Abstract
Background
Deep vein thrombosis (DVT) is common among patients with stroke. However, the incidence of DVT in acute ischemic stroke patients treated with thrombolytic therapy and the risk factors associated with this condition are unknown.
Objective
This study aimed to investigate the incidence and risk factors of DVT after thrombolysis in patients with acute ischemic stroke.
Settings and Methods
We retrospectively reviewed the medical records of all patients with acute ischemic stroke who underwent ultrasonic examination after intravenous thrombolysis between April 2017 and December 2019 at the stroke center of the First Hospital of Jilin University, China. Color duplex ultrasound was used to diagnosis DVT in all patients within 72 h after intravenous thrombolysis. Univariate and multivariate logistic regression analyses were performed to identify the risk factors for DVT.
Results
Overall, 474 patients were included in the study. Of these, 75 (15.8%) developed DVT (95% confidence interval 12.5–19.1). Older age was the risk factor that most significantly affected the development of DVT (p = 0.001). Compared to patients younger than 60 years old, those aged 60–69 years and ≥70 years had a higher risk of DVT, at rates of 2.201 (95% CI: 1.033–4.689; p < 0.05) and 4.241 (95% CI: 2.016–8.922; p < 0.001) times higher, respectively. Patients with higher triglyceride levels (odds ratio 0.545, 95% CI: 0.372–0.799, p = 0.002) and longer activated partial thromboplastin time (OR 0.927, 95% CI: 0.862–0.996, p = 0.040) were less likely to have DVT.
Conclusion
DVT is a common complication among patients undergoing intravenous thrombolysis after acute ischemic stroke. Advanced age may increase the occurrence of DVT to some extent. For these patients, safe antiplatelet therapy should be explored and implemented as soon as possible.
Keywords: acute ischemic stroke, deep vein thrombosis, incidence, risk factors, thrombolytic therapy
Introduction
Acute ischemic stroke is a major cause of mortality and morbidity worldwide. Patients admitted to the hospital with a stroke of recent onset are at risk of deep vein thrombosis (DVT),1 even complicated by pulmonary emboli, which is a frequent, potentially fatal complication among incapacitated stroke patients. DVT is mainly caused by abnormal blood coagulation in the deep veins and venous reflux disease, which often occurs in the lower extremities. It is commonly seen in the muscular calf veins, fibular veins, and posterior tibial veins.2 The incidence rate of DVT among post-stroke patients varies from 10% to 75%, depending on the method of diagnosis and the time of evaluation.3
The most effective treatment for acute ischemic stroke is intravenous thrombolytic therapy with recombinant tissue-type plasminogen activator (rt-PA). It can effectively activate plasminogen within the fibrinolytic system, to maximally dissolve the thrombus. As a result, thrombolytic therapy may be regarded as a protective factor against DVT. However, a study revealed that thrombolytic therapy did not protect against DVT, with 21% of patients treated with rt-PA developing DVT,4 at a rate almost equal to that of stroke patients managed without thrombolysis. In addition, several cross-sectional studies have shown that the incidence of DVT among patients with ischemic stroke treated with thrombolytic therapy was slightly higher than that among patients who did not receive thrombolytic therapy.5–7 According to a meta-analysis, older age, diabetes mellitus, atrial fibrillation, paralysis, a history of deep vein puncture, and infection, are some of the risk factors for DVT in stroke patients.8 To date, no study has investigated the risk factors for DVT among patients with stroke following intravenous thrombolytic therapy.
Therefore, in the present study, we evaluated the incidence and risk factors for DVT and analyzed the possible causes in patients with acute ischemic stroke after thrombolytic therapy with the aim of early prevention in the future.
Materials and Methods
Study Design and Participants
We retrospectively reviewed 777 consecutive acute ischemic stroke patients treated with rt-PA during a three-year period (April 2017-December 2019) at our stroke center. The eligible patients were required to meet the following criteria: (1) age ≥18 years; (2) hospitalization with a primary diagnosis of acute ischemic stroke, with the diagnosis being made according to the Guidelines for the Early Management of Patients with Acute Ischemic Stroke;9 (3) stroke onset time <4.5 h and receipt of intravenous thrombolytic therapy with rt-PA; (4) evaluation via ultrasound examination during hospitalization; and (5) informed consent obtained from the patient or an authorized representative. We excluded patients who did not undergo ultrasound examination or died during hospitalization, those for whom we could not access a complete medical record, or those with a previous history of DVT. As catheterization could lead to vascular endothelial damage, a risk factor for thrombosis,10 patients who underwent interventional therapy via femoral artery puncture were also excluded.
