estimable, missing, or unknown. The reduction in tumour volume per investigator assessment is summarised in the appendix (pp 12–13).
Table 2:
Tumour response by EZH2 mutation status in the modified intention-to-treat population as assessed by the IRC and investigators
|
EZH2mut (n=45) |
EZH2WT (n=54) |
|||
|---|---|---|---|---|
| IRC-assessed | Investigator-assessed | IRC-assessed | Investigator-assessed | |
| Objective response rate* | 31 (69%; 53–82) | 35 (78%; 63–89) | 19 (35%; 23–49) | 18 (33%; 21–48) |
| Overall disease control rate† | 44 (98%) | 45 (100%) | 37 (69%) | 34 (63%) |
| Best overall response | ||||
| Complete response | 6 (13%) | 4 (9%) | 2 (4%) | 3 (6%) |
| Partial response | 25 (56%) | 31 (69%) | 17 (31%) | 15 (28%) |
| Stable disease | 13 (29%) | 10 (22%) | 18 (33%) | 16 (30%) |
| Progressive disease | 1 (2%) | 0 | 12 (22%) | 16 (30%) |
| Not estimable or unknown | 0 | 0 | 5 (9%) | 4 (7%) |
Data are n (%; 95% CI) or n (%). IRC=independent radiology committee.
Objective response rate includes patients with a complete or partial response.
Overall disease control rate includes patients with a complete response, partial response, or stable disease.
Figure 1: IRC-assessed change in tumour volume from baseline.
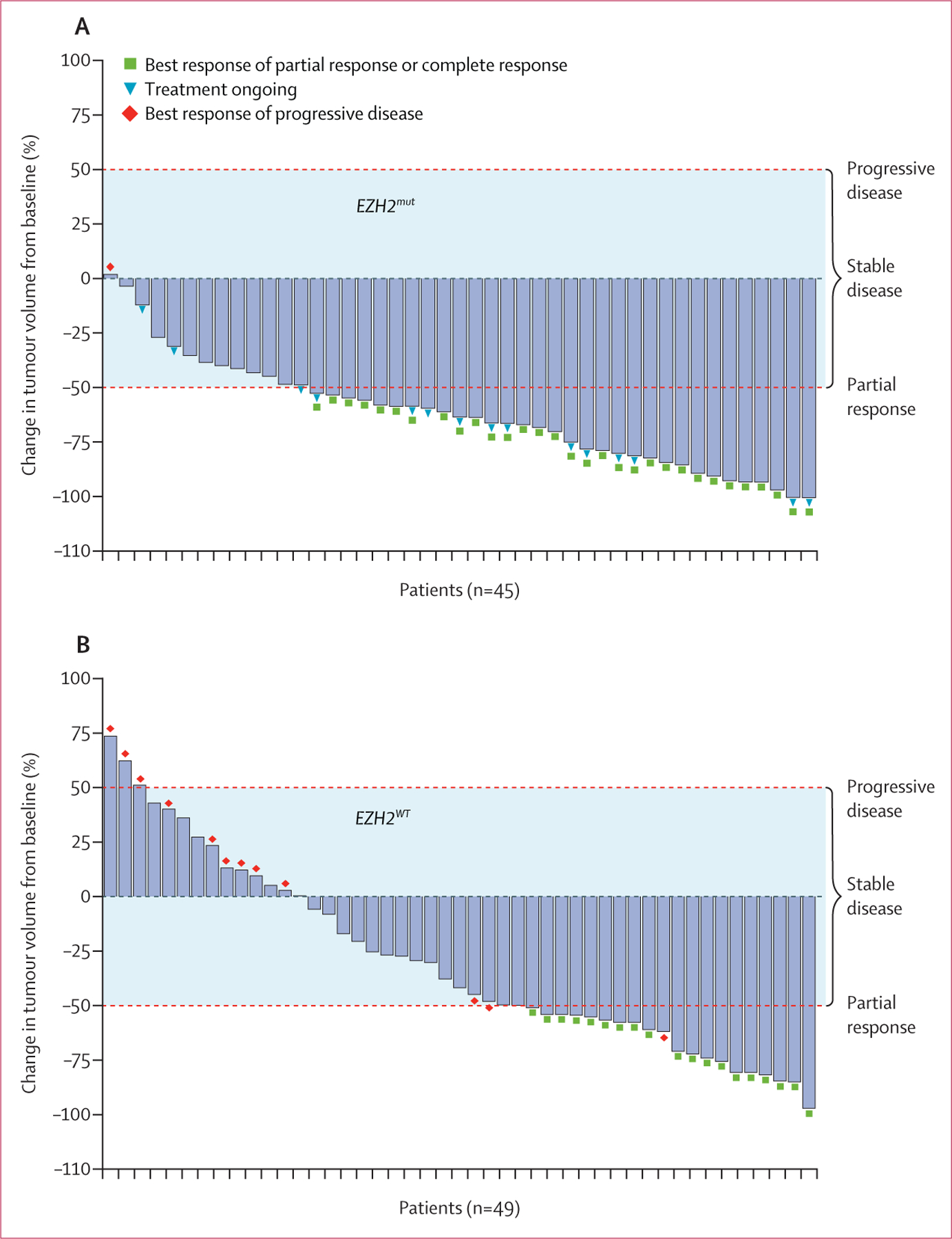
(A) Patients with EZH2mut follicular lymphoma. (B) Patients with EZH2WT follicular lymphoma. Tumour responses were not estimable, missing, or unknown in five patients in the EZH2WT cohort. Tumour volume was calculated as the sum of the product of perpendicular diameters according to the 2007 International Working Group criteria for non-Hodgkin lymphoma.21 Dashed red lines represent thresholds for progressive disease (≥50% increase in tumour volume) and partial response (≥50% reduction in tumour volume). The shaded area represents tumour volume changes that correspond to stable disease (<50% increase or decrease in tumour volume). IRC=independent radiology committee.
