Abstract
Background and Aims
Emerging evidence points to a link between creeping fat and the pathogenesis of Crohn’s disease [CD]. Non-invasive assessment of the severity of creeping fat on cross-sectional imaging modality has seldom been investigated. This study aimed to develop and characterize a novel mesenteric creeping fat index [MCFI] based on computed tomography [CT] in CD patients.
Methods
MCFI was developed based on vascular findings on CT in a retrospective cohort [n = 91] and validated in a prospective cohort [n = 30]. The severity of creeping fat was graded based on the extent to which mesenteric fat extended around the intestinal circumference using the vessels in the fat as a marker. The accuracy of MCFI was assessed by comparing it with the degree of creeping fat observed in surgical specimens. The relationship between MCFI and fibrostenosis was characterized by determining if these correlated. The accuracy of MCFI was compared with other radiographic indices [i.e. visceral to subcutaneous fat area ratio and fibrofatty proliferation score].
Results
In the retrospective cohort, MCFI had moderate accuracy in differentiating moderate–severe from mild fibrostenosis (area under the receiver operating characteristic [ROC] curve [AUC] = 0.799; p = 0.000). ROC analysis in the retrospective cohort identified a threshold MCFI of > 3 which accurately differentiated fibrostenosis severity in the prospective cohort [AUC = 0.756; p = 0.018]. An excellent correlation was shown between MCFI and the extent of fat wrapping in specimens in the prospective cohort [r = 0.840, p = 0.000]. Neither visceral to subcutaneous fat area ratio nor fibrofatty proliferation score correlated well with the degree of intestinal fibrosis.
Conclusions
MCFI can accurately characterize the extent of mesenteric fat wrapping in surgical specimens. It may become another non-invasive measure of CD fibrostenosis.
Keywords: Creeping fat, intestinal fibrosis, Crohn’s disease
1. Introduction
Mesenteric fat wrapping around the inflamed gut, known as creeping fat [CF], is a hallmark of Crohn’s disease [CD]. Although it has long been recognized that accumulation of mesenteric adipose tissue [MAT] is associated with CD, the role of fat wrapping has remained elusive. Increasing evidence suggests that CF is associated with transmural fibrosis and stricture formation in CD.1–6 Macroscopically, bowel strictures and CF are topographically coupled in CD.2 Microscopically, mesenteric mesenchymal abnormalities closely resemble those observed in the submucosa and both sets of abnormalities extend into the intestinal longitudinal muscle,3 thereby generating the characteristic transmural appearance of CD. In keeping with this, it appears that progressive stricture formation is also associated with fat wrapping and mesenteric abnormalities. In CD, the thickness of muscularis mucosae adjacent to CF is significantly greater than that adjacent to normal mesentery.4 Based on this, it is feasible that CF may contribute at a pathobiological level to the fibrostenotic phenotype in CD.5,6
Fibrotic stricture formation is a significant clinical problem in CD. The current lack of targeted anti-fibrotic therapies reflects a deficit in our understanding of intestinal fibrogenesis. Given the association between CF and intestinal manifestations of CD, it is feasible that CF could represent another therapeutic target.4 Further characterization of the relationship between CF and intestinal fibrogenesis in CD could provide therapeutic opportunities in the pharmacological management of intestinal strictures.
Unfortunately, CF can only be directly observed during surgery or pathologically. Cross-sectional imaging on computed tomography [CT] or magnetic resonance imaging has the potential of non-invasively determining the extent and severity of CF. Previous studies7 defined CF qualitatively as an increased volume of fat adjacent to an affected bowel segment. This approach is subjective with both potential intra-/inter-observer variability. More recently, the visceral to subcutaneous fat area ratio on CT assessment was used as an index of mesenteric disease activity.8 However, this approach is also prone to error due to the inclusion of retroperitoneal fat under the heading of visceral adiposity. Moreover, this index does not directly and selectively assess fat wrapping.
Expansion of adipose tissue deposits is associated with angiogenesis9 and often macroscopically coupled with new blood vessel formation. Neo- or hypervascularity can be indirectly assessed by CT enterography [CTE]. Patients with CF often have prominent peri-enteric vasculature on radiological assessment [i.e. comb sign]. Coffey et al. recently developed a mesenteric disease activity index that was partly based on the thickness of the mesentery and the ability to identify vascular pedicles.2 Given that mesenteric vessels are coupled with CF, and that these vessels are visible on CTE, the purpose of this investigation was to develop a mesenteric creeping fat index [MCFI] in CD, based upon the extent of vessel presence around the bowel lumen. Once developed, we assessed the role of MCFI for the characterization of CD fibrostenosis compared with other reported radiographic indices such as visceral to subcutaneous fat area ratio and fibrofatty proliferation score.
