Abstract
Background
Slow heart rate recovery (HRR) after exercise is associated with autonomic dysfunction and increased mortality. What HRR criterion at 1-minute after a 6-minute walk test (6MWT) best defines pulmonary impairment?.
Study Design and Methods
A total of 5008 phase 2 COPDGene (NCT00608764) participants with smoking history were included. A total of 2127 had COPD and, of these, 385 were followed-up 5-years later. Lung surgery, transplant, bronchiectasis, atrial fibrillation, heart failure and pacemakers were exclusionary. HR was measured from pulse oximetry at end-walk and after 1-min seated recovery. A receiver operator characteristic (ROC) identified optimal HRR cut-off. Generalized linear regression determined HRR association with spirometry, chest CT, symptoms and exacerbations.
Results
HRR after 6MWT (bt/min) was categorized in quintiles: ≤5 (23.0% of participants), 6–10 (20.7%), 11–15 (18.9%), 16–22 (18.5%) and ≥23 (18.9%). Compared to HRR≤5, HRR≥11 was associated with (p<0.001): lower pre-walk HR and 1-min post HR; greater end-walk HR; greater 6MWD; greater FEV1%pred; lower airway wall area and wall thickness. HRR was positively associated with FEV1%pred and negatively associated with airway wall thickness. An optimal HRR ≤10 bt/min yielded an area under the ROC curve of 0.62 (95% CI 0.58–0.66) for identifying FEV1<30%pred. HRR≥11 bt/min was the lowest HRR associated with consistently less impairment in 6MWT, spirometry and CT variables. In COPD, HRR≤10 bt/min was associated with (p<0.001): ≥2 exacerbations in the previous year (OR=1.76[1.33–2.34]); CAT≥10 (OR=1.42[1.18–1.71]); mMRC≥2 (OR=1.42[1.19–1.69]); GOLD 4 (OR=1.98[1.44–2.73]) and GOLD D (OR=1.51[1.18–1.95]). HRR≤10 bt/min was predicted COPD exacerbations at 5-year follow-up (RR=1.83[1.07–3.12], P=0.027).
Conclusion
HRR≤10 bt/min after 6MWT in COPD is associated with more severe expiratory flow limitation, airway wall thickening, worse dyspnoea and quality of life, and future exacerbations, suggesting that an abnormal HRR≤10 bt/min after a 6MWT may be used in a comprehensive assessment in COPD for risk of severity, symptoms and future exacerbations.
Keywords: autonomic dysfunction, chest computed tomography, COPD exacerbation, exercise, spirometry
Background
Chronic obstructive pulmonary disease (COPD) is associated with autonomic dysfunction1,2 that predicts increased mortality.3,4 Heart rate recovery (HRR) after exercise is an easily-acquired measure of autonomic dysfunction.5 Abnormal HRR after maximal exercise, such as a cardiopulmonary exercise test (CPET), predicts overall mortality in various populations,5–7 and associates with pulmonary function abnormalities.8 In addition, in COPD, prevalence of impaired HRR increases with greater GOLD-defined severity of expiratory flow limitation.9
The 6-minute walk test (6MWT) is a simple test of functional performance that is used widely in COPD patient assessment. Six-minute walk distance (6MWD) associates negatively with COPD hospitalizations, exacerbations and mortality10 and also predicts survival in patients with heart failure,11 idiopathic pulmonary fibrosis12 and pulmonary hypertension.13 Previous studies used values of ≤12, ≤13, ≤14 or ≤16 bt/min as criterion to identify abnormal HRR in cardiovascular disease patients,5,12,14 but a criterion to define a suitable HRR cut-off after 6MWT in COPD is not well established.
A previous study indicated that HRR≤14 bt/min after a symptom-limited incremental exercise test strongly predicted mortality in 147 COPD patients.7 Rodriguez et al found, in 101 COPD patients, that HRR≤14 bt/min after 6MWT associated with higher exacerbation risk during the subsequent 12 months.15 Criteria defining normality in these studies were based on small sample sizes (<150 COPD patients)7,15 and were not associated with pulmonary function or symptoms.15 In COPD, exercise may be limited by respiratory symptoms prior to reaching cardiovascular limits, often with substantial heart rate reserve; therefore, COPD patients with more severe obstruction or greater symptom burden may exhibit lower HRR following a 6MWT. Furthermore, autonomic function may be associated with changes that affect vagal afferent innervation of the respiratory tract16,17 reflected in anatomic chest CT variables. Establishing a criterion HRR following 6MWT in COPD that is associated with lung function, dyspnoea and quality of life and future exacerbations (as opposed to cut-offs established in other disease states or testing modalities) has the potential to provide insight into health status and guide management. We therefore aimed to identify an HRR abnormality criterion after 6MWT that associated independently with COPD, COPD severity, symptoms, and predicted future exacerbations. To achieve this, we analysed data from the longitudinal cohort study, the Genetic Epidemiology of COPD (COPDGene).
Methods
Study Design and Population
This was a retrospective analysis of COPDGene18 (NCT00608764) participants at 5 years (phase 2; 2012–2017) and 10 years (Phase 3; 2017–2019) after their initial study visit. The 5-year visit was the first visit at which HRR was recorded. COPDGene enrolled current and former smokers with ≥10 pack-years smoking history and never smokers. Participants were either Non-Hispanic White or African American and aged 45–90 years at their 5-year visit. A detailed description of the study design and inclusion/exclusion criteria is available.18 Those with complete 6MWT and lung function data were included in the analysis and those with lung surgery, transplant, bronchiectasis, atrial fibrillation, heart failure, and pacemakers were excluded. Participants underwent 6MWT, post-bronchodilator spirometry, quantitative computed tomography (CT), and questionnaires to assess symptoms and medical history. The COPDGene protocol was approved by institutional review boards at 21 participating centres (see online supplement) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Measurements
6MWT was performed at all sites according to ATS guidelines19 and as described in the COPDGene manual of procedures. Heart rate and SpO2 were measured using a pulse oximeter after 10 minutes of seated rest prior to the walk, immediately at walk’s end, and 1-minute after the end of the walk. At the end of the walk, participants were seated as quickly but as safely as possible. HRR (independent variable) was defined as the difference in HR between end-walk and 1-min recovery. HRR grades were identified according to quintiles of HRR: HRR≤5 bt/min, 6–10 bt/min, 11–15 bt/min, 16–22 bt/min and HRR ≥23 bt/min.
Exposures included smoking history, exacerbation history, and medication use. Outcome variables included the scores of the modified Medical Research Council dyspnoea questionnaire (mMRC) and COPD assessment test (CAT). Exacerbation frequency during the year prior to the 5-year or 10-year follow-up visits, with exacerbation defined as acute worsening of COPD symptoms that resulted either in prescription of a course of antibiotics or oral corticosteroids, or in hospitalization (ie, moderate or severe exacerbations).20 Frequent exacerbations were defined as ≥2 exacerbations in the previous year.20,21 Outcomes also included COPD severity, assessed by GOLD spirometric stage, and COPD Group by assessment of symptoms and risk of exacerbations based on the 2020 GOLD guidelines, eg GOLD group D were those with mMRC ≥2 or CAT ≥10 and exacerbation frequency ≥2 in the previous year.20
Spirometry was performed using an EasyOne® Spirometer (ndd, Zurich, Switzerland) before and after administration of a short-acting β2-agonist (albuterol) according to ATS/ERS recommendations.22 Spirometry studies were reviewed centrally by COPDGene to ensure multicentre quality control.18 The greatest combined FEV1 and FVC measurements of three acceptable efforts was reported. Lung diffusing capacity for carbon monoxide (DLCO) was measured by using the EasyOne Pro system (ndd Medical Technologies, Inc.) in accordance with European Respiratory Society/American Thoracic Society standards for measurement of single-breath carbon monoxide uptake in the lung.23 DLCO percent predicted values were calculated by using Global Lung Initiative reference equations,24 with DLCO values adjusted for haemoglobin and altitude.
