In their Matters Arising, Verstraete et al.1 assert that the conclusions we draw in our manuscript (Wei et al.2) are not supported by the data presented. Based on seven cryo-EM structures of ATP Citrate Lyase (ACLY) in different liganded states, we concluded that both the reaction of ATP, citrate and CoA substrates and the formation of acetyl-CoA and OAA products occur in the ASH domain of ACLY. We proposed the following model for ACLY catalysis: CoA first binds to the ACLY CSH domain, which induces a structural rearrangement of ACLY, resulting in CoA translocation to the ASH domain. Once bound to the ASH domain, CoA reacts with ASH-bound citrate and ATP to form acetyl-CoA and OAA products. Once acetyl-CoA product is formed in the ASH domain, another structural rearrangement of ACLY unloads acetyl-CoA through binding to the CSH domain before acetyl-CoA leaves ACLY. Instead, Verstraete et al.1 contend that, as previously proposed in Verschueren et al.3, the citryl-CoA intermediate is formed in the ASH domain, followed by a ~35 Å movement of the intermediate into the CSH module where cleavage to acetyl-CoA and OAA products occurs. Although we cannot exclude the model for ACLY catalysis proposed by Verstraete et al.1, we believe that our alternative model is also consistent with the data reported by Verschueren et al.3, Verstraete et al.1 and Wei et al.2.
The specific concerns raised Verstraete et al.1 are (1) The reduced catalytic activity of ACLY mutants within the CSH module (H976, R976, D1026 and R1065) supports the catalytic roles of these residues, (2) The data reported in Wei et al.2 are not sufficient to claim that formation of acetyl-CoA and OAA products occurs in the ASH domain, (3) Even though we observed CoA bound to the CSH domain in the reported structure for ACLY in complex with CoA (PDB 6POE), a role of the CSH module in citryl-CoA cleavage is not considered, and (4 and 5) the phospho-citryl-CoA intermediate in the structure of the ACLY-E599Q mutant (PDB 6UUW) is incorrectly modeled.
Response to (1)
The mutational sensitivity of residues H975, R976, D1026 and R1065 within the human ACLY CSH module indeed argues strongly for their functional importance. However, based on the D2-symmetric 3.0 Å ACLY product structure with acetyl-CoA and OAA reported in Wei et al.2 (PDB 6UI9), we propose that these residues participate in binding of regulatory OAA and CoA molecules rather than play direct catalytic roles.
