Abstract
Ginkgolides are the most important components of Ginkgo biloba extracts, whose lactone can be hydrolyzed in the aqueous environment. Although the hydrolyzed products have complex structures and their functions are not well-understood, opening the lactone ring is an important strategy in producing novel derivatives of ginkgolide. The preparation of a single pure aminolyzed ginkgolide for the study of its bioactivity and understanding of the process of aminolysis are challenging. To obtain stable aminolyzed products, four amide derivatives (2–5) of ginkgolide B (GB, 1) were prepared via the ring-opening reaction of its lactone with propylamine. These products were purified and fully identified by high-resolution mass spectrometry (HRMS) and nuclear magnetic resonance (NMR) spectroscopy and were further evaluated for their ability to inhibit the PAF-induced platelet aggregation of rabbit platelets in vitro. Compound 2, which was obtained by selective aminolysis of the lactone ring C of GB, showed a much better inhibitory activity of platelet aggregation (IC50, 15 nM) than the parent compound GB (IC50, 442 nM). The other three products (3–5), which were obtained by the aminolysis of lactone rings C and F of GB, did not show platelet aggregation inhibitory activity. The results greatly extended our understanding of the chemistry of GB and provided important structural information for the exploration and development of new drugs based on ginkgolides in G. biloba.
1. Introduction
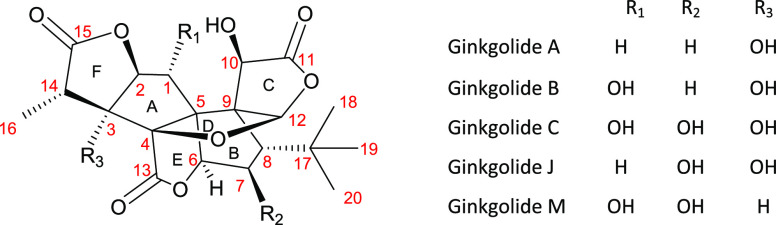
The extracts of leaves of Ginkgo biloba are being used worldwide to promote blood circulation, improve various cognitive functions, and reduce inflammation.1 Ginkgolides are the key bioactive components of G. biloba extracts that have a caged skeleton consisting of six five-membered rings, a spiro[4,4]-nonane carbocyclic ring (A, B), a tetrahydrofuran ring (D), three lactones (C, E, F), and a unique t-Bu group (Figure 1).2,3
Figure 1.
Structures of ginkgolides.
Lactone aminolysis is a common transformation that allows the direct conversion of lactones into the corresponding amides that are more stable and show better bioactivity than the corresponding acids or esters.4,5 Hu et al. prepared amide derivatives of 10-oxaginkgolide A via opening ring C with the amine.6 Vogensen et al. also observed the C ring opening during modification at the 7-position of ginkgolide C.7 Besides, aminolysis of the lactone ring has the potential to significantly improve the solubility and bioactivity, which is an important way to discover drug candidates with better activity. Therefore, lactone aminolysis is a crucial aspect of the chemical study of ginkgolides.
On the other hand, diterpene ginkgolides meglumine injection has been approved for clinical application in China. It is mainly composed of a series of ring-opening mixtures formed by the aminolysis reaction of ginkgolides A, B, C, and K and methylglucamine (data unpublished). More importantly, the ring-opening compounds possess good solubility and clinical effects. Therefore, to find compounds with better biological activity, it is important to carry out a research on the ring-opening products of ginkgolides.
Platelet-activating factor (PAF) is a phospholipid that was originally discovered as an IgE-sensitized rabbit basophil-derived substance responsible for platelet aggregation. The cellular effects of PAF are mediated by a specific G-protein-coupled receptor (PAFR). PAF and PAFR have been implicated in a wide range of diseases and disorders.8 Currently, PAFR is a well-established therapeutic target, and an array of structurally diverse PAFR antagonists has been identified with diverse pharmaceutical activities including treatment of inflammatory diseases, anti-platelet aggregation activity, inhibition of human bronchial smooth muscle activation, and regulation of pulmonary inflammation.9−11 It is known that ginkgolides, particularly ginkgolide B (GB), are strong antagonists of the PAFR.2
In this study, GB was chosen as the research target for the ring-opening reaction. Although previous studies have revealed that GB (1) is a superior antagonist of platelet activating factors and extensive studies have already been performed for GB (1) and its analogs,8,12−23 the structures and bioactivities of GB (1) ring-opening products have not been reported. Herein, we designed and synthesized a series of stable ring-opening products of GB (1) with amine via amide formation and evaluated their ability to inhibit the PAF-induced platelet aggregation of rabbit platelets.