Exploratory outcomes included CT scans variables. CT scan at phase 2 COPDGene were performed at full inspiration (200 mAs), and at the end of normal expiration (50 mAs).18 Airway wall thickening was assessed by segmental airway wall thickness (AWT) calculated as average values in six segmental bronchi, segmental wall area percentage (WA%=[outer bronchus area-airway luminal area]/outer bronchus area×100) obtained at the same sites as used for airway wall thickness, and the square root of wall area of a 10-mm perimeter airway (Pi10).25–27 Emphysema percentage was defined as percentage of lung voxels ≤−950 Hounsfield units (HU) on an end-inspiratory CT scan, and gas trapping percentage was defined as percentage of lung voxels ≤−856 HU on expiratory CT.27,28
Statistical Analyses
Data are presented as mean±SD, median (interquartile range) or number (percent). Univariate analyses were performed using one-way ANOVA, Kruskal–Wallis tests, independent-samples Student’s t or Mann–Whitney test for continuous variables and chi-square test or z-test for proportions, as appropriate. Associations between HRR or HRR quintile (independent variables) and pulmonary function and quantitative CT (dependent variables) were assessed by multivariable generalized linear regression models. Emphysema percentage and gas trapping percentage were natural log transformed to account for skewed distributions. Estimates of linear trend across increasing HRR quintiles were performed by including median of each quintile as a continuous variable (providing a P-for-trend value). The optimal HRR cut-off point (highest sum of sensitivity and specificity) to identify FEV1<30%pred was selected using receiver operating characteristic (ROC) analysis. Relationships between HRR cut-off (independent variable) and exacerbations and symptoms (dependent variables) were assessed by logistic regression models. At the 5-year visit (phase 2) relationships between HRR and exacerbation frequency ≥2 in the previous year, CAT ≥10 and mMRC ≥2 were assessed. COPD patients with available data were followed-up at the 10-year visit (phase 3), where the relationship between HRR at phase 2 and exacerbations in the year prior to phase 3 was assessed. Multivariable models were adjusted for race, sex, age, body mass index (BMI), smoking history, cardiovascular disease (CVD) history and (for CT measures) CT scanner type. All statistical analyses were performed using SPSS (IBM SPSS V.25.0, Armonk, NY, USA). Alpha level was set at P<0.05, with Bonferroni correction where needed to account for multiple comparisons. Figures were created using GraphPad Software (GraphPad Prism V.8.0 for Windows, San Diego, California USA).
Results
Subject Characteristics
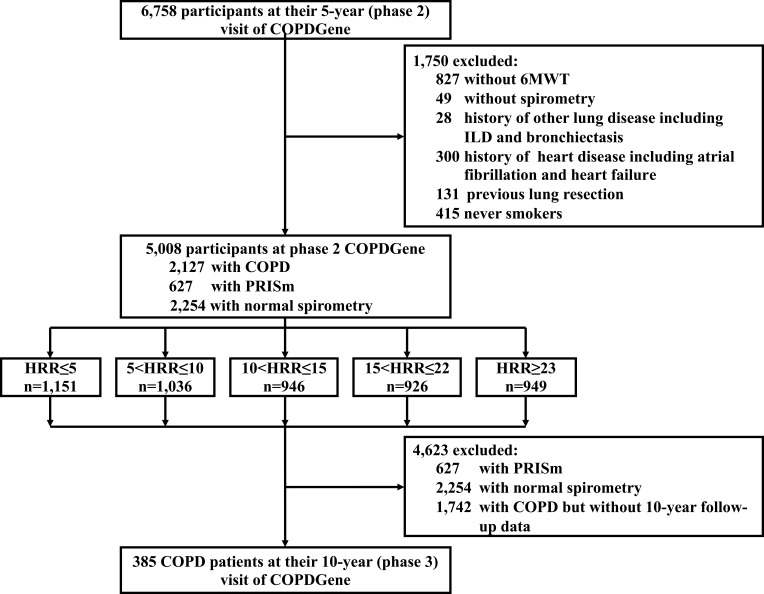
10,311 non-Hispanic white and African American participants were initially enrolled in COPDGene (phase 1). Of these, 6758 were followed-up at phase 2 (5-year visit). Of those, 1750 were excluded from this analysis (Figure 1): 876 of these did not conduct 6MWT or spirometry; and 874 were excluded on the basis of history of other lung disease including interstitial lung disease (ILD) and bronchiectasis, or history of heart disease including atrial fibrillation and heart failure, or previous lung resection or being a never-smoker. 5008 participants were included for analysis, of whom 2127 (42.5%) had COPD, 627 had preserved ratio with impaired spirometry (PRISm), and 2254 had normal spirometry. 385 of the COPD patients with 5.5±0.5 years of follow-up duration were also assessed at the 10-year visit of COPDGene. The remaining participants had either not yet reached their 10-year visit date or were lost to follow-up.
Figure 1.
Study design.
Abbreviation: PRISm, Preserved Ratio Impaired Spirometry defined as FEV1/FVC≥70% but FEV1<80%.
To identify variables associated the HRR across the whole cohort, we compared HRR characteristics with the reference group of HRR ≤5 bt/min (Table 1). Compared with HRR ≤5 bt/min, individuals with 6≤HRR≤10 bt/min had greater 6MWD, BMI, end-walk HR and DLCO%pred and slightly lower Pi10 from quantitative CT (Table 1). However, spirometry, and most other 6MWT- and CT-related variables were not statistically different between the first 2 quintiles of HRR (Table 1).
Table 1.
Baseline Participant Characteristics in Quintiles of Heart Rate Recovery (HRR, bt/min)
| Variables | HRR≤5 | 6≤HRR≤10 | 11≤HRR≤15 | 16≤HRR≤22 | HRR≥23 | Total | P value |
|---|---|---|---|---|---|---|---|
| N (% of total) | 1151 (23.0%) | 1036 (20.7%) | 946 (18.9%) | 926 (18.5%) | 949 (18.9%) | 5008 | – |
| Demographics | |||||||
| Age (years) | 64.3±8.8 | 65.6±8.5* | 65.5±8.4* | 64.6±8.5 | 64.0±8.2 | 64.8±8.5 | <0.001 |
| Sex Male, n (% of quintile) | 613 (53.3%) | 530 (51.2%) | 482 (51.0%) | 437 (47.2%)* | 447 (47.1%)* | 2509 (50.1%) | 0.018 |
| BMI (kg/m2) | 28.1±6.2 | 29.2±6.3* | 29.1±6.1* | 29.3±6.4* | 29.0±6.6* | 28.9±6.3 | <0.001 |
| Race, Non-Hispanic White, n (%) | 674 (58.6%) | 730 (70.5%)* | 712 (75.3%)* | 668 (72.1%)* | 639 (67.3%)* | 3423 (68.4%) | <0.001 |
| Smoking status, n (% of quintile) | |||||||
| Former smoker | 616 (53.5%) | 604 (58.3%) | 597 (63.1%)* | 567 (61.2%)* | 594 (62.6%)* | 2978 (59.5%) | <0.001 |
| Current smoker | 535 (46.5%) | 432 (41.7%) | 349 (36.9%)* | 359 (38.8%)* | 355 (37.4%)* | 2030 (40.5%) | <0.001 |
| Smoking (pack-years) | 46±25 | 44±23 | 43±23 | 42±22* | 40±21* | 43±23 | <0.001 |
| Comorbidities, n (% of quintile) | |||||||
| Cardiovascular disease | 171 (14.9%) | 135 (13.0%) | 133 (14.1%) | 102 (11.0%)* | 80 (8.4%)* | 621 (12.4%) | <0.001 |
| Hypertension | 603 (52.4%) | 566 (54.6%) | 471 (49.8%) | 440 (47.5%) | 427 (45.0%)* | 2507 (50.1%) | <0.001 |
| Diabetes | 214 (18.6%) | 209 (20.2%) | 150 (15.9%) | 159 (17.2%) | 122 (12.9%)* | 854 (17.1%) | <0.001 |
| 6-min walk test | |||||||
| 6MWD (m) | 362.9±118.8 | 388.5±113.8* | 412.0±111.0* | 425.2±114.0* | 446.0±124.8* | 404.7±120.2 | <0.001 |
| HR pre-walk (bt/min) | 75±13 | 74±12 | 73±12* | 73±12* | 71±11* | 73±12 | <0.001 |
| SpO2 pre-walk (%) | 96.0±3.3 | 96.0±2.9 | 96.1±2.6 | 96.4±2.3* | 96.5±2.6* | 96.2±2.8 | <0.001 |
| HR end-of-walk (bt/min) | 86±17 | 94±16* | 98±16* | 102±15* | 112±16* | 98±18 | <0.001 |
| HR 1-min post (bt/min) | 87±17 | 86±16 | 85±15* | 83±15* | 79±15* | 84±16 | <0.001 |
| SpO2 end-of-walk (%) | 93.2±6.2 | 93.6±5.0 | 93.8±5.2 | 94.1±5.1* | 93.1±6.2 | 93.5±5.6 | 0.001 |
| SpO2 1-min post (%) | 95.6±4.2 | 95.9±3.5 | 96.1±3.4* | 96.6±3.1* | 96.8±2.8* | 96.2±3.5 | <0.001 |
| Pulmonary function | |||||||
| Post-BD FEV1%pred (%) | 75.6±24.6 | 76.9±24.9 | 79.8±23.9* | 80.7±22.9* | 83.9±22.6* | 79.2±24.0 | <0.001 |
| Post-BD FVC%pred (%) | 85.8±17.7 | 86.0±17.6 | 87.7±17.4 | 88.2±17.3* | 90.9±17.1* | 87.6±17.5 | <0.001 |
| Post-BD FEV1/FVC (%) | 66.3±15.2 | 66.9±15.5 | 68.3±14.3* | 69.1±13.4* | 70.0±13.2* | 68.0±14.5 | <0.001 |
| DLCO%pred (%) | 70.5±22.0 | 74.5±21.1* | 77.2±21.6* | 76.9±20.5* | 77.2±21.1* | 75.1±21.4 | <0.001 |
| Quantitative chest CT | |||||||
| Emphysema (%) | 1.8 (0.5–6.8) | 1.8 (0.5–5.9) | 1.6 (0.5–5.1) | 1.5 (0.5–4.7) | 1.2 (0.3–3.6)* | 1.5 (0.4–5.2) | <0.001 |
| Gas trapping (%) | 16.3 (6.9–32.9) | 14.3 (5.7–28.0) | 12.9 (6.3–27.9)* | 12.5 (5.5–28.0)* | 12.1 (5.3–23.2)* | 13.5 (5.9–28.4) | <0.001 |
| Pi10 (mm) | 2.36±0.62 | 2.28±0.57* | 2.24±0.57* | 2.22±0.53* | 2.20±0.55* | 2.27±0.57 | <0.001 |
| WA (%) | 51.2±8.6 | 50.6±8.2 | 49.9±8.4* | 49.3±8.1* | 48.8±8.3* | 50.0±8.4 | <0.001 |
| AWT (mm) | 1.06±0.23 | 1.05±0.22 | 1.03±0.22 | 1.01±0.21* | 0.99±0.21* | 1.03±0.22 | <0.001 |
Notes: Data are presented as mean±SD or median (25th and 75th percentiles), or number (percent). *P<0.05 (with Bonferroni correction) for comparison with individuals with HRR≤5 bt/min, obtained from one-way ANOVA or Kruskal–Wallis tests or z-test (for proportions).
