Abstract
Context
Anorexia nervosa (AN) is prevalent in adolescent girls and is associated with bone impairment driven by hormonal alterations in nutritional deficiency.
Objective
To assess the impact of estrogen replacement with and without recombinant human insulin-like growth factor-1 (rhIGF-1) administration on bone outcomes.
Design
Double-blind, randomized, placebo-controlled 12-month longitudinal study.
Participants
Seventy-five adolescent and young adult women with AN age 14 to 22 years. Thirty-three participants completed the study.
Intervention
Transdermal 17-beta estradiol 0.1 mg/day with (i) 30 mcg/kg/dose of rhIGF-1 administered subcutaneously twice daily (AN-IGF-1+) or (ii) placebo (AN-IGF-1−). The dose of rhIGF-1 was adjusted to maintain levels in the upper half of the normal pubertal range.
Main Outcome Measures
Bone turnover markers and bone density, geometry, microarchitecture, and strength estimates.
Results
Over 12 months, lumbar areal bone mineral density increased in AN-IGF-1− compared to AN-IGF-1+ (P = 0.004). AN-IGF-1+ demonstrated no improvement in areal BMD in the setting of variable compliance to estrogen treatment. Groups did not differ for 12-month changes in bone geometry, microarchitecture, volumetric bone mineral density (vBMD), or strength (and results did not change after controlling for weight changes over 12 months). Both groups had increases in radial cortical area and vBMD, and tibia cortical vBMD over 12 months. Levels of a bone resorption marker decreased in AN-IGF-1− (P = 0.042), while parathyroid hormone increased in AN-IGF-1+ (P = 0.019). AN-IGF-1− experienced irregular menses more frequently than did AN-IGF-1+, but incidence of all other adverse events did not differ between groups.
Conclusions
We found no additive benefit of rhIGF-1 administration for 12 months over transdermal estrogen replacement alone in this cohort of young women with AN.
Keywords: anorexia nervosa, adolescents, bone density, bone microarchitecture, IGF-1, estradiol
Anorexia nervosa (AN) is a condition characterized by altered body image, fear of weight gain, and nutritional restriction leading to weight loss (1). AN occurs in 0.3% to 4% of adolescent and young adult women and is associated with an increased risk for mental and medical morbidity, including increased fracture risk (2,3). The onset of AN during adolescence is particularly problematic, as this is a critical time for bone accrual, with peak bone mass, a predictor of fracture risk in later life, being reached between the ages of 18 to 22 years (4). Young women with AN have lower bone mineral density (BMD) and reduced bone accrual rates compared to normal-weight controls, and adult women who develop AN during adolescence have lower BMD than those who develop AN in adulthood despite a similar duration of amenorrhea, indicating an impact on peak bone mass acquisition (5,6).
Hormonal changes caused by nutritional deficiency are key contributors to bone impairment in AN. Hypothalamic amenorrhea occurs in about a third of adolescents with AN, and duration of amenorrhea correlates negatively with BMD (7,8). Suppression of the hypothalamic-pituitary-gonadal axis causes low levels of estrogen, a hormone that inhibits bone resorption and remodeling and maintains bone formation (9). Therefore, estrogen deficiency is a critical determinant of low BMD (9). Another hormonal alteration in AN is a reduction in levels of insulin-like growth factor-1 (IGF-1), a bone trophic hormone (10). IGF-1 levels are positively associated with levels of bone formation markers and BMD measures in AN, suggesting that lower IGF-1 also contributes to low BMD in AN (6,11).
While weight restoration improves BMD, not all women recover, and many women with adolescent-onset AN never achieve normative bone measures, indicating a need for therapeutic intervention (11). We have shown in a randomized-controlled trial that adolescent girls with AN receiving transdermal physiologic 17-β-estradiol (with a cyclic progestogen) for 18 months demonstrate increases in spine and hip BMD at a rate that is equivalent to age-matched normal-weight controls, an effect not observed in the placebo group (12). This resulted in maintenance of BMD Z-scores in those receiving physiologic transdermal 17-β-estradiol. However, residual bone deficits persisted (12), possibly because estrogen replacement did not address other hormonal alterations in AN that contribute to low BMD, such as low IGF-1 levels. Importantly, we have shown that short-term recombinant human IGF-1 (rhIGF-1) administration in doses that increase IGF-1 levels to the upper half of the normal range increases levels of bone formation markers without increasing levels of bone resorption markers in adolescents with AN (13). Similarly, rhIGF-1 administration in adults with AN increases levels of bone formation markers and when given with oral estrogen increases spine and hip BMD (14).The impact on bone health of combined transdermal physiologic 17-β-estradiol replacement with long-term rhIGF-1 administration has not been previously studied in adolescents with AN.
We performed a 12-month randomized, double-blind, placebo-controlled study to assess the impact of physiologic estrogen replacement with and without rhIGF-1 administration on bone turnover markers, bone density, geometry, microarchitecture, and strength estimates in adolescent and young adult women (14 to 22 years old) with AN. We hypothesized that compared to estrogen monotherapy, rhIGF-1 co-administration in adolescent and young adult women with AN would increase bone formation and BMD, and improve bone geometry, microarchitecture, and strength.
Participants and Methods
Participant selection
Seventy-five females with AN between the ages of 14 and 22 years with a bone age of at least 14 years were enrolled. Participants were required to meet the Diagnostic and Statistical Manual of Mental Disorders (fourth or fifth edition) criteria, depending on time of enrollment, for either AN or atypical AN, and the study psychologist confirmed the diagnosis prior to enrollment. To establish the criterion of low weight in AN, participants were required to be below 90% on 2 of the following three measures: percentage median body mass index (%mBMI) for age, percentage median body weight for age, or percentage median body weight for age and height, based on Centers for Disease Control and Prevention growth charts (15). Participants with atypical AN were required to have demonstrated significant weight loss. The study did not assume clinical care for participants, but participants were encouraged to continue with or begin treatment for AN and its comorbidities as advised by their healthcare providers.
Exclusion criteria included contraindications to estrogen therapy (ie, a personal history of migraines with aura or thromboembolism, a personal or family history of conditions that may increase risk for thromboembolism, personal history or a first-degree relative with history of an estrogen-dependent cancer), a history of conditions known to impact bone metabolism (eg, Cushing’s syndrome, diabetes mellitus, pituitary disease, untreated thyroid disease, renal failure), bone fracture within the previous 6 months, and past or current use of medications known to affect bone metabolism (such as bisphosphonates or long-term steroids). Of note, past or current use of oral estrogen or progestin-containing compounds was not an exclusion criterion given their limited impact on BMD in AN (16); however, participants using estrogen/progestin medications at the time of the screening visit were asked to discontinue usage for at least 2 months before the baseline visit. Further exclusion criteria included pregnancy, suicidality, substance abuse, psychosis, hematocrit below 30% (indicative of anemia), potassium below 3.0 mmol/L (suggesting active purging), blood glucose below 50 mg/dL (concerning for severe restriction and potential for increased risk of hypoglycemia with IGF-1 replacement), and other causes of hypoestrogenism as indicated by high levels of follicle-stimulating hormone (FSH) (premature ovarian failure) or abnormal thyroid-stimulating hormone (TSH) levels.
Participants were recruited through referrals from eating disorder providers and treatment center and via outreach to healthcare providers in primary care practices. The Partners Healthcare Institutional Review Board approved this study. Informed consent was obtained from participants ≥18 years and from parents of participants <18 years old. Informed assent was obtained from participants <18 years.
Experimental protocol
This study was registered with ClinTrials.gov (NCT01301183). Participants were seen at our institution’s Translational and Clinical Research Center. At the study screening visit, a study provider completed a detailed medical history and physical examination, and the participant had a urine pregnancy test and blood draw for hematocrit, potassium, glucose, FSH, and TSH. Participants were weighed to the nearest 0.1 kg on an electronic scale in a hospital gown, and height was measured to the nearest millimeter in triplicate with a wall-mounted stadiometer. A left hand and wrist X-ray were taken to determine bone age. Only girls with a bone age of at least 14 years were included in the study to exclude girls who were still actively growing to prevent premature epiphyseal fusion from estradiol administration.
