To the Editor: Vaccine-induced immune thrombotic thrombocytopenia (VITT), also known as thrombosis with thrombocytopenia syndrome, is a rare but potentially fatal complication of vector-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines.1-3 The clinical picture and the serologic findings in patients with VITT resemble heparin-induced thrombocytopenia.1-3 Several groups have reported the presence of platelet factor 4 (PF4)–reactive antibodies in patients with VITT.1-3 IgG from patients with VITT induces platelet activation and aggregation by cross-linking Fcγ receptor IIA on platelets.1 PF4 is a tetrameric protein that is released from platelet alpha granules on activation. VITT antibodies bind to the heparin-binding site on PF4.4 The link between vaccination and the formation of anti-PF4 antibodies is yet to be determined. A proposed mechanism includes cross-reactivity between anti–SARS-CoV-2 and anti-PF4 antibodies.5 In the current study, we investigated the correlation between anti–PF4–heparin antibodies and anti–SARS-CoV-2 antibodies in vaccinated health care workers (healthy controls) and in vaccinated patients with clinically suspected VITT.
The level of anti–PF4–heparin antibodies was measured with the use of an enzyme-linked immunosorbent assay (ELISA), and the levels of antibodies against various antigenic sites of the SARS-CoV-2 spike protein (spike trimer, receptor-binding domain [RBD], subunit 1 [S1] domain, and subunit 2 [S2] domain) and against nucleocapsid protein were measured with the use of a bead-based assay (Luminex). Antibodies were measured in 101 healthy controls 2 weeks after the first dose of ChAdOx1 nCoV-19 (Oxford–AstraZeneca) had been administered and in 59 patients with clinically suspected VITT between 11 and 22 days after the first dose had been administered. The ability of the sera to activate platelets was tested with the use of a modified heparin-induced platelet aggregation assay. Details of the methods are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
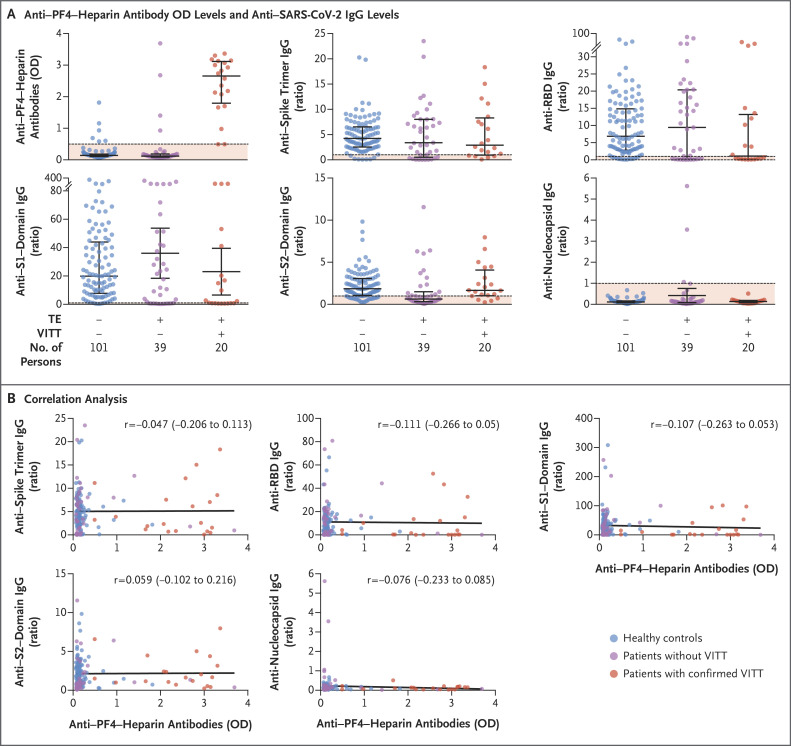
VITT was confirmed in 20 of 59 patients (34%) on the basis of a positive PF4 ELISA and a positive modified heparin-induced platelet aggregation assay (Table S1 in the Supplementary Appendix). The level of anti–PF4–heparin antibodies was higher among the patients with confirmed VITT than among the healthy controls and the patients who did not have VITT (Figure 1A and Table S1). The 95% confidence intervals for the differences between the groups are presented in Table S2; these confidence intervals were not adjusted for multiplicity and therefore cannot be used to infer effects. The levels of antibodies against spike trimer, RBD, S1 domain, and nucleocapsid protein were similar in the three groups. The levels of antibodies against S2 domain were lower among the patients who did not have VITT than among the persons in the other two groups. We did not find any correlation between the level of anti–PF4–heparin antibodies and the level of anti–SARS-CoV-2 IgG antibodies in any of the three groups (Figure 1B and Table S3).
Figure 1. Antibody Levels and Correlation Analysis.
Panel A shows the anti–platelet factor 4 (PF4)–heparin antibody optical density (OD) levels and anti–SARS-CoV-2 IgG levels against spike trimer, receptor-binding domain (RBD), subunit 1 (S1) domain, subunit 2 (S2) domain, and nucleocapsid protein in the healthy controls, the patients without vaccine-induced immune thrombotic thrombocytopenia (VITT), and the patients with confirmed VITT. Panel B shows the correlation analysis between the level of anti–PF4–heparin antibodies and the level of anti–SARS-CoV-2 IgG antibodies against spike trimer, RBD, S1 domain, S2 domain, and nucleocapsid protein in the healthy controls, the patients without VITT, and the patients with confirmed VITT. Anti–PF4–heparin antibodies were quantified with the use of an enzyme-linked immunosorbent assay. Anti–SARS-CoV-2 IgG antibodies were quantified with the use of a bead-based assay (Luminex). Each dot in the figure represents an individual person, and the numbers of persons tested is shown in Panel A. The dashed lines in Panel A indicate the cutoff values, and the solid lines in Panel B indicate the correlation coefficient (r). TE denotes thrombotic event.
Moreover, the levels of anti–SARS-CoV-2 antibodies did not differ substantially between vaccinated persons without complications (i.e., the healthy controls) and patients with VITT. Similarly, Scully et al.2 reported that the levels of antibodies to spike protein and RBD in patients with VITT were in the same range as those of the recipients of one dose of ChAdOx1 nCoV-19. Furthermore, our study did not show a correlation between anti–PF4–heparin antibodies and anti–SARS-CoV-2 antibodies in patients with VITT. Although a preprint publication suggested that spike protein shares an immunogenic epitope with PF4, purified anti-PF4 and anti–PF4–heparin antibodies from patients with VITT did not show cross-reactivity to recombinant SARS-CoV-2 spike protein.5
Our results do not support the hypothesis that the immune response against SARS-CoV-2 proteins leads to the formation of anti-PF4 antibodies in patients with VITT. However, we cannot exclude the possibility of cross-reactivity between a subgroup of anti–SARS-CoV-2 antibodies and a subgroup of anti-PF4 antibodies. A better understanding of the link between vaccination and VITT is necessary for the development of more targeted therapies.
Supplementary Appendix
Disclosure Forms
This letter was published on August 25, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica 2021;106:2170-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021. July 7 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 5.Greinacher A, Selleng K, Mayerle J, et al. Anti-SARS-CoV-2 spike protein and anti-platelet factor 4 antibody responses induced by COVID-19 disease and ChAdOx1 nCov-19 vaccination. April 9, 2021. (https://www.researchsquare.com/article/rs-404769/v1). preprint.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.