2. Results and Discussion
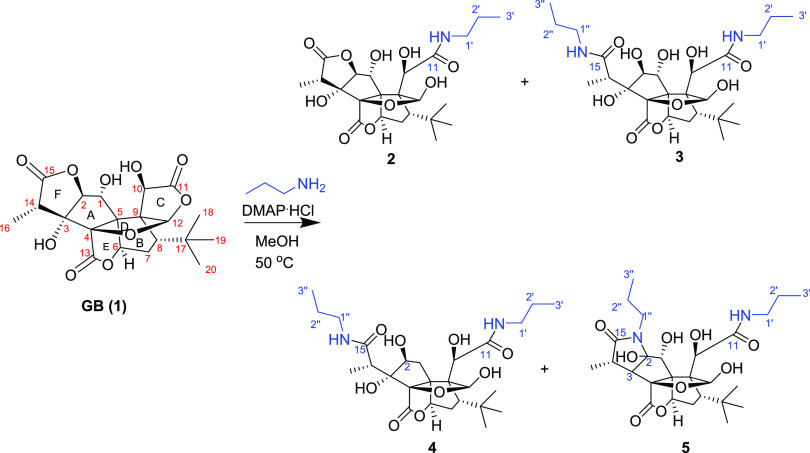
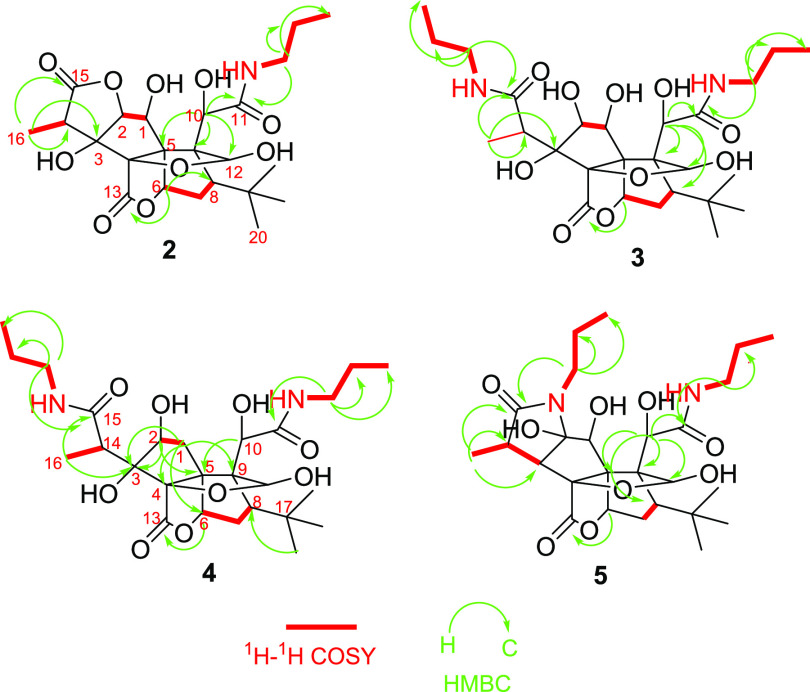
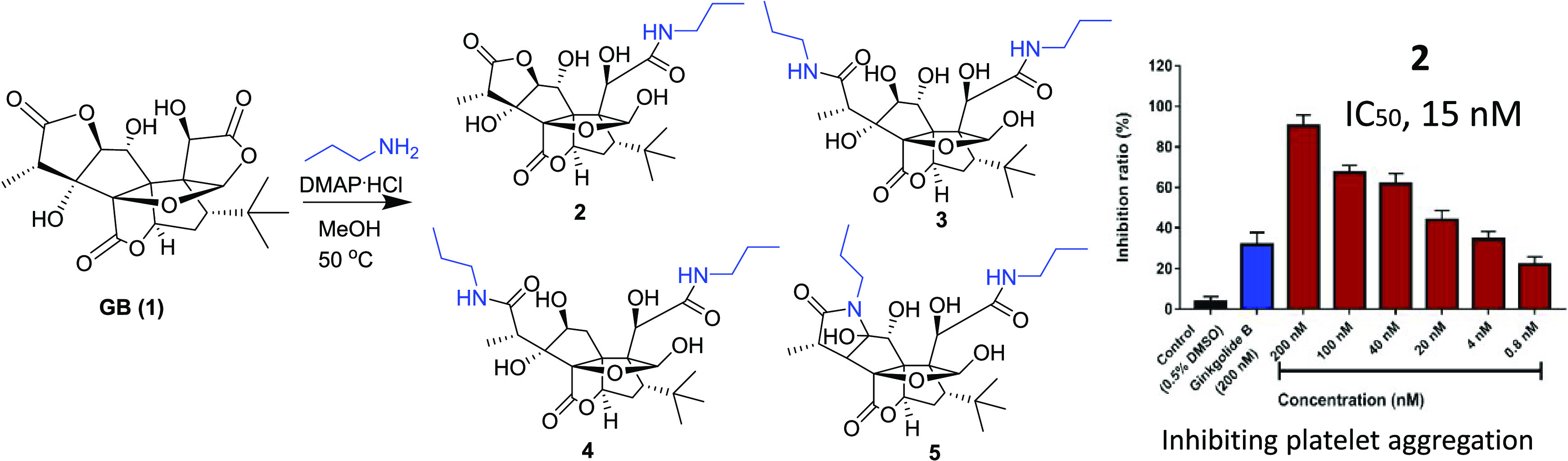
We chose propylamine and DMAP.HCl as catalysts and carried out the reaction with GB (1) in methanol at 50 °C. The reaction was monitored by liquid chromatography–mass spectrometry (LC–MS) (Scheme 1). Four main peaks were observed at the m/z values of 541, 482, 523, and 525 (Figure S1, Supporting Information). All of the products (2–5) were isolated using the high-performance liquid chromatography coupled with a diode-array detector and an evaporative light scattering detector method (HPLC-DAD-ELSD). Compound 2 is a white powder and gave an [M – H]− ion peak at m/z 482.2056 (calcd for C23H32NO10, 482.2081) in its negative-ion HRESIMS, suggesting that one molecule of propylamine was introduced into GB (1). The IR spectrum of 2 showed the presence of ester (1774 cm–1) and amide (1641 cm–1) groups. Compared to GB (1), the 1H NMR and 13C NMR spectra of 2 showed additional signals attributed to propylamine at δH/δC 3.18/42.3, 1.54/23.3, and 0.93/11.8 (Table 1). Based on the HMBC spectrum, the long-range correlation from the proton of propylamine (δH 3.18) to a carbonyl group (δC 177.3) that can be assigned to C-11 according to its correlation with H-10 (δH 4.82) indicated that ring C was opened and formed an amide with propylamine. The carbonyl signals at δC 176.8 and 180.2 were assigned to C-13 and C-15, respectively, according to the HMBC correlations between δc 180.2 and δH 1.22 (H-16), 4.49 (H-2), and between δC 176.8 and δH 5.34 (H-6) (Figure 2). A key NOESY correlation was observed between H-12 and H-18, indicating that H-12 and the t-Bu group are in the cis orientation, which is the same as that of GB.