Response to (2)
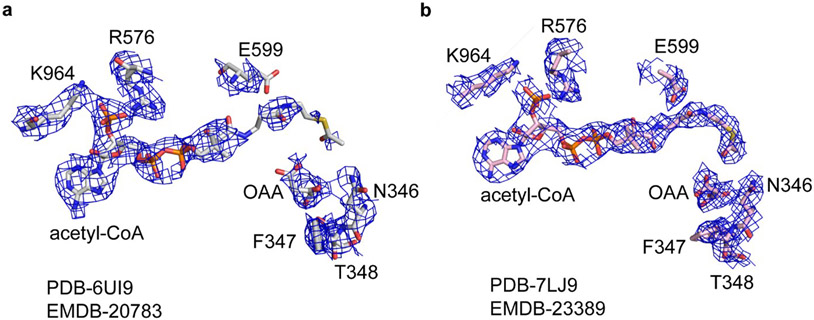
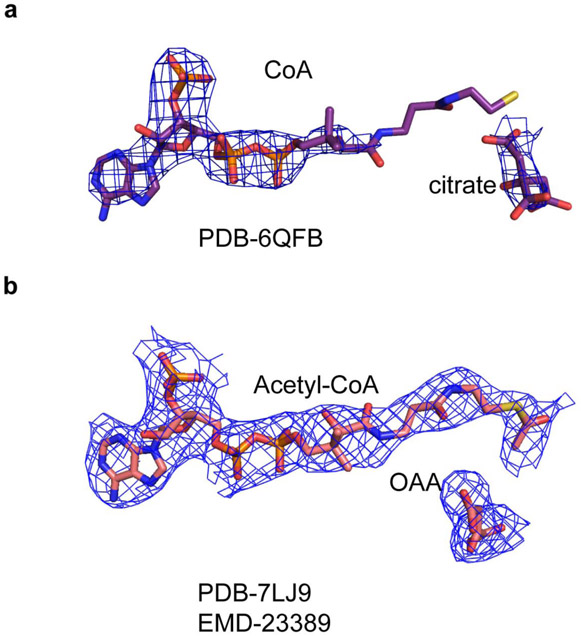
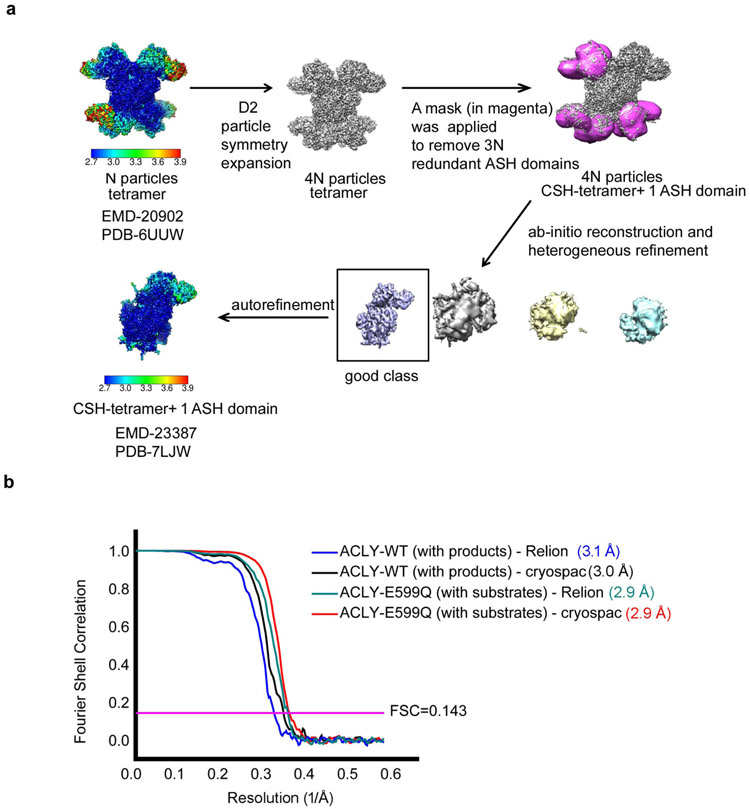
Verstraete et al.1 argue that observing acetyl-CoA and OAA products in the ASH domain of ACLY does not demonstrate that their cleavage occurs at this site. Furthermore, the authors contend that the experimental density for the acetyl-thioester moieties of acetyl-CoA in the ASH active site is poorly defined. We believe that our D2-symmetric 3.0 Å ACLY product structure with acetyl-CoA and OAA (PDB 6UI9) shows compelling density for acetyl-CoA in the ASH domain (Figure 1a) and that the more compromised density for the mercapto group of acetyl-CoA bound to the ASH domain likely reflects the structural variability of the ASH domain relative to the CSH domain2. Indeed, the mercapto group of CoA and the citrate molecule in the ASH domain of the crystal structure reported in Verschueren et al.3 (PDB 6QFB) is also poorly resolved in the electron density map (Extended Data Figure 1a). Nonetheless, to increase confidence that acetyl-CoA and OAA are correctly modeled in the ASH domain, we carried out non-uniform refinement together with particle symmetry expansion, particle subtraction and local refinement with Cryosparc (Extended Data Figure 2, Supplementary Table 1) on the cryo-EM density corresponding to the ACLY ASH domain of our ACLY–OAA–acetyl-CoA-D2 structure (PDB 6UI9)(Figure 1)4,5. This resulted in improved cryo-EM density for acetyl-CoA and OAA products in the ASH domain (Figure 1b). We have therefore updated the deposited atomic model and EM density map in the wwPDB and EMDB, which can be accessed via accession codes PDB-7LJ9 and EMBD-23389, respectively. While we also observe cryo-EM density in the CSH module, we believe this density corresponds to a regulatory OAA molecule. To note, we do not observe compelling density corresponding to acetyl-CoA in the CSH module, despite the significantly higher local resolution of the CSH module. In our view, these data are consistent with formation of acetyl-CoA and OAA in the ASH domain of ACLY as we originally proposed2, although we agree that they are not sufficient to prove this model.
