Abstract
Accurate discrimination between safe and dangerous stimuli is essential for survival. Prior research has begun to uncover the neural structures that are necessary for learning this discrimination, but exploration of brain regions involved in this learning process has been mostly limited to males. Recent findings show sex differences in discrimination learning, with reduced fear expression to safe cues in females compared to males. Here, we used male and female Sprague Dawley rats to explore neural activation, as measured by Fos expression, in fear and safety learning related brain regions. Neural activation after fear discrimination (Discrimination) was compared between males and females, as well as with fear conditioned (Fear Only) and stimulus presented (Control) conditions. Correlations of discrimination ability and neural activation were also calculated. We uncovered a correlation between central amygdala (CeA) activation and discrimination abilities in males and females. Anterior medial bed nucleus of the stria terminalis (BNST) was the only region where sex differences in Fos counts were observed in the Discrimination condition, and the only region where neural activation significantly differed between Fear Only and Discrimination conditions. Together, these findings indicate the importance of fear expression circuitry in mediating discrimination responses and generate important questions for future investigation.
Keywords: safety learning, sex differences, fear discrimination, fos, BNST, amygdala
1. Introduction
The expression of fear or anxiety in environments where it is not appropriate or under conditions that would not typically elicit fear in a healthy individual is a hallmark feature of post-traumatic stress disorder (PTSD) [1]. One case in which fear may be expressed inappropriately is in the presence of information indicating safety. Accurate discrimination between safety and danger allows an individual to respond flexibly and only express fear when threat is most likely to occur. Cues present when aversive stimuli are expected, but do not occur, may become safety signals. Safety signals predict the absence of the aversive stimuli and become inhibitors of responses associated with the aversive stimuli including fear.
People [2], monkeys [3] and rodents [4] are all able to discriminate between danger and safety in laboratory settings. Yet difficulty utilizing learned safety signals has been observed in a number of clinical PTSD studies [5–7]. Despite the increased prevalence of PTSD in women compared to men, much of the research on fear discrimination has focused on males [8,9]. In humans, fear discrimination is more sensitive to trauma history in females compared with males [10,11]. Translational research regarding sex differences in rodent fear discrimination is relatively limited, but a burgeoning area of exploration. Reports, including work from our own lab, indicate greater discrimination in females compared with males, with female rodents able to modulate fear to a safety signal more rapidly than males [12,13].
Compared to fear conditioning and extinction learning and recall, which are closely related areas of research, there is limited work on the neural circuitry of cued fear discrimination. A better understanding of the neural mechanisms that underlie this learning will provide critical information relevant to disorders in fear modulation. Based on existing information about the neural structures involved in fear learning and fear discrimination, particular brain regions are the focus of this work. This includes prefrontal regions known to be involved in fear modulation, subregions of the insular cortex (IC) likely involved in the integration of fear and safety information, and the amygdala and bed nucleus of the stria terminalis (BNST), regions of fear learning and expression.
The prelimbic (PL) and infralimbic (IL) subregions of the prefrontal cortex are known to be involved in promotion of fear and fear inhibition in extinction learning, respectively [14,15]. PL and IL modulation of fear occurs via projections to specific amygdala subregions, as well as inputs from amygdala, thalamus and hippocampus [15]. PL and IL are frequently identified in safety learning research, although reports on the necessity of each subregion vary [16–21]. Beyond the medial prefrontal cortex, the orbitofrontal cortex (OFC) plays a role in switching between states of fear and safety [22]. IC is also known to be a region of emotion learning and salience detection (for review, see [23]). In prior research, we found that posterior (pIC), but not anterior (aIC) or medial (mIC), IC was necessary acquisition of a conditioned fear inhibitor, but that pIC was not necessary for the prerequisite fear discrimination learning in males [24]. In sum, the PL, IL, OFC and IC, which all innervate the amygdala, are components of the executive circuitry underlying fear discrimination.
It is important to look at regions known to be involved in fear learning and expression, such as the basolateral amygdala (BLA) [25–28], the central amygdala (CeA) [29–31], and the bed nucleus of the stria terminalis (BNST) [32–35]. BLA and BNST may play critical roles in safety learning. Many studies have in fact found evidence that safety signals impact neuronal responding in the BLA, in single unit recordings [36,37], as well as spine morphology and synapse size and strength [38,39]. Similarly, safety training alters BNST activation, although the direction of change may depend on the behavioral paradigm used [33,40]. Together, these brain regions implicated in fear learning, safety learning, and expression may be key nodes in a brain wide safety learning circuit, which is the basis of this work [41].
Here we explore the brain regions involved in acquisition of fear discrimination in males and females using Fos as a marker of neural activation. Quantifying Fos, the protein product of immediate early gene c-fos, which is induced in response to a large range of stimuli, is widely used as a proximate measure of a neuron’s recent activity [42,43]. Since the neural basis of fear discrimination is largely unknown, we compare fear discrimination neural activation patterns to animals that are not given a safety cue (a Fear Only group) and to animals that are never shocked (a cue only Control group). This approach allows for observation of brain regions that may be activated specifically due to fear discrimination compared to fear learning without safety information or the sensory experience of cue exposure. We analyzed Fos in several ways to 1) test if fear discrimination conditioning differentially activates regions compared to fear conditioning and controls, 2) test for potential sex differences in Fos levels, and 3) to correlate regional Fos with fear discrimination learning.
2. Materials And Methods
2.1. Animals
Sprague Dawley rats were obtained from Taconic Biosciences (Hudson, NY). Adult males (7-8 weeks old) and females (10-12 weeks old) were weight-matched, arriving at 225-250g, so that down force on the footshock grids would be comparable. Rats were housed in same-sex pairs in plastic tub cages with free access to food and water. Males and females were housed in isosexual pairs within the same colony room, where they were kept on a 12-hour light/dark cycle. All animals had 7 days to acclimate to colony housing before any experimental procedures took place. Each of 3 experimental groups included 8 male and 8 female rats. All experimental procedures were reviewed and approved by the Boston College Institutional Animal Care and Use Committee.
2.2. Apparatus
All behavioral conditions were performed in the same apparatus used previously: a 15 × 12 × 27in (L x W x H) light and sound-attenuating chamber with a fan for ventilation and background noise (~55dB) housed a 10 × 11 × 6in (L x W x H) chamber made of black plastic with wire mesh lids with a stainless steel grid floor [13,22,24,44,45]. A 1.2 mA, 0.5s scrambled foot shock was delivered via shocker Model H13-15, Coulbourn Instruments. Digital video cameras (Model VX-5000, Microsoft, Redmond, VA) were used to record behavior, with infrared blocking filters removed. Infrared LEDs illuminated the chambers, allowing for video observation of freezing as a behavioral measure of fear, and detected with ANY-Maze computer software (version 4.99, Stoelting, Wood Dale, IL). Stimuli were delivered through a flashing white LED light, 264.0 Lux, 20ms on/off, and a white noise pip was 10ms duration, 3 Hz interval, 75 dB. Cue presentations in all behavioral conditions were preceded by a 5 s, 1 kHz tone (75 dB) to correspond with the paradigm used for discrimination conditioning. Assignment of stimuli as danger (CS+) or safe (CS−) was counterbalanced, and no effect of cue was observed, as reported previously [13,22,24,44,45].