Scheme 1. The Reaction of GB (1) with Propylamine at 50 °C.
Table 1. 1H NMR Spectroscopic Data (δ in ppm, J in Hz) of Compounds 1–5 in CD3OD.
| position | GB (1) | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1α | 4.19 (d, 7.8) | 4.56 (d, 7.8) | 4.44 (s) | 2.67 (d, 16.2) | 4.51 (s) |
| 1β | 2.74 (dd, 16.2, 4.8) | ||||
| 2 | 4.58 (d, 7.8) | 4.49 (d, 7.8) | 4.01 (s) | 4.01 (d, 4.8) | |
| 3 | 2.35 (d, 9.6) | ||||
| 6 | 5.40 (d, 4.2) | 5.34 (d, 5.4) | 5.54 (d, 6.0) | 4.70 (d, 5.4) | 5.35 (d, 5.4) |
| 7α | 2.12 (dt, 13.8, 5.1) | 2.34 (dt, 13.8, 5.3) | 2.39 (dt, 13.8, 6.0) | 2.44 (dt, 13.8, 5.5) | 2.28 (dt, 13.8, 5.3) |
| 7β | 2.27 (dd, 13.8, 4.8) | 1.91(dd, 13.8, 4.8) | 1.90 (dd, 13.8, 6.0) | 1.86 (dd, 13.8, 6.0) | 1.86 (dd, 13.8, 4.8) |
| 8 | 1.92 (dd, 13.8, 4.8) | 1.78 (dd, 13.8, 4.8) | 1.84 (dd, 6.0, 1.0) | 1.86 (dd, 13.8, 6.0) | 1.81 (dd, 13.8, 4.8) |
| 10 | 5.10 (s) | 4.83 (s) | 4.74 (s) | 4.73 (s) | 4.82 (s) |
| 12 | 6.08 (s) | 5.89 (s) | 5.67 (s) | 5.67 (s) | 5.88 (s) |
| 14 | 3.04 (m) | 3.91 (m) | 3.10 (m) | 3.10 (m) | 3.42 (m) |
| 16 | 1.23 (d, 7.2) | 1.22 (d, 7.2) | 1.44 (d, 7.2) | 1.45 (d, 7.2) | 1.22 (d, 7.8) |
| 18–20 | 1.11 (s) | 1.08 (s) | 108 (s) | 1.09 (s) | 1.09 (s) |
| 1′ | 3.18 (m) | 3.21 (m) | 3.20 (m) | 3.25 (m) | |
| 2′ | 1.54 (m) | 1.57 (m) | 1.56 (m) | 1.67 (m) | |
| 3′ | 0.93 (t, 7.2) | 0.93(t, 7.2) | 0.96(t, 7.8) | 0.93(t, 7.2) | |
| 1″ | 3.18 (m) | 3.10 (m) | 3.15 (m) | ||
| 2″ | 1.53 (m) | 1.54 (m) | 1.50 (m) | ||
| 3″ | 0.91 (t, 7.2) | 0.94 (t, 7.8) | 0.88 (t, 7.2) |
Figure 2.