Abbreviations: HRR, heart rate recovery; BMI, body mass index; SpO2, oxygen saturation by pulse oximeter; 6MWD, 6-minute walk distance; BD, bronchodilator; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO%pred, percentage of predicted DLCO, adjusted for haemoglobin concentration and altitude; CT, computed tomography; Pi10, square root wall area of a 10-mm perimeter airway; WA, segmental wall area percentage; AWT, segmental airway wall thickness.
However, participants in the 3rd quintile (11≤HRR≤15 bt/min) had many variables that were significantly different from reference (HRR≤5 bt/min). Those with HRR≥11 bt/min were older, with greater BMI and 6MWD, lower pre-walk HR and 1-min post HR, greater end-walk HR and SpO2, less impaired spirometry (FEV1%pred, FEV1/FVC), greater DLCO%pred, less gas trapping, Pi10 and airway wall thickness. Therefore, 6MWT, spirometry and CT variables were significantly less impaired in participants at HRR≥11 bt/min compared with those in the first two quintiles. Above this quintile, there were few additional significant findings: greater pack-years smoking, greater pre-walk and end-walk SpO2, greater FVC%pred and lower airway wall thickness (at HRR≥16 bt/min), and less emphysema (at HRR≥23 bt/min). In ROC analysis, an optimal HRR≤10 beat/min yielded an area under the curve (AUC) of 0.62 (95% CI 0.58–0.66) for identifying FEV1<30%pred. This cut-off corresponded to a sensitivity of 62% and specificity of 58%. Overall, in smokers with and without COPD, HRR≥11 bt/min was the lowest HRR associated with consistently less impairment in 6MWT, spirometry and CT variables.
Spirometry, Chest CT and HRR
Associations between measures of pulmonary function and HRR in the whole cohort were further assessed by multivariable generalized linear regression models, adjusted for age, sex, race, BMI, smoking status and cardiovascular disease history. When modelled as a continuous variable, HRR was independently associated with spirometry (Table 2). In categorical analyses, compared with HRR≤5 bt/min, pulmonary function variables (FEV1%pred, FVC%pred, FEV1/FVC and DLCO%pred) were greater (each P<0.001) when HRR≥11 bt/min, and increased progressively across HRR quintiles above this level (each P for trend <0.001) (Table 2).
Table 2.
Adjusted Associations Among Pulmonary Function, Quantitative CT and Heart Rate Recovery (HRR, bt/min)
| Dependent Variables | β (95% CI) HRR as a Continuous Variable | Mean Difference (95% CI) by Quintile of HRR | |||||
|---|---|---|---|---|---|---|---|
| HRR≤5 (n=1151) | 6≤HRR≤10 (n=1036) | 11≤HRR≤15 (n=946) | 16≤HRR≤22 (n=926) | HRR≥23 (n=949) | P for Trend | ||
| FEV1%pred | 0.187 (0.136 to 0.238)# | 0 (ref) | 1.772 (−0.219 to 3.764) | 4.684 (2.638 to 6.730)# | 5.159 (3.101 to 7.217)# | 7.984 (5.942 to 10.027)# | <0.001 |
| FVC%pred | 0.134 (0.097 to 0.171)# | 0 (ref) | 0.756 (−0.682 to 2.194) | 2.492 (1.015 to 3.969)# | 2.998 (1.512 to 4.483)# | 5.372 (3.898 to 6.846)# | <0.001 |
| FEV1/FVC (%) | 0.070 (0.041 to 0.099)# | 0 (ref) | 0.861 (−0.279 to 2.000) | 2.310 (1.140 to 3.480)# | 2.471 (1.294 to 3.648)# | 3.153 (1.985 to 4.322)# | <0.001 |
| DLCO%pred (%) | 0.096 (0.051 to 0.141)# | 0 (ref) | 2.605 (0.828 to 4.382)* | 4.614 (2.787 to 6.441)# | 4.062 (2.235 to 5.889)# | 4.503 (2.695 to 6.310)# | <0.001 |
| Ln emphysema (%) | 0.002 (−0.002 to 0.005) | 0 (ref) | −0.015 (−0.148 to 0.118) | 0.017 (−0.121 to 0.154) | 0.075 (−0.064 to 0.213) | 0.010 (−0.130 to 0.151) | 0.618 |
| Ln gas trapping (%) | −0.001 (−0.003 to 0.001) | 0 (ref) | −0.041 (−0.127 to 0.044) | −0.015 (−0.103 to 0.073) | −0.073 (−0.163 to 0.017) | −0.068 (−0.159 to 0.023) | 0.124 |
| Pi10 (mm) | −0.002 (−0.004 to −0.001)# | 0 (ref) | −0.066 (−0.113 to −0.019) * | −0.084 (−0.132 to −0.036)* | −0.110 (−0.159 to −0.061)# | −0.121 (−0.171 to −0.072)# | <0.001 |
| WA (%) | −0.043 (−0.060 to −0.026)# | 0 (ref) | −0.599 (−1.257 to 0.059) | −1.047 (−1.727 to −0.366)* | −1.611 (−2.298 to −0.923)# | −1.723 (−2.420 to −1.026)# | <0.001 |
| AWT (mm) | −0.001 (−0.001 to −0.001)# | 0 (ref) | −0.015 (−0.031 to 0.001) | −0.023 (−0.039 to −0.006)* | −0.039 (−0.056 to −0.021)# | −0.042 (−0.060 to −0.025)# | <0.001 |
Notes: Data are presented as Mean difference (95% CI). Emphysema percentage and gas trapping percentage were natural log (Ln) transformed to account for their skewed distributions. *P<0.05, #P<0.001, assessed by multivariate generalized linear regression models adjusted for age, sex, race, BMI, smoking status, CVD history, and CT scanner type for CT outcomes. P for trend were estimated by generalized linear models including the median of each quartile as a continuous variable.
Abbreviations: HRR, heart rate recovery; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO%pred, percentage of predicted DLCO, adjusted for haemoglobin concentration and altitude; CT, computed tomography; Pi10, square root wall area of a 10-mm perimeter airway; WA, segmental wall area percentage; AWT, segmental airway wall thickness.
To identify the locus of these associations we performed a sub-group analysis of HRR in COPD, PRISm and normal spirometry. We found that the associations were driven largely by the COPD group, because there were no consistent associations between HRR quintiles and pulmonary function variables in PRISm and normal spirometry groups, assessed separately (Table 3). Only FVC%pred was weakly associated (β = 0.043, 95% CI = 0.008 to 0.079) with HRR as a continuous variable in normal spirometry smokers. In the COPD sub-group, however, all pulmonary function variables were significantly associated with HRR considered as a continuous variable, or as a trend across quintiles (P<0.05) (Table 3).
Table 3.