Diagnosis of DVT
DVT was diagnosed by physicians using color Doppler ultrasound on both lower extremities in all patients approximately 72 h after intravenous thrombolysis. Confirmatory ultrasound examination was performed among patients with obvious signs of DVT (such as swelling, pitting edema, redness, tenderness, etc) 72 h after thrombolysis. The main outcome in the present study was either symptomatic or asymptomatic DVT in the lower limb veins bilaterally.
Data Collection
We collected data regarding patients’ demographics, medical history, laboratory test results, and clinical outcomes. Intravenous thrombolytic therapy was instituted by administrating rt-PA (Boehringer Ingelheim Pharma GmbH, Ingelheim am Rhein, Germany) at a dose of 0.9 mg/kg. We investigated age, sex, body mass index, blood pressure, baseline National Institutes of Health Stroke Scale score, activities of daily living, comorbidities, smoking and drinking, pharmacological history (reported by the patient himself), onset-to-needle time, door-to-needle time and ten laboratory parameters before thrombolysis as potential risk factors. We also evaluated the incidence of pneumonia during hospitalization and the length of stay in the hospital.
Ethics
We conducted the study in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the First Hospital of Jilin University, Changchun, China. All participants provided written informed consent.
Statistical Analysis
All continuous variables are expressed as the mean±standard deviation, whereas categorical variables are presented as numbers and percentages. Continuous variables were analyzed using the independent samples t-test and the Mann–Whitney U-test. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Univariate variables with p values less than or equal to 0.1 were retained in a multivariate logistic regression analysis of predictors of DVT. Receiver operating characteristic (ROC) curve analysis was performed by determining the area under the ROC curve (AUC) to evaluate the performance of the logistic regression model. All statistical analyses were performed using SPSS Statistics for Windows, version 22.0 (IBM Corporation, Armonk, NY, USA). Analysis items with p < 0.05 were considered statistically significant.
Results
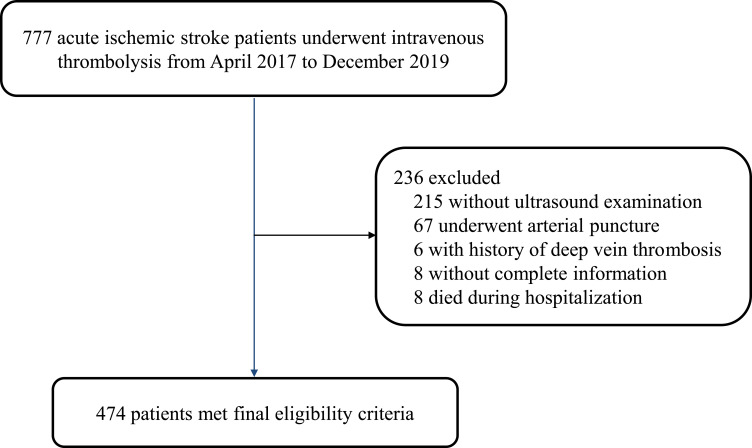
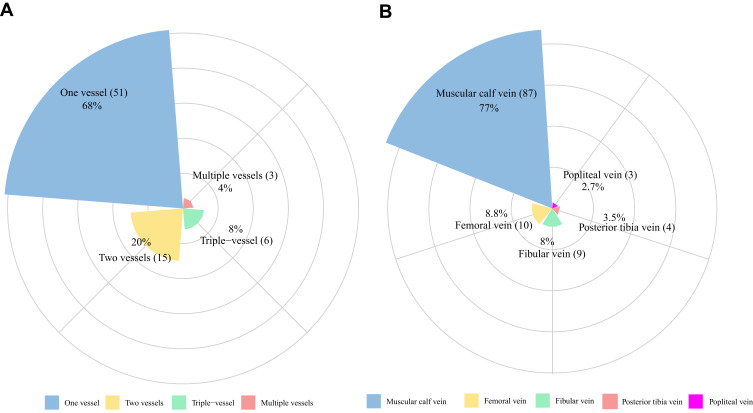
A total of 474 patients were included in the analysis (Figure 1). Among them, 75 patients (44 men and 31 women) developed DVT (15.8%, 95% confidence Interval [CI]: 12.5–19.1) based on the results of the ultrasound. Approximately two-thirds of patients (68%) developed a clot in a single blood vessel, while the rest experienced mixed-type DVT (Figure 2A). A total of 113 extremities were found to have DVT. The most frequently involved vessels were muscular calf veins, and approximately 77.0% of cases of thrombosis were located in the muscular calf veins (Figure 2B). Among the patients who underwent interventional treatment via femoral arterial puncture, 41.8% (28/67) developed DVT.