Key 1H–1H COSY and HMBC correlations of compounds 2–5.
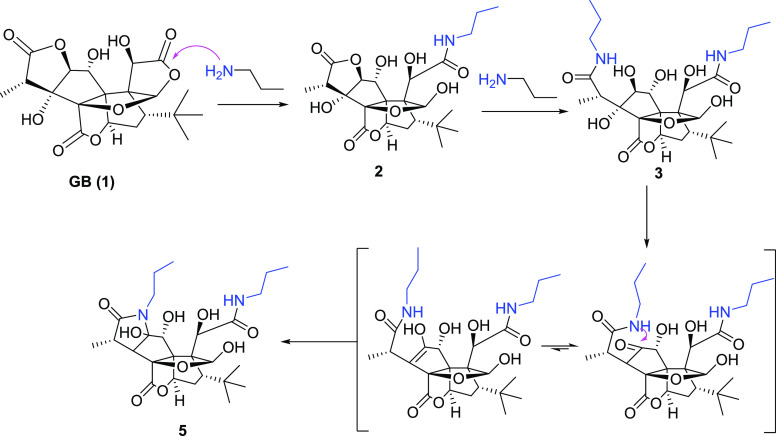
Compound 3 is a white powder and gave an [M – H]− ion peak at m/z 541.2766 (calcd for C26H41N2O10, 541.2767) in the negative-ion HRESIMS, suggesting that two molecules of propylamine were introduced into GB (1). In the 1D and 2D NMR spectra of 3, propylamine group signals were observed at δH/δC 3.18/42.0, 41.9; 1.54/23.6, 23.5; and 0.93/11.8, 11.7. One propylamine molecule was attached to C-11 (δC 174.7) on the basis of the HMBC correlation between δC 174.7 and δH 4.76 (H-10), and another propylamine group was attached to C-15 (δC 179.5) assigned by the correlation between δC 179.5 and δH 1.45 (H-16) in the HMBC spectra. Another carbonyl signal at δC 176.2 (C-13) was correlated with δH 5.56 (H-6) in the HMBC spectra, suggesting that 3’s lactone ring E remained. The formation of 3 can be explained by the plausible mechanism proposed in Scheme 2 in which the amide formed from the nitrogen of propylamine attacked the carbonyl groups of the lactone rings C and F.
Scheme 2. Proposed Reaction Pathway for the Synthesis of 2, 3, and 5.
Similarly, compound 4 was obtained as a white powder and gave an [M – H]− ion peak at m/z 525.2820 (calcd for C26H41N2O9, 525.2818) in the negative-ion HRESIMS, with one fewer oxygen atom than that of 3. The 1D and 2D NMR spectra of 4 are similar to those of 3, except that the oxygenated methine (C-1) in 3 was replaced by a methylene group at δC 43.6 (C-1) and δH 2.67 (1H, d, J = 16.6 Hz, H-1α), and 2.74 (1H, dd, J = 16.6, 4.9 Hz, H-1β) in 4. 1H–1H COSY correlations of H-1/H-2 as well as HMBC correlations from H-1α to C-1, C-3, C-4, C-5, and C-9; from H-1β to C-5, C-6, and C-9; and from H-2 to C-3, C-4, and C-5 confirmed these assignments. NOESY correlation of H-2/H-16 indicated that the hydroxyl group at C-2 is in β-orientation, which is the same with that of compound 3. Two propylamine units were attached to C-11 and C-15 on the basis of HMBC correlations from H-1′ to C-11 and H-1″ to C-15, respectively. Thus, the structure of 4 was elucidated as shown.
Compound 5 is a white powder and gave an [M – H]−ion peak at m/z 523.2669 (calcd for C26H39N2O9, 523.2661) in the negative-ion HRESIMS. Similar to 3, two propylamine molecules in 5 were observed in the NMR spectra of 5. The tertiary hydroxyl group at C-3 in 3 was replaced by a proton in 5 that was assigned to H-3 (δH 2.33, d, J = 9.6 Hz), corresponding to the carbon signal at δC 60.5, on the basis of the 1H–1H COSY correlations of H-3/H-14 (δH 3.42, q)/H-16 (δH 1.21, d, J = 7.2 Hz). Another major change in the NMR spectra was the replacement of the secondary hydroxyl group at C-2 (δC 88.4) in 3 by a tertiary hydroxyl at C-2 (δC 98.7) in 5. The HMBC correlations from H-1″ to C-2 and C-15 indicated that the lactone ring F in GB (1) was replaced by a lactam ring in 5. The formation of 5 can be explained by the plausible mechanism proposed in Scheme 2. Here, the C2-C3 dehydration of 3 is different from the common C3-C14 dehydration in ginkgolides and may lead to form keto-enol tautomers.24,25 Subsequently, the nitrogen of amide attacked the ketone to obtain 5 (Scheme 2).