Adjusted Associations Among Pulmonary Function, Quantitative CT and Heart Rate Recovery (HRR, bt/min) in Subgroups: Patients with COPD, PRISm and Normal Spirometry
| Dependent Variables | β (95% CI) HRR as a Continuous Variable | Mean Difference (95% CI) by Quintile of HRR | |||||
|---|---|---|---|---|---|---|---|
| HRR≤5 | 6≤HRR≤10 | 11≤HRR≤15 | 16≤HRR≤22 | HRR≥23 | P for Trend | ||
| COPD Patients | (n=548) | (n=479) | (n=379) | (n=373) | (n=348) | ||
| FEV1%pred (%) | 0.199 (0.124 to 0.274)# | 0 (ref) | 0.484 (−2.231 to 3.199) | 2.202 (−0.699 to 5.102) | 4.248 (1.334 to 7.162)* | 7.675 (4.701 to 10.650)# | <0.001 |
| FVC%pred (%) | 0.172 (0.107 to 0.237)# | 0 (ref) | 0.755 (−1.605 to 3.115) | 2.136 (−0.384 to 4.657) | 3.460 (0.927 to 5.992)* | 6.677 (4.091 to 9.262)# | <0.001 |
| FEV1/FVC (%) | 0.070 (0.041 to 0.108)* | 0 (ref) | −0.340 (−1.786 to 1.106) | 0.513 (−1.032 to 2.058) | 1.707 (0.155 to 3.259)* | 2.524 (0.940 to 4.108)* | <0.001 |
| DLCO%pred (%) | 0.124 (0.051 to 0.198)* | 0 (ref) | 3.684 (0.987 to 6.380)* | 3.900 (1.015 to 6.786)* | 3.556 (0.704 to 6.429)* | 5.554 (2.633 to 8.474)# | 0.001 |
| Ln emphysema (%) | 0.001 (−0.004 to 0.006) | 0 (ref) | −0.019 (−0.183 to 0.145) | 0.099 (−0.077 to 0.275) | 0.090 (−0.090 to 0.270) | −0.014 (−0.200 to 0.172) | 0.810 |
| Ln gas trapping (%) | −0.002 (−0.004 to 0.001) | 0 (ref) | −0.004 (−0.097 to 0.088) | 0.030 (−0.069 to 0.128) | 0.001 (−0.102 to 0.103) | −0.114 (−0.220 to −0.008)* | 0.054 |
| Pi10 (mm) | −0.002 (−0.004 to −0.000)* | 0 (ref) | −0.095 (−0.164 to −0.025)* | −0.025 (−0.100 to 0.050) | −0.104 (−0.180 to 0.027)* | −0.137 (−0.217 to −0.058)* | 0.002 |
| WA (%) | −0.045 (−0.072 to −0.018)* | 0 (ref) | −1.187 (−2.135 to −0.239)* | −0.546 (−1.565 to 0.474) | −1.698 (−2.740 to −0.655)* | −2.162 (−3.241 to −1.084)# | <0.001 |
| AWT (mm) | −0.001 (−0.002 to −0.001)# | 0 (ref) | −0.036 (−0.061 to −0.011)* | −0.027 (−0.054 to −0.000) | −0.048 (−0.075 to −0.020)* | −0.062 (−0.091 to −0.034)# | <0.001 |
| PRISm Patients | (n=161) | (n=127) | (n=118) | (n=117) | (n=104) | ||
| FEV1%pred (%) | 0.003 (−0.045 to 0.051) | 0 (ref) | −0.674 (−2.464 to 1.116) | −1.100 (−2.947 to 0.746) | 0.124 (−1.704 to 1.952) | 0.560 (−1.339 to 2.458) | 0.405 |
| FVC%pred (%) | 0.002 (−0.051 to 0.056) | 0 (ref) | −0.526 (−2.503 to 1.451) | −0.665 (−2.705 to 1.375) | 0.329 (−1.690 to 2.348) | 0.378 (−1.719 to 2.476) | 0.534 |
| FEV1/FVC (%) | 0.000 (−0.031 to 0.030) | 0 (ref) | −0.153 (−1.285 to 0.979) | −0.523 (−1.691 to 0.645) | −0.104 (−1.260 to 1.052) | 0.136 (−1.065 to 1.337) | 0.815 |
| DLCO%pred (%) | −0.039 (−0.153 to 0.074) | 0 (ref) | −0.139 (−4.211 to 3.932) | 2.976 (−1.192 to 7.143) | 3.339 (−0.785 to 7.464) | −1.007 (−5.311 to 3.298) | 0.868 |
| Ln emphysema (%) | 0.008 (−0.002 to 0.017) | 0 (ref) | −0.059 (−0.389 to 0.272) | 0.016 (−0.334 to 0.367) | 0.388 (0.037 to 0.739) * | 0.224 (−0.143 to 0.591) | 0.050 |
| Ln gas trapping (%) | 0.005 (−0.001 to 0.011) | 0 (ref) | −0.083 (−0.297 to 0.131) | −0.070 (−0.292 to 0.152) | 0.001 (−0.224 to 0.227) | 0.127 (−0.114 to 0.367) | 0.245 |
| Pi10 (mm) | 0.002 (−0.001 to 0.005) | 0 (ref) | 0.055 (−0.060 to 0.169) | 0.034 (−0.087 to 0.156) | 0.014 (−0.108 to 0.135) | 0.069 (−0.058 to 0.196) | 0.452 |
| WA (%) | −0.007 (−0.055 to 0.040) | 0 (ref) | 1.485 (−0.223 to 3.192) | 0.655 (−1.158 to 2.467) | −0.308 (−2.124 to 1.508) | 0.442 (−1.462 to 2.346) | 0.813 |
| AWT (mm) | 0.000 (−0.001 to 0.002) | 0 (ref) | 0.042 (−0.003 to 0.086) | 0.027 (−0.020 to 0.075) | −0.004 (−0.052 to 0.043) | 0.015 (−0.034 to 0.065) | 0.935 |
| Normal Spirometry Participants | (n=442) | (n=430) | (n=449) | (n=436) | (n=497) | ||
| FEV1%pred (%) | 0.029 (−0.007 to 0.065) | 0 (ref) | 0.353 (−1.183 to 1.890) | 0.467 (−1.058 to 1.993) | 0.671 (−0.856 to 2.207) | 1.093 (−0.391 to 2.085) | 0.134 |
| FVC%pred (%) | 0.043 (0.008 to 0.079)* | 0 (ref) | −0.651 (−2.147 to 0.846) | 0.273 (−1.212 to 1.758) | 0.386 (−1.110 to 1.882) | 1.298 (−0.146 to 2.743) | 0.020 |
| FEV1/FVC (%) | −0.011 (−0.026 to 0.004) | 0 (ref) | 0.852 (0.220 to 1.483)* | 0.178 (−0.448 to 0.805) | 0.219 (−0.413 to 0.850) | −0.130 (−0.740 to 0.479) | 0.145 |
| DLCO%pred (%) | 0.015 (−0.035 to 0.065) | 0 (ref) | −0.137 (−2.272 to 1.998) | 1.073 (−1.055 to 3.201) | 0.859 (−1.279 to 2.998) | 0.076 (−1.981 to 2.133) | 0.838 |
| Ln emphysema (%) | 0.006 (0.002 to 0.010)* | 0 (ref) | 0.029 (−0.140 to 0.198) | 0.163 (−0.005 to 0.331) | 0.186 (0.018 to 0.355)* | 0.219 (0.052 to 0.386)* | 0.004 |
| Ln gas trapping (%) | 0.002 (−0.001 to 0.005) | 0 (ref) | −0.062 (−0.180 to 0.056) | 0.122 (0.004 to 0.241) * | −0.003 (−0.122 to 0.115) | 0.088 (−0.029 to 0.205) | 0.080 |
| Pi10 (mm) | −0.001 (−0.002 to 0.000) | 0 (ref) | −0.038 (−0.088 to 0.013) | −0.048 (−0.099 to 0.002) | −0.056 (−0.107 to −0.005)* | −0.041 (−0.091 to 0.010) | 0.151 |
| WA (%) | −0.022 (−0.043 to −0.001)* | 0 (ref) | −0.242 (−1.112 to 0.628) | −0.679 (−1.544 to 0.186) | −0.928 (−1.796 to −0.061)* | −0.622 (−1.480 to 0.236) | 0.099 |
| AWT (mm) | −0.001 (−0.001 to 0.000)* | 0 (ref) | 0.000 (−0.022 to 0.022) | −0.007 (−0.029 to 0.015) | −0.020 (−0.042 to 0.002) | −0.015 (−0.037 to 0.007) | 0.075 |
Notes: Data are presented as Mean difference (95% CI). The emphysema percentage, and gas trapping percentage were natural log transformed because of their skewed distributions. *P<0.05, #P<0.001, assessed by multivariate generalized linear regression models adjusted for age, sex, race, BMI, smoking status, CVD history, and CT scanner type for CT outcomes. β = standardized coefficient represents the change of the dependent variable for 1 bt/min change in HRR. P for trend were estimated by generalized linear models including the median of each quartile as a continuous variable.