The baseline visit was scheduled within 8 weeks of the screening visit. Physical exercise and activity in the prior year were assessed with the Modifiable Activity Questionnaire (17). The Modifiable Activity Questionnaire captures average hours per week of both leisure time and occupational physical activity in the preceding 12 months. Further, a detailed history was obtained to determine average hours per week of both weight-bearing and nonweight-bearing directed physical exercise. Dual energy X-ray absorptiometry (DXA) performed at the screen or baseline visit to assess areal BMD (aBMD) and bone mineral content of the spine, hip, radius, and whole body, as well as body composition. High-resolution peripheral quantitative computed tomography (HRpQCT) was used to assess bone geometry, microarchitecture, and strength estimates at the nondominant distal radius and tibia. Fasting blood samples were drawn for glucose, calcium, phosphorus, 25(OH) vitamin D (25OHD), parathyroid hormone (PTH), IGF-1, insulin-like growth factor binding protein 3 (IGF-BP3), estradiol, a marker of bone formation [N-terminal propeptide of type 1 procollagen (P1NP)], a marker of bone resorption [N-telopeptide (NTX)].
After the baseline visit, participants were randomly assigned by the Massachusetts General Hospital Research Pharmacy to the active (AN-IGF-1+) or placebo (AN-IGF-1−) arms of the study. All participants received 17-beta estradiol 0.1 mg/day delivered by a patch (Vivelle-Dot™; Noven Pharmaceuticals Corporation, Miami, FL, USA) twice weekly continuously, with micronized progesterone (Prometrium) given at a dose of 100 mg daily orally for the first 10 days of each month (to prevent endometrial hyperplasia from unopposed estrogen administration). The AN-IGF-1+ group received rhIGF-1 subcutaneous injections [Increlex (Ipsen Biopharmaceuticals, Inc); INN mecasermin] at a starting dose of 30 mcg/kg/dose twice daily (14), and the dose was titrated up for a maximum of two 25% dose increases over the course of study participation, to a maximum of 46.88 mcg/kg/dose twice daily, to maintain IGF-1 levels in the upper half of the normal range for pubertal stage. rhIGF-1 is only approved to treat severe primary IGF-1 deficiency in children below 18 years of age, so its use in this study was off-label. The AN-IGF-1− group received placebo injections with sham dose increases as necessary. All participants received 1000 to 1200 mg of elemental calcium daily in 2 divided doses, and 400 to 1200 IU of vitamin D daily, depending on repletion needs indicated by 25OHD levels. Those with 25OHD below 20 ng/mL received 50 000 IU/week supplementation for 4 to 8 weeks, and then supplementation was reduced to 400 to 1200 IU/day depending on repeat 25OHD level. To monitor study medication compliance, participants completed medication calendars. Study investigators collected these calendars and any unused study medications at follow-up study visits before dispensing new supplies. Additionally, participants self-reported missed medications to study investigators at all follow-up visits.
Participants completed follow-up visits at 1, 3, 4.5, 6, 7.5, 9, 10.5, and 12 months after baseline. All baseline study procedures were repeated at the 6- and 12-month visits. Other visits included an interval medical history, physical examination, phlebotomy for glucose and IGF-1 levels, and a pregnancy test.
Areal bone density and body composition assessments
DXA (Hologic 4500 A, Apex software version 13.3; Hologic Inc, Waltham, MA, USA) was used to assess bone mineral content and BMD of the lumbar spine, total hip, femoral neck, radius, and whole body, as well as measures of body composition (fat and lean mass). Z-scores were calculated using the standard pediatric database for those younger than 19 years old and the standard adult database for those 19 and older to allow assessments using the same database at the conclusion of the 12-month period (18). The coefficients of variation for BMD, fat mass, and lean mass for our institution are 0.8% to 1.1%, 2.1%, and 1.0%, respectively.
Bone size, geometry, and microarchitecture assessment
HRpQCT (XtremeCT; Scanco Medical AG, Bassersdorf, Switzerland) was used to measure bone geometry, microarchitecture, and volumetric BMD (vBMD) of the nondominant distal radius and tibia while strength estimates (stiffness and failure load) were obtained using micro finite element analysis via mathematical modeling of the effects of simulated mechanical load on bone. In cases of previous acute fracture at a nondominant site, the nonfractured dominant side was assessed. Per scan protocol, 2D scout views were utilized to align the slices at 9.5 mm and 22.5 mm from the radius and tibia endplates, respectively. We used fixed distances as linear growth is almost complete in girls by the bone age of 14 years (19). Extended cortical analysis quantified porosity and thickness of cortical bone. The reproducibility of these measures at our center is listed in Supplementary Table 1 in (20).
Biochemical analysis
Screening and safety labs (FSH, TSH, hematocrit, potassium, and glucose) were assessed at the hospital laboratory. Colorimetric assays were used to measure calcium [LabCorp Esoteric Testing, Burlington, NC, USA; sensitivity 0.8 mg/dL; intraassay coefficient of variation (CV) 0.9%-3.0%] and phosphorus (LabCorp Esoteric Testing; sensitivity 6.0 pg/mL; intraassay CV 0.9%-3.0%), and an immunochemiluminometric assay was used to assess 25OHD levels (LabCorp Esoteric Testing; sensitivity 4.0 ng/mL; intraassay CV 4.8%-7.7%). A chemiluminescent immunoassay was used to measure PTH (Beckman Coulter, Fullerton, CA, USA; sensitivity 1 pg/mL; intraassay CV 1.6%-2.6%). IGF-1 was assayed via liquid chromatography/mass spectrometry (Quest Diagnostics, Nichols Institute, San Juan Capistrano, CA, USA; sensitivity 15.6 ng/mL; intraassay CV 3.5%-15%). Chemiluminescence was used to measure IGF-BP3 (Immunodiagnostic Systems, Inc., Scottsdale, AZ, USA; sensitivity 50 ng/mL; intraassay CV 1.92%) and estradiol (Beckman Coulter; sensitivity 20 pg/mL; intraassay CV 2.0%-4.2%). Radioimmunoassay was used for P1NP (Orion Diagnostics, Espoo, Finland; sensitivity 0.7 μg/L; intraassay CV 3.5%-5.3%). NTX was assessed with enzyme immunoassay (Alere Osteomark, Scarborough, ME, USA; sensitivity 5 nM bone collagen equivalents; intraassay CV 4.6%).
All blood samples were drawn at least 2 h postprandial and not necessarily in the morning due to constraints with participant availability.
Statistical analysis
All data were initially assessed for normality and are reported as mean ± standard error of the mean (parametric data) or median with interquartile range (non-parametric data). For our primary longitudinal analysis, a repeated measures data analysis of variance with a shared baseline was used to estimate the treatment effect at 6 and 12 months for cortical, trabecular, and total vBMD at the radius and tibia, lumbar spine, total hip, femoral neck, and 1/3 radius aBMD. For study completers, we also ran between- and within-group comparisons. Depending on data distribution, we used the Student t-test or Wilcoxon rank sum test for between-group analysis. For within-group assessments (baseline vs 6- or 12-month data), we used the paired t-test or Wilcoxon sign rank test (depending on data distribution). We further used multivariable regression to control for confounding variables, including age, height, and the baseline measure of the specific bone parameter being assessed (as this may impact the rate of change over time). To elucidate the effects of changes in weight and in other biomarkers such as IGF-1 and PTH on bone parameters, we included these covariates in multivariable regression models. Correlations between longitudinal changes in bone and biochemical parameters were evaluated using Pearson or Spearman correlation, depending on data distribution. A 2-tailed P-value below 0.05 was considered significant.