Figure 1.
Cryo-EM density of acetyl-CoA and OAA in the ACLY active site. (a) Cryo-EM density corresponding to acetyl-CoA and OAA in the ASH domain of the ACLY–OAA–acetyl-CoA-D2 structure (PDB- 6UI9/EMD-20783) reported in Wei et al.2. (b) Cryo-EM density corresponding to acetyl-CoA and OAA in the ASH domain after non-uniform refinement together with particle symmetry expansion, particle subtraction and local refinement in Cryosparc (PDB-7LJ9/EMD-23389)4,5. The figure was made in Pymol with a contour level of 2.0 σ applied. Important acetyl-CoA and OAA contacting residues, and catalytic E599, with their corresponding cryo-EM densities are shown for reference.
Response to (3)
Our model for ACLY catalysis involves the initial binding of CoA to the CSH domain prior to its translocation to the ASH domain for product formation, consistent with CoA binding to both the ASH and CSH domains as observed in our ACLY–citrate–CoA structure (PDB 6POE)2 and by Verschueren et al.3. The use of regulatory or allosteric sites to load substrates prior to translocation to catalytic sites in metabolic enzymes is seen in other metabolic enzymes, for example with NADP+ in human glucose-6-phosphatedehydrogenase6 and we therefore favor our model over the one proposed by Verstraete et al.1 , since it rationalizes how CoA could bind to two distinct sites of ACLY.
Response to (4) and (5)
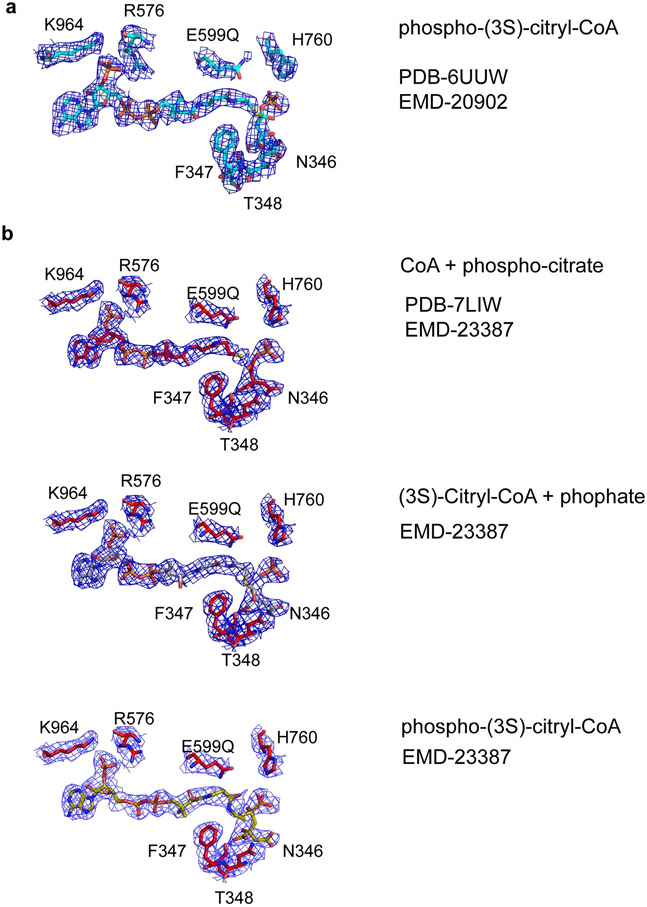
To address the concern that the phospho-citryl-CoA intermediate (ligand QHD) in the structure of the ACLY-E599Q mutant is incorrectly modeled, we carried out non-uniform and local refinement on the cryo-EM density corresponding to the ACLY ASH domain of the ACLY-E599Q–ATP–citrate–CoA-D2 structure (PDB-6UUW)4,5 (Extended Data Figure 2, Supplementary Table 1). This resulted in improved ligand density in the ASH active site, which could be best modeled as CoA + phospho-citrate, although at the current resolution we cannot exclude the additional presence of other reaction intermediates, such as phosphate + (3S)-citryl-CoA, and phospho-(3S)-citryl-CoA (Figure 2)7. We were also able to correct the stereochemistry of the modeled ligand QHD. The updated atomic model and EM density map have been deposited in the wwPDB and EMDB, respectively, with accession codes PDB-7LIW and EMBD-23387.