Abbreviations: HRR, heart rate recovery; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO%pred, percentage of predicted DLCO, adjusted for haemoglobin concentration and altitude; CT, computed tomography; Pi10, square root wall area of a 10-mm perimeter airway; WA, segmental wall area percentage; AWT, segmental airway wall thickness; PRISm, Preserved Ratio Impaired Spirometry defined as FEV1/FVC ≥ 70% but FEV1 < 80%.
We also aimed to explore associations between pulmonary morphology measured by quantitative CT, and HRR in the whole cohort. Multivariable generalized linear regression models were adjusted for age, sex, race, BMI, smoking status, cardiovascular disease history, and CT scanner type. Using HRR as a continuous variable, HRR was independently negatively associated with wall area percentage, airway wall thickness and Pi10 (each P<0.001) but not with emphysema or gas trapping (Table 2). This association was also observed in categorical analyses, with wall area percentage, airway wall thickness and Pi10 being consistently less impaired at HRR≥11 bt/min compared with reference (HRR≤5 bt/min; each P<0.05; Table 2). There was progressive decrease in wall area percentage, airway wall thickness and Pi10 with increase in HRR quintile (each P for trend <0.001), but not with emphysema or gas trapping (each P for trend >0.124; Table 2). These findings were again corroborated in subgroup analysis (Table 3) where, in COPD patients, wall area percentage, airway wall thickness and Pi10, but not emphysema or gas trapping, were associated with HRR when HRR was considered as continuous or as categorical variable with HRR≥6 bt/min. Wall area percentage and airway wall thickness were significantly negatively associated with HRR as continuous variable in PRISm subjects, however these associations were extremely weak (β < −0.022; Table 3).
COPD Symptoms, Severity and HRR
Finding significant associations between HRR and anatomic and functional variables in the COPD sub-group, we then assessed associations with COPD symptoms. Multivariable logistic regression models were adjusted for age, sex, race, BMI, smoking status and cardiovascular disease history. When HRR was modelled as a continuous variable, HRR was independently associated with severe symptoms and exacerbation frequency ≥2 (Table 4). In categorical analyses, compared with HRR≤5 bt/min, odds ratios (ORs) of severe symptoms were decreased (each P<0.05) when HRR≥11 bt/min, and decreased progressively across HRR quintiles above this level (each P for trend <0.001) (Table 4).
Table 4.
Associations Between Severe Symptoms and Frequent Exacerbator Status and Heart Rate Recovery (HRR, bt/min) in Patients with COPD
| Dependent Variables | OR (95% CI) HRR as a Continuous Variable | OR (95% CI) by Quintile of HRR | |||||
|---|---|---|---|---|---|---|---|
| HRR≤5 (n=548) | 6≤HRR≤10 (n=479) | 11≤HRR≤15 (n=379) | 16≤HRR≤22 (n=373) | HRR≥23 (n=348) | P for Trend | ||
| Exacerbation frequency ≥ 2 | 0.987 (0.976 to 0.998)* | 1 (reference) | 1.043 (0.732 to 1.504) | 0.613 (0.394 to 0.955)* | 0.632 (0.406 to 0.984)* | 0.439 (0.305 to 0.796)# | <0.001 |
| mMRC ≥ 2 | 0.986 (0.979 to 0.993)# | 1 (reference) | 0.844 (0.653 to 1.091) | 0.667 (0.508 to 0.876)* | 0.667 (0.507 to 0.879)* | 0.620 (0.468 to 0.821)* | <0.001 |
| CAT ≥ 10 | 0.985 (0.977 to 0.992)# | 1 (reference) | 0.832 (0.633 to 1.094) | 0.718 (0.540 to 0.957)* | 0.650 (0.487 to 0.867)* | 0.568 (0.425 to 0.759)# | <0.001 |
Notes: Data are presented as Odds ratio (95% CI). *P<0.05, #P<0.001, assessed by multivariate logistic regression models adjusted for age, sex, race, BMI, smoking status, and CVD history. P for trend were estimated by logistic regression models including the median of each quartile as a continuous variable.
Abbreviations: HRR, heart rate recovery; CAT, COPD assessment test; mMRC, modified Medical Research Council dyspnoea scale; OR, odds ratio.
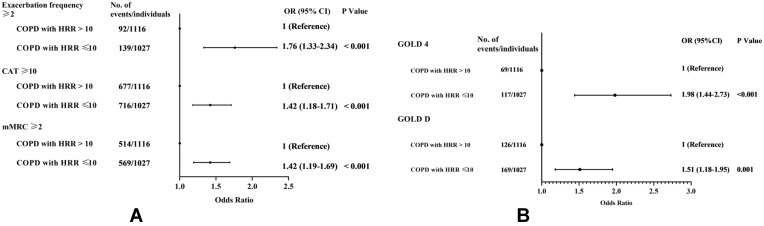
In univariable and multivariable analyses, significant differences among 6MWT, pulmonary function and CT variables in COPD patients were first observed at HRR≥11 bt/min. We therefore explored the association between HRR≤10 bt/min and COPD severity. After adjusting for age, sex, race, BMI, smoking status and cardiovascular disease history, HRR≤10 bt/min was independently associated (P<0.001) with: Exacerbation frequency ≥2 in the previous year (OR=1.76[1.33–2.34]); CAT ≥10 (OR=1.42[1.18–1.71]); and mMRC ≥2 (OR=1.42[1.19–1.69]) (Figure 2A). Compared to those with HRR≥11 bt/min, COPD patients with HRR≤10 bt/min had 1.97 times the risk of very severe obstruction (OR for GOLD 4 =1.98[1.44–2.73]) and 1.51 times the risk of having worse symptoms combined with ≥2 exacerbations in the previous year (OR for GOLD D=1.51[1.18–1.95]) (Figure 2B).
Figure 2.
Odds ratio of COPD severity based on abnormal heart rate recovery (HRR≤10 bt/min). Multivariable logistic regression model for patients with COPD (n=2127) at baseline, adjusting for age, sex, race, BMI and smoking status. (A) Associations between abnormal HRR and severe symptoms. (B) Associations between abnormal HRR and COPD severity grades.
Abbreviations: HRR, heart rate recovery; CAT, COPD assessment test; mMRC, modified Medical Research Council dyspnoea scale; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
HRR Predicts Exacerbations at 5-Years
We then assessed the prognostic value of HRR 10≤bt/min for future exacerbations. Logistic regression models assessed association between HRR≤10 bt/min and exacerbations in the year preceding the phase 3 visit in 385 COPD patients. Risk ratios (RR) of future exacerbations predicted by abnormal HRR are presented in Table 5. RR was essentially constant in the crude model, and across 5 models adjusting for a range of variables (Table 5). After adjusting for sex, age, BMI, race, smoking status, cardiovascular disease history, FEV1%pred, DLCO%pred and 6MWD, HRR≤10 bt/min was associated with exacerbations risk ratio (RR=1.83[1.07–3.12], P=0.027) (Table 5).
Table 5.
Risk Ratio (RR) for Exacerbations in the Year Prior to 5-Year Follow-Up in 385 Patients with COPD Heart Rate Recovery (HRR) ≤10 bt/min Compared to Those with HRR ≥11 bt/min (Reference)
| Variable | RR | 95% CI | P value |
|---|---|---|---|
| Crude | 1.759 | 1.121–2.758 | 0.014 |
| Model 1 | 1.747 | 1.111–2.745 | 0.016 |
| Model 2 | 1.762 | 1.118–2.775 | 0.015 |
| Model 3 | 1.775 | 1.126–2.798 | 0.014 |
| Model 4 | 1.823 | 1.148–2.894 | 0.011 |
| Model 5 | 1.827 | 1.071–3.115 | 0.027 |
Notes: Logistic regression model for COPD patients followed-up for 5-years (n=385). Crude: Single factor for logistic regression. Model 1: Adjusted for sex. Model 2: Adjusted for sex, age. Model 3: Adjusted for sex, age, body mass index (BMI). Model 4: Adjusted for sex, age, body mass index (BMI), race, smoking status, CVD. Model 5: Adjusted for sex, age, body mass index (BMI), race, smoking status, CVD, FEV1%pred, DLCO%pred, 6MWD.
COPD Characteristics Stratified by HRR
Having identified an optimal cut-off of HRR≤10 bt/min was associated with airway morphology, disease severity, dyspnoea, quality of life, exacerbation frequency and risk of future exacerbations in COPD, we compared the characteristics of COPD patients at phase 2 (n=2127) with HRR≤10 bt/min and ≥11 bt/min. Compared with COPD patients with HRR≥11 bt/min, those with HRR≤10 bt/min had significantly (P<0.01): Worse pulmonary function; worse 6MWT physiology (including lower 6MWD, greater pre-walk-HR, lower end-walk-HR, and less recovery of SpO2); worse emphysema and airway morphology on chest CT; more severe COPD symptoms; greater exacerbation frequency; and greater combination of inhaled long-acting beta-agonist, inhaled long-acting muscarinic antagonist and inhaled corticosteroid use (Table 6).