Figure 1.
Study population flow diagram.
Figure 2.
Distribution and probability of DVT in different low limb vessels. (A) The number of vessels involved with DVT. (B) Distribution of thrombus in different low limb vessels.
Table 1 shows the demographic characteristics of the study population. Patients with DVT were older than those without DVT (69.83 ± 9.75 vs 61.35 ± 11.49 years, p < 0.001). In terms of sex, women were more likely to have DVT (41.3% vs 27.8%, p < 0.05). Regarding the comorbidities, atrial fibrillation and smoking were over-represented among patients with and without DVT. There was no statistically significant difference between the two groups of patients in taking antihypertensive agents, hypoglycemic agents, antiplatelet agents and stains in the past. Only two patients who did not develop DVT had previously taken anticoagulant agents. Moreover, patients with DVT had more severe cases of stroke and poorer mobility. Laboratory test results showed that the level of high-density lipoprotein cholesterol was higher in patients with DVT. However, patients with higher triglyceride levels and longer activated partial thromboplastin time were less likely to have DVT. The incidence of pneumonia was higher among patients with DVT than among those without DVT (20.0% vs 7.5%, p = 0.001), Patients with DVT stayed longer in the hospital (median, 11 days [range, 9–15] vs 10 days [range, 8–12], p < 0.001).
Table 1.
Demographic and Clinical Characteristics of Study Population at Baseline
| With DVT (N=75) | Without DVT (N=399) | Statistic | P | |
|---|---|---|---|---|
| Age, years | 69.83±9.75 | 61.35±11.49 | 5.991 | <0.001 |
| Female, n (%) | 31 (41.3) | 111 (27.8) | 5.495 | 0.019 |
| Length of stay, d | 11 (9,15) | 10 (8,12) | −3.369 | 0.001 |
| Pneumonia, n (%) | 15 (20.0) | 30 (7.5) | 11.446 | 0.001 |
| Smoking | 26 (34.7) | 190 (47.6) | 4.270 | 0.039 |
| Drinking | 28 (37.3) | 157 (39.3) | 0.108 | 0.743 |
| Comorbidities, n (%) | ||||
| Ischemic stroke | 15 (20.0) | 98 (24.6) | 0.723 | 0.395 |
| Hypertension | 43 (57.3) | 241 (60.4) | 0.247 | 0.619 |
| Diabetes | 17 (22.7) | 103 (25.8) | 0.331 | 0.565 |
| Dyslipidemia | 27 (36.0) | 167 (41.9) | 0.895 | 0.344 |
| Coronary artery disease | 3 (4.0) | 30 (7.5) | 1.207 | 0.272 |
| Atrial fibrillation | 17 (22.7) | 37 (9.3) | 11.219 | 0.001 |
| Pharmacological history, n (%) | ||||