Figure 2.
Cryo-EM density of a reaction intermediate in the ACLY active site. (a) Cryo-EM density (2.5 σ) corresponding to modeled phosphor-(3S)-citryl-CoA in the ASH domain of the ACLY-E599Q–ATP–citrate–CoA-D2 structure (PDB- 6UUW/EMD-20902) reported in Wei et al.2. (b) Cryo-EM density (3.5 σ) corresponding to the reaction intermediate in the ASH domain after non-uniform refinement together with particle symmetry expansion, particle subtraction and local refinement in Cryosparc (PDB-7LIW/EMD-23387) 4,5. While the cryo-EM density can be best modeled as CoA + phospho-citrate (top, PDB-7LIW), at the current resolution we cannot exclude the additional presence of phosphate + (3S)-citryl-CoA (middle), and phospho-(3S)-citryl-CoA (bottom). The figure was made in Pymol. Important contacting residues, and catalytic E599, with their corresponding cryo-EM densities are shown for reference.
Verstraete et al.1 also report new data to support their model for ACLY catalysis including (1) Biochemical data that an isolated CSH module added in trans can complete the ACLY reaction of a catalytically inactive ACLY-D026A mutant, (2) A crystal structure of a CSH module incubated with acetyl-CoA and OAA resulting in one of four CSH modules containing a modeled (3S)-citryl-CoA molecule, and (3) A crystal structure of an archaeal ACLY Asp541 mutant incubated with substrates, resulting in CSH modules containing additional electron density modeled as (3S)-citryl-CoA.
Response to additional data
(1) While the isolated CSH module is able to catalyze cleavage of citryl-CoA to OAA and acetyl-CoA, in our view that does not necessarily demonstrate that the CSH module carries out the same chemistry in the context of the intact ACLY enzyme. (2) The observation that the isolated CSH module is able to form a stable complex with citryl-CoA is not surprising given that the CSH module can also bind citrate, OAA, CoA and acetyl-CoA2,3. The observation that the CSH module can synthesize citryl-CoA from acetyl-CoA and OAA is consistent with the reversibility of enzymes and the demonstration by Verstraete et al.1 that the CSH module can convert citryl-CoA to OAA and acetyl-CoA. (3) The crystal structure of an archaeal ACLY Asp541 mutant incubated with substrates showing citryl-CoA bound to the CSH module is consistent with a defect of the mutant in producing acetyl-CoA and OAA products. However, this result does not prove that the CSH domain of the archaeal ACLY directly participates in chemistry to produce OAA and acetyl-CoA products. For example, citryl-CoA could be formed in the ASH domain and then translocated to the CSH module for release. It is also possible that the archaeal and human ACLY enzymes have diverged in their molecular mechanisms of product formation. In line with this idea, we previously reported that orthologous N-terminal acetyltransferase enzymes in archaea and human differ in their mechanism of N-terminal acetylation8 .
Overall, we think that further studies are required to unambiguously resolve the catalytic mechanism of ACLY.