Table 6.
Characteristics of Patients with COPD Stratified by a Criterion Heart Rate Recovery (HRR) After 6MWT of 10 bt/min
| Variables | COPD with Abnormal HRR ≤10 bt/min (n=1027) | COPD with Normal HRR ≥11 bt/min (n=1100) | Total (n=2127) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 67.5±8.5 | 67.5±8.1 | 67.5±8.3 | 0.958 |
| Sex, Male, n (%) | 570 (28.3%) | 602 (26.8%) | 1172 (55.1%) | 0.720 |
| BMI (kg/m2) | 27.6±6.2 | 28.1±6.1 | 27.9±6.2 | 0.069 |
| Race, Non-Hispanic White, n (%) | 743 (34.9%) | 857 (40.3%) | 1600 (75.2%) | 0.003 |
| Smoking status, n (%) | ||||
| Ex-smoker | 631 (29.7%) | 707 (33.2%) | 1338 (62.9%) | 0.177 |
| Current smoker | 396 (18.6%) | 393 (18.5%) | 789 (37.1%) | 0.177 |
| Smoking (pack-years) | 51±26 | 49±24 | 50±25 | 0.107 |
| 6-min walk test | ||||
| 6MWD (m) | 350.3±118.7 | 396.6±122.8 | 374.3±123.0 | <0.001 |
| HR pre-walk (bt/min) | 76±13 | 73±12 | 75±13 | <0.001 |
| SpO2 pre-walk (%) | 95.0±3.7 | 95.6±3.1 | 95.3±3.4 | 0.001 |
| HR end-walk (bt/min) | 91±17 | 104±17 | 98±18 | <0.001 |
| HR 1-min post (bt/min) | 87±16 | 83±15 | 85±16 | <0.001 |
| SpO2 end-walk (%) | 91.9±6.0 | 92.1±6.0 | 92.0±6.0 | 0.531 |
| SpO2 1-min post (%) | 94.6±4.6 | 95.5±3.9 | 95.1±4.2 | <0.001 |
| Pulmonary function | ||||
| Post-BD FEV1%pred (%) | 59.7±22.5 | 64.1±22.4 | 62.0±22.6 | <0.001 |
| Post-BD FVC%pred (%) | 81.9±19.5 | 85.4±19.8 | 83.7±19.7 | <0.001 |
| Post-BD FEV1/FVC (%) | 53.8±12.7 | 55.5±11.7 | 54.7±12.3 | 0.001 |
| DLCO%pred (%) | 62.6±22.1 | 66.1±21.5 | 64.4±21.9 | 0.001 |
| Quantitative chest CT | ||||
| Emphysema (%) | 5.9 (1.9–16.6) | 5.0 (1.6–14.5) | 5.4 (1.7–15.3) | 0.008 |
| Gas trapping (%) | 31.1 (18.3–51.5) | 29.6 (15.8–46.3) | 30.4 (16.9–49.2) | 0.005 |
| Pi10 (mm) | 2.6±0.6 | 2.5±0.6 | 2.5±0.6 | 0.035 |
| WA (%) | 53.6±8.3 | 52.6±8.2 | 53.1±8.2 | 0.011 |
| AWT (mm) | 1.11±0.24 | 1.07±0.22 | 1.09±0.23 | 0.001 |
| COPD assessment | ||||
| Exacerbations/year | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0.036 |
| Exacerbation frequency ≥2, n (%) | 139 (6.5%) | 92 (4.3%) | 231 (10.9%) | <0.001 |
| mMRC | 2 (0–3) | 1 (0–3) | 2 (0–3) | <0.001 |
| CAT | 15 (8–22) | 12 (7–19) | 13 (7–20) | <0.001 |
| CAT ≥10, n (%) | 716 (33.7%) | 677 (31.8%) | 1393 (65.5%) | <0.001 |
| mMRC ≥2, n (%) | 569 (26.8%) | 514 (24.2%) | 1083 (50.9%) | <0.001 |
| Medication, n (%) | ||||
| Non-medication | 336 (15.8%) | 453 (21.3%) | 789 (37.1%) | <0.001 |
| LABA use | 11 (0.5%) | 8 (0.4%) | 19 (0.9%) | 0.400 |
| LAMA use | 99 (4.7%) | 88 (4.1%) | 187 (8.8%) | 0.182 |
| LABA+LAMA use | 18 (0.8%) | 20 (0.9%) | 38 (1.8%) | 0.909 |
| ICS+LABA or LAMA use | 168 (7.9%) | 164 (7.7%) | 332 (15.6%) | 0.357 |
| LABA+LAMA+ ICS use | 206 (9.7%) | 177 (8.3%) | 383 (18.0%) | 0.017 |
| Others | 189 (8.9%) | 190 (8.9%) | 379 (17.8%) | 0.496 |
Note: Data are presented as mean±SD or median (interquartile range), or number (percent).
Abbreviations: HRR, heart rate recovery; BMI, body mass index; SpO2, oxygen saturation by pulse oximeter; 6MWD, 6-minute-walk distance; BD, bronchodilator; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO%pred, percentage of predicted DLCO, adjusted for haemoglobin concentration and altitude; CT, computed tomography; Pi10, square root wall area of a 10-mm perimeter airway; WA%, segmental wall area percentage; AWT, segmental airway wall thickness; CAT, COPD assessment test; mMRC, modified Medical Research Council dyspnoea scale; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LABA, inhaled long-acting beta-agonist; LAMA, inhaled long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
Discussion
This is the first study to show that HRR≤10 bt/min after 6MWT in COPD patients was an important cut-off which was significantly associated with impaired 6MWT distance, spirometry and DLCO and CT variables of airway morphology. This is also the first study to show that low HRR was independently associated with airway wall thickening in smokers with or without COPD, suggesting airway morphologic changes may influence autonomic function. Using HRR≤10 bt/min cut-off, we showed increased odds in COPD patients of worse dyspnoea and quality of life and increased exacerbation frequency. In addition, in a subgroup of 385 COPD patients, we also showed that HRR≤10 bt/min was a significant predictor of exacerbations during the year preceding the 5 year follow-up visit. HRR≤10 bt/min at 1-minute after a 6MWT in COPD is, therefore, proposed as an important tool to evaluate the clinical manifestation of severe outcomes in COPD.
Seshadri et al (2004) were the first to characterize HRR in COPD patients. In 627 participants, they found that HRR≤12 bt/min after incremental exercise testing in smokers and non-smokers was associated with spirometric abnormalities, such as lower FEV1%pred.8 This was corroborated by Gupta et al (2013), who found that COPD patients with HRR≤12 bt/min after target-HR-limited or symptom-limited incremental cycle ergometry had lower FEV1%pred than those with HRR≥13 bt/min, and that abnormal HRR increased in frequency together with GOLD-defined COPD severity.9 Both studies used the HRR≤12 bt/min criterion established by Cole et al (1999) who - from a prospective cohort referred for exercise testing - showed that this cut-off associated with mortality in cardiovascular disease patients.5 It was suggested that autonomic dysfunction (reflected in elevated heart rate, reduced baroreflex sensitivity, reduced heart rate variability and/or prolonged QTc interval) would also result in low HRR following exercise.29 However, the HRR≤12 bt/min criterion value may not be suitable after 6MWT in COPD patients, who commonly reach symptom limitation before reaching cardiovascular limitation during exercise.30 Lower peak HR during exercise would predispose towards smaller HRR. Indeed, our data show in COPD patients stratified on HRR≤10 bt/min (Table 6), that pre-walk HR is significantly higher (by 3 bt/min) and end-walk HR is significantly lower (by 15 bt/min) than those with HRR≥11 bt/min. These patients also had worse spirometry, DLCO, airway wall thickening and symptom and exacerbation burden. This is consistent with the notion that more severe COPD patients are less able to increase HR during 6MWT and, therefore, less able to recover by ≥11 bt/min. We suggest that HRR≤10 bt/min can be used to identify COPD patients at greater risk for worse dyspnoea, quality of life and future exacerbations.
COPD is characterized by poorly-reversible expiratory airflow limitation, caused by emphysema and/or airway remodeling.31,32 Airway wall thickening and emphysema provide independent contributions to expiratory flow limitation and gas exchange impairment in COPD.26 Lower FEV1 is associated with both greater airway wall thickness33,34 and with emphysema and gas trapping.35,36 We used CT to assess airway remodeling33 and emphysema37 in relation to 6MWT performance and HRR. We measured wall area percentage, airway wall thickness and Pi10, which collectively reflect wall thickening across the entire bronchial tree and negatively associate with FEV1.35 We demonstrated that wall area percentage, airway wall thickness and Pi10, but not measures of emphysema, decreased progressively with increasing HRR, and were independently negatively associated with HRR≤10 bt/min in smokers with or without COPD. As emphysema and impaired DLCO are more strongly associated with exercise-induced arterial hypoxemia, it might be hypothesized that HRR recovery would be more impaired in those with greater gas exchange abnormalities.38 In accordance with this suggestion, DLCO was negatively associated with HRR, but we found no relation between HRR and emphysema on CT. In addition, although 1515 (30.3%) participants experienced at least mild exercise induced arterial desaturation (SpO2<92%), this was only very weakly associated with HRR (β=0.016, 95% CI 0.005–0.027, r2=0.002) (c.f.39). Our results are therefore consistent with a greater autonomic disturbance in airway predominant disease rather than in emphysema.