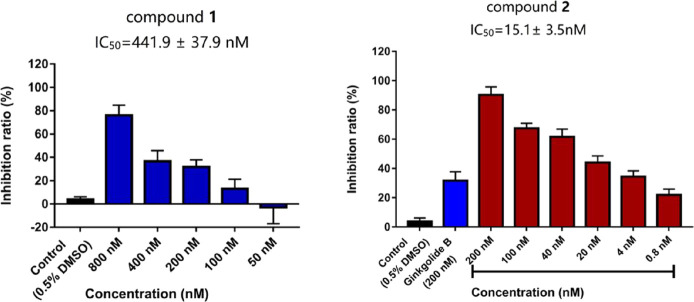
Each compound was tested for the PAF receptor binding inhibitory activity at a concentration of 20 μM according to Koch’s experimental method.26 One derivative (2) showed a much better inhibitory activity than GB (1), while the other three compounds (3–5) exhibited lower activities. The concentrations required to half-maximally inhibit PAF-induced aggregation were calculated based on the results of the concentration dependence experiments (Figure 3). The IC50 value of GB (1) and 2 is 441.93 ± 37.89 and 15.10 ± 3.50 nM, respectively (Table 3). These results indicated that the lactone ring F is very important for the platelet aggregation inhibitory activity of GB derivatives. Meanwhile, in the presence of lactone ring F, the opening of lactone ring C is beneficial for increasing the activity.
Figure 3.
Effect of compounds 1 and 2 on PAF-induced aggregation of rabbit platelets in PRP. Data represented as mean values ± S.D. of three independent experiments.
Table 3. Anti-Platelet Aggregation Effects of Compounds 1–5.
| compounds | IC50 (nM) |
|---|---|
| GB (1) | 441.9 ± 37.9 |
| 2 | 15.1 ± 3.5 |
| 3 | >20,000 |
| 4 | >20,000 |
| 5 | >20,000 |
3. Conclusions
In summary, four GB (1) amide derivatives (2–5) were synthesized, characterized, and evaluated on platelet aggregation activity. The biological screening results indicated that compound 2, a GB derivative by selective aminolysis of lactone ring C, showed a much better inhibitory activity for platelet aggregation than the parent compound GB (1). The nanomolar level of the IC50 value of 2 (15.1 ± 3.5 nM) suggested that 2 is a very potent inhibitor of platelet aggregation and can serve as a new candidate for the development of a drug for the treatment of platelet aggregation. Compounds 3–5 were obtained by di-aminolysis of lactone rings C and F with the various modes and did not show platelet aggregation inhibitory activities. Thus, our results provide a powerful tool for the understanding of GB (1) amide derivatives as well as important structural information for the exploration and development of new drugs based on ginkgolides in G. biloba. More bioactive amide derivatives of GB will be synthesized via opening lactone rings C and E using different primary or secondary amines and aliphatic or aromatic amines in future studies.
4. Experimental Section
4.1. Materials
Ginkgolide B (GB) was kindly provided by Livzon Pharmaceutical Group Inc. (Zhuhai, China). It was extracted from natural sources and has a purity of 91.2%. Platelet activating factor (PAF, 1-O-octadecyl-2-O-acetyl-sn-glycero-3-phosphoryl-choline) was purchased from Bachem (Heidelberg, Germany), and heparin (5000 IU/mL) was obtained from Braun AG (Melsungen, Germany). DMSO, bovine serum albumin (BSA) fraction V, and sodium citrate solution were acquired from Sigma (Deisenhofer, Germany). All other chemicals were obtained from Merck (Darmstadt, Germany).
PAF was diluted in DMSO (2 mg/mL) and stored at 20 °C. Stocks of buffer salt solutions were kept at 4 °C and stored for up to 1 month. Working dilutions of buffers were prepared at weekly intervals with the exception of albumin, glucose, and NaHCO3 that were added at the day of use. The final composition of the buffer (pH 7.4) used for the dilution of platelet-rich plasma (PRP) was as follows: NaCl (137 mM), MgCl2 (2.1 mM), CaCl2 (1.36 mM), KCl (2.7 mM), NaH2PO4 (0.42 mM), NaHCO3 (15.6 mM), d-glucose (1 g/L), and heparin (2 IU/mL). For the dilution of the PAF stock, the above buffer was supplemented with 2.5 g/L BSA but did not contain heparin.
The 1D and 2D NMR experiments were performed using a Bruker Ascend 600 NMR spectrometer (600 MHz for 1H and 150 MHz for 13C, Bruker, Germany) with the residual solvent signal as the internal reference. High-resolution mass spectra (HRMS) were obtained using an Agilent 6230 electrospray ionization (ESI, Agilent, USA) time-of-flight (TOF) mass spectrometer. Optical rotations were measured using a Rudolph Research Autopol I automatic polarimeter. IR spectra were measured on a Bruker Alpha II series FT-IR spectrometer (KBr). All of the chemicals were purchased from 9dingchem Co., Ltd. (Shanghai, China), unless otherwise specified.