2.3. Behavioral Conditions
Conditioning for the Discrimination group consisted of 15 presentations each of shock-paired (CS+) or unpaired (CS−) cues, for a total of 45 minutes in the session. Each trial was signaled by a common element (X), a 5 s, 1 kHz tone (75 dB) immediately followed by a 15 s discrete auditory (white noise pips) or visible (flashing LED light) CS. We adapted our procedure from the AX+/BX− procedure of Myers and Davis [4]. In extensive pilot studies we found that placing the transfer stimulus, X, before the CS facilitated the rate at which the CS− inhibited behavioral freezing in summation tests while reducing the impact of external inhibition. These issues are directly addressed previously [4,24]. Trials were presented in a quasi-random order, so that no cue occurred more than twice in succession. There was a fixed 70 s inter-trial-interval. Assignment of the light or pip as CS+ or CS− was counterbalanced in each experiment, and equally represented in each treatment condition. CS− trials were omitted in the Fear Only condition and inter-trial intervals were extended to maintain equal time in the conditioning apparatus. The Control condition received the same auditory and visual stimuli as in CS+/CS− fear discrimination conditioning, but no shocks were presented.
2.4. Estrous Phase Testing
Immediately prior to perfusion and tissue collection, all females were tested for estrous phase via vaginal smear with sterile saline, as estrogen and estrous phase impact fear discrimination [46,47]. Estrous phase was verified on unstained slides at 10X magnification. Phase verification and procedure were performed as in [48].
2.5. Tissue Collection and Fos Immunohistochemistry Procedures
After conditioning, rats were moved to a quiet room, where they remained undisturbed for 1 hour. Next, all animals were perfused with 0.01 M heparinized phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were dissected and post-fixed in 4% paraformaldehyde at 4°C for 24h and before being transferred to 30% sucrose. Brains were then sliced into 40μm sections at −20°C and stored in cryoprotectant-filled well plates at 4°C. Immediate early gene product Fos was identified via immunohistochemistry (IHC) as a neural marker of activation.
Fos was visualized as previously [49]. Free floating sections were blocked with 2% normal donkey serum in PBS-T (0.01% Triton-X100) and incubated overnight in rabbit anti-c-fos antibody at 1:5000 (Millipore, ABE457). The following morning, sections were washed and incubated in biotinylated donkey anti-rabbit secondary antibody at 1:200 (Jackson ImmunoResearch). Secondary was visualized using the avidin-biotin complex method (ABC Elite Kit, Vector Labs) with chromogen (Vector SG Peroxidase Substrate Kit, Vector Laboratories). At the completion of the reaction, slices were floated onto glass slides, dehydrated, cleared, coverslipped with Permount, and left to dry for 48 hours. Sections were imaged at 10x using a Zeiss Axioimager Z2 light microscope with an AxioCam HRc digital camera. Fos positive cells were quantified within a standardized size area for each region based on atlas images [50]. The cell counter plug-in on ImageJ software was used to automate Fos quantification, and parameters were verified by manual cell counts. For each brain region of interest, 2 sections per animal were analyzed.
2.6. Brain Regions of Analysis
2.7. Data Analysis
Freezing was analyzed as percent time freezing during the 15 s CS presentations and as percentage during the entire 45-minute session. A discrimination index was calculated as freezing to CS− divided by freezing to CS+ times 100, so that a value of 100 reflected no discrimination between the CS+ and CS−, and values less than 100 indicated reduced fear to the CS− compared to the CS+, as previously [13]. Discrimination index was used in correlation analysis with Fos data to explore how well neural activation predicted discrimination.
Group differences in behavioral freezing were evaluated by analyses of variance (ANOVA) with sex and treatment condition as a between-subjects factors; cue type and trial block were within-subjects factors. Sex differences in freezing were evaluated with a 2-way ANOVA of cue or trial block by sex. 2-tailed, unpaired t-tests were used to compare freezing in males and females during Fear Only conditioning, as well as to compare discrimination indices in the Discrimination condition. A 3-way ANOVA of cue by sex by trial block (5 blocked of 3 trials) was used to assess fear and discrimination learning across the training session. A 1-way ANOVA was used to assess the role of estrus phase on discrimination in females. Freezing differences across behavioral conditions were evaluated with a 2-way ANOVA of condition by sex. Fos counts in each brain region were analyzed with a 2 (Sex) by 3 (Condition) ANOVA of average Fos counts per brain region. For the Discrimination condition, Fos was compared to discrimination abilities using Pearson’s r correlation between region Fos and discrimination index.
Main effects and interactions were deemed significant with p < 0.05 and between-subjects post hoc comparisons were made with Sidak’s correction. Correlations between Fos and discrimination index were analyzed with Pearson’s r. Discrimination index and Fos counts in each brain region were checked for outliers with Grubb’s test with alpha set to 0.05. All analyses were made using GraphPad Prism 8.
3. Results
3.1. Behavioral Results
3.1.1. CS+/CS− Fear Discrimination Conditioning
One male rat from the Discrimination Condition, with significantly greater fear to the CS− compared to the CS+, was identified as an outlier and excluded from all analyses. Sex differences were apparent in CS+/CS− fear discrimination conditioning, as previously reported [13]. Looking at average freezing to each cue during CS+/CS− fear discrimination conditioning (Figure 1A), a two-way ANOVA revealed main effects of both cue, F (1, 13) = 52.64, p < 0.0001, and sex, F (1, 13) = 12.42, p = 0.0037, but no cue by sex interaction, F (1, 13) = 0.74, p = 0.40. Post hoc analyses showed that males (n = 7) and females (n = 8) displayed significantly different freezing to both cues, CS+, p = 0.02, and CS−, p = 0.003. Both males and females significantly discriminated between the CS+ and CS−, p = 0.0015 and p < 0.0001, respectively. Looking at discrimination index, females displayed significantly lower discrimination index, representative of greater discrimination, compared to males, t (13) = 2.58, p = 0.023 (Figure 1B). This trend was evident early in conditioning. A 3-way within subjects ANOVA of sex by cue by trial block (5 blocks of 3 cue trials) identified main effects of cue, F (1, 13) = 52.64, p < 0.0001, trial block, F (4, 52) = 5.4, p = 0.001, sex, F (1, 13) = 12.42, p = 0.0037, and a significant trial by sex interaction, F (4, 52) = 2.69, p = 0.04 (Figure 1C). Post hoc comparisons revealed that females significantly discriminated between the CS+ and CS− on trial blocks 2 and 3, ps < 0.02, while males did not significantly discriminate between the CS+ and CS− on any trial blocks. Males and females displayed significantly different freezing to the CS− on trial block 2, p = 0.016. To investigate a role of estrus phase, we performed a one-way ANOVA, which revealed no main effect of cycle phase on discrimination, F (3, 4) = 1.85, p = 0.28. Importantly, since cycle phase was not the main focus of this work, this analysis is underpowered, with only 1 to 3 animals in each of the 4 cycle phases.
Figure 1. Freezing behavior.