Vagal afferent innervation of the respiratory tract is thought to be activated in COPD by alterations in airway mechanical stimuli, inflammatory and noxious sensory stimuli,16,17 increasing bronchoconstriction and susceptibility to cough. Similarly, sympathetic activity is enhanced in COPD patients, and is known to be affected by several mechanisms associated with COPD, including: Recurrent hypoxemia; hypercapnia; increased intrathoracic pressure alterations; respiratory effort; systemic inflammation; pulmonary circulation; and use of beta-sympathomimetics resulting in augmented cardiac afterload. Indeed, anticholinergic bronchodilators reduce HRR after exercise in COPD patients,40 and we found greater combination of LABA, LAMA and inhaled corticosteroids use in those with abnormal HRR. However, although patients did not temporarily abstain from long-acting bronchodilator medications for this study, the 6MWT was conducted after albuterol administration for spirometry assessment in all participants, meaning beta-agonist load should have been high in all subjects. In addition, chronic inflammation is associated with autonomic dysfunction in middle-aged and elderly subjects with no apparent heart disease.41 Taken together, our findings support that chronic inflammation and/or an association between bronchial and cardiac vagal tone are reflected in an association between airway wall thickness and abnormal HRR in COPD patients. Therefore, HRR≤10 bt/min cut-off might provide a prognostic feature of airway-dominant COPD.42 Further study is warranted to identify mechanisms involved in associations among HRR, parasympathetic nervous activity and airway wall thickness in COPD. Indeed, the association between HRR and airway abnormalities remains even when diagnostic criteria (eg COPD or non-COPD) are considered. This suggests that HRR may represent a treatable trait,43 mediated by an underlying airway and/or autonomic pathophysiology, that could be a target to improve outcomes agnostic of diagnostic phenotype.
The GOLD ABCD assessment tool20,44 identifies highest risk COPD patients using exacerbation frequency ≥2 in the previous year, combined with significant dyspnoea assessed by CAT ≥10 or mMRC ≥2. GOLD D patients have the worst health status and outcomes.20 To our knowledge, this is the first study to show that HRR≤10 bt/min was independently associated with GOLD D COPD classification. COPD patients with HRR≤10 bt/min have greater risk for symptoms and frequent exacerbations than those with HRR≥11 bt/min. We also demonstrated that HRR≤10 bt/min was a predictor for future exacerbation at 5-year follow-up in 385 COPD patients.
Previous studies have associated HRR≤14 bt/min with future exacerbation risk15 or mortality in COPD.7 These studies, however, found no association between HRR and pulmonary function or symptoms. HRR≤10 bt/min after 6MWT, on the other hand, identified associations with both COPD severity and future exacerbation. This, again, supports the notion that HRR≤10 bt/min after 6MWT may further inform COPD prognosis.
Our study has several limitations. Firstly, we do not have mortality data to assess association with HRR at different cut-off values. At the time of writing, only a modest fraction of COPDGene participants have been followed up at their 10-year visit (phase 3), introducing potential for selection bias in our exacerbation outcome data. There also remains chance bias due to the multiple outcomes examined. Secondly, common medications such as β-agonists may alter the HR and HRR response to 6MWT. However, the type and amount of medication use is a Co-variate of COPD severity; therefore, adjusting for medication confounds analyses for associations between HRR and COPD severity. We also sought to adjust for peak HR in our models. Adjustment of peak HR tended to increase the strength of association between HRR and some model variables, eg the association between HRR and FEV1%pred increased from β=0.194 to β=0.318 (P<0.001 for both). However, peak HR is used in the calculation of HRR, and its inclusion introduced variance of the coefficients without major impact on the primary study conclusions. For this reason, peak HR was not included in the presented models. In addition, exacerbations in the previous year are known to be the primary predictor of subsequent exacerbations. Since we did not measure exacerbations prior to our follow-up window, we are not able to assess the independence of HRR as a predictor of exacerbations at 5-year follow up. Finally, patients with HRR≤10 bt/min are also included in groups with greater cut-off values (eg, ≤12–16 bt/min), which introduces bias when attempting to identify which cut-off value to use. Here we chose to report the lowest value, because COPD is predisposed to lower end-walk HR values than some other patient populations. Although not shown, using ≤12, ≤14 or ≤16 bt/min as more granular criteria cut-off values, did not change the main finding that HRR≤10 bt/min was the lowest HRR criterion identifying severe impairments in clinical outcomes.
Conclusions
In conclusion, in this retrospective study we demonstrated that HRR≤10 bt/min after 6MWT in COPD was associated with: 1) worse lung function and airway wall thickening; 2) worse dyspnoea and quality of life; and 3) greater exacerbation burden; and was 4) prognostic of future exacerbations. These findings suggest that HRR of ≤10 bt/min may be useful as part of a comprehensive longitudinal assessment in COPD patients to determine risk for disease severity, symptoms and future exacerbations.
Acknowledgments
Other contributions: The authors would like to thank the COPDGene study participants and all investigators at the investigative sites from the COPDGene study for their support of this study.
Funding Statement
The COPDGene study is supported by grants from the National Heart, Lung, and Blood Institute (NIH/NHLBI R01HL089897, R01HL089856 [(Rossiter, Adami], U01HL089897, U01HL089856 [(Crapo, Silverman; COPDGene]).
Abbreviations
6MWT, Six-minute walk test; 6MWD, Six-minute walk distance; ATS, American Thoracic Society; AWT, Airway wall thickness; BD, Bronchodilator; BMI, Body mass index; CAT, COPD assessment test; CT, Computed tomography; COPD, Chronic obstructive pulmonary disease; PRISm, Preserved Ratio Impaired Spirometry (FEV1/FVC≥70% and FEV1<80%); CVD, Cardiovascular disease; CPET, Cardiopulmonary exercise test; FEV1, Forced expiratory volume in one second; FEV1%pred, Percentage of predicted FEV1; FVC, Forced vital capacity; FVC%pred, Percentage of predicted FVC; DLCO, Diffusing capacity of the lungs for carbon monoxide; DLCO%pred, Percentage of predicted DLCO, adjusted for haemoglobin concentration and altitude; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HRR, Heart rate recovery; HRV, Heart rate variability; HU, Hounsfield units; ICS, Inhaled corticosteroids; ILD, Interstitial lung disease; LABA, Inhaled long-acting beta-agonist; LAMA, Inhaled long-acting muscarinic antagonist; mMRC, modified Medical Research Council dyspnoea questionnaire; Pi10, Square root of the wall area of a 10-mm perimeter airway; WA%, Wall area percentages.
Declaration
Notation of prior abstract publication/presentation: Results of this study have been presented at the European Respiratory Society VIRTUAL CONGRESS 2020.
Guarantor Statement
Harry B. Rossiter is the guarantor of the content of the manuscript, including the data and analysis.
Role of the Sponsors
The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Data Sharing Statement
The datasets used and analysed during the current study are available from the COPDGene study on reasonable request.
Ethics Approval and Consent to Participate
The clinical study was approved by the Institutional Review Boards of each centre participating in the COPDGene study (NCT00608764). Informed consent about the study procedures was signed and obtained from all the subjects before participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Summary conflict of interest statements: The authors report funding from the NIH in direct support of this work; other support, including consultancy, advisory board fees and contracted research from industry is outside the submitted work. A full list of disclosures is provided.
Dongxing Zhao has no disclosures to report.
Asghar Abbasi is supported by a postdoctoral fellowship from the Tobacco-Related Disease Research Program (28FT-0017).
Richard Casaburi reports personal fees from Glaxo Smith Kline, Boehringer Ingelheim, Astra Zeneca, Regeneron and Genentech and contracted clinical research support from Astra Zeneca, Boehringer Ingelheim, Glaxo Smith Kline, Genentech and Regeneron outside the submitted work.
Alessandra Adami is supported by a grant from NIH/NHLBI (R01HL151452).
Nicholas B. Tiller is supported by a postdoctoral fellowship from the Tobacco-Related Disease Research Program (T31FT1692).
Wei Yuan has no disclosures to report.
Christopher Yee has no disclosures to report.
Nicholas Jendzjowsky reports grant funding from AazeinTx, outside the submitted work; is a Parker B Francis Fellowship Recipient; reports US provisional patent, application no. 59263173-179 outside the submitted work; has patent 62/534,638 pending to University of Calgary.