| Antihypertensive agents | 37(49.3) | 171(42.9) | 1.075 | 0.300 |
| Hypoglycemic agents | 15(20.0) | 63(15.8) | 0.814 | 0.367 |
| Antiplatelet agents | 7(9.3) | 41(10.3) | 0.062 | 0.804 |
| Stains | 12(16.0) | 82(20.6) | 0.823 | 0.364 |
| Baseline values | ||||
| SBP, mmHg | 150.35±20.35 | 151.73±18.60 | −0.583 | 0.560 |
| DBP, mmHg | 87.00±12.88 | 89.21±10.99 | −1.555 | 0.121 |
| BMI, kg/m2 | 23.90±1.87 | 24.20±2.96 | −0.845 | 0.399 |
| NIHSS, median (IQR) | 10 (6,13) | 7 (5,11) | −3.830 | <0.001 |
| ADL, median (IQR) | 50 (30,80) | 70 (30,80) | −3.033 | 0.002 |
| ONT, min | 177.38±62.53 | 188.71±59.44 | −1.501 | 0.134 |
| DNT, min | 52.83±16.87 | 53.20±25.99 | −0.121 | 0.904 |
| Laboratory parameters | ||||
| Platelet count (*109/L) | 199.40±53.87 | 201.65±49.57 | −0.355 | 0.723 |
| Triglyceride (mmol/L) | 1.32±0.67 | 1.87±1.36 | −3.462 | 0.001 |
| Cholesterol (mmol/L) | 4.83±1.12 | 5.00±0.98 | −1.333 | 0.183 |
| LDL-C (mmol/L) | 2.92±0.87 | 3.04±0.74 | −1.278 | 0.202 |
| HDL-C (mmol/L) | 1.35±0.37 | 1.25±0.30 | 2.540 | 0.011 |
| Glucose (mmol/L) | 8.01±2.90 | 8.09±3.14 | −0.209 | 0.834 |
| PT, s | 11.23±0.79 | 11.04±0.78 | 1.955 | 0.051 |
| APTT, s | 28.85±3.54 | 29.88±3.98 | −2.100 | 0.036 |
| TT, s | 14.59±2.22 | 14.30±2.31 | 0.984 | 0.326 |
| Fibrinogen (g/l) | 3.15±0.68 | 3.11±0.70 | 0.400 | 0.689 |
Abbreviations: DVT, deep vein thrombosis; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; NIHSS, National Institute of Health Stroke Scale; IQR, interquartile range; ADL, activities of daily living; ONT, onset-to-needle time; DNT, door-to-needle time; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time.
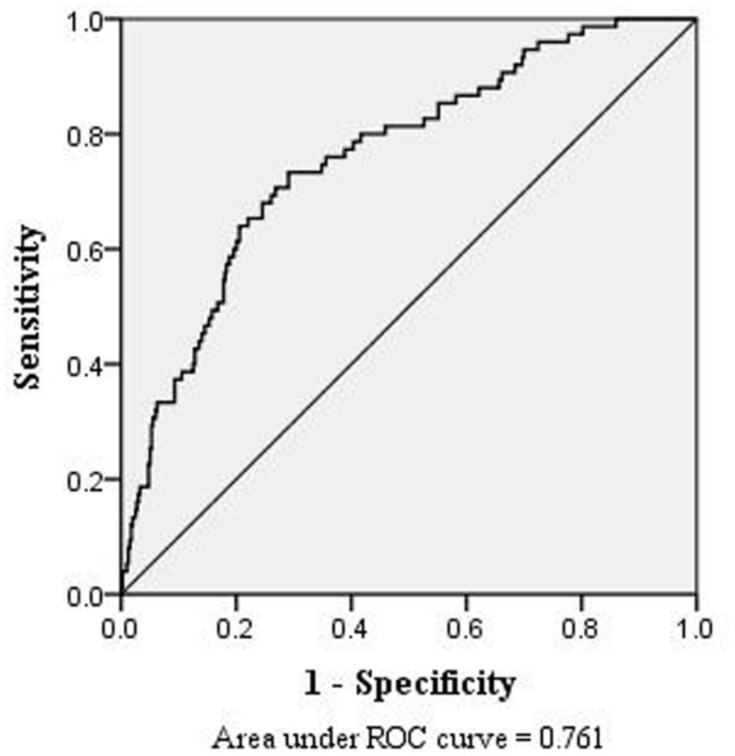
In the multivariate logistic regression analysis, compared to patients younger than 60 years old, those aged 60–69 years and ≥ 70 years had a higher risk of DVT, at rates of 2.201 (95% CI: 1.033–4.689; p < 0.05) and 4.241 (95% CI: 2.016–8.922; p < 0.001) times higher, respectively (Table 2). Higher triglyceride levels and longer activated partial thromboplastin time were associated with a lower risk of DVT. ROC curve analysis was performed based on the multivariate regression model (Figure 3). The corresponding AUC was 0.761 (95% CI: 0.702–0.819). The quartile of estimated probabilities was evaluated using the Hosmer-Lemeshow goodness of fit test (p = 0.767).