Results
Participant enrollment and attrition
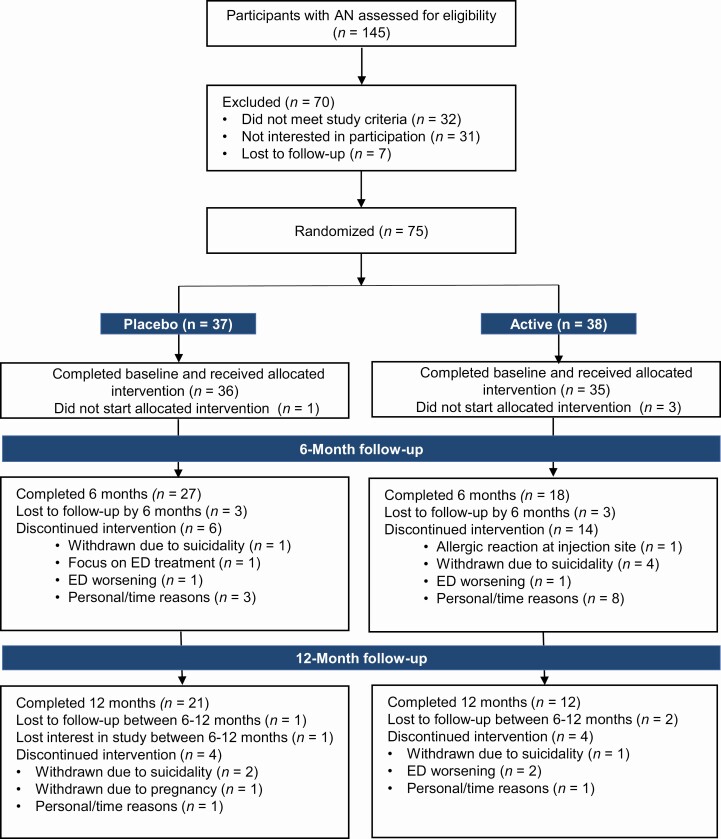
Seventy-five participants were randomized with 37 assigned to AN-IGF-1− and 38 to AN-IGF-1+. The average age of participants was 19.4 years old, and they were predominantly white and non-Hispanic (Table 1). Forty-three participants (27 in the AN-IGF-1− group and 18 in the AN-IGF-1+ group) completed the 6-month visit, and 33 participants (21 in the AN-IGF-1− group and 12 in the AN-IGF-1+ group) completed the 12-month visit (Fig. 1).
Table 1.
Baseline and changes over 12 months in clinical characteristics, body composition and biochemical parameters
| Baseline measures | Within group change over 12 months [mean (95% confidence interval)] | P-value comparing changes between groups over 12 months | |||
|---|---|---|---|---|---|
| AN-IGF-1− (n = 37) | AN-IGF-1+ (n = 38) | AN-IGF-1− (n = 21) | AN-IGF-1+ (n = 12) | ||
| Clinical characteristics | |||||
| Age (years) | 19.4 ± 0.4 | 19.4 ± 0.3 | — | — | — |
| Bone age (years) | 18.0 (16.6 to 18.0) | 18.0 (16.3 to 18.0) | — | — | — |
| Height (cm) | 165.9 ± 1.0 | 163.1 ± 0.9a | 0.1 (−0.3, 0.4) | 0.8 (−0.0, 1.5) | 0.061 |
| Race (% white /% African American /% more than 1 race) | 89.2/0.0/10.8 | 89.5/2.6/7.9 | — | — | — |
| Ethnicity (% non-Hispanic/% Hispanic) | 89.2/10.8 | 97.4/2.6 | — | — | — |
| Weight (kg) | 51.4 ± 0.9 | 49.7 ± 0.8 | 3.1 (1.0, 5.1) | 1.7 (−1.5, 4.9) | 0.627 |
| BMI (kg/m2) | 18.7 ± 0.3 | 18.7 ± 0.3 | 1.1 (0.3, 1.9) | 0.5 (−0.8, 1.7) | 0.562 |
| BMI Z-Score | −0.92 (−1.54 to −0.56) | −0.96 (−1.81 to −0.54) | 0.45 (0.07, 0.83) | −0.02 (−0.67, 0.63) | 0.489 |
| % median BMI for age | 87.4 ± 1.4 | 87.2 ± 1.2 | 4.6 (0.8, 8.4) | 1.6 (−4.1, 7.2) | 0.562 |
| Age of menarche (years) | 13.0 (12.0 to 14.0) | 13.0 (12.0 to 14.0) | — | — | — |
| Duration of amenorrhea (months) | 4.00 (0.00 to 11.50) | 3.00 (0.00 to 8.50) | — | — | — |
| Weight-bearing exercise activity (hours per week) | 3.33 (1.52 to 6.47) | 3.65 (1.23 to 6.45) | 0.16 (−4.38, 4.69) | −2.90 (−7.59, 1.80) | 0.669 |
| DXA measures of body composition | |||||
| Lean mass (kg) | 37.5 ± 0.6 | 36.7 ± 0.6 | 1.5 (0.5, 2.5) | 0.6 (−0.5, 1.6) | 0.185 |
| Lean mass (%) | 71.9 ± 0.9 | 72.0 ± 0.8 | −2.0 (−4.3, 0.2) | −0.2 (−3.3, 2.9) | 0.307 |
| Fat mass (kg) | 13.1 ± 0.6 | 12.7 ± 0.5 | 2.0 (0.3, 3.6) | 0.9 (−1.6, 3.4) | 0.464 |
| Fat mass (%) | 24.6 ± 0.9 | 24.6 ± 0.8 | 2.1 (−0.2, 4.5) | 0.2 (−3.1, 3.5) | 0.319 |
| Biochemical parameters | |||||
| 25(OH) vitamin D (ng/mL) | 33.0 (27.5 to 45.3) | 30.5 (24.4 to 39.5) | −6.7 (−12.6, −1.3) | −0.6 (−8.3, 7.0) | 0.082 |
| Calcium (mg/dL) | 9.44 ± 0.05 | 9.42 ± 0.07 | −0.16 (−0.34, 0.03) | 0.19 (−0.24, 0.63) | 0.111 |
| Phosphorus (mg/dL) | 4.09 ± 0.08 | 4.01 ± 0.08 | −0.26 (−0.51, 0.00) | −0.25 (−0.90, 0.39) | 0.878 |
| IGF-1 (ng/mL) | 266.8 ± 14.3 | 249.7 ± 15.3 | −32.9 (−67.4, 1.7) | 101.9 (11.3, 192.5) | 0.004 |
| IGF-1 Z-score | −0.1 (−1.0 to 0.4) | −0.3 (−1.0 to 0.2) | −0.1 (−0.5, 0.3) | 1.1 (0.1, 2.0) | 0.019 |
| IGF-BP3 (ng/mL) | 5073.7 ± 172.3 | 4988.5 ± 172.2 | −517.0 (−625.0, −408.9) | −251.9 (−1045.3, 541.5) | 0.441 |
| IGF-1/IGF-BP3 | 0.054 (0.041 to 0.062) | 0.053 (0.037 to 0.063) | −0.002 (−0.009, 0.006) | 0.030 (0.003, 0.057) | 0.011 |
| P1NP (μg/L) | 86.7 (63.3 to 131.7) | 98.7 (62.5 to 147.5) | −12.1 (−33.4, 9.3) | −39.5 (−89.2, 10.1) | 0.280 |
| NTX (nM BCE) | 14.5 (10.8 to 19.1) | 15.4 (10.5 to 19.8) | −3.5 (−6.8, −0.1) | −1.0 (−7.7, 5.6) | 0.167 |
| PTH (pg/mL) | 21.5 ± 9.2 | 17.4 ± 1.7 | −0.2 (−5.9, 5.4) | 10.6 (2.9, 18.2) | 0.021 |
Baseline data are presented as mean ± standard error of the mean or median (interquartile range); changes over 12 months are presented as mean (95% confidence interval). Bolded change over 12 months denotes significant within-group difference.
Abbreviations: BCE, bone collagen equivalents; IGF-1, insulin like growth factor-1, IGFBP-3: insulin like growth factor binding protein 3; NTX: N-telopeptide; P1NP: N-terminal propeptide of type 1 procollagen; PTH: parathyroid hormone
a Denotes significant baseline between-group difference.
Figure 1.
Consort diagram: number of adolescent and young women with anorexia nervosa recruited for the study and attrition over the 12-month study course.
We sought differences between completers and noncompleters in each group. Within the AN-IGF-1− group, those who completed the study did not differ from noncompleters in clinical characteristics or biochemical parameters at baseline (data not shown). However, AN-IGF-1− completers had significantly lower lumbar spine, whole-body, and whole-body/less head BMD than noncompleters, as well as significantly lower trabecular thickness, trabecular vBMD, and total bone vBMD of the tibia (data not shown). Within the AN-IGF-1+ group, completers were taller than noncompleters, but completers and noncompleters did not differ for other clinical, biochemical, or bone parameters (data not shown).