The median duration of response was 10·9 months (95% CI 7·2–not estimable [NE]) in the EZH2mut cohort (n=31) and 13·0 months (5·6–NE) in the EZH2WT cohort (n=19; figure 2A; appendix pp 14–15). Data on duration of response analysed in subgroups can be found in the appendix (pp 5–6). Of the 31 patients in the EZH2mut cohort who had an objective response, 19 (61%) had a response lasting for 6 months or more, seven (23%) for 12 months or more, and six (19%) for 18 months or more. Of the 19 patients in the EZH2WT cohort who had an objective response, ten (53%) had a response lasting for 6 months or more, seven (37%) for 12 months or more, and four (21%) for 18 months or more. 11 patients (three from the EZH2mut cohort and eight from the EZH2WT cohort) completed 2 years of treatment and entered the roll-over study (TRuST); two of these patients have since relapsed (one from the EZH2mut cohort and one from the EZH2WT cohort). Of these 11 patients, four had complete responses (two from the EZH2mut cohort and two from the EZH2WT cohort), five had partial responses (one from the EZH2mut cohort and four from the EZH2WT cohort), and two had stable disease (two from the EZH2WT cohort). Duration of response per investigator assessment is summarised in the appendix (pp 16–17). 25 patients in the EZH2mut cohort and 32 patients in the EZH2WT cohort had progression-free survival events. Median progression-free survival was 13·8 months (95% CI 10·7–22·0) for the EZH2mut cohort and 11·1 months (3·7–14·6) for the EZH2WT cohort (figure 2B). Data on progression-free survival in patient subgroups are summarised in the appendix (pp 5, 7).
Figure 2: IRC-assessed outcomes in the modified intention-to-treat population by EZH2 mutation status.
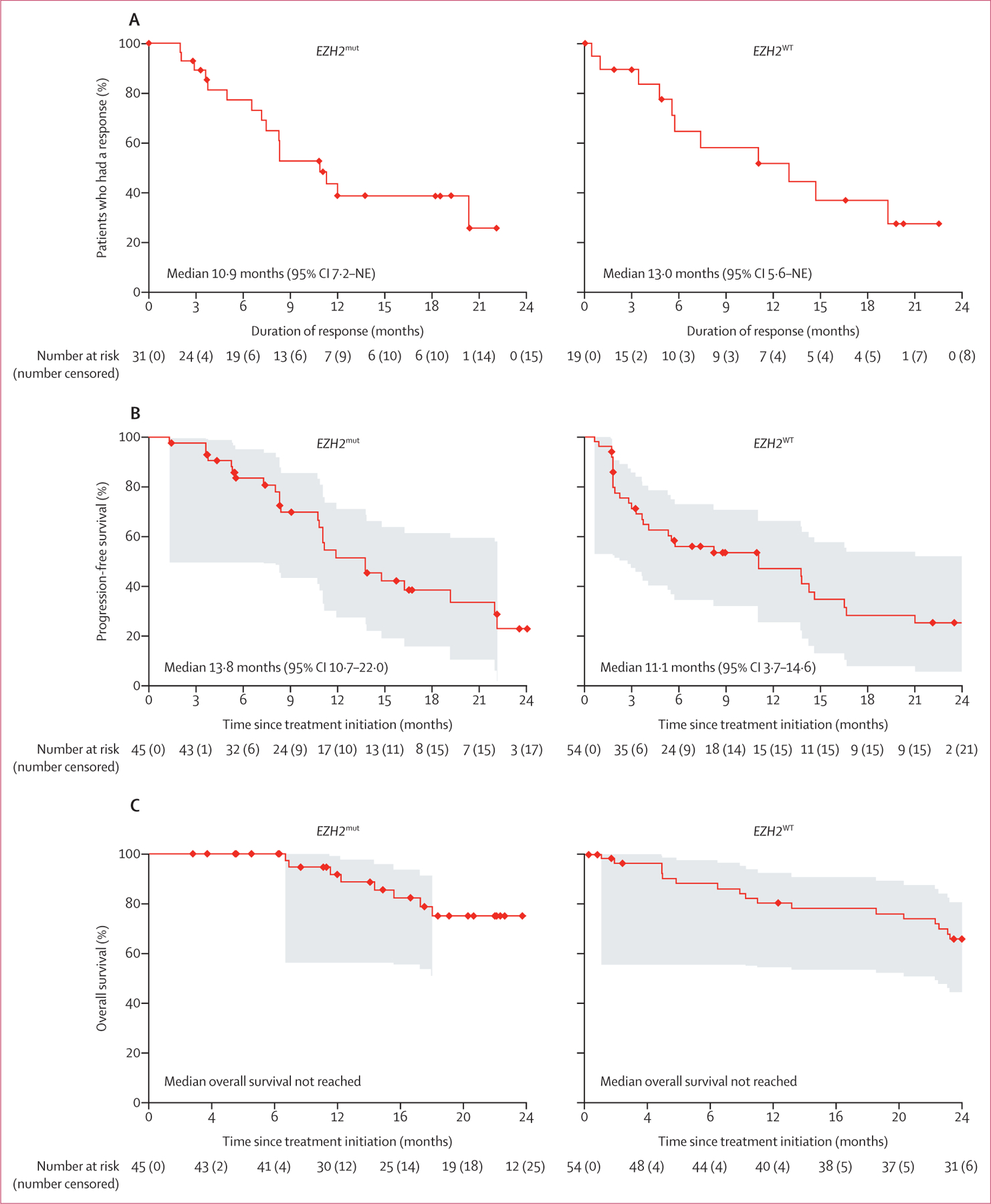
(A) Duration of response. (B) Progression-free survival. (C) Overall survival. The shaded areas represent 95% simultaneous confidence bands. IRC=independent radiology committee. NE=not estimable.
In prespecified exploratory analyses, the median time to first response was 3·7 months (IQR 1·9–5·5) in the EZH2mut cohort and 3·7 months (2·2–8·3) in the EZH2WT cohort. There were eight deaths in the EZH2mut cohort and 21 in the EZH2WT cohort; median overall survival was not reached in either cohort (95% CI was NE–NE in the EZH2mut cohort and 24·9–NE in the EZH2WT cohort; figure 2C). Overall disease control rate is reported in table 2.