4.2. Chemistry
GB (1) derivatives were synthesized following the procedure described below. Propylamine (30 μL, 0.3661 mmol) and DMAP.HCl (8.0 mg, 0.0505 mmol) were added to a solution of GB (1, 100 mg, 0.2356 mmol) in methanol (2 mL). The mixture was stirred at 50 °C for 6 h, the solvent was removed under reduced pressure, and the residue was purified by the high-performance liquid chromatography coupled with a diode-array detector and an evaporative light scattering detector method (HPLC-DAD-ELSD) to obtain 2 (40 mg, 35%), 3 (22 mg, 17%), 4 (12 mg, 9.7%), and 5 (9 mg, 7.3%).
2, white solid. [α]24D −67.1 (c = 0.39, MeOH); IR (KBr) vmax 3401, 2960, 1774, 1641 cm–1;1H and 13C NMR data, see Tables 1 and 2; HRMS (ESI-TOF) m/z [M – H]− calcd for C23H32NO10, 482.2081, found 482.2056.
Table 2. 13C NMR Spectroscopic Data of Compounds 1–5 in CD3OD.
| position | GB (1) | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 75.5 | 75.5 | 80.5 | 43.6 | 79.3 |
| 2 | 93.3 | 95.2 | 88.4 | 83.9 | 98.7 |
| 3 | 84.7 | 84.8 | 86.7 | 87.4 | 60.5 |
| 4 | 100.2 | 100.7 | 104.1 | 104.0 | 96.7 |
| 5 | 69.2 | 68.2 | 78.5 | 73.9 | 75.8 |
| 6 | 80.7 | 81.4 | 84.0 | 93.6 | 82.5 |
| 7 | 38.1 | 37.8 | 36.5 | 36.8 | 37.0 |
| 8 | 50.5 | 54.8 | 58.8 | 58.2 | 55.1 |
| 9 | 73.5 | 73.3 | 70.3 | 68.8 | 68.5 |
| 10 | 70.7 | 71.2 | 72.0 | 72.9 | 71.4 |
| 11 | 175.2 | 177.3 | 174.7 | 174.5 | 177.3 |
| 12 | 111.9 | 110.9 | 110.6 | 111.5 | 110.1 |
| 13 | 172.7 | 175.1 | 176.3 | 176.2 | 179.4 |
| 14 | 43.3 | 43.9 | 41.5 | 41.2 | 40.3 |
| 15 | 178.4 | 180.2 | 179.6 | 179.7 | 176.8 |
| 16 | 8.1 | 8.1 | 13.9 | 14.2 | 16.2 |
| 17 | 33.3 | 34.3 | 34.6 | 34.5 | 34.3 |
| 18–20 | 29.5 | 30.6 | 30.5 | 30.5 | 30.7 |
| 1′ | 42.3 | 42.0 | 41.9 | 42.3 | |
| 2′ | 23.3 | 23.6 | 23.6 | 23.3 | |
| 3′ | 11.8 | 11.8 | 11.8 | 11.8 | |
| 1″ | 41.9 | 41.9 | 42.3 | ||
| 2″ | 23.5 | 23.5 | 23.0 | ||
| 3″ | 11.7 | 11.7 | 11.8 |
3, white solid. [α]24D +8.3 (c = 0.30, MeOH); IR (KBr) vmax 3422, 1781, 1646, 1641 cm–1, 1H and 13C NMR data, see Tables 1 and 2; HRMS (ESI-TOF) m/z [M – H]− calcd for C26H41N2O10, 541.2767, found 541.2766.
4, white solid. [α]24D −14.4 (c = 0.79, MeOH); IR (KBr) vmax 3419, 2961, 1775, 1639 cm–1, 1H and 13C NMR data, see Tables 1 and 2; HRMS (ESI-TOF) m/z [M – H]− calcd for C26H41N2O9, 525.2818, found 525.2820.
5, white solid. [α]24D −35.9° (c = 0.27, MeOH); IR (KBr) vmax 3738, 2964, 1771, 1645 cm–1, 1H and 13C NMR data, see Tables 1 and 2; HRMS (ESI-TOF) m/z [M – H]− calcd for C26H39N2O9, 523.2661, found 523.2669.