Baseline characteristics
Baseline clinical characteristics, body composition measures, aBMD, HRpQCT measures, and biochemical parameters are presented in Tables 1 and 2, respectively. Regarding the use of oral contraceptives, in the AN-IGF-1−: 56.8% never used, 24.3% were past users (prior to 6 months before study enrollment), 13.5% were current users, and 5.4% were past and current users. Similarly, within the AN-IGF-1+, 36.8% had never used, 42.1% used in the past, 7.9% were current users, and 13.2% past and current users (P = 0.157 between groups). AN-IGF-1− were taller than AN-IGF-1+, but groups did not differ for other clinical characteristics or body composition. Groups also did not differ in aBMD measures even after controlling for height. AN-IGF-1− had significantly higher radial trabecular area than AN-IGF-1+, but this difference was no longer significant after controlling for height. The groups did not differ for other measures of bone geometry, microarchitecture, vBMD, or strength estimates at the radius and tibia after controlling for height. Additionally, groups did not differ in baseline levels of IGF-1, calcium, phosphorus, and 25OHD. Participants who completed the 6- and/or 12-month study visit (AN-IGF-1− n = 24, AN-IGF-1+ n = 16) had baseline assessments of IGF-BP3, estradiol, P1NP, NTX, and PTH, and groups did not differ in these measures. Baseline estradiol level was 55.4 (27.1-93.7) pg/mL in AN-IGF-1− and 42.6 (20.8-145.3) pg/mL in AN-IGF-1+ (P = 0.662).
Table 2.
Baseline and changes over 12 months in DXA, HRpQCT, and finite element analysis measures
| Baseline measures | Within group change over 12 months [mean (95% confidence interval)] | P-values comparing changes between groups over 12 months | |||||
|---|---|---|---|---|---|---|---|
| AN-IGF-1− | AN-IGF-1+ | AN-IGF-1− | AN-IGF-1+ | Unadjusted | Adjusted for baseline age, height, and bone measure | Adjusted for baseline age, height, bone measure, and 12-month weight change | |
| DXA | n = 36 | n = 37 | n = 21 | n = 12 | |||
| 1/3 radius BMD (g/cm2) | 0.677 ± 0.009 | 0.664 ± 0.007 | 0.004 (−0.003, 0.011) | 0.000 (−0.015, 0.015) | 0.736 | 0.932 | 0.936 |
| 1/3 radius BMD Z-score | −0.133 ± 0.173 | −0.413 ± 0.129 | −0.005 (−0.114, 0.104) | −0.027 (−0.273, 0.219) | 0.276 | 0.772 | 0.810 |
| FN BMD (g/cm2) | 0.757 ± 0.020 | 0.775 ± 0.018 | 0.011 (−0.010, 0.032) | 0.014 (−0.014, 0.041) | 0.849 | 0.420 | 0.396 |
| FN BMD Z-score | −1.24 ± 0.19 | −1.09 ± 0.17 | −0.016 (−0.262, 0.231) | 0.101 (−0.146, 0.347) | 0.470 | 0.350 | 0.278 |
| Total hip BMD (g/cm2) | 0.861 ± 0.019 | 0.882 ± 0.017 | 0.024 (0.007, 0.040) | 0.016 (−0.007, 0.038) | 0.487 | 0.898 | 0.967 |
| Total hip BMD Z-score | −0.786 ± 0.169 | −0.614 ± 0.145 | 0.155 (−0.005, 0.315) | 0.091 (−0.101, 0.282) | 0.555 | 0.790 | 0.914 |
| LS BMD (g/cm2) | 0.862 (0.742 to 0.960) | 0.896 (0.807 to 0.953) | 0.043 (0.025, 0.061) | 0.010 (−0.004, 0.024) | 0.004 | 0.018 | 0.035 |
| LS BMD Z-score | −1.31 ± 0.22 | −1.08 ± 0.17 | 0.280 (0.085, 0.475) | 0.045 (−0.083, 0.174) | 0.028 | 0.064 | 0.109 |
| WB BMD (g/cm2) | 0.998 ± 0.015 | 1.01 ± 0.01 | 0.019 (0.009, 0.030) | 0.008 (−0.007, 0.024) | 0.283 | 0.469 | 0.321 |
| WB BMD Z-score | −1.21 ± 0.19 | −1.16 ± 0.13 | 0.110 (−0.059, 0.279) | 0.009 (−0.170, 0.188) | 0.547 | 0.670 | 0.503 |
| HRpQCT | n = 35 | n = 33 | n = 19 | n = 12 | |||
| Distal radius | |||||||
| Bone geometry | |||||||
| Cort. area (mm2) | 43.0 (36.0 to 50.0) | 43.9 (40.8 to 49.9) | 3.8 (0.9, 6.7) | 2.4 (0.0, 4.8) | 0.401 | 0.965 | 0.861 |
| Trab. area (mm2) | 215.7 (194.8 to 242.7) | 197.2 (170.1 to 223.9)a | −3.7 (−7.9, 0.4) | −1.8 (−4.2, 0.7) | 0.839 | 0.390 | 0.890 |
| Cort. thickness (mm) | 0.650 (0.550 to 0.774) | 0.700 (0.590 to 0.795) | 0.053 (0.011, 0.094) | 0.039 (−0.002, 0.080) | 0.599 | 0.623 | 0.517 |
| Bone microarchitecture | |||||||
| Cort. porosity (%) | (n = 34) 1.13 (0.67 to 1.39) | 1.29 (0.68 to 1.78) | −0.74 (−2.10, 0.62) | 0.00 (−0.20, 0.19) | 0.568 | 0.684 | 0.853 |
| Trab. number (1/mm) | 1.92 ± 0.05 | 1.91 ± 0.05 | −0.02 (−0.12, 0.07) | −0.10 (−0.18, −0.02) | 0.202 | 0.318 | 0.344 |
| Trab. separation (mm) | 0.444 (0.402 to 0.518) | 0.452 (0.413 to 0.493) | 0.005 (−0.020, 0.030) | 0.023 (−0.002, 0.048) | 0.301 | 0.360 | 0.400 |
| Trab. thickness (mm) | 0.067 (0.060 to 0.078) | 0.066 (0.062 to 0.074) | 0.002 (−0.002, 0.005) | 0.002 (−0.007, 0.010) | 0.714 | 0.598 | 0.670 |
| vBMD | |||||||
| Cort. vBMD (mgHA/cm3) | 812.9 (780.9 to 844.8) | 827.4 (789.1 to 859.8) | 26.1 (12.0, 40.1) | 16.9 (5.0, 28.9) | 0.530 | 0.620 | 0.636 |
| Trab. vBMD (mgHA/cm3) | 160.0 ± 6.7 | 155.3 ± 5.2 | 1.5 (−2.3, 5.3) | −5.1 (−23.3, 13.1) | 0.394 | 0.642 | 0.626 |
| Total vBMD (mgHA/cm3) | 284.0 ± 9.0 | 294.1 ± 8.6 | 10.4 (1.4, 19.5) | 5.7 (−16.1, 27.5) | 0.855 | 0.997 | 0.928 |
| Strength estimates | |||||||
| Stiffness (kN/mm) | 65.6 (54.1 to 83.1) | 65.1 (57.1 to 74.2) | 2.3 (−1.2, 5.8) | 0.8 (−3.3, 4.9) | 0.584 | 0.745 | 0.790 |
| Failure load (kN) | 3.58 ± 0.16 | 3.35 ± 0.93 | 0.10 (0.00, 0.24) | 0.03 (−0.12, 0.19) | 0.417 | 0.679 | 0.759 |
| Distal tibia | (n = 36) | (n = 33) | n = 21) | (n = 12) | |||
| Bone geometry | |||||||
| Cort. area (mm2) | 102.7 (90.5 to 119.9) | 102.2 (96.2 to 118.5) | 1.4 (−0.1, 2.9) | 3.1 (−1.1, 7.2) | 0.574 | 0.309 | 0.365 |
| Trab. area (mm2) | 539.1 ± 12.2 | 509.9 ± 14.4 | −0.8 (−2.2, 0.5) | −2.9 (−6.1, 0.2) | 0.203 | 0.371 | 0.890 |
| Cort. thickness (mm) | 1.06 ± 0.03 | 1.09 ± 0.03 | 0.02 (−0.00, 0.03) | 0.04 (−0.01, 0.08) | 0.409 | 0.294 | 0.336 |
| Bone microarchitecture | |||||||
| Cort. porosity (%) | 2.56 (2.05 to 3.14) | 2.51 (1.81 to 3.27) | −0.07 (−0.20, 0.07) | 0.01 (−0.49, 0.51) | 0.750 | 0.778 | 0.738 |
| Trab. number (1/mm) | 1.77 ± 0.04 | 1.82 ± 0.05 | 0.03 (−0.02, 0.08) | −0.04 (−0.17, 0.09) | 0.297 | 0.474 | 0.534 |