Extended Data
Extended Data Fig. 1.
Revised Cryo-EM density for ACLY with products.
Extended Data Fig. 2.
Workflow of data analysis.
Supplementary Material
Acknowledgements
Molecular graphics and structural analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing. This work was supported by NIH grants R35 GM118090 and P01 AG031862 to R.M.
Footnotes
Competing interests
The authors declare no conflict of interests.
METHODS
A description of the methods is available as Supplementary Note.
REPORTING SUMMARY
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
DATA AVAILIBILTY
A revised cryo-EM map of the ACLY ASH domain of the ACLY–OAA–acetyl-CoA complex and the associated coordinate were generated from micrographs corresponding to ACLY–OAA–acetyl-CoA-D2 (PDB-6UI9) and EMBD-20783 reported in Wei et al.2, and were deposited with local refinement as accession codes PDB-7LJ9 and EMBD-23389 to the wwPDB and EMDB, respectively; and without local refinement as accession codes PDB-7LLA and EMBD-23413 to the wwPDB and EMDB, respectively. A revised cryo-EM map of the ACLY ASH domain of the ACLY-E599Q–ATP–citrate–CoA complex and the associated coordinate were generated from micrographs corresponding to ACLY-E599Q–ATP–citrate–CoA-D2 (PDB-6UUW) and EMBD-20902 reported in Wei et al.2 and deposited with accession codes PDB-7LIW and EMBD-23387 to the wwPDB and EMDB, respectively.
REFERENCES
- 1.Verstraete K, Verschueren KHG, Dansercoer A & Savvides SN Acetyl-CoA is produced by the Citrate Synthase Homology module of ATP-citrate lyase. Nat Struct Mol Biol (2021). [DOI] [PubMed] [Google Scholar]
- 2.Wei X, Schultz K, Bazilevsky GA, Vogt A & Marmorstein R Molecular basis for acetyl-CoA production by ATP-citrate lyase. Nat Struct Mol Biol 27, 33–41, doi: 10.1038/s41594-019-0351-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verschueren KHG et al. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature 568, 571–575, doi: 10.1038/s41586-019-1095-5 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Punjani A, Zhang H & Fleet DJ Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nature methods 17, 1214–1221, doi: 10.1038/s41592-020-00990-8 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Zhou M et al. Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev 29, 2349–2361, doi: 10.1101/gad.272278.115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au SW, Gover S, Lam VM & Adams MJ Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 8, 293–303, doi: 10.1016/s0969-2126(00)00104-0 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Wei X, Schultz K, Bazilevsky GA, Vogt A & Marmorstein R Author Correction: Molecular basis for acetyl-CoA production by ATP-citrate lyase. Nat Struct Mol Biol 27, 511–513, doi: 10.1038/s41594-020-0421-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liszczak G & Marmorstein R Implications for the evolution of eukaryotic amino-terminal acetyltransferase (NAT) enzymes from the structure of an archaeal ortholog. Proc Natl Acad Sci U S A 110, 14652–14657, doi: 10.1073/pnas.1310365110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A revised cryo-EM map of the ACLY ASH domain of the ACLY–OAA–acetyl-CoA complex and the associated coordinate were generated from micrographs corresponding to ACLY–OAA–acetyl-CoA-D2 (PDB-6UI9) and EMBD-20783 reported in Wei et al.2, and were deposited with local refinement as accession codes PDB-7LJ9 and EMBD-23389 to the wwPDB and EMDB, respectively; and without local refinement as accession codes PDB-7LLA and EMBD-23413 to the wwPDB and EMDB, respectively. A revised cryo-EM map of the ACLY ASH domain of the ACLY-E599Q–ATP–citrate–CoA complex and the associated coordinate were generated from micrographs corresponding to ACLY-E599Q–ATP–citrate–CoA-D2 (PDB-6UUW) and EMBD-20902 reported in Wei et al.2 and deposited with accession codes PDB-7LIW and EMBD-23387 to the wwPDB and EMDB, respectively.