Objective response rates in the POD24, double-refractory, and refractory to rituximab subgroups are in the appendix (p 5). Objective response in additional subgroups is shown in the appendix (p 6). Of the three patients with grade 3b or transformed follicular lymphoma in the EZH2mut cohort, all had a response (all partial). Two (33%) of the six patients with grade 3b or transformed follicular lymphoma in the EZH2WT cohort had a response (both partial). One (20%) of five patients in the EZH2mut cohort and six (38%) of 16 patients in the EZH2WT cohort who had relapsed after receipt of a PI3K inhibitor or an immunomodulatory drug had an objective response. In a post-hoc exploratory analysis, deepening responses were observed with continued treatment; of patients who had a best response of complete response, five (83%) of six in the EZH2mut cohort and one (50%) of two in the EZH2WT cohort had a partial response before the complete response (appendix pp 14–15).
98 (99%) of 99 patients had one or more treatment-emergent adverse events of any grade and treatment-related adverse events occurred in 80 (81%) patients (table 3). Serious treatment-emergent adverse events occurred in 27 (27%) of 99 patients, with the most common events being sepsis, general physical health deterioration, and anaemia (two patients each [2%]). Four (4%) patients had a serious treatment-related adverse event (neutropenia, pancytopenia, and transient global amnesia in one patient each, and arrhythmia and myelodysplastic syndrome in one patient). Treatment-related adverse events of grade 3 or worse severity included thrombocytopenia (three [3%]), neutropenia (three [3%]), and anaemia (two [2%]; table 3). Treatment-emergent adverse events leading to tazemetostat dose reductions occurred in nine (9%) of 99 patients and treatment-emergent adverse events leading to dose interruptions of tazemetostat occurred in 27 (27%). Eight (8%) patients discontinued tazemetostat because of a treatment-emergent adverse event, five (5%) of which were treatment related (appendix p 8). Myelodysplastic syndrome in one patient was reported at 15·3 months after starting tazemetostat and acute myeloid leukaemia was reported in one patient at 25·8 months after starting tazemetostat (appendix p 9). Four patients died within 30 days of the last dose of study treatment (three due to progressive disease and one due to chronic kidney disease); there were no treatment-related deaths.