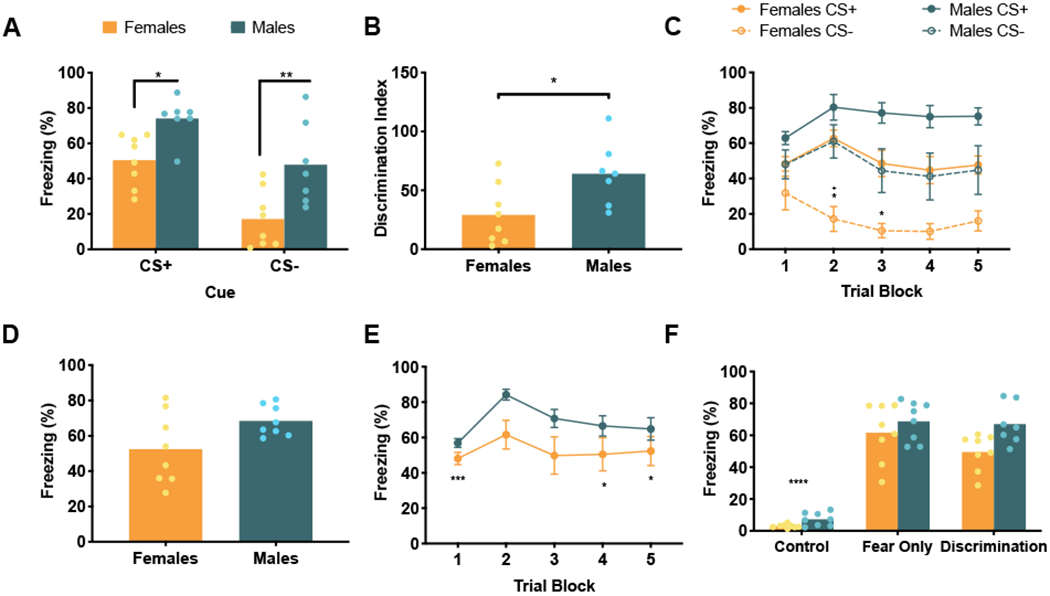
(A) Mean (and individual replicates) freezing to CS+ and CS− of animals in the Fear Discrimination condition. Both males and females significantly discriminated between CS+ and CS− (significance not marked, ps < 0.0015). Females froze significantly less than males to both CS+ (p = 0.02) and CS− (p = 0.003). (B) Mean (and individual replicates) discrimination indices (time freezing to CS− / time freezing to CS+ x 100) of the Fear Discrimination animals during conditioning. Females had a significantly reduced discrimination index compared to males (p = 0.023). (C) Freezing averages (±SEM) to CS+ and CS− across blocks of three trials in conditioning. Females significantly discriminated between CS+ and CS− in trial blocks 2 and 3 (ps < 0.02), while males did not significantly discriminate on any trial blocks. Females also displayed less freezing to CS− than males on trial block 2 (p = 0.016). *significant difference between CS+ and CS− within sex, +significant difference between males and females to CS−. (D) Mean (with individual replicates) fear to danger cue, CS+, for animals in the Fear Only condition. Males and females did not significantly differ in freezing to CS+ (p = 0.06). (E) Mean (±SEM) freezing to CS+ of Fear Only animals across blocks of three trials in conditioning. Freezing was significantly different in trial blocks 1, 4 and 5 compared to trial 2 (ps <0.025). (F) Mean (and individual replicates) percent time freezing during the entire 45 minute conditioning session for all behavior conditions. Control animals froze significantly less than both Fear Only and Discrimination animals (ps < 0.0001).
* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
3.1.2. Fear Conditioning
In the Fear Only condition, a two-tailed t-test comparing average freezing to the cue in males (n = 8) and females (n = 8) found trending, but not significant differences between males and females, t (14) = 2.07, p = 0.06, which is similar to the effect found on the CS+ cue in the Discrimination condition (Figure 1D). Despite the variation of fear in females, this did not appear to depend on estrus phase. No females in the fear condition were in estrus. Comparing fear to the cue in females in proestrous, diestrus, and metestrus cycle phases in a one-way ANOVA, there was no main effect of Estrous phase, F (2, 5) = 0.31, p = 0.75. Freezing to cue presentations during conditioning across trial blocks, as in the Discrimination condition, a 2-way ANOVA of sex by trial block found a main effect of trial block, F (4, 56) = 5.47, p = 0.0009, but no significant main effect of sex, F (1, 14) = 4.47, p = 0.053, and no sex by trial block interaction, F (4, 56) = 0.81, p = 0.53 (Figure 1E). Post hoc analyses showed significantly different freezing during trial blocks 1 (p = 0.0004), 4 (p = 0.024), and 5 (p = 0.025) compared to trial block 2.
3.1.3. All Behavioral Conditions
In analysis of percentage of time freezing during the entire 45 minute conditioning session, including cues and ITIs, for all behavioral conditions, a 2-way ANOVA found main effects of Condition, F (2, 41) = 131.1, p < 0.0001, and Sex, F (1, 41) = 8.36, p = 0.006, but no Condition by Sex interaction, F (2, 41) = 1.42, p = 0.25 (Figure 1F). As expected, post hoc analyses revealed significantly increased freezing in Fear Only and Discrimination conditions compared to Control animals (ps < 0.0001). Control animals did not express freezing to the cues or conditioning context.
3.2. Fos Results
3.2.1. Prefrontal Cortex
Prelimbic (PL)
One female in the Control condition was not analyzed for PL Fos due to damaged brain slices. Therefore, analysis of PL Fos included: Control Females (n = 7), Control Males (n = 8), Fear Only Females (n = 8), Fear Only Males (n = 8), Discrimination Females (n = 8), Discrimination Males (n = 7). Analysis of PL Fos revealed a main effect of condition, but no main effect of sex or sex by condition interaction (see Table 2). Post hoc comparisons found that PL was significantly increased in Fear Only (p = 0.023) and Discrimination (p = 0.0002) conditions compared to Control animals, while Fos in Fear Only and Discrimination conditions did not significantly differ, but was trending toward significance (p = 0.07), with increased Fos in the Discrimination condition compared to Controls (Figure 2B). Examining the Discrimination condition more closely, we aimed to uncover if Fos in any brain region of interest directly correlated with animals’ ability to discrimination between CS+ and CS−, as measured by the discrimination index. Pearson r correlation was calculated between discrimination index and PL Fos (Table 3). Across all Discrimination animals, PL Fos and discrimination index trended toward a significant correlation (p = 0.077), where there were higher levels of Fos in PL in animals with lower discrimination indices – demonstrating greater discrimination (Figure 2C).
Table 2.
Results of a 2 (Sex) by 3 (Condition) ANOVA of average Fos counts for each brain region. Regions in bold represent significant main effects.
| Brain Region | Condition | Sex | Interaction |
|---|---|---|---|
| PL | F (2, 40) = 8.66 *** | F (1, 40) = 1.14 | F (2, 40) = 0.23 |
| IL | F (2, 40) = 4.85 * | F (1, 40) = 0.08 | F (2, 40) = 0.41 |
| vlOFC | F (2, 39) = 0.97 | F (1, 39) = 1.24 | F (2, 39) = 0.23 |
| aIC | F (2, 41) = 1.68 | F (1, 41) = 0.16 | F (2, 41) = 1.34 |
| mIC | F (2, 40) = 3.54 * | F (1, 40) = 0.0009 | F (2, 40) = 0.73 |
| pIC | F (2, 41) = 1.70 | F (1, 41) = 0.65 | F (2, 41) = 1.68 |
| BLA | F (2, 41) = 9.02 *** | F (1, 41) = 1.46 | F (2, 41) = 0.79 |
| CeA | F (2, 41) = 19.64 **** | F (1, 41) = 0.017 | F (2, 41) = 2.36 |
| BNST | F (2, 39) = 13.56 **** | F (1, 39) = 6.59 * | F (2, 39) = 0.53 |
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001
Figure 2. Fos in Prefrontal Cortex.