4.3. Bioassay
4.3.1. Preparation of Platelet-Rich Plasma (PRP) and Platelet-Poor Plasma (PPP)
Blood was collected from the central ear artery of female White New Zealand rabbits (body weight between 3 and 4 kg). To improve blood flow, the ear was slightly rubbed with a xylol-wetted cotton pad. The artery was punctured with a hypodermic needle, and the freely flowing blood was dispensed into a centrifuge tube containing 1 mL of sodium citrate for each 9 mL of blood. The citrated blood was mixed well by gentle inversion. A blood sample (2 mL) was drawn into vacutainer tubes containing 3.2% sodium citrate (200 μL). Platelet-rich plasma (PRP) was obtained as a supernatant after centrifuging the blood at 100g for 15 min at room temperature. The remaining blood was further centrifuged at 1600g for 5 min to obtain platelet-poor plasma (PPP).
4.3.2. Assay of Platelet Aggregation
Platelet aggregation in PRP was measured using AggRAM (Helena Platelet aggregometer) by following the change of the light transmission of a PRP sample (1 mL) in a plastic cuvette. The platelets were continuously stirred (800 rpm) and maintained at a temperature of 37 °C. The aggregation process was recorded using a single-channel XY-recorder. Before each measurement, 0 and 100% aggregations were standardized with a cuvette containing 1 mL of PPP. Aggregation was triggered using PAF (10 nM) after incubating platelets with each compound with different concentrations. The maximum rate of platelet aggregation (RPA%) within 5 min was acquired by AggRAM. Inhibition of platelet aggregation versus a solvent control was calculated in percent. Half-maximal inhibition concentrations (IC50) were determined by nonlinear regression analysis using the GraphPad Prism 6.0 software package (GraphPad Software, San Diego, CA, USA).
Acknowledgments
This work was funded by the Science and Technology Development Fund, Macau SAR (0082/2019/A2). We thank Professor Guo-Zheng Cui in Zunyi Medical University for the suggestion to evaluate the bioactivity.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01682.
1D and 2D NMR spectra and IR spectra of compounds 1–5 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Abad M. J.; Bedoya L. M.; Bermejo P. An update on drug interactions with the herbal medicine ginkgo biloba. Curr. Drug Metabiol. 2010, 11, 171–181. 10.2174/138920010791110818. [DOI] [PubMed] [Google Scholar]
- Stroømgaard K.; Nakanishi K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew. Chem., Int. Ed. 2004, 43, 1640–1658. 10.1002/anie.200300601. [DOI] [PubMed] [Google Scholar]
- Nakanishi K. Terpene trilactones from Gingko biloba: from ancient times to the 21st century. Bioorg. Med. Chem. 2005, 13, 4987–5000. 10.1016/j.bmc.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Proinsias K. ó.; Sessler J. L.; Kurcoń S.; Gryko D. New hydrophobic vitamin B12 derivatives via ring-opening reactions of c-lactone. Org. Lett. 2010, 12, 4674–4677. 10.1021/ol102008n. [DOI] [PubMed] [Google Scholar]
- Gao J.; Chen M.; Ren X. C.; Zhou X. B.; Shang Q.; Lu W. Q.; Luo P.; Jiang Z. H. Synthesis and cardiomyocyte protection activity of crocetin diamide derivatives. Fitoterapia 2017, 121, 106–111. 10.1016/j.fitote.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Hu L. H.; Chen Z. L.; Xie Y. Y. Synthesis and biological activity of amide derivatives of ginkgolide A. J. Asian Nat. Prod. Res. 2001, 3, 219–227. 10.1080/10286020108041394. [DOI] [PubMed] [Google Scholar]
- Vogensen S. B.; Strømgaard K.; Shindou H.; Jaracz S.; Suehiro M.; Ishii S.; Shimizu T.; Nakanishi K. Preparation of 7-substituted ginkgolide derivatives: potent platelet activating factor (PAF) receptor antagonists. J. Med. Chem. 2003, 46, 601–608. 10.1021/jm0203985. [DOI] [PubMed] [Google Scholar]
- Lordan R.; Tsoupras A.; Zabetakis I.; Demopoulos C. A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414–4446. 10.3390/molecules24234414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland I. K.; O’Toole R. F.; Smith J. A.; Bissember A. C. Progress in the Development of Platelet-Activating Factor Receptor (PAFr) Antagonists and Applications in the Treatment of Inflammatory Diseases. ChemMedChem 2018, 13, 1873–1884. 10.1002/cmdc.201800401. [DOI] [PubMed] [Google Scholar]
- Antigoni M.; Vasiliki D. P.; George M. S.; Constantinos A. D.; Gregor S.; Aikaterini K. A.; Panagiotis G.; Joannis K. K.; Athanassios I. P. Substituted pyridine-quinoline ligands as building blocks for neutral rhodium(III) complexes. Synthesis, structural characterization studies and anti-platelet activity towards the Platelet-Activating Factor (PAF). Polyhedron 2020, 178, 114336. 10.1016/j.poly.2019.114336. [DOI] [Google Scholar]