Table 3:
Summary of the safety profile of tazemetostat in the modified intention-to-treat population (n=99)
| Treatment-emergent adverse events |
Treatment-related adverse events |
|||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Nausea | 23 (23%) | 0 | 0 | 19 (19%) | 0 | 0 |
| Diarrhoea | 18 (18%) | 0 | 0 | 12 (12%) | 0 | 0 |
| Alopecia | 17 (17%) | 0 | 0 | 14 (14%) | 0 | 0 |
| Cough | 16 (16%) | 0 | 0 | 2 (2%) | 0 | 0 |
| Asthenia | 15 (15%) | 3 (3%) | 0 | 13 (13%) | 1 (1%) | 0 |
| Fatigue | 15 (15%) | 2 (2%) | 0 | 11 (11%) | 1 (1%) | 0 |
| Upper respiratory tract infection | 15 (15%) | 0 | 0 | 1 (1%) | 0 | 0 |
| Bronchitis | 15 (15%) | 0 | 0 | 3 (3%) | 0 | 0 |
| Abdominal pain | 12 (12%) | 1 (1%) | 0 | 2 (2%) | 0 | 0 |
| Headache | 12 (12%) | 0 | 0 | 5 (5%) | 0 | 0 |
| Vomiting | 11 (11%) | 1 (1%) | 0 | 6 (6%) | 0 | 0 |
| Back pain | 11 (11%) | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | 10 (10%) | 0 | 0 | 2 (2%) | 0 | 0 |
| Anaemia | 9 (9%) | 4 (4%) | 1 (1%) | 7 (7%) | 2 (2%) | 0 |
| Thrombocytopenia | 5 (5%) | 4 (4%) | 1 (1%) | 5 (5%) | 3 (3%) | 0 |
| Neutropenia | 3 (3%) | 3 (3%) | 1 (1%) | 3 (3%) | 2 (2%) | 1 (1%) |
| Leucopenia | 2 (2%) | 1 (1%) | 0 | 2 (2%) | 1 (1%) | 0 |
| Hypophosphataemia | 2 (2%) | 1 (1%) | 0 | 2 (2%) | 1 (1%) | 0 |
| Hypertriglyceridaemia | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 0 |
| Increased serum amylase | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 0 |
| Presyncope | 0 | 1 (1%) | 0 | 0 | 1 (1%) | 0 |
| Pancytopenia | 0 | 0 | 1 (1%) | 0 | 0 | 1 (1%) |
| Increased serum aminotransferase | 0 | 1 (1%) | 0 | 0 | 1 (1%) | 0 |
| Myelodysplastic syndrome | 0 | 1 (1%) | 0 | 0 | 1 (1%) | 0 |
| Dyspnoea | 5 (5%) | 3 (3%) | 0 | 1 (1%) | 0 | 0 |
| Dizziness | 6 (6%) | 1 (1%) | 0 | 3 (3%) | 0 | 0 |
| Upper abdominal pain | 5 (5%) | 1 (1%) | 0 | 4 (4%) | 0 | 0 |
| Urinary tract infection | 5 (5%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Hypogammaglobulinaemia | 3 (3%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Hypertension | 3 (3%) | 1 (1%) | 0 | 0 | 0 | 0 |
| Hyperkalaemia | 2 (2%) | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 0 |
| Lung infection | 2 (2%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Pleural effusion | 2 (2%) | 1 (1%) | 0 | 0 | 0 | 0 |
| Herpes zoster | 1 (1%) | 1 (1%) | 0 | 0 | 0 | 0 |
| Sepsis | 0 | 0 | 2 (2%) | 0 | 0 | 0 |
| General physical health deterioration | 0 | 0 | 2 (2%) | 0 | 0 | 0 |
| Non-cardiac chest pain | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Hyperuricaemia | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 0 | 0 |
| Prolonged QT interval on electrocardiogram | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Hypothyroidism | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Lymphopenia | 1 (1%) | 0 | 1 (1%) | 0 | 0 | 0 |
| Lower gastrointestinal haemorrhage | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Hypokalaemia | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Acute myeloid leukaemia | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Malignant melanoma | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Chronic kidney disease | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Bile duct obstruction | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Ascites | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Acute pancreatitis | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Empyema | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Cerebrovascular accident | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Osmotic demyelination syndrome | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Post-herpetic neuralgia | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Syncope | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Hypoxia | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Hypercalcaemia | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Hyperglycaemia | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Hyponatraemia | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Increased blood pressure | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Femoral artery occlusion | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Subclavian vein thrombosis | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Squamous cell carcinoma | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Constrictive pericarditis | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
Data are n (%) or n. We report any grade 1–2 adverse event occurring in at least 10% of patients. All grade 3 and 4 events are reported. There were no deaths due to adverse events.