Table 2.
Multivariate Logistic Regression Analysis of Potential Risk Factors for DVT After Thrombolysis
| Adjusted Odds of Experiencing DVT | |||
|---|---|---|---|
| OR | 95% CI | P | |
| Age | 0.001 | ||
| <60 | 1.000 | (Reference) | |
| 60~69 | 2.201 | 1.033–4.689 | 0.041 |
| ≥70 | 4.241 | 2.016–8.922 | <0.001 |
| Female | 1.355 | 0.742–2.474 | 0.323 |
| Atrial fibrillation | 1.320 | 0.619–2.816 | 0.472 |
| Smoking | 1.003 | 0.547–1.840 | 0.991 |
| NIHSS | 0.560 | ||
| 0~4 | 1.000 | (Reference) | |
| 5~14 | 0.924 | 0.426–2.003 | 0.840 |
| >14 | 1.461 | 0.477–4.472 | 0.507 |
| ADL | 0.197 | ||
| >60 | 1.000 | (Reference) | |
| 40~60 | 2.295 | 0.898–5.865 | 0.083 |
| <40 | 1.441 | 0.714–2.909 | 0.308 |
| Triglyceride | 0.545 | 0.372–0.799 | 0.002 |
| HDL-C | 1.530 | 0.626–3.743 | 0.351 |
| PT | 1.014 | 0.701–1.467 | 0.939 |
| APTT | 0.927 | 0.862–0.996 | 0.040 |
Abbreviations: OR, odds ratio; CI, confidence interval; TG, triglyceride; NIHSS, National Institute of Health Stroke Scale; ADL, activities of daily living; HDL-C, high density lipoprotein cholesterol; PT, prothrombin time; APTT, activated partial thromboplastin time.
Figure 3.
The area under receiver operation characteristics (ROC) curves showing that age, triglyceride and APTT had a good diagnostic value in predicting DVT.
Discussion
In the present study, the incidence rate of DVT among patients with acute ischemic stroke after thrombolysis was 15.8% (75/474). This rate was slightly lower than the 21% (6/29) incidence of DVT reported among patients treated with rt-PA by Baloqun et al.4 The main reason may be that the author included less research subjects and performed ultrasound scans about 2 weeks after the patients were admitted to the hospital, which increased the detection rate of asymptomatic DVT.
The results of the present study confirmed that thrombolytic therapy does not reduce the incidence of DVT among patients with stroke. DVT after thrombolysis may be mainly due to the short half-life of rt-PA (only about 5 min), after which the blood soon returns to a hypercoagulable state. A massive response of the markers of coagulation activation and fibrin formation is induced by thrombolysis with rt-PA and persists for up to 72 h.11 The three main factors of thrombosis are disturbances in the blood flow pattern, blood clotting factors that promote coagulation, and vascular endothelial injury.12 Intravenous thrombolysis activates the coagulation mechanism of the body and causes platelet activation and aggregation.11,13 After recanalization, due to the partial damage of re-perfused vascular endothelial cells, the local embolus is not completely cleared. Exposure of the thrombus’ lipid core further promotes platelet aggregation, and even the imbalance in the fibrinolytic system caused by thrombolytic drugs may lead to re-occlusion of recanalized vessels and formation of fresh thrombi. On the other hand, fibrin thrombi bind to large amounts of thrombin during thrombus formation. Once the thrombi dissolve, feedback activation occurs, and a large amount of thrombin is released, resulting in a state of hypercoagulation after thrombus dissolution, which further leads to thrombus formation. Immobility is another key factor.14 In particular, the patients were monitored at a stroke unit and stayed in bed strictly for 24 h after intravenous thrombolysis.