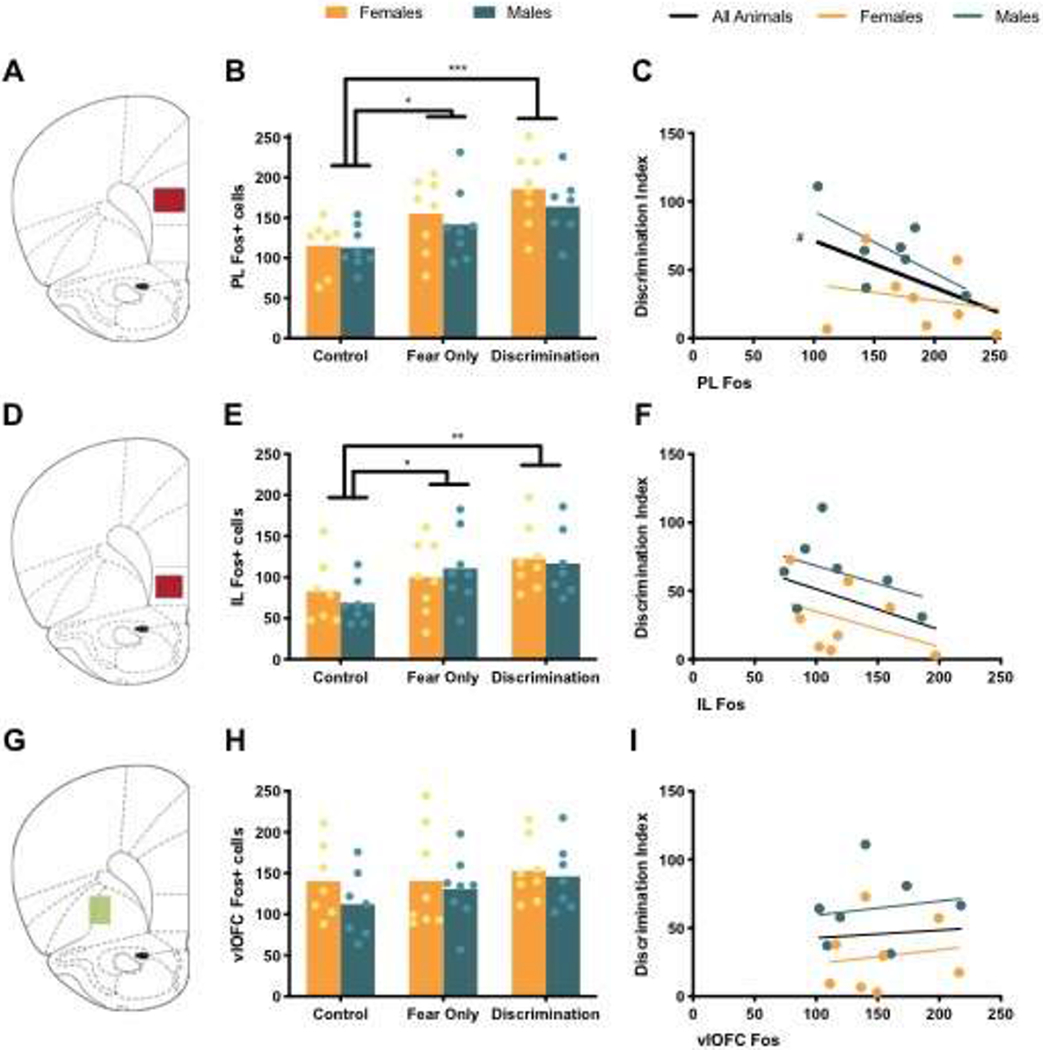
(A) Atlas image at 3.20 mm from Bregma with representative PL area of analysis in red. (B) Mean (with individual replicates) Fos positive cells in the PL. (C) Correlation between PL Fos and discrimination index, # indicates trending effect, p = 0.077. (D) Atlas image at 3.20 mm from Bregma with representative IL area of analysis in red. (E) Mean (with individual replicates) Fos positive cells in the IL. (F) Correlation between IL Fos and discrimination index. (G) Atlas image at 3.20 mm from Bregma with representative vlOFC area of analysis in green. (H) Mean (with individual replicates) Fos positive cells in the vlOFC. (I) Correlation between vlOFC Fos and discrimination index.
#p < 0.10, *p < 0.05, **p < 0.01, p < 0.001
Table 3.
Pearson’s r correlation (and corresponding p-value) between brain region and discrimination index across all animals in the Discrimination condition, and by sex. Regions in bold represent trending or significant correlations.
| Brain Region | All Animals | Female | Males |
|---|---|---|---|
| PL | r = −0.47, p = 0.077# | r = −0.21, p = 0.617 | r = −0.65, p = 0.116 |
| IL | r = −0.37, p = 0.174 | r = −0.40, p = 0.329 | r = −0.40, p = 0.374 |
| vlOFC | r = 0.069, p = 0.808 | r = 0.15, p = 0.727 | r = 0.16, p = 0.739 |
| aIC | r = 0.27, p = 0.340 | r = −0.13, p = 0.758 | r = 0.019, p = 0.968 |
| mIC | r = −0.14, p = 0.631 | r = −0.39, p = 0.342 | r = 0.36, p = 0.433 |
| pIC | r = −0.29, p = 0.290 | r = −0.25, p = 0.552 | r = 0.19, p = 0.689 |
| BLA | r = −0.06, p = 0.837 | r = 0.24, p = 0.567 | r = 0.30, p = 0.512 |
| CeA | r = −0.59, p = 0.019 * | r = −0.63, p = 0.091# | r = −0.50, p = 0.256 |
| BNST | r = 0.13, p = 0.659 | r = −0.65, p = 0.084# | r = 0.11, p = 0.843 |
p < 0.1 (trending),
p < 0.05 (significant).
Infralimbic (IL)
As in PL, brain sections from one female from the Control condition were unable to be analyzed for IL Fos. Analysis of average IL Fos revealed a main effect of condition, but no main effect of sex or sex by condition interaction (Table 2), with reduced Fos in Control animals compared to both Fear Only (p = 0.04) and Discrimination (p = 0.004) conditions (Figure 2E). There was no significant difference between Fear Only and Discrimination conditions (p = 0.32). Pearson r correlation did not find a significant correlation between IL Fos and discrimination index (Figure 2F; Table 3).
Ventral Lateral Orbital Frontal Cortex (vlOFC)
vlOFC Fos counts for one male in the Control condition was identified as an outlier by Grubbs’ test, alpha set to 0.05, and was therefore excluded from analyses. The resulting groups were as follows: Control Females (n = 8), Control Males (n = 7), Fear Only Females (n = 8), Fear Only Males (n = 8), Discrimination Females (n = 8), Discrimination Males (n = 7). Unlike the subregions of vmPFC, no significant main effects were found in average vlOFC Fos (Table 2; Figure 2H). There was also no significant correlation between vlOFC Fos and discrimination index (Table 3; Figure 2I).
3.2.2. Insular Cortex
Anterior (aIC)
Analysis of average Fos in aIC revealed no main effects of condition or sex, and no sex by condition interaction (Table 2, Figure 3B). There was also no significant correlation between aIC Fos and discrimination index (Table 3, Figure 3C).
Figure 3. Fos in Insular Cortex.