David MacDonald has no disclosures to report.
Ken Kunisaki reports grants from NIH, during the conduct of the study, personal fees from Nuvaira for data safety and monitoring board services, and contracted clinical research support from Sanofi outside the submitted work.
William Stringer reports research funding from AstraZeneca and consultancy for GlaxoSmithKline outside the submitted work.
Janos Porszasz reports contracted clinical research support with United Therapeutics, Genentech and Regeneron outside the submitted work.
Barry Make reports (related to the general topic of COPD over the last three years) grants from NHLBI, Pearl Research, Circassia, GlaxoSmithKline and AstraZeneca; advisory board fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Verona, Third Pole, and Phillips; consulting fees from AstraZeneca; medical board member, grants, non-financial support, grant funds provided to and controlled by National Jewish Health for/from Astra Zeneca, grants and CME activity for/from Glaxo Smith Kline, CME activity for Wolters Kluwer Health, Spiration, CME activity for Sunovion, Mt Sinai, Web MD, National Jewish Health, Novartis, American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, Medscape, Ultimate Medical Academy, Eastern Pulmonary Society, Catamount Medical, Eastern VA Medical Center, and Academy Continued Health Care Learning, grants from Pearl Research (funds provided to and controlled by National Jewish Health), medical advisory board for Verona, Boehringer Ingelheim, Theravance, Phillips, and Science 24/7, non-financial support from Circassia, personal fees from Third Pole and Takeda, and grants from NHLBI, outside the submitted work.
Russ Bowler has no disclosures to report.
Harry Rossiter is supported by grants from NIH (R01HL151452, P50HD098593, R01DK122767, P2CHD086851) and the Tobacco Related Disease Research Program (T31IP1666). He reports consulting fees from Omniox Inc., and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech and Regeneron.
The authors report no other potential conflicts of interest for this work.
References
- 1.Stewart AG, Waterhouse JC, Howard P. Cardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary disease. Eur Respir J. 1991;4(10):1207–1214. [PubMed] [Google Scholar]
- 2.Stewart AG, Marsh F, Waterhouse JC, Howard P. Autonomic nerve dysfunction in COPD as assessed by the acetylcholine sweat-spot test. Eur Respir J. 1994;7(6):1090–1095. [PubMed] [Google Scholar]
- 3.La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(9101):478–484. doi: 10.1016/S0140-6736(97)11144-8 [DOI] [PubMed] [Google Scholar]
- 4.Tsuji H, Venditti FJ Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.CIR.90.2.878 [DOI] [PubMed] [Google Scholar]
- 5.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804 [DOI] [PubMed] [Google Scholar]
- 6.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132(7):552–555. doi: 10.7326/0003-4819-132-7-200004040-00007 [DOI] [PubMed] [Google Scholar]
- 7.Lacasse M, Maltais F, Poirier P, et al. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir Med. 2005;99(7):877–886. doi: 10.1016/j.rmed.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Seshadri N, Gildea TR, McCarthy K, Pothier C, Kavuru MS, Lauer MS. Association of an abnormal exercise heart rate recovery with pulmonary function abnormalities. Chest. 2004;125(4):1286–1291. doi: 10.1378/chest.125.4.1286 [DOI] [PubMed] [Google Scholar]
- 9.Gupta M, Bansal V, Chhabra SK. Abnormal heart rate recovery and chronotropic incompetence on exercise in chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(3):117–126. doi: 10.1177/1479972313493097 [DOI] [PubMed] [Google Scholar]
- 10.Celli B, Tetzlaff K, Criner G, et al. The 6-Minute-Walk Distance Test as a Chronic Obstructive Pulmonary Disease Stratification Tool. Insights from the COPD Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016;194(12):1483–1493. doi: 10.1164/rccm.201508-1653OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalin LP, Arena R, Labate V, Bandera F, Lavie CJ, Guazzi M. Heart rate recovery after the 6 min walk test rather than distance ambulated is a powerful prognostic indicator in heart failure with reduced and preserved ejection fraction: a comparison with cardiopulmonary exercise testing. Eur J Heart Fail. 2013;15(5):519–527. doi: 10.1093/eurjhf/hfs216 [DOI] [PubMed] [Google Scholar]
- 12.Swigris JJ, Swick J, Wamboldt FS, et al. Heart rate recovery after 6-min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest. 2009;136(3):841–848. doi: 10.1378/chest.09-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groepenhoff H, Vonk-Noordegraaf A, van de Veerdonk MC, Boonstra A, Westerhof N, Bogaard HJ. Prognostic relevance of changes in exercise test variables in pulmonary arterial hypertension. PLoS One. 2013;8(9):e72013. doi: 10.1371/journal.pone.0072013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minai OA, Nguyen Q, Mummadi S, Walker E, McCarthy K, Dweik RA. Heart rate recovery is an important predictor of outcomes in patients with connective tissue disease-associated pulmonary hypertension. Pulm Circ. 2015;5(3):565–576. doi: 10.1086/682432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez DA, Kortianou EA, Alison JA, et al. Heart Rate Recovery After 6-min Walking Test Predicts Acute Exacerbation in COPD. Lung. 2017;195(4):463–467. doi: 10.1007/s00408-017-0027-0 [DOI] [PubMed] [Google Scholar]
- 16.Mazzone SB, Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev. 2016;96(3):975–1024. doi: 10.1152/physrev.00039.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belvisi MG, Birrell MA, Khalid S, et al. Neurophenotypes in Airway Diseases. Insights from Translational Cough Studies. Am J Respir Crit Care Med. 2016;193(12):1364–1372. doi: 10.1164/rccm.201508-1602OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 20.GOLD Science Committee. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2020 Report. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available from: http://www.goldcopd.org. Accessed August23, 2021.
- 21.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 23.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 24.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127(3):270–277. [DOI] [PubMed] [Google Scholar]
- 25.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC [DOI] [PubMed] [Google Scholar]
- 26.Patel BD, Coxson HO, Pillai SG, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(5):500–505. doi: 10.1164/rccm.200801-059OC [DOI] [PubMed] [Google Scholar]
- 27.Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr RG, Berkowitz EA, Bigazzi F, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. Copd. 2012;9(2):151–159. doi: 10.3109/15412555.2012.654923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gestel AJ, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis. 2010;2(4):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troosters T, Vilaro J, Rabinovich R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;20(3):564–569. doi: 10.1183/09031936.02.02092001 [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 32.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6 [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309–1315. doi: 10.1164/rccm.200601-037OC [DOI] [PubMed] [Google Scholar]
- 34.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120 [DOI] [PubMed] [Google Scholar]
- 35.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201(3):W460–470. doi: 10.2214/AJR.12.10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostridge K, Williams NP, Kim V, et al. Relationship of CT-quantified emphysema, small airways disease and bronchial wall dimensions with physiological, inflammatory and infective measures in COPD. Respir Res. 2018;19(1):31. doi: 10.1186/s12931-018-0734-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurlbeck WM, Müller NL. Emphysema: definition, imaging, and quantification. AJR Am J Roentgenol. 1994;163(5):1017–1025. doi: 10.2214/ajr.163.5.7976869 [DOI] [PubMed] [Google Scholar]
- 38.Barbera JA, Ramirez J, Roca J, Wagner PD, Sanchez-Lloret J, Rodriguez-Roisin R. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(4 Pt 1):895–901. doi: 10.1164/ajrccm/141.4_Pt_1.895 [DOI] [PubMed] [Google Scholar]
- 39.Shiroishi R, Kitagawa C, Miyamoto N, et al. Heart rate recovery after the 6-min walk test is related to 6-min walk distance and percutaneous oxygen saturation recovery in patients with COPD. Respirology. 2015;20(4):671–673. doi: 10.1111/resp.12510 [DOI] [PubMed] [Google Scholar]
- 40.Yuan W, Nie S, Wang H, Xu Q, Jia N. Anticholinergics aggravate the imbalance of the autonomic nervous system in stable chronic obstructive pulmonary disease. BMC Pulm Med. 2019;19(1):88. doi: 10.1186/s12890-019-0848-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25(5):363–370. doi: 10.1016/j.ehj.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 42.Subramanian DR, Gupta S, Burggraf D, et al. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J. 2016;48(1):92–103. doi: 10.1183/13993003.01878-2015 [DOI] [PubMed] [Google Scholar]
- 43.Sterk PJ. Chronic diseases like asthma and COPD: do they truly exist? Eur Respir J. 2016;47(2):359–361. doi: 10.1183/13993003.01930-2015 [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Roisin R, Rabe KF, Vestbo J, Vogelmeier C, Agustí A. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20th Anniversary: a brief history of time. Eur Respir J. 2017;50(1):1700671. doi: 10.1183/13993003.00671-2017 [DOI] [PubMed] [Google Scholar]