| Trab. separation (mm) | 0.474 (0.443 to 0.531) | 0.461 (0.414 to 0.515) | −0.009 (−0.022, 0.005) | 0.005 (−0.037, 0.047) | 0.708 | 0.674 | 0.749 |
| Trab. thickness (mm) | 0.087 ± 0.002 | 0.087 ± 0.003 | −0.002 (−0.004, 0.001) | −0.001 (−0.008, 0.006) | 0.837 | 0.663 | 0.731 |
| vBMD | |||||||
| Cort. vBMD (mgHA/cm3) | 863.2 ± 5.2 | 867.4 ± 5.5 | 10.1 (5.8, 14.4) | 9.0 (2.4, 15.6) | 0.762 | 0.712 | 0.722 |
| Trab. vBMD (mgHA/cm3) | 183.7 ± 5.9 | 186.8 ± 5.8 | 0.1 (−2.9, 3.1) | −4.4 (−13.4, 4.6) | 0.155 | 0.236 | 0.264 |
| Total vBMD (mgHA/cm3) | 297.2 ± 6.8 | 305.8 ± 7.3 | 2.8 (−1.3, 6.9) | 1.9 (−6.9, 10.6) | 0.940 | 0.680 | 0.695 |
| Strength estimates | |||||||
| Stiffness (kN/mm) | 203.0 ± 5.6 | 194.8 ± 5.9 | −1.0 (−4.2, 2.2) | −0.1 (−8.2, 8.1) | 0.812 | 0.774 | 0.827 |
| Failure load k(N) | 10.2 ± 0.3 | 9.8 ± 0.3 | 0.0 (−0.2, 0.1) | 0.0 (−0.3, 0.3) | 0.845 | 0.829 | 0.812 |
Baseline data are presented as mean ± standard error of the mean or median (interquartile range); changes over 12 months are presented as mean (95% confidence interval). Bolded change over 12 months denotes significant within-group difference. Bolded P-value denotes significant between-group difference.
Abbreviations: BMD, bone mineral density; Cort, cortical; DXA, dual energy X-ray absorptiometry; FN, femoral neck; HRpQCT, high resolution peripheral quantitative computed tomography; LS, lumbar spine; Trab, trabecular; vBMD, volumetric bone mineral density; WB, whole body.
a Denotes significant baseline between-group difference.
Groups did not differ e in self-reported symptoms of depression, anxiety, or eating disorder behaviors (data not shown). They also did not differ in number of prior eating disorder-related hospitalizations or duration since AN diagnosis. However, duration since reaching their lowest body weight was shorter in AN-IGF-1+ (15.7 months) compared to AN-IGF-1− (25.8 months) (P = 0.048).
Changes in clinical characteristics and body composition over 6- and 12-months
Six- and 12-month changes in clinical characteristics and body composition are shown in Supplementary Table 2 (20) (6-month changes) and Table 1 (12-month changes) respectively. Over 6-months, the AN-IGF-1− group had significant within-group increases in weight, BMI, %mBMI, and fat mass, with significant differences compared to the AN-IGF-1+ group. Over 12 months, the AN-IGF-1− group again had significant within-group increases in weight, BMI, BMI Z-score, %mBMI, and lean and fat mass but without any between-group differences.
Completers Analysis
Changes in areal bone mineral density and HRpQCT measures over 6 months
Six-month changes in aBMD
The AN-IGF-1− group demonstrated within-group increases in spine, total hip, and whole-body BMD, and a decrease in radius BMD Z-score (Supplementary Table 3 (20)). The AN-IGF-1+ group showed within-group increases in whole body BMD only. After controlling for age, height, and the specific bone measure at baseline, groups differed significantly for changes in radius BMD over 6 months, with the AN-IGF-1+ group doing better for this measure. When we added 6-month changes in weight to this regression model, the between-group difference in radius BMD was no longer significant.
Six-month changes in HRpQCT parameters
At the distal radius, both groups demonstrated within-group increases in cortical area, thickness and vBMD, as well as in bone stiffness, with no differences noted between groups (Supplementary Table 3 (20)). AN-IGF-1− also had increases in trabecular thickness, total vBMD, and failure load, while AN-IGF-1+ had a decrease in trabecular number. At the distal tibia, both groups demonstrated within-group increases in cortical vBMD; AN-IGF-1− had increases in trabecular number, and AN-IGF-1+ had decreases in trabecular area; no between-group differences were noted after controlling for baseline age, height and the specific bone measure with or without 6-month weight change.
Changes in areal bone mineral density and HRpQCT measures over 12 months
Twelve-month changes in aBMD
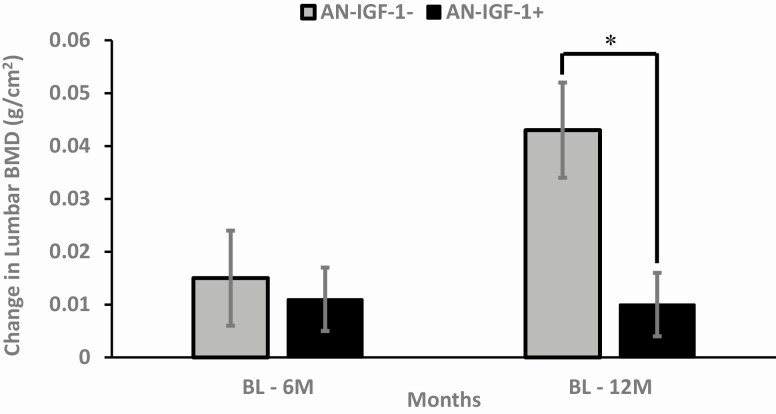
The AN-IGF-1− group demonstrated within-group increases in spine BMD and BMD Z-score, and total hip, whole body, and whole-body/less head BMD (Table 2). These increases were not observed in the AN-IGF-1+ group. The groups differed significantly for changes in lumbar spine BMD over 12 months (Fig. 2), with greater effects seen in the AN-IGF-1− group (P = 0.018 after controlling for age, height, and baseline bone measure). When we added changes in weight to the regression model, the between-group difference in change in spine BMD remained significant. However, when change in PTH was added to this model, with and without change in weight, this difference was no longer significant.
Figure 2.
Six- and 12-month changes in lumbar spine bone mineral density (BMD) in AN-IGF-1− (gray bars) and AN-IGF-1+ (black bars). AN-IGF-1− had significant increases in lumbar BMD over 12 months compared to AN-IGF-1+. *Denotes significant between-group difference.
Twelve-month changes in HRpQCT measures
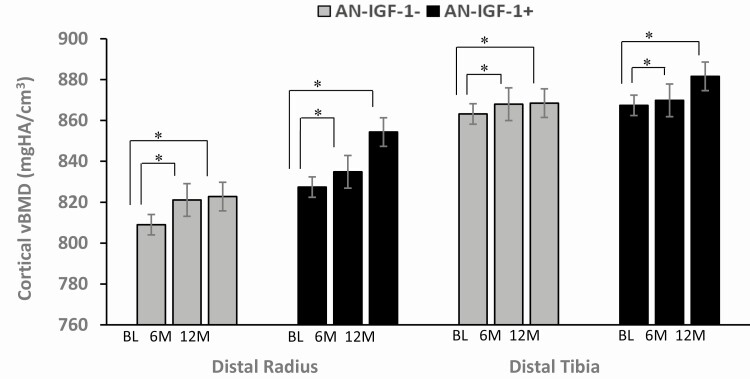
At the distal radius, AN-IGF-1− had within-group increases in cortical area and thickness, cortical and total vBMD, and failure load, while AN-IGF-1+ had within-group increases in cortical area and vBMD and a decrease in trabecular number; no differences were noted between groups for any of these measures (Table 2). At the distal tibia, both groups demonstrated within-group increases in cortical vBMD, with no differences noted between groups for this and other HRpQCT measures. Our results did not change after controlling for baseline age, height, the respective bone measure with or without 12-month weight changes, and with or without 12-month change in PTH. Changes in cortical vBMD at both sites are shown in Figure 3.