Intravenous thrombolysis is also associated with an increased likelihood of DVT prophylaxis.15 One case suggested that a delay in the commencement of antithrombotic treatment after intravenous thrombolysis could have been a determinant of DVT.16 According to the current guidelines put forth by the American Heart Association/American Stroke Association, antiplatelet agents were started 24 h after thrombolysis, aiming to minimize the possibility of symptomatic intracranial hemorrhage, and early initiation of antiplatelet therapy may be considered under certain circumstances.17 However, the trade-off between the risk of bleeding and the benefit of prophylaxis against DVTs using heparin, and the optimal time and dose to administer anticoagulants after thrombolysis require further investigation.18 The results of a meta-analysis of 11 studies including 2082 cases showed that compared to patients who underwent standard antithrombotic therapy (after 24 h), patients with acute ischemic stroke who underwent early antithrombotic therapy within 24 h after receiving intravenous alteplase thrombolytic therapy had higher proportion of 90-day favorable outcomes without increased risks of symptomatic intracranial hemorrhage and mortality.19
The incidence rate of DVT was 41.8% among patients who underwent interventional therapy (such as arterial thrombolysis, endovascular suppository, or endovascular stent placement) through femoral arterial puncture. Vascular endothelial injury leads to the release of subendothelial tissue factors and reduces venous flow, which activates the clotting cascade, resulting in thrombus formation.20 Previous studies have shown that both peripheral and central catheterization increases the risk of catheter-related thrombosis.10,21
Age, particularly ≥ 65 years, is closely related to the incidence of DVT.8,22 With the increase in age, the production of proteins C and S decreases faster than that of clotting factors, such that the blood gradually enters a state of hypercoagulability among the elderly.23 Other studies have also shown that advanced age is significantly associated with DVT.24,25 Among elderly patients undergoing thrombolysis, more high-quality prospective studies are needed to verify the safety of early antithrombotic therapy.
Patients with DVT had lower triglyceride levels than those without DVT, which is consistent with the results of two other studies.7,26 We also found decreased triglyceride levels in a murine model of DVT.27 This could be due to the fact that a decrease in triglyceride levels leads to the low availability of acetyl CoA, which further affects the tricyclic acid cycle and finally leads to perturbed energy metabolism pathways in DVT. In addition, the APTT of patients with DVT in this study is shorter than those without DVT. A lower APTT is common in hypercoagulable state and thromboembolic diseases. Studies have shown that a low APTT added to the risk of thrombotic events,28,29 which is consistent with the results of our study.
Meanwhile, we should also consider the role of care manager in such a context. Studies have shown that care managers have an inseparable relationship with patients and doctors, and exert a positive influence on patient health and self-management.30 At this time, the care managers play the role of planner and educator. Use the Wells score to classify patients, provided health education to the patient and family members, such as the importance and methods of preventing DVT. The study of Serpici A et al also confirmed that nurse-led training improved the DVT knowledge and self-care practices of patients.31
Limitations
The present study had some limitations. First, due to the inherent limitations of the retrospective nature of this study, we were unable to determine the causal relationship between thrombolysis and the occurrence of DVT. Beside, the pharmacological history was reported by the patient himself, which may cause recall bias. Second, as the most important limitation of our study, due to the irregular determination of D-dimer levels, we did not analyze this common risk factor and predictor of DVT. Thrid, we only performed ultrasound examination in stroke patients within 72 h after thrombolysis and symptomatic DVT, which led to the non-detection of the occurrence of asymptomatic DVT beyond 72 h after thrombolysis. The incidence of DVT in the present study may be lower than the actual incidence. Fourth, we did not perform a specific comparative analysis of stroke patients who underwent interventional therapy through femoral arterial catheterization. Future studies can further compare the differences between patients with DVT after catheterization and patients who did not undergo catheterization and explore whether catheter-related factors influence the occurrence of DVT.
Conclusion
The incidence rate of DVT among patients with acute ischemic stroke after thrombolysis was 15.8%. Thrombolysis does not reduce the incidence of DVT among stroke patients and may increase the occurrence of DVT to some extent. Advanced age is the most significant risk factor of DVT. The occurrence of DVT will increase the incidence of pneumonia during hospitalization, prolong the length of stay in the hospital and bring additional burden to patients.