Discussion
Our results have shown that tazemetostat, an investigational EZH2 inhibitor, has anti-tumour activity in patients with relapsed or refractory follicular lymphoma (objective response rate of 69% [95% CI 53–82; 31 of 45 patients] in the EZH2mut cohort and 35% [23–49; 19 of 54 patients] in the EZH2WT cohort). The high objective response rate observed in the EZH2mut cohort, which included six complete responses, underscores the importance of the EZH2 mutation in the pathogenesis of some cases of follicular lymphoma. Furthermore, the objective response rate for patients with double refractory disease was high, suggesting the potential of EZH2 inhibition in managing difficult-to-treat follicular lymphomas. The responses in both EZH2 cohorts were durable. A small number of patients had a partial response that deepened to a complete response with continued treatment. These findings, in addition to the median time to first response being 3·7 months in both cohorts, are consistent with results observed with other epigenetic therapies, for which responses have been gradual and emerging.22,23 These data suggest that, with an extended dosing interval, inhibition of EZH2 not only sustains responses, but can transform partial responses into complete responses in some patients. Importantly, tazemetostat was well tolerated. The low prevalence of treatment-related adverse events and the low proportion of patients requiring dose reductions or discontinuing therapy due to treatment-related adverse events allowed for a longer duration on therapy.
Although this phase 2 study was not designed to compare outcomes in patients with different EZH2 mutational statuses, patients with EZH2mut follicular lymphoma had a higher objective response rate than did those with EZH2WT follicular lymphoma. Despite a higher proportion of poor risk features in patients in the EZH2WT cohort, we believe that the nearly doubled objective response rate for the EZH2mut cohort was mainly driven by the EZH2 mutation. In-vitro studies have shown that treatment with EZH2 inhibitors reduced viability in both EZH2WT and EZH2mut lymphoma cell lines; however, viability was much more reduced in the EZH2mut cells.10 EZH2 is required to maintain the proliferation and survival of the germinal centre B cells from which follicular lymphoma is derived;6 this reliance on EZH2 exists in most cases of follicular lymphoma, regardless of EZH2 mutation status. In patients with EZH2mut follicular lymphoma, aberrantly active EZH2 drives lymphomagenesis by preventing the expression of genes that would normally become active as B cells exit the germinal centre. Since EZH2mut drives transformation, tumours with EZH2mut have an increased dependency on this protein, explaining their greater responsiveness to EZH2 inhibitors. The mechanism of action of tazemetostat in patients with EZH2WT follicular lymphoma is not yet fully understood. Approximately 70% of patients with follicular lymphoma harbour two or more different mutations in chromatin-modifying proteins.24 In patients with EZH2WT follicular lymphoma, somatic mutations in proteins such as CREBBP and KMT2D can prevent the activation of genes that would otherwise promote B-cell exit from the germinal centre.16–18 We believe that in these patients, the ability of EZH2 to maintain transcriptional repression, and ultimately the germinal centre, proceeds relatively unchecked, thus rendering such lymphomas susceptible to EZH2 inhibition. In addition, amplification of EZH2 has been reported in 15% of patients with follicular lymphoma and was not correlated with mutation status.25
Other than sparse guidance related to the use of PI3K inhibitors, there are no established treatment recommendations for patients with follicular lymphoma in the third line and beyond,2,26 with response rates decreasing with each successive line of therapy.27,28 European treatment guidelines mention the approval of the PI3K inhibitor idelalisib in patients with double refractory follicular lymphoma,26 and the US Food and Drug Administration (FDA) has approved the PI3K inhibitors copanlisib and duvelisib, in addition to idelalisib.2 However, treatment options in the third-line setting or beyond are scarce. In this setting in our study, tazemetostat has shown a clinically meaningful benefit combined with a favourable safety profile, but with a novel mechanism of action. Through the inhibition of EZH2, tazemetostat reprogrammes cell differentiation, reduces B-cell proliferation, promotes anti-tumour immunity, and permits the expression of tumour suppressor genes.29 On Jan 23, 2020, tazemetostat received accelerated approval from the FDA for the treatment of patients with locally advanced or metastatic epithelioid sarcoma ineligible for complete resection. Furthermore, on June 18, 2020, the FDA granted accelerated approval for the use of tazemetostat in the treatment of adult patients with relapsed or refractory follicular lymphoma whose tumours are positive for an EZH2 mutation, as detected by an FDA-approved test, and who have received at least two previous systemic therapies, and for adult patients with relapsed or refractory follicular lymphoma who have no satisfactory alternative treatment options. Tazemetostat is also being studied in other molecularly driven haematological malignancies and solid tumours, including prostate cancer (eg, NCT02601950, NCT04179864, and NCT04204941).
Our study is limited by having a non-randomised, single-arm study design, and was not designed to compare outcomes in patients with EZH2mut follicular lymphoma versus those in patients with EZH2WT follicular lymphoma. Subgroup analyses are limited by small patient numbers. For example, in analyses of the POD24 subgroup, the lower objective response rate in patients in the EZH2WT cohort than in patients in the EZH2mut cohort could be due to a higher number of patients with grade 3b or transformed follicular lymphoma in the EZH2WT cohort.