Figure 3.
Cortical volumetric bone mineral density (vBMD) of the distal radius and tibia at baseline, 6 months, and 12 months. Both AN-IGF-1− (gray bars) and AN-IGF-1+ (black bars) had significant within-group increases in cortical vBMD of both sites over 6 months and 12 months. No between-group differences were observed. *Denotes significant within-group difference.
Intent to treat analysis
In repeated measures intention-to-treat analysis where we estimated the treatment effect at 6 and 12 months for BMD and its corresponding Z-score at the 1/3 radius, lumbar spine, total hip, whole body (by DXA), and total, cortical, and trabecular vBMD at the radius and tibia (by HRpQCT), we did not find any significant differences between groups (all P > 0.05).
Changes in biochemical parameters
IGF-1 and IGF-BP3
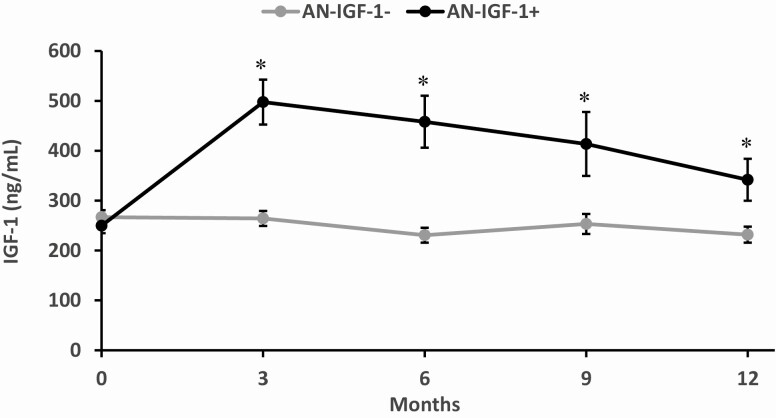
As expected, AN-IGF-1+ (but not AN-IGF-1−) had within-group increases in IGF-1 levels, IGF-1 Z-scores, and the ratio of IGF-1/IGFBP-3 over 6 and 12 months, with significant between-group differences in these measures (Supplementary Table 2 (20); Table 1). Fig. 4 shows changes in IGF-1 levels over 12 months in the 2 groups.
Figure 4.
IGF-1 responses by randomization group over the 12-month study course. Results are shown as mean ± standard error of the mean. AN-IGF-1+ (black dots) had significantly higher IGF-1 levels than AN-IGF-1− (gray dots) at all time points after baseline. *Denotes significant between-group difference.
Estradiol
Only AN-IGF-1− had significant within-group 12-month increase in estradiol levels of 55.8 (5.8-127.7) pg/mL (P = 0.012). AN-IGF-1+ did not demonstrate significant 12-month changes in estradiol (12.8 (−33.9-34.0) pg/mL; P = 0.520). Neither AN-IGF-1− nor AN-IGF-1+ demonstrated significant 6-month changes in estradiol levels (45.6 (−25.2 to 75.6) pg/mL, P = 0.108, and 28.5 (−1.8 to 73.6) pg/mL, P = 0.244, respectively). No between-group differences were noted for changes in estradiol levels over 6 or 12 months (P = 0.978 and P = 0.112, respectively).
Bone turnover markers
Levels of P1NP, a marker of bone formation, did not change significantly within groups over 6 or 12 months, and no between-group differences were noted. NTX, a marker of bone resorption, showed a trend for a decrease in the AN-IGF-1− group (P = 0.057) but remained unchanged in the AN-IGF-1+ group.
Parathyroid hormone
AN-IGF-1+ had a greater within-group increase in PTH than AN-IGF-1− at 6 and 12 months, with a significant difference from AN-IGF-1− over 12 months (and a similar trend over 6 months)
25(OH) vitamin D/calcium/phosphorus
25OHD levels decreased in the AN-IGF-1− group; however, this was still in the sufficient range at 12 months (mean 31.9 ± 8.4 ng/mL). 25OHD levels remained unchanged and in the sufficient range in the AN-IGF-1+ group. There were no significant changes in the calcium and phosphorus levels in either group over 12 months.
Correlation of changes in bone endpoints with changes in biochemical parameters (whole group)
Changes in radial and tibial cortical vBMD correlated negatively with changes in NTX (Rho = -0.36, P = 0.047 and Rho = -0.47, P = 0.007, respectively), while changes in whole-body BMD correlated negatively with changes in P1NP (Rho = -0.41, P = 0.022). Changes in lumbar BMD were negatively correlated with changes in PTH (Rho = −0.38, P = 0.039).
Adverse events
Percentages of participants in each group reporting adverse events are detailed in Table 3. More participants in the AN-IGF-1− group experienced irregular menses than in the AN-IGF1+ group, but groups did not differ in incidence of other adverse events, including adverse events expected to be related to rhIGF-1, such as hypoglycemia and headache. While the percentage of each group experiencing a suicide attempt or increased suicidal ideation during study course was not significantly different, 8.1% of AN-IGF-1− and 13.2% of AN-IGF-1+ experienced these. To note, this patient population as a whole is prone to suicidal ideation and behavior, and these side effects are not associated with rhIGF-1. Similarly, while not significantly different, 10.8% of AN-IGF-1− and 18.4% of AN-IGF-1+ were hospitalized during participation. There were 5 hospitalization events among AN-IGF-1− and 8 among AN-IGF-1+ over the course of study (all related to the underlying diagnosis of AN).
Table 3.
Percentages of AN-IGF-1− and AN-IGF-1+ reporting side effects and adverse events
| AN-IGF-1− (n = 37), % | AN-IGF-1+ (n = 38), % | P-value | |
|---|---|---|---|
| Likely related to study medications or procedures | |||
| Bruising at injection site | 2.7 | 2.6 | 0.985 |
| Rash at injection site | 0 | 5.3 | 0.096 |
| Swelling at injection site | 0 | 2.6 | 0.241 |
| Dizziness after injection | 2.7 | 0 | 0.232 |
| Syncope during injection attempt | 0 | 2.6 | 0.241 |
| Irritation at patch site | 5.4 | 2.6 | 0.537 |
| Irregular menses | 8.1 | 0 | 0.037 |
| Breast tenderness | 5.4 | 0 | 0.090 |
| Vasovagal reaction during blood draw | 8.1 | 2.6 | 0.282 |
| Low fasting blood sugar | 2.7 | 2.6 | 0.985 |
| Possibly related to study medications or procedures | |||
| Stuttering, confusion, and difficulty concentrating after injection | 0 | 2.6 | 0.241 |
| Dizziness with jitteriness | 5.4 | 0 | 0.090 |
| Excessive fatigue | 2.7 | 0 | 0.232 |
| Headaches | 8.1 | 5.3 | 0.621 |
| Acne | 2.7 | 0 | 0.232 |
| Mood swings | 2.7 | 2.6 | 0.985 |
| Hot flashes | 2.7 | 0 | 0.232 |
| Bloating | 2.7 | 0 | 0.232 |
| Constipation | 5.4 | 5.3 | 0.978 |
| Unrelated to study medications or procedures | |||
| Suicide attempt | 5.4 | 7.9 | 0.665 |
| Suicidal ideation | 2.7 | 5.3 | 0.568 |
| Hospitalization related to eating disorder | 10.8 | 18.4 | 0.349 |
| Bradycardia | 2.7 | 2.6 | 0.985 |
| Panic attacks | 2.7 | 0 | 0.232 |
| Cardiovascular palpitations | 5.4 | 0 | 0.090 |
| Femoral stress reaction | 2.7 | 0 | 0.232 |
| Elbow fracture | 0 | 2.6 | 0.241 |
| Abrasion needing sutures | 2.7 | 0 | 0.232 |
| Dehydration | 0 | 2.6 | 0.241 |
| Allergic reaction to peanuts | 2.7 | 0 | 0.232 |
| Optic pressure | 2.7 | 0 | 0.232 |
| Abdominal pain | 2.7 | 0 | 0.232 |
| Shoulder and neck pain | 0 | 2.6 | 0.241 |
| Lightheadedness | 0 | 2.6 | 0.241 |
| Tremors | 2.7 | 0 | 0.232 |
| Loose stool | 2.7 | 0 | 0.232 |
| Oily hair | 2.7 | 0 | 0.232 |
Bolded P-value means P < .05.