Future Directions
Among elderly patients or patients undergoing interventional therapy, high-quality randomized controlled studies with large sample sizes should be conducted to explore the safety and effectiveness of antiplatelet therapy within 24 h after thrombolysis. Further use the DVT risk assessment scale combine with laboratory test results and other risk factors for early prediction of DVT.
Funding Statement
The authors disclosed receipt of the following financial support for the publication of this article: Natural Science Foundation of Jilin Province, China (20200201314JC), Jilin Province Department of Finance (JLSWSRCZX2020-080).
Disclosure
Chunping Ni and Xiuli Yan are co-correspondence authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Dennis M, Sandercock P, Graham C, Forbes J, Forbes J on behalf of the CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. The Clots in Legs Or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1–90. doi: 10.3310/hta19760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388(10063):3060–3073. doi: 10.1016/S0140-6736(16)30514-1 [DOI] [PubMed] [Google Scholar]
- 3.Khan MT, Ikram A, Saeed O, et al. Deep vein thrombosis in acute stroke – a systemic review of the literature. Cureus. 2017;9:e1982. doi: 10.7759/cureus.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baloqun IO, Roberts LN, Patel R, Pathansali R, Kalra L, Arya R. Clinical and laboratory predictors of deep vein thrombosis after acute stroke. Thromb Res. 2016;142:33–39. doi: 10.1016/j.thromres.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 5.Ji R, Li G, Zhang R, Hou H, Zhao X, Wang Y. Higher risk of deep vein thrombosis after hemorrhagic stroke than after acute ischemic stroke. J Vasc Nurs. 2019;37:18–27. doi: 10.1016/j.jvn.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 6.Ha SH, Kim YJ, Heo SH, Chang DI, Kim BJ. Prediction of deep vein thrombosis by ultrasonography and D-dimer in Asian patients with ischemic stroke. BMC Neurol. 2020;20:257. doi: 10.1186/s12883-020-01842-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Shi Y, Dong Y, Dong Q, Ye T, Fang K. Clinical risk factors of asymptomatic deep venous thrombosis in patients with acute stroke. Clin Appl Thromb Hemost. 2019;25:1076029619868534. doi: 10.1177/1076029619868534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WJ, Shi ZX, Zhang CY, Hu Z. Risk factors of deep venous thrombosis in stroke patients: a Meta-analysis. Chin J Nurs. 2019;54:989–994. doi: 10.3761/j.issn.0254-1769.2019.07.006 [DOI] [Google Scholar]
- 9.Furie KL, Jayaraman MV. 2018 Guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:509–510. doi: 10.1161/STROKEAHA.118.020176 [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Wan G, Zhu B. Incidence and risk factors of symptomatic thrombosis related to peripherally inserted central catheter in patients with lung cancer. J Adv Nurs. 2021;77:1284–1292. doi: 10.1111/jan.14666 [DOI] [PubMed] [Google Scholar]
- 11.Pilato F, Calandrelli R, Profice P, et al. Pulmonary embolism in a stroke patient after systemic thrombolysis: clinical decisions and literature review. J Stroke Cerebrovasc Dis. 2013;22:e667–70. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Shaikhouni A, Baum J, Lonser RR. Deep vein thrombosis prophylaxis in the neurosurgical patient. Neurosurg Clin N Am. 2018;29:567–574. doi: 10.1016/j.nec.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Fassbender K, Dempfle CE, Mielke O, et al. Changes in coagulation and fibrinolysis markers in acute ischemic stroke treated with recombinant tissue plasminogen activator. Stroke. 1999;30:2101–2104. doi: 10.1161/01.str.30.10.2101 [DOI] [PubMed] [Google Scholar]
- 14.Dizon MAM, De Leon JM. Effectiveness of initiating deep vein thrombosis prophylaxis in patients with stroke: an integrative review. J Neurosci Nurs. 2018;50:308–312. doi: 10.1097/JNN.0000000000000385 [DOI] [PubMed] [Google Scholar]