Tazemetostat showed anti-tumour activity in the treatment of heavily pretreated patients with relapsed or refractory follicular lymphoma, with durable responses seen in both the EZH2WT cohort and the EZH2mut cohort. In addition, the higher objective response rate observed in the EZH2mut cohort compared with that in the EZH2WT cohort further illustrates the importance of the EZH2 mutation in some cases of follicular lymphoma. Although the observed anti-tumour activity of tazemetostat in relapsed or refractory follicular lymphoma suggests that this drug can be used as a single agent in this patient population, tazemetostat’s tolerability and potential immunomodu lating properties make it an attractive candidate for combination use. For example, tazemetostat might be able to enhance the sensitivity and immunogenicity of follicular lymphoma to lenalidomide and rituximab when given in combination. In addition, given that the PI3K signalling pathway is known to promote oncogenesis by regulating the epigenome,30 tazemetostat could be used in combination with PI3K inhibitors to potentially synergistically increase the anti-tumour efficacy of these two distinctly different treatments. Combination treatment with tazemetostat plus lenalidomide and rituximab will be evaluated in a confirmatory phase 3 study of patients with relapsed or refractory follicular lymphoma (NCT04224493). With its ability to produce clinically meaningful and durable responses, favourable safety profile, and unique mechanism of action, tazemetostat represents a new therapeutic option for patients with follicular lymphoma.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles on clinical trials published between database inception and April 3, 2020, with no language restrictions. First, we used the search terms “epigenetic OR epigenetics” AND “lymphoma”, which produced 25 results. Of these 25, only two clinical trials included patients with follicular lymphoma. Second, we used the search terms “EZH2” AND “lymphoma”. Of the four results generated, three focused on patients with follicular lymphoma. Given the paucity of results, we supplemented our literature search with a description of key studies relevant to the medical literature on this topic. Among studies of epigenetic therapies, investigators concluded that epigenetic mechanisms might cause the change in CD20 expression following rituximab treatment. Two phase 2 studies evaluated the safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in patients with non-Hodgkin lymphoma or chronic lymphocytic leukaemia, including patients with follicular lymphoma, and found clinical activity in heavily pretreated patients with follicular lymphoma and a manageable toxicity profile, although grade 3–4 thrombocytopenia and any grade diarrhoea were noted. The histone deacetylase inhibitor vorinostat was investigated in a small, phase 1 study of ten patients with malignant lymphoma. Results from a study of PRMD1 expression in follicular lymphoma might suggest a biological basis for which pan-histone deacetylase inhibitors might have activity in patients with non-functional CREB-binding protein. Investigators found that follicular lymphoma tumours with non-functional CREB-binding protein were unable to upregulate PRDM1 expression, despite a functional capacity to activate IL-21–phosphorylated STAT3 signalling, and pan-histone deacetylase inhibitors restored PRDM1 expression. The bromodomain inhibitor OTX015, another epigenetic modulator, was investigated in a phase 1 study of patients with lymphoma or multiple myeloma; however, the study included only two patients with follicular lymphoma. Among studies identified in our PubMed search that included “EZH2”, two evaluated the prognostic significance of EZH2 mutation status in patients with follicular lymphoma, and the third summarised data from a phase 1 study of tazemetostat in patients with relapsed or refractory B-cell non-Hodgkin lymphoma or advanced solid tumours.
Added value of this study
Treatment options are urgently needed for patients with relapsed or refractory follicular lymphoma. As an epigenetic therapy and a first-in-class EZH2 inhibitor, tazemetostat has a novel mechanism of action for the treatment of follicular lymphoma. Results from this phase 2 study show that tazemetostat can produce clinically meaningful and durable responses and has a favourable safety profile in heavily pretreated patients with follicular lymphoma. These results support the promising anti-tumour activity and favourable tolerability of tazemetostat observed in the phase 1 setting.
Implications of all the available evidence
Other than abexinostat, tazemetostat is the only epigenetic regulator tested in patients with follicular lymphoma with published data. Tazemetostat is unique in that it targets a specific protein (EZH2) rather than epigenetic modification in general (ie, acetylation). Although the observed anti-tumour activity of tazemetostat in patients with relapsed or refractory follicular lymphoma suggests that this drug can be used as a single agent in this patient population, tazemetostat’s tolerability makes it an attractive candidate for combination use. Future studies should examine concomitant use of tazemetostat with agents commonly used in the treatment of patients with follicular lymphoma.
Acknowledgments
This study was funded by Epizyme. We thank the patients and their caregivers and families for their participation in this study. We acknowledge Preeti Joshi, Kat Cosmopoulos, and Neil Michaud, of Epizyme, for their contributions to, and review of, this manuscript. Medical writing assistance was provided by Tiffany DeSimone of Ashfield Healthcare Communications (Lyndhurst, NJ, USA), a UDG Healthcare company, and was funded by Epizyme.