Discussion
This is the first study evaluating the combined effect of transdermal 17-β estradiol (mostly antiresorptive) and rhIGF-1 (a bone trophic hormone) on bone end points in adolescent and young adult women with AN in a double-blind placebo-controlled randomized trial. The therapies investigated to date in adolescents with AN include low-magnitude mechanical stimulation, combined estrogen-progestin contraceptive pills, transdermal 17-β estradiol (with a cyclic progestin), dehydroepiandrosterone, and bisphosphonates (21-26). Most of these therapies have shown only modest improvements in bone outcomes, and none have shown catch-up bone accrual during the physiological window of bone accrual in this young population. Teriparatide, which is a bone anabolic agent, showed an increase in BMD in adult women with AN, but no data are available in adolescents (26). In this study we did not find any additive benefit of using rhIGF-1 at a dose of 30 to 46.88 mcg/kg twice a day for 12 months over the use of transdermal 17-β estradiol alone (except for the 6-month change in radial aBMD), despite a demonstrated increase in IGF-1 levels in this group.
We found that the AN-IGF-1− group had greater increases in lumbar spine BMD and BMD Z-scores than the AN-IGF-1+ group. This difference was likely consequent to weight gain in the AN-IGF-1− group, which was not seen in the AN-IGF-1+ group. Weight gain in AN is one of the most efficient ways to increase bone density at the spine (27). Also, the lumbar spine consists of primarily trabecular bone, which responds to estrogen replacement (12), and we found an increase in estradiol levels in the AN-IGF-1− group but not the AN-IGF-1+ group at 12-months. The magnitude of increase in estradiol levels at 6 and 12 months in the IGF-1- group (but not the IGF-1+ group) in this study is consistent with increases reported in other studies of transdermal estradiol administration in conditions of functional hypothalamic amenorrhea (28,29). The lack of increase in estradiol levels in the rhIGF-1+ group is concerning for lack of adherence to the transdermal estradiol regimen in the AN-IGF-1+ group, particularly for the 12-month completers. However, in both groups, the range of change in estradiol levels was wide, concerning for widespread lack of adherence to the treatment regimen. Another possibility is that labs were drawn at random times in relation to last patch application, and a decrease in estradiol delivery on the last day on the patch may have contributed to the substantial variability in estradiol levels. Further, whereas rhIGF-1 administration increases IGF-1 levels alone, weight gain would normalize or improve levels of many other hormones that may contribute to low BMD in AN, such as high levels of cortisol and peptide YY (30, 31) and low levels of leptin (32). This maybe another reason why increases in lumbar spine BMD measures were evident in the AN-IGF-1− group, but not the AN-IGF-1+ group (12), although differences between groups persisted after controlling for weight changes over time.
Of note, PTH levels increased significantly in the AN-IGF-1+ group. Sustained elevations in PTH may be deleterious to bone by increasing bone resorption, and in fact, the decrease in NTX, a bone resorption marker, observed in the AN-IGF-1− group (as expected with estrogen replacement), was not observed in the AN-IGF-1+ group. In patients with acromegaly who have high IGF-1 levels, urinary calcium excretion is high, which may lead to increases in PTH levels to maintain normocalcemia (33). Moreover, estrogen decreases the osteoclastic actions of PTH and the lack of increase in estrogen levels in the AN-IGF-1+ group may explain why NTX levels did not decrease in this group (34). Further, anabolic effects of PTH on bone require normal systemic growth hormone (GH) secretion (35). It is possible that exogenous IGF-1 administration led to a decrease in endogenous GH levels by negative feedback and subsequently decreased local IGF-1 secretion in bone. This likely negated the bone anabolic effects of PTH.
Thus, both weight gain and the increase in estradiol level, observed preferentially in the AN-IGF-1− group, and the increase in PTH, observed preferentially in the AN-IGF-1+ group, may have contributed to greater increases in lumbar spine BMD measures in the AN-IGF-1− group but not the AN-IGF-1+ group. In fact, differences between the 2 groups were abolished after controlling for both increases in weight and in PTH levels.
Another possibility is that completers in the AN-IGF-1− group were enriched to demonstrate greater improvements in BMD than the AN-IGF-1+ group because they started with lower baseline BMD than the noncompleters. However, differences between groups persisted after also controlling for baseline BMD.
Our study results differ from those reported in a study in adult women with AN, where IGF-1 supplementation combined with oral estrogen-progestin led to an increase in BMD compared to a double placebo group (14). It is likely that the dose of IGF-1 supplementation, although similar to that used in the adult study, was low for this younger population, which typically has higher IGF-1 levels than adults. We did titrate the dose of rhIGF-1 to achieve IGF-1 levels in the upper half of the normal range. However, per study design, only 2 dose increments were performed for any participant, and further increases may have been necessary to attain optimal IGF-1 levels. However, overall, IGF-1 levels were significantly higher in the AN-IGF-1+ group than the AN-IGF-1− group, which makes this explanation less likely.
Importantly, contrary to our expectation of balancing the severity of illness in a randomized study, our subjects in the AN-IGF-1+ group had more severe disease suggested by numerically greater number of AN-related hospitalizations in this group during the study and greater study attrition. Disease severity is reflected in body weight status, and this group did not have the weight gain over 6 and 12 months observed in the rhIGF-1− group and was thus more likely to have had a greater extent of other hormonal alterations (such as higher cortisol and peptide YY and lower leptin) over the study duration with deleterious effects on bone. In addition, this group was more likely to have reduced activity levels over the study course and to have been prescribed medications such as antidepressants that deleteriously affect bone (36).
Further, if rhIGF-1 administration led to increases in bone remodeling overall in this young sample, with an increase in both bone formation and resorption, this may have negated the antiresorptive effects of 17-β estradiol observed in the AN-IGF-1− group, particularly if the AN-IGF-1+ group was not adherent to the treatment regimen with transdermal estradiol (as suggested by the lack of increase in estradiol levels in this group and the documentation of missed patches by self-report at each study visit (Supplementary Table 4 (20)). Interestingly, we did not observe the expected increase in P1NP in the AN-IGF-1+ group, which may reflect overall sicker participants. It may also reflect lack of adherence to the rhIGF-1 injections in this group notwithstanding the increase in IGF-1 levels, in that adherence to the regimen between visits may have been lower than in the week or so before the visit (when the impending visit may have resulted in improved adherence). Finally, if increasing the IGF-1 levels to the upper half of the normal range suppressed endogenous GH levels, a lack of direct GH-mediated effects on bone may have contributed to the lack of efficacy of rhIGF-1 in this study.
An additional consideration is that the study in adults of rhIGF-1 and oral estrogen-progestin demonstrated an effect of combination therapy on bone outcomes compared to the double placebo group but not the group that received oral estrogen-progestin alone, and the latter is more consistent with our study results. Finally, it is possible that addition of rhIGF-1 is more relevant when the mode of estrogen administration is oral, which is associated with a reduction in systemic IGF-1 levels from hepatic first pass effects. However, addition of rhIGF-1 may be less relevant when the mode of estrogen administration is transdermal 17β-estradiol, which does not undergo hepatic first pass and is not associated with a reduction in IGF-1 levels (12,29).