- Liu G.; Mateer S. W.; Hsu A.; Goggins B. J.; Tay H.; Mathe A.; Fan K.; Neal R.; Bruce J.; Burns G.; Minahan K.; Maltby S.; Fricker M.; Foster P. S.; Wark P. A. B.; Hansbro P. M.; Keely S. Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the NLRP3 inflammasome. Mucosal Immunol. 2019, 12, 862–873. 10.1038/s41385-019-0163-3. [DOI] [PubMed] [Google Scholar]
- Xia S.-h.; Fang D.-c. Pharmacological action and mechanisms of ginkgolide B. Chin. Med. J. 2007, 120, 922–928. 10.1097/00029330-200705020-00013. [DOI] [PubMed] [Google Scholar]
- Wang G. G.; Chen Q. Y.; Li W.; Lu X. H.; Zhao X. Ginkgolide B increases hydrogen sulfide and protects against endothelial dysfunction in diabetic rats. Croat. Med. J. 2015, 56, 4–13. 10.3325/cmj.2015.56.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; He Z.; Ke J.; Li S. M.; Wu X. R.; Lian L.; He X. W.; He X. S.; Hu J. C.; Zou Y. F.; Wu X. J.; Lan P. PAF receptor antagonist ginkgolide B inhibits tumourigenesis and angiogenesis in colitis-associated cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 432–440. [PMC free article] [PubMed] [Google Scholar]
- Hu L.; Chen Z.; Xie Y.; Jiang Y.; Zhen H. Alkyl and alkoxycarbonyl derivatives of ginkgolide B: synthesis and biological evaluation of PAF inhibitory activity. Bioorg. Med. Chem. 2000, 8, 1515–1521. 10.1016/S0968-0896(00)00085-7. [DOI] [PubMed] [Google Scholar]
- Ding H. X.; Zhang Z. C.; Cao S. N.; Xu Y.; Yu J. G. Transformation of multi-component ginkgolide into ginkgolide B by Coprinus comatus. BMC Biotechnol. 2015, 15, 17–11. 10.1186/s12896-015-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. M.; Xu Y.; Yuan J. Ginkgolide B ameliorates NLRP3 inflammasome activation after hypoxic-ischemic brain injury in the neonatal male rat. Int. J. Dev. Neurosci. 2018, 69, 106–111. 10.1016/j.ijdevneu.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Guo C. L.; Zhang J. B.; Zhang P. Y.; Si A. Y.; Zhang Z. L.; Zhao L. P.; Lv F. H.; Zhao G. A. Ginkgolide B ameliorates myocardial ischemia reperfusion injury in rats via inhibiting endoplasmic reticulum stress. Drug Des., Dev. Ther. 2019, Volume 13, 767–774. 10.2147/DDDT.S179101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lou C. J.; Lu H. B.; Ma Z. G.; Liu C.; Zhang Y. Q. Ginkgolide B enhances gemcitabine sensitivity in pancreatic cancer cell lines via inhibiting PAFR/NF-κB pathway. Biomed. Pharmacother. 2019, 109, 563–572. 10.1016/j.biopha.2018.10.084. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Sun J.; Chen B. D.; Zhao Y. Y.; Gong H.; You Y.; Qi R. M. Ginkgolide B inhibits platelet and monocyte adhesion in TNF alpha-treated HUVECs under laminar shear stress. BMC Complementary Med. Ther. 2018, 18, 220. 10.1186/s12906-018-2284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P. D.; Mungur R.; Zhou H. J.; Hassan M.; Jiang S. N.; Zheng J. S. Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both in vivo and in vitro. Neural Regener. Res. 2018, 13, 1204–1211. 10.4103/1673-5374.232476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. M.; Pan J.; Zhang W. N.; Hui A. L.; Guo W. Q.; Huang L.; Zhu Q. J.; Chen Y. Synthesis, in silico and in vivo blood brain barrier permeability of ginkgolide B cinnamate. Fitoterapia 2015, 106, 110–114. 10.1016/j.fitote.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Xu L.; Zhang D.; Hu B.; Luo Q.; Han D.; Li J. B.; Shen C. W. Cardioprotection of ginkgolide B on myocardial ischemia/reperfusion-induced inflammatory injury via regulation of A20-NF-κB Pathway. Front Immunol. 2018, 9, 2844. 10.3389/fimmu.2018.02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. K.; Iskander G.; Black D. S.; Kumar N. An efficient lactamization of fimbrolides to novel 1,5-dihydropyrrol-2-ones. Tetrahedron Lett. 2007, 48, 2287–2290. 10.1016/j.tetlet.2007.01.165. [DOI] [Google Scholar]
- Pereira U. A.; Moreira T. A.; Barbosa L. C. A.; Maltha C. R. A.; Bomfim I. S.; Maranhao S. S.; Moraes M. O.; Pessoa C.; Barros-Nepomuceno F. W. A. Rubrolide analogues and their derived lactams as potential anticancer agents. Medchemcomm 2016, 7, 345–352. 10.1039/C5MD00459D. [DOI] [Google Scholar]
- Koch E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine 2005, 12, 10–16. 10.1016/j.phymed.2004.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.