- 15.Douds GL, Hellkamp AS, Olson DM, et al. Venous thromboembolism in the Get With The Guidelines-Stroke acute ischemic stroke population: incidence and patterns of prophylaxis. J Stroke Cerebrovasc Dis. 2014;23:123–129. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 16.Delgado MG, Mauri G, Vega J. Massive pulmonary thromboembolism after intravenous stroke thrombolysis. BMJ Case Rep. 2012;bcr1020115008. doi: 10.1136/bcr.10.2011.5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhou YC, Zhu WD, et al. The risk of postoperative hemorrhage and efficacy of heparin for preventing deep vein thrombosis and pulmonary embolism in adult patients undergoing neurosurgery: a systematic review and meta-analysis. J Investig Med. 2017;65:1136–1146. doi: 10.1136/jim-2016-000235 [DOI] [PubMed] [Google Scholar]
- 19.Li X, Tong X, Li -J-J, et al. Meta-analysis of safety and efficacy of early antithrombotic therapy in patients with acute ischemic stroke after intravenous alteplase thrombolysis. Chin J Stroke. 2008;13:430–436. doi: 10.3969/j.issn.1673-5765.2018.05.005 [DOI] [Google Scholar]
- 20.Turpie AGG, Esmon C. Venous and arterial thrombosis – pathogenesis and the rationale for anticoagulation. Thromb Haemost. 2011;105:586–596. doi: 10.1160/TH10-10-0683 [DOI] [PubMed] [Google Scholar]
- 21.Evans NS, Ratchford EV. Catheter-related venous thrombosis. Vasc Med. 2018;23:411–413. doi: 10.1177/1358863X18779695 [DOI] [PubMed] [Google Scholar]
- 22.Liu LP, Zheng HG, Wang DZ, et al. Risk assessment of deep-vein thrombosis after stroke: a prospective study using clinical factors. CNS Neurosci Ther. 2014;20:403–410. doi: 10.1111/cns.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danish SF, Burnett MG, Stein SC. Prophylaxis for deep venous thrombosis in patients with craniotomies: a review. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.4.2 [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Dai B, Yao Y, Song K, Chen D, Jiang Q. Chronic kidney dysfunction can increase the risk of deep vein thrombosis after total hip and knee arthroplasty. Biomed Res Int. 2017;2017:8260487. doi: 10.1155/2017/8260487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita Y, Nakatsuka H, Namba Y, et al. The incidence of pulmonary embolism and deep vein thrombosis and their predictive risk factors after lower extremity arthroplasty: a retrospective analysis based on diagnosis using multidetector CT. J Anesth. 2015;29:235–241. doi: 10.1007/s00540-014-1891-x [DOI] [PubMed] [Google Scholar]
- 26.Dai X, Ding W, Li H, et al. Associations of serum lipids and deep venous thrombosis risk after total knee arthroplasty in patients with primary knee osteoarthritis. Int J Low Extrem Wounds. 2020;19:51–56. doi: 10.1177/1534734619868123 [DOI] [PubMed] [Google Scholar]
- 27.Sung Y, Spagou K, Kafeza M, et al. Deep vein thrombosis exhibits characteristic serum and vein wall metabolic phenotypes in the inferior vena cava ligation mouse model. Eur J Vasc Endovasc Surg. 2018;55:703–713. doi: 10.1016/j.ejvs.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 28.Zakai NA, Ohira T, White R, Folsom AR, Cushman M. Activated partial thromboplastin time and risk of future venous thromboembolism. Am J Med. 2008;121:231–238. doi: 10.1016/j.amjmed.2007.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hron G, Eichinger S, Weltermann A, Quehenberger P, Halbmayer WM, Kyrle PA. Prediction of recurrent venous thromboembolism by the activated partial thromboplastin time. J Thrombosis Haemostasis. 2006;4:752–756. doi: 10.1111/j.1538-7836.2006.01868.x [DOI] [PubMed] [Google Scholar]
- 30.Ciccone MM, Aquilino A, Cortese F, et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc Health Risk Manag. 2010;6:297–305. doi: 10.2147/VHRM.S9252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serpici A, Gürsoy A. Nurse-led patient training improves deep vein thrombosis knowledge and self-care practices. J Vasc Nurs. 2018;36:53–63. doi: 10.1016/j.jvn.2018.03.002 [DOI] [PubMed] [Google Scholar]