We also assessed changes in bone vBMD, geometry, and microarchitecture across the randomization groups and did not find any significant differences between the AN-IGF-1+ and AN-IGF-1− groups both at the weight bearing tibia and nonweight bearing radius for these endpoints. Girls with AN and amenorrhea have adverse outcomes for cortical microarchitecture (37,38), and a study in normal-weight oligoamenorrheic athletes demonstrated greater percentage increases in cortical area and thickness and total or cortical vBMD after transdermal 17-β estradiol replacement therapy vs the pill or no estrogen (39). In our current study, consistent with the study in athletes, the AN-IGF-1− group had increases in cortical area and thickness, total and cortical vBMD at the radius, and cortical vBMD at the tibia, likely reflecting the effect of 17-β estradiol. Effects were less robust in the AN-IGF+ 1- group, which also showed an increase in cortical vBMD at both sites, possibly reflecting the lesser increase in estradiol levels in this group. This is further substantiated by the association of changes in cortical vBMD at the radius and tibia with decreases in NTX, a marker of bone resorption, suggesting a primary estrogen effect.
The limitations of the study must be acknowledged. This is a small study with significant attrition, particularly in the AN-IGF-1+ group. Of note, this group was sicker with many more hospitalizations over the duration of the study than the placebo group. Participants were not always allowed or able to continue study medications during hospitalization and inpatient eating disorder treatments, increasing nonadherence during these time periods. Additionally, the use of injectable medication twice a day in adolescents is challenging, and the demands of the study medication regimen likely contributed to the significant attrition observed in this study. More discrete characterization of the clinical course of the participants’ disease may help better interpret our study results, given the potential for a significant confounding effect of such variables. We used surrogate markers such as hospitalizations, weight loss or gain, and adverse events such as suicidal ideation or attempts to characterize disease course in our study sample. Additionally, results may be confounded by a lack of adherence to study medications, particularly to estrogen therapy as indicated by the wide range of change in estradiol levels over study course and self-report of missing patches by the participants (Supplementary Table 4 (20)). Moreover, the bone turnover markers were not always assessed in the morning, and hence, these results must be interpreted with caution.
Overall, this is the first year-long study evaluating the benefit of transdermal 17-β estradiol and rhIGF-1 on bone endpoints in adolescents and young adult women, and our study demonstrates effects attributable to transdermal 17-β estradiol alone, without an additive effect of rhIGF-1, likely because of the confounding effect of a sicker active group that did not gain weight and demonstrated significant attrition over 12 months.
Acknowledgments
Financial Support: This research was supported by grants R01DK062249 (MM, AK), Global Foundation of Eating Disorder (VS), and K24HD07184 (MM). Recombinant human insulin like growth factor-1 was provided by Ipsen pharmaceuticals.
Additional Information
Disclosures: MM is a consultant for Sanofi and has served on the scientific advisory board for Abbvie and Ipsen. Other authors do not report any relevant conflicts.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2. Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2011;68(7):714-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson JG, Cohen P, Kasen S, Brook JS. Eating disorders during adolescence and the risk for physical and mental disorders during early adulthood. Arch Gen Psychiatry. 2002;59(6):545-552. [DOI] [PubMed] [Google Scholar]
- 4. Teegarden D, Proulx WR, Martin BR, et al. . Peak bone mass in young women. J Bone Miner Res. 1995;10(5):711-715. [DOI] [PubMed] [Google Scholar]
- 5. Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68(3):548-554. [DOI] [PubMed] [Google Scholar]
- 6. Soyka LA, Misra M, Frenchman A, et al. . Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87(9):4177-4185. [DOI] [PubMed] [Google Scholar]
- 7. Vale B, Brito S, Paulos L, Moleiro P. Menstruation disorders in adolescents with eating disorders-target body mass index percentiles for their resolution. Einstein (Sao Paulo). 2014;12(2):175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84(12):4489-4496. [DOI] [PubMed] [Google Scholar]
- 9. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Misra M, Miller KK, Bjornson J, et al. . Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88(12):5615-5623. [DOI] [PubMed] [Google Scholar]
- 11. Mumford J, Kohn M, Briody J, et al. . Long-term outcomes of adolescent anorexia nervosa on bone. J Adolesc Health. 2019;64(3):305-310. [DOI] [PubMed] [Google Scholar]
- 12. Misra M, Katzman D, Miller KK, et al. . Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26(10):2430-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Misra M, McGrane J, Miller KK, et al. . Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45(3):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87(6):2883-2891. [DOI] [PubMed] [Google Scholar]
- 15. Ogden CL, Kuczmarski RJ, Flegal KM, et al. . Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45-60. [DOI] [PubMed] [Google Scholar]
- 16. Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15(3):135-143. [DOI] [PubMed] [Google Scholar]
- 17. Kriska AM, Knowler WC, LaPorte RE, et al. . Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401-411. [DOI] [PubMed] [Google Scholar]
- 18. Kalkwarf HJ, Zemel BS, Gilsanz V, et al. . The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087-2099. [DOI] [PubMed] [Google Scholar]
- 19. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423-441. [DOI] [PubMed] [Google Scholar]
- 20. Singhal V. Effect of Transdermal Estradiol and Recombinant Insulin-Like Growth Factor-1 on Bone Endpoints of Young Women with Anorexia Nervosa [ draft version]. Harvard Dataverse; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DiVasta AD, Feldman HA, Rubin CT, et al. . The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporos Int. 2017;28(4):1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DiVasta AD, Feldman HA, O’Donnell JM, Long J, Leonard MB, Gordon CM. Impact of adrenal hormone supplementation on bone geometry in growing teens with Anorexia Nervosa. J Adolesc Health. 2019;65(4):462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon CM, Grace E, Emans SJ, et al. . Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87(11):4935-4941. [DOI] [PubMed] [Google Scholar]
- 24. DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014;29(1):151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golden NH, Iglesias EA, Jacobson MS, et al. . Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(6):3179-3185. [DOI] [PubMed] [Google Scholar]
- 26. Fazeli PK, Wang IS, Miller KK, et al. . Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014;99(4):1322-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Misra M, Prabhakaran R, Miller KK, et al. . Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93(4):1231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ackerman KE, Singhal V, Baskaran C, et al. . Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53(4):229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singhal V, Ackerman KE, Bose A, Flores LPT, Lee H, Misra M. Impact of route of estrogen administration on bone turnover markers in oligoamenorrheic athletes and its mediators. J Clin Endocrinol Metab. 2019;104(5):1449-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Misra M, Prabhakaran R, Miller KK, et al. . Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab. 2008;93(4):1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Misra M, Miller KK, Almazan C, et al. . Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89(10):4972-4980. [DOI] [PubMed] [Google Scholar]
- 32. Misra M, Miller KK, Cord J, et al. . Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92(6):2046-2052. [DOI] [PubMed] [Google Scholar]
- 33. Kamenický P, Blanchard A, Gauci C, et al. . Pathophysiology of renal calcium handling in acromegaly: what lies behind hypercalciuria? J Clin Endocrinol Metab. 2012;97(6):2124-2133. [DOI] [PubMed] [Google Scholar]
- 34. Liu BY, Wu PW, Bringhurst FR, Wang JT. Estrogen inhibition of PTH-stimulated osteoclast formation and attachment in vitro: involvement of both PKA and PKC. Endocrinology. 2002;143(2):627-635. [DOI] [PubMed] [Google Scholar]
- 35. Hock JM, Fonseca J. Anabolic effect of human synthetic parathyroid hormone-(1-34) depends on growth hormone. Endocrinology. 1990;127(4):1804-1810. [DOI] [PubMed] [Google Scholar]
- 36. DiVasta AD, Feldman HA, O’Donnell JM, Long J, Leonard MB, Gordon CM. Effect of exercise and antidepressants on skeletal outcomes in adolescent girls with anorexia nervosa. J Adolesc Health. 2017;60(2):229-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faje AT, Karim L, Taylor A, et al. . Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013;98(5):1923-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singhal V, Tulsiani S, Campoverde KJ, et al. . Impaired bone strength estimates at the distal tibia and its determinants in adolescents with anorexia nervosa. Bone. 2018;106:61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ackerman KE, Singhal V, Slattery M, et al. . Effects of estrogen replacement on bone geometry and microarchitecture in adolescent and young adult oligoamenorrheic athletes: a randomized trial. J Bone Miner Res. 2020;35(2):248-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.