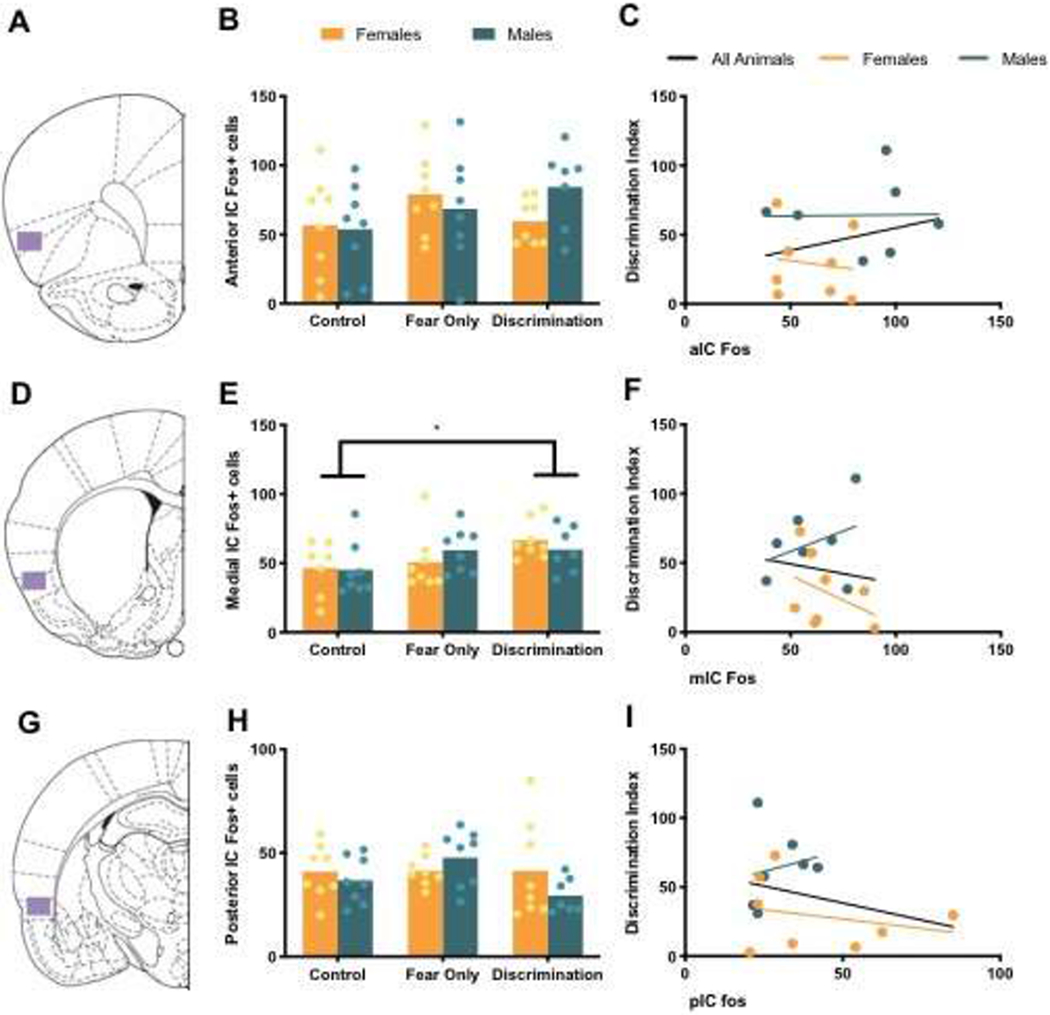
(A) Atlas image at 3.20 mm from Bregma with representative aIC area of analysis in purple. (B) Mean (with individual replicates) Fos positive cells in aIC. (C) Correlation between aIC Fos and discrimination index. (D) Atlas image at 0.20 mm from Bregma with representative mIC area of analysis in purple. (E) Mean (with individual replicates) Fos positive cells in mIC. (F) Correlation between mIC Fos and discrimination index. (G) Atlas image at −2.56 mm from Bregma with representative pIC area of analysis in purple. (H) Mean (with individual replicates) Fos positive cells in the pIC. (I) Correlation between pIC Fos and discrimination index. * p < 0.05
Medial (mIC)
Fos measurements from mIC were not obtained for one female in the Control condition due to damaged brain slices. Therefore, analysis of mIC Fos included: Control Females (n = 7), Control Males (n = 8), Fear Only Females (n = 8), Fear Only Males (n = 8), Discrimination Females (n = 8), Discrimination Males (n = 7). A main effect of condition, but no main effect of sex and no sex by condition interaction, was found in average Fos measures in mIC (Table 2). Post hoc analysis revealed significantly different mIC Fos in Control and Discrimination groups (p = 0.011), but not Fear Only compared to Discrimination animals (p = 0.2) or Controls (p = 0.16; Figure 3E). There was no significant correlation between mIC Fos and discrimination Index (Table 3; Figure 3F).
Posterior (pIC)
As in aIC, no main effects of condition or sex, or a sex by condition interaction were found in analysis of average pIC Fos (Table 2; Figure 3H). There were also no significant correlations between pIC Fos and discrimination index (Table 3; Figure 3I).
3.2.3. Extended Amygdala
Basolateral Amygdala (BLA)
Analysis of BLA Fos revealed a main effect of condition, but no main effect of sex and no sex by condition interaction (Table 2). Post hoc analyses showed significantly increased Fos positive cells in BLA in Fear Only (p = 0.001) and Discrimination (p = 0.0004) conditions compared to Control animals (Figure 4B). Pearson r correlation analysis of BLA Fos and discrimination index found no significant correlations (Table 3; Figure 4C).
Figure 4. Fos in the Extended Amygdala.
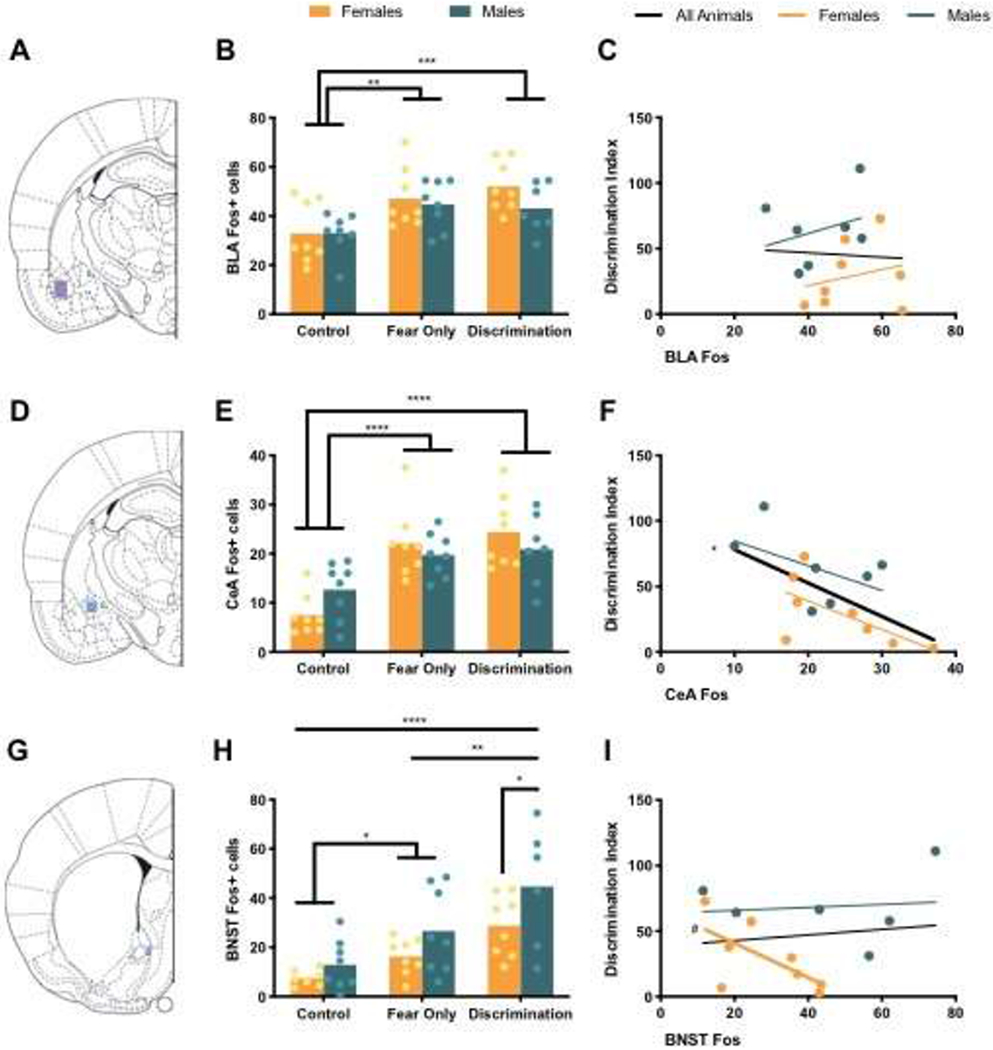
(A) Atlas image at −2.56 mm from Bregma with representative BLA area of analysis in purple. (B) Mean (with individual replicates) Fos positive cells in BLA. (C) Correlation between BLA Fos and discrimination index. (D) Atlas image at −2.56 mm from Bregma with representative CeA area of analysis in blue. (E) Mean (with individual replicates) Fos positive cells in CeA. (F) Correlation between CeA Fos and discrimination index. (G) Atlas image at 0.20 mm from Bregma with representative anterior medial BNST area of analysis in blue. (H) Mean (with individual replicates) Fos positive cells in the BNST. (I) Correlation between BNST Fos and discrimination index. #p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Central Amygdala CeA
As in BLA, CeA Fos analysis revealed a main effect of condition, with significantly increased Fos in shocked conditions – Fear Only and Discrimination (ps < 0.0001) – compared to Control animals (Figure 4E), but no significant difference in Fos between Fear Only and Discrimination conditions (p = 0.43). No main of effect of sex or interaction of sex and condition was found in CeA Fos (Table 2). A significant correlation between CeA Fos and discrimination index was apparent, Pearson r = −0.59 and p = 0.02, such that higher CeA Fos indicates a lower discrimination index, or greater discrimination between the CS+ and CS− (Figure 4F).