Footnotes
Declaration of interests
FM has received honoraria from Bristol Myers Squibb and Janssen, and has served as a consultant or adviser to Celgene, Bayer, Abbvie, Verasteem, Gilead, Servier, Roche-Genentech, and Epizyme. HT has received honoraria from Merck and Servier and has served as a consultant or adviser to Roche-Genentech, Janssen, and Karyopharm. AC has received honoraria from Celgene, Takeda, Gilead, and Janssen. PM has received honoraria and educational support from, and has served on advisory boards for, Takeda, Janssen, Roche, Gilead, Celgene, and Epizyme. TP has received research funding from Pharmacyclics and Abbvie, and has served as a consultant or adviser to Genentech, Gilead, Bayer, Seattle Genetics, and Pharmacyclics. SAs has received honoraria from Roche-Genentech, Janssen, Abbvie, and Gilead. CLB has received research funding from Janssen, Novartis, Epizyme, Xynomics, and Bayer, and has served as a consultant or adviser to Juno-Celgene, Seattle Genetics, and Kite. VR has received research funding from ArgenX, and has served as a consultant or adviser to Gilead, Infinity, Merck Sharp & Dohme, Bristol Myers Squibb, Epizyme, Nanostring, Incyte, Roche, AstraZeneca, and Servier. GLD has received honoraria and educational support from, and has served on advisory boards for, Takeda, Astellas, Genzyme, Roche, and Abbvie. MD has served as a consultant or adviser to Novartis, Roche, Amgen, Takeda, Merck Sharp & Dohme, Celgene, and Janssen, and has received research funding from Novartis, Roche, Amgen, Takeda, Merck Sharp & Dohme, and Celgene. WJ has served as a consultant or adviser to AstraZeneca, Janssen, Loxo, and Sandoz, and has received research funding from AstraZeneca, Bayer, Celgene, Celtrion, Epizyme, Gilead, Incyte, Janssen, Loxo, Morphosys, Novo Nordisk, Roche, Sandoz, Servier, Takeda, and TG Therapeutics. SO’s institution receives research funding from Abbvie, Amgen, Bristol Myers Squibb, AstraZeneca, Beigene, Celgene, Epizyme, Merck Sharp & Dohme, Pharmacyclics, Janssen-Cilag, and Roche. SO has served as a consultant or adviser to Abbvie, AstraZeneca, CSL, Merck Sharp & Dohme, Roche-Genentech, Janssen-Cilag, Gilead, Mundipharma, Novartis, and Takeda. JR has served as a consultant or adviser to Takeda, Bristol Myers Squibb, Seattle Genetics, and Novartis, owns stock in GlaxoSmithKline and AstraZeneca, has received honoraria from Takeda, and has received research funding from Takeda, Pfizer, ADC Therapeutics, Celgene, and AstraZeneca. JY, JW, DA, and SAg are salaried employees of, and own stock in, Epizyme. GS has served as a consultant or adviser to Abbvie, Amgen, Autolus, Celgene, Gilead, Epizyme, Janssen, Karyopharm, Kite, Merck, Morphosys, Novartis, Roche, Servier, and Takeda. All other authors declare no competing interests.
Data sharing
Individual participant data that underlie the results reported in this Article (after deidentification) will be made available on a case-by-case basis, including the study protocol and statistical analysis plan. Data availability will begin 9 months after publication and end 36 months after publication. To gain access, data requestors should submit a proposal to ISTgrants@epizyme.com. Proposals will be reviewed by an independent review committee made for this purpose.
Contributor Information
Prof Franck Morschhauser, Groupe de Recherche sur les formes Injectables et les Technologies Associées, CHU de Lille, Université de Lille, Lille, France.
Hervé Tilly, Department of Hematology and INSERM U1245, Centre Henri Becquerel and Rouen University, Rouen, France.
Aristeidis Chaidos, Centre for Haematology, Department of Immunology and Inflammation, Faculty of Medicine, Imperial College London, Hammersmith Hospital & Imperial College Healthcare NHS Trust, London, UK.
Pamela McKay, Department of Hematology, Beatson West of Scotland Cancer Centre, Glasgow, UK.
Tycel Phillips, Division of Hematology and Oncology, University of Michigan, Ann Arbor, MI, USA.
Sarit Assouline, Division of Hematology, Jewish General Hospital, Montreal, QC, Canada, Department of Oncology, McGill University, Montreal, QC, Canada.
Connie Lee Batlevi, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Phillip Campbell, Department of Clinical Haematology, Barwon Health, University Hospital Geelong, Geelong, VIC, Australia.
Vincent Ribrag, Hematology Department, Gustave Roussy, Villejuif, France.
Prof Gandhi Laurent Damaj, Hematology Institute, Hematologie Clinique, University Hospital School of Medicine, Caen, France.