Bed Nucleus of the Stria Terminalis (BNST)
Damaged tissue slices for BNST prevented Fos collection from one Control female and one Discrimination Male. Fos analysis of BNST included: Control Females (n = 7), Control Males (n = 8), Fear Only Females (n = 8), Fear Only Males (n = 8), Discrimination Females (n = 8), Discrimination Males (n = 6). Anterior medial BNST Fos was the only brain region of our selected regions of interest where we found a significant main effect of sex. There was also a significant main effect of condition in BNST Fos, but no sex by condition interaction (Table 2). Post hoc analyses revealed significantly less Fos positive cells in females of the Discrimination condition compared to males in the Discrimination condition (p = 0.04), but no sex differences were found in Fear Only (p = 0.14) or Control (p = 0.45) conditions. Between conditions, there was significantly increased BNST Fos in the Fear Only condition compared to Controls (p = 0.027) and in Discrimination compared to both the Control (p < 0.0001) and Fear Only (p = 0.004) conditions (Figure 4H). Discrimination index did not correlate with BNST Fos (Table 3). However, the correlation between BNST Fos and discrimination in female discrimination animals was trending toward significance (p = 0.08), such that more BNST Fos indicated a lower discrimination index, or better discrimination (Figure 4I).
4. Discussion
While the investigation of a safety learning circuit is far from complete, the work here aimed to identify brain regions differentially activated by safety learning compared to fear learning and controls, and explore sex differences in activation in these regions. Sex differences were only found in the anterior medial BNST. As expected, many brain regions showed differential activation to shock conditions (Discrimination and Fear Only) compared to our cue only (Control) condition, including PL, IL, BLA, and CeA. Differential activation was also observed in mIC, with more Fos in the Discrimination condition than Controls, and anterior medial BNST, with significantly different activation in each behavioral condition. Of these regions, Fos in the CeA correlated with discrimination. Overall, this is an important start to uncovering the extent of the brain mechanisms that mediate safety learning.
4.1. Prefrontal Cortex
Fear Only and Discrimination conditions had increased Fos in PL and IL compared to Controls. We predicted that these regions process danger information in a discrimination paradigm due to prior research that found reduction of fear to a danger cue after inhibition of either PL or IL [21]. Studies have also reported reduced GABA transmission in PL reduced discrimination, while chronic disinhibition of IL via blockade of GABA synthesis enhanced conditioned safety learning [17,18,51]. Recent work also suggests that ventral hippocampus projections to PL, but not IL, mediate inhibition of fear [20]. Here, the correlation between PL and discrimination index only trended toward significance.
On the side of safety processing, our lab and others previously found evidence that vlOFC is necessary for a reduction of fear in the presence of safety signals [22,52]. OFC is anatomically connected for involvement in fear modulation—receiving sensory and amygdala inputs and sending projections to IC, amygdala, and striatum [53,54]. However there was no effect of vlOFC across conditions or sex, and Fos in vlOFC did not correlate with discrimination index.
4.2. Insular Cortex
IC is a region known to involved in salience detection, emotional learning and responding to multisensory stimuli [55]. We previously found a necessity of pIC, but not aIC or mIC, in the acquisition of a conditioned inhibition of fear but not to early discrimination, as measured by a summation test [24]. Here there were no differences in pIC Fos across conditions suggesting that pIC may contribute to transitioning a discriminatory CS− into a conditioned inhibitor. In contrast, we found a main effect of condition in mIC Fos, with increased Fos in the Discrimination condition compared to Controls. This may reflect greater demand on the IC “salience network” which is engaged by changing and unexpected sensory in puts which are present in the discrimination conditioning [56]. Interestingly IC function and salience processes are dysregulated in PTSD [57–59].
4.3. Extended Amygdala
There was increased Fos in the BLA in all shocked conditions (Fear Only and Discrimination) compared to Controls. However, Fos in BLA did not differ between males and females or correlate with discrimination index. Although there is evidence that some lateral amygdala neurons respond specifically to safe cues, many of these neurons exhibit reduced firing to the safe cue, which would not be evident in Fos analyses [37]. Interestingly, in the CeA Fos positively correlated with discrimination behavior in males and females such that animals with greater discrimination had increased CeA Fos. Interestingly, the CeA is composed of heterogeneous cell groups, some of which are critical inhibitors of CeA output [60,61]. Although not directly measurable with the current approach, greater CeA Fos could reflect a specific recruitment of fear-inhibiting CeA neurons.
That no sex difference was found in any correlation with discrimination could potentially indicate that different populations of neurons are activated in males and females rather than different numbers of neurons. CeA is known to contain receptors for estrogen, progesterone, and androgen, which may be capable of modulating emotional behavior [62]. Identifying the activation of neurons with these sex-related hormone receptors may be informative in determining if CeA activation is impacting discrimination in sex-specific ways.
In our study, only the anterior medial BNST had significantly different activation in animals trained in a fear discrimination paradigm compared to animals that underwent fear conditioning without a safe cue. The activation of BNST in safety learning fits with prior Fos evidence that medial and ventral BNST regions all show increased activation in safety trained animals compared to naïve animals [33]. Anterior medial BNST receives projections from IL and is densely connected to amygdala structures, particularly the CeA [63,64]. Anterior medial BNST may regulate the activity and output of the ventral and lateral aspects of the BNST which tend to promote fear and anxiety expression [34,65]. BNST also exhibited a sex difference in the number of Fos positive cells, which fits with ample evidence that sex-specific modulation of anxiety may depend on different mechanisms within the BNST (for review, see [62]).