Michael Dickinson, Department of Clinical Haematology, Peter MacCallum Cancer Centre, Royal Melbourne Hospital, Melbourne, VIC, Australia, Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, VIC, Australia.
Prof Wojciech Jurczak, Department of Hematology, Maria Sklodowska-Curie National Research Institute of Oncology, Kraków, Poland.
Maciej Kazmierczak, Department of Hematology and Bone Marrow Transplantation, Poznań University of Medical Sciences, Poznań, Poland.
Prof Stephen Opat, Haematology Department, Monash University, Melbourne, VIC, Australia.
Prof John Radford, Department of Medical Oncology, University of Manchester, Manchester, UK, NIHR Manchester Clinical Research Facility, Manchester Academic Health Science Centre, The Christie NHS Foundation Trust, Manchester, UK.
Anna Schmitt, Department of Hematology, Institut Bergonié, Bordeaux, France.
Jay Yang, Clinical Development, Epizyme, Cambridge, MA, USA.
Jennifer Whalen, Clinical Development, Epizyme, Cambridge, MA, USA.
Shefali Agarwal, Clinical Development, Epizyme, Cambridge, MA, USA.
Deyaa Adib, Clinical Development, Epizyme, Cambridge, MA, USA.
Prof Gilles Salles, Department of Hematology, Lyon-Sud Hospital, University of Lyon, Pierre-Bénite, France.
References
- 1.National Cancer Institute. Adult non-Hodgkin lymphoma treatment (PDQ)—health professional version. https://www.cancer.gov/types/lymphoma/hp/adult-nhl-treatment-pdq#link/_552_toc (accessed April 3, 2020).
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. B-cell lymphomas. Version 4. 2019. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf (accessed April 3, 2020).
- 3.Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med 2018; 379: 934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017; 377: 1331–44. [DOI] [PubMed] [Google Scholar]
- 5.Korfi K, Araf S, Bewicke-Copley F, et al. Longitudinal analysis of diagnostic-relapse biopsies of diffuse large B cell lymphoma suggest that relapse is mediated by distinct mechanisms in ABC and GCB lymphoma. Hematol Oncol 2019; 37 (suppl 2): S142–43 (abstr). [Google Scholar]
- 6.Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature 2011; 469: 343–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bödör C, Grossmann V, Popov N, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 2013; 122: 3165–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 2010; 42: 181–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson SK, Kawano S, Minoshima Y, et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther 2014; 13: 842–54. [DOI] [PubMed] [Google Scholar]
- 10.Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013; 23: 677–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caganova M, Carrisi C, Varano G, et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J Clin Invest 2013; 123: 5009–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Béguelin W, Teater M, Gearhart MD, et al. EZH2 and BCL6 cooperate to assemble CBX8-BCOR complex to repress bivalent promoters, mediate germinal center formation and lymphomagenesis. Cancer Cell 2016; 30: 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015; 527: 249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zingg D, Arenas-Ramirez N, Sahin D, et al. The histone methyltransferase Ezh2 controls mechanisms of adaptive resistance to tumor immunotherapy. Cell Rep 2017; 20: 854–67. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Brea LT, Yu J. Immune modulatory functions of EZH2 in the tumor microenvironment: implications in cancer immunotherapy. Am J Clin Exp Urol 2019; 7: 85–91. [PMC free article] [PubMed] [Google Scholar]
- 16.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol 2015; 15: 172–84. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med 2015; 21: 1190–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasqualucci L, Khiabanian H, Fangazio M, et al. Genetics of follicular lymphoma transformation. Cell Rep 2014; 6: 130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasqualucci L, Dominguez-Sola D, Chiarenza A, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 2011; 471: 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Italiano A, Soria J-C, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 2018; 19: 649–59. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–86. [DOI] [PubMed] [Google Scholar]
- 22.Silverman LR, Fenaux P, Mufti GJ, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer 2011; 117: 2697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen L, Kantarjian H, Guo Y, et al. DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol 2010; 28: 605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci USA 2015; 112: E1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huet S, Xerri L, Tesson B, et al. EZH2 alterations in follicular lymphoma: biological and clinical correlations. Blood Cancer J 2017; 7: e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27 (suppl 5): v83–90. [DOI] [PubMed] [Google Scholar]
- 27.Galaznik A, Bell JA, Hamilton L, et al. Treatment patterns in newly treated and relapse/refractory patients with follicular lymphoma in routine clinical care—a United States electronic medical record database study. Blood 2017; 130 (suppl 1): 4059. [Google Scholar]
- 28.Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol 2019; 184: 660–63. [DOI] [PubMed] [Google Scholar]
- 29.Brach D, Johnston-Blackwell D, Drew A, et al. EZH2 Inhibition by tazemetostat results in altered dependency on B-cell activation signaling in DLBCL. Mol Cancer Ther 2017; 16: 2586–97. [DOI] [PubMed] [Google Scholar]
- 30.Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer 2017; 1868: 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