4.4. Limitations
This study sought to start the lengthy process of establishing a large-scale understanding of the neural circuits that are active when learning to discriminate between danger and safety. In selecting a design, we felt compelled to include three conditioning groups and both male and female subjects. The resulting n = 8 is likely underpowered to detect real, but relatively small effects across treatments or regions of interest. Future studies may therefore consider eliminating no-shock control groups and increasing the number of males and females. An unfortunate consequence of the experimental design is that interesting correlations between Fos and behavior, or between multiple regions of interest did reach significance when controlling for the large number of post hoc comparisons. These data were not presented here, but are publicly available [66]. For the dependent measure, Fos and the regions of interest were selected so our results would compare to the substantial prior art. However, Fos counts cannot identify 1) cell types, 2) cell anatomical connectivity, 3) or temporal specificity. As noted above, it is likely that treatment and sex differences may be evident when looking at specific cell groups, circuits and times. Specifically, we cannot untangle whether different neurons responded the CS+, CS−, or both. Thus, failure to observe discrimination effects in the current data should not be interpreted as reflecting a true lack of involvement in the regions studied.
Further, the brain regions explored in this study only touch upon the plethora of regions that are implicated in fear learning and fear discrimination. Subregions of the striatum have been explored as a site of fear discrimination. Nucleus accumbens (NAcc) lesions were not found to effect acquisition of a safety signal, but there is evidence of enhanced synaptic responding to a safety cue in the caudate putamen [67,68] and a role for NAcc in very rapid behavioral responses to danger and safety cues [69]. There is also evidence that dorsolateral striatum mediates extinction learning and both NAcc and dorsolateral striatum dopamine release are mediated by estradiol and progesterone in females [70–72]. Similarly, there is evidence that activation of the ventral tegmental area (VTA) is involved in successful fear discrimination, and there are known sex differences in the VTA dopaminergic systems [73–75]. Existing work also indicates a role of dopamine in safety learning, specifically binding to D1- and D2-receptors in the BLA [76]. While the work presented here covers many brain regions involved in fear discrimination, it is a noncomprehensive exploration.
It is worth noting that the behavioral sex difference in discrimination observed here, and in our prior work, is consistent with some, but not all, other preclinical discrimination studies in rodents [12,16,46,77] and largely conflicts human literature, where males display greater discrimination than females [10,11]. Although women are more likely to be diagnosed with PTSD than men [8,9], in PTSD, both men and women display aberrant fear discrimination [7,78,79]. Given that in our paradigm, both male and female rats exhibited discrimination and comparable levels of Fos, in most regions, the larger apparent effect in females may be a consequence of parameters that reveal subtle differences in the behavioral expression of fear and its inhibition. Together these limitations should be considered when comparing the current findings other investigations and clinical application.
4.5. Conclusion
With this work, we aimed to test a hypothetical circuitry underlying sex differences in fear discrimination by exploring Fos activation in regions likely involved in the inhibition of fear by a safety signal. As previously, female rats demonstrated greater discrimination between fear CS+ and safe CS− during conditioning. CeA and BNST, structures situated to directly regulate the proximate mediators of fear behaviors, emerged as interesting sites of future investigation. CeA Fos correlated with discrimination index and BNST Fos was greater in male rats that exhibited poorer fear discrimination. Future investigations of cell types, subregions and functional connectivity are needed to more fully understand the brain learns to modulate fear.
Table 1.
Regions of Fos Analysis
| Brain region | Location from Bregma (mm) | Area analyzed (μm2) |
|---|---|---|
| PL | 3.72 to 2.76 | 570 |
|
|
||
| IL | 3.72 to 2.76 | 460 |
|
|
||
| vlOFC | 4.20 to 3.00 | 570 |
|
|
||
| aIC | 4.20 to 3.00 | 570 |
|
|
||
| mIC | 0.48 to −0.24 | 570 |
|
|
||
| pIC | −1.80 and −2.80 | 570 |
|
|
||
| BLA | 2.04 to −3.36 | 570 |
|
|
||
| CeA | 2.04 to −3.36 | 190 |
|
|
||
| BNST | 0.48 to −0.24 | 230 |
HIGHLIGHTS.
The neural bases of sex differences in fear discrimination are not well understood.
We quantified neural activity and fear discrimination in male and female rats.
Fos levels in the central amygdala (CeA) correlated with discrimination behavior.
Greater Fos in the anterior medial bed nucleus of the stria terminalis (BNST) after discrimination in males may represent an important area for future investigation.
Acknowledgements
Funding for this research was provided by NIH Grant MH093412 and the Brain and Behavior Research Foundation grant No. 19417 to J.P.C. The authors of this paper declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rauch SL et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47, 769–776. [DOI] [PubMed] [Google Scholar]
- [2].Jovanovic T et al. (2005). Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biol Psychiatry 57, 1559–1564. [DOI] [PubMed] [Google Scholar]
- [3].Winslow JT, Noble PL, and Davis M (2008). AX+/BX− discrimination learning in the fear-potentiated startle paradigm in monkeys. Learn Mem 15, 63–66. [DOI] [PubMed] [Google Scholar]
- [4].Myers KM and Davis M (2004). AX+, BX− discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem 11, 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Costanzo M et al. (2016). Psychophysiological Investigation of Combat Veterans with Subthreshold Post-traumatic Stress Disorder Symptoms. Mil Med 181, 793–802. [DOI] [PubMed] [Google Scholar]
- [6].Jenewein J et al. (2016). Altered Pain Perception and Fear-Learning Deficits in Subjects With Posttraumatic Stress Disorder. J Pain 17, 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jovanovic T, Kazama A, Bachevalier J, and Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kessler RC, Sonnega A, Bromet E, Hughes M, and Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- [9].Kilpatrick DG et al. (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gamwell K et al. (2015). Fear conditioned responses and PTSD symptoms in children: Sex differences in fear-related symptoms. Dev Psychobiol 57, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lonsdorf TB et al. (2015). Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J Psychiatry Neurosci 40, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Day HL, Reed MM, and Stevenson CW (2016). Sex differences in discriminating between cues predicting threat and safety. Neurobiol Learn Mem 133, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foilb AR, Bals J, Sarlitto MC, and Christianson JP (2018). Sex differences in fear discrimination do not manifest as differences in conditioned inhibition. Learn Mem 25, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sierra-Mercado D, Padilla-Coreano N, and Quirk GJ (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sotres-Bayon F and Quirk GJ (2010). Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Day HLL, Suwansawang S, Halliday DM, and Stevenson CW (2020). Sex differences in auditory fear discrimination are associated with altered medial prefrontal cortex function. Sci Rep 10, 6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kreutzmann JC and Fendt M (2020). Chronic inhibition of GABA synthesis in the infralimbic cortex facilitates conditioned safety memory and reduces contextual fear. Transl Psychiatry 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, and Gordon JA (2014). Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 17, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meyer HC and Bucci DJ (2014). The contribution of medial prefrontal cortical regions to conditioned inhibition. Behav Neurosci 128, 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meyer HC et al. (2019). Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sangha S, Robinson PD, Greba Q, Davies DA, and Howland JG (2014). Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology 39, 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sarlitto MC, Foilb AR, and Christianson JP (2018). Inactivation of the Ventrolateral Orbitofrontal Cortex Impairs Flexible Use of Safety Signals. Neuroscience 379, 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gogolla N (2017). The insular cortex. Curr Biol 27, R580–R586. [DOI] [PubMed] [Google Scholar]
- [24].Foilb AR, Flyer-Adams JG, Maier SF, and Christianson JP (2016). Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiol Learn Mem 134 Pt B, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].LeDoux JE (2000). Emotion circuits in the brain. Annu Rev Neurosci 23, 155–184. [DOI] [PubMed] [Google Scholar]
- [26].LeDoux JE (2014). Coming to terms with fear. Proc Natl Acad Sci U S A 111, 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Quirk GJ, Repa C, and LeDoux JE (1995). Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039. [DOI] [PubMed] [Google Scholar]
- [28].Rogan MT, Stäubli UV, and LeDoux JE (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607. [DOI] [PubMed] [Google Scholar]
- [29].LeDoux JE, Iwata J, Cicchetti P, and Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maren S (2001). Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24, 897–931. [DOI] [PubMed] [Google Scholar]
- [31].Swanson LW and Petrovich GD (1998). What is the amygdala. Trends Neurosci 21, 323–331. [DOI] [PubMed] [Google Scholar]
- [32].Campeau S and Davis M (1995). Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 15, 2312–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Campeau S et al. (1997). Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience 78, 1087–1104. [DOI] [PubMed] [Google Scholar]
- [34].Davis M, Walker DL, Miles L, and Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Walker DL and Davis M (2008). Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 213, 29–42. [DOI] [PubMed] [Google Scholar]
- [36].Genud-Gabai R, Klavir O, and Paz R (2013). Safety signals in the primate amygdala. J Neurosci 33, 17986–17994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sangha S, Chadick JZ, and Janak PH (2013). Safety encoding in the basal amygdala. J Neurosci 33, 3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ostroff LE, Cain CK, Bedont J, Monfils MH, and Ledoux JE (2010). Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci U S A 107, 9418–9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ostroff LE, Cain CK, Jindal N, Dar N, and Ledoux JE (2012). Stability of presynaptic vesicle pools and changes in synapse morphology in the amygdala following fear learning in adult rats. J Comp Neurol 520, 295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Christianson JP et al. (2011). Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry 70, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Foilb AR and Christianson JP, ‘Brain Mechanisms for Learning and Using Safety Signals’, In: Sangha S and Foti D (Eds.), Neurobiology of Abnormal Emotion and Motivated Behaviors, Vol.pp. 204–222. [Google Scholar]
- [42].Dragunow M and Faull R (1989). The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29, 261–265. [DOI] [PubMed] [Google Scholar]
- [43].McReynolds JR, Christianson JP, Blacktop JM, and Mantsch JR (2018). What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders. Neurobiol Stress 9, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen VM, Foilb AR, and Christianson JP (2016). Inactivation of ventral hippocampus interfered with cued-fear acquisition but did not influence later recall or discrimination. Behav Brain Res 296, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Foilb AR and Christianson JP (2016). Serotonin 2C receptor antagonist improves fear discrimination and subsequent safety signal recall. Prog Neuropsychopharmacol Biol Psychiatry 65, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Toufexis DJ, Myers KM, Bowser ME, and Davis M (2007). Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci 27, 9729–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Trask S, Reis DS, Ferrara NC, and Helmstetter FJ (2020). Decreased cued fear discrimination learning in female rats as a function of estrous phase. Learn Mem 27, 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cora MC, Kooistra L, and Travlos G (2015). Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol Pathol 43, 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rogers-Carter MM et al. (2018). Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci 21, 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Paxinos G and Watson C, The Rat Brain in Stereotaxic Coordinates, Sixth Edition: Hard Cover Edition. Academic Press, 2007, pp 456 [Google Scholar]
- [51].Piantadosi PT and Floresco SB (2014). Prefrontal cortical GABA transmission modulates discrimination and latent inhibition of conditioned fear: relevance for schizophrenia. Neuropsychopharmacology 39, 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ray MH, Hanlon E, and McDannald MA (2018). Lateral orbitofrontal cortex partitions mechanisms for fear regulation and alcohol consumption. PLoS One 13, e0198043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ongür D and Price JL (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10, 206–219. [DOI] [PubMed] [Google Scholar]
- [54].Price JL (2007). Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci 1121, 54–71. [DOI] [PubMed] [Google Scholar]
- [55].Rodgers KM, Benison AM, Klein A, and Barth DS (2008). Auditory, somatosensory, and multisensory insular cortex in the rat. Cereb Cortex 18, 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Menon V and Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hughes KC and Shin LM (2011). Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother 11, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Paulus MP and Stein MB (2006). An insular view of anxiety. Biol Psychiatry 60, 383–387. [DOI] [PubMed] [Google Scholar]
- [59].Szeszko PR and Yehuda R (2019). Magnetic resonance imaging predictors of psychotherapy treatment response in post-traumatic stress disorder: A role for the salience network. Psychiatry Res 277, 52–57. [DOI] [PubMed] [Google Scholar]
- [60].Amano T, Unal CT, and Paré D (2010). Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13, 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Haubensak W et al. (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Toufexis D (2007). Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol 19, 461–473. [DOI] [PubMed] [Google Scholar]
- [63].Dong HW, Petrovich GD, and Swanson LW (2001). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38, 192–246. [DOI] [PubMed] [Google Scholar]
- [64].Wood M et al. (2018). Infralimbic prefrontal cortex structural and functional connectivity with the limbic forebrain: a combined viral genetic and optogenetic analysis. Brain Struct Funct [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Duvarci S, Bauer EP, and Paré D (2009). The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci 29, 10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Foilb AR (2019). Sex Differences and the Neural Correlates of Safety Learning. PhD, Boston College, http://hdl.handle.net/2345/bc-ir:108467 [Google Scholar]
- [67].Josselyn SA, Falls WA, Gewirtz JC, Pistell P, and Davis M (2005). The nucleus accumbens is not critically involved in mediating the effects of a safety signal on behavior. Neuropsychopharmacology 30, 17–26. [DOI] [PubMed] [Google Scholar]
- [68].Rogan MT, Leon KS, Perez DL, and Kandel ER (2005). Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron 46, 309–320. [DOI] [PubMed] [Google Scholar]
- [69].Ray MH, Russ AN, Walker RA, and McDannald MA (2020). The Nucleus Accumbens Core is Necessary to Scale Fear to Degree of Threat. J Neurosci 40, 4750–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Goodman J, Gabriele A, and Packard MG (2017). Differential effects of neural inactivation of the dorsolateral striatum on response and latent extinction. Behav Neurosci 131, 143–148. [DOI] [PubMed] [Google Scholar]
- [71].Goodman J and Packard MG (2018). The role of the dorsal striatum in extinction: A memory systems perspective. Neurobiol Learn Mem 150, 48–55. [DOI] [PubMed] [Google Scholar]
- [72].Yoest KE, Quigley JA, and Becker JB (2018). Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav 104, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gillies GE, Virdee K, McArthur S, and Dalley JW (2014). Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience 282, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jo YS, Heymann G, and Zweifel LS (2018). Dopamine Neurons Reflect the Uncertainty in Fear Generalization. Neuron 100, 916–925.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yan R, Wang T, and Zhou Q (2019). Elevated dopamine signaling from ventral tegmental area to prefrontal cortical parvalbumin neurons drives conditioned inhibition. Proc Natl Acad Sci U S A 116, 13077–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ng KH, Pollock MW, Urbanczyk PJ, and Sangha S (2018). Altering D1 receptor activity in the basolateral amygdala impairs fear suppression during a safety cue. Neurobiol Learn Mem 147, 26–34. [DOI] [PubMed] [Google Scholar]
- [77].Greiner EM, Norris MR, Müller I, Ng KH, and Sangha S (2018). Sex differences in fear regulation and reward seeking behaviors in a fear-safety-reward discrimination task. bioRxiv 390377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jovanovic T et al. (2009). Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res 167, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jovanovic T et al. (2013). Acute stress disorder versus chronic posttraumatic stress disorder: inhibition of fear as a function of time since trauma. Depress Anxiety 30, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]