2. Methods
2.1. Patients and study design
This cross-sectional study included retrospective and prospective cohorts. In the retrospective study, the feasibility and reliability of MCFI was determined and a threshold for distinguishing degrees of intestinal fibrosis was established. In the prospective study, we tested the hypothesis that MCFI correlated with macroscopic fat wrapping and validated the relationship between MCFI and intestinal fibrosis. The study was approved by the institutional ethics review board. Written informed consent was required for all patients in the prospective cohort but waived in the retrospective cohort.
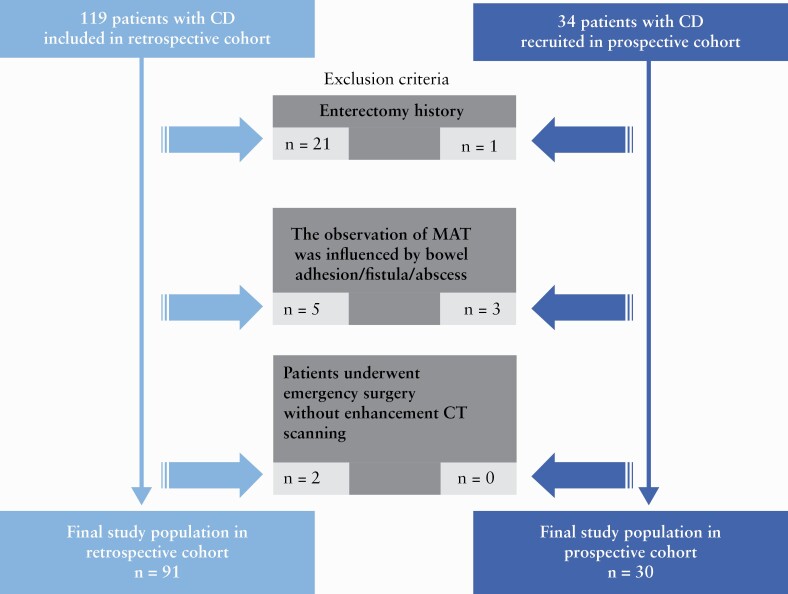
The retrospective cohort included 119 consecutive CD patients who underwent surgery during the interval from January 2014 to November 2018. The inclusion criteria were as follows: [a] patients with an established diagnosis of CD based on standard clinical, endoscopic, imaging and histological criteria; [b] available preoperative abdominal CT within 3 months of surgery for symptomatic bowel strictures; and [c] availability of a histopathological surgical [i.e. post-resection] specimen of a segment of the intestine corresponding to a matching abnormality on abdominal CT. Exclusion criteria were as follows: [a] patients with a history of prior enterectomy; [b] inability to differentiate mesenteric disease manifestations on abdominal CT due to coexistence of pathologies such as intestinal adhesion/fistula/abscess; or [c] patients who underwent emergency surgery without enhanced CT scanning [Figure 1]. A prospective cohort was also included involving 34 consecutive CD patients, who underwent surgery between December 2018 and July 2019 and satisfied the inclusion criteria.
Figure 1.
Flow diagram of the study population. CD, Crohn’s disease; MAT, mesenteric adipose tissue
2.2. Bowel lesions selected for radiological and histological assessment
The study adopted the following criteria when defining a bowel stricture: [a] localized luminal narrowing [a luminal diameter reduction of at least 50%, measured relative to the normal adjacent bowel loop], and [b] bowel wall thickening [a 25% increase in wall thickness relative to the adjacent non-affected bowel].10 In each patient, the bowel segment with the most luminal narrowing and/or the most wall thickening on CTE images was selected for inclusion in the study, using the matched radiological and surgical evaluation described in previous studies [Supplementary Material].11,12 The MCFI and the macroscopic CF were evaluated in the mesenteric region corresponding to the bowel stricture but away from the penetrating diseases.
2.3. CT image analysis
All patients underwent preoperative abdominal and pelvic CTE examination [Supplementary Materials]. Target segments on CTE were pre-marked by a radiologist with 20 years of experience in bowel CT.
2.3.1. Semi-quantification of CF
Similar to the study by Coffey et al.,2 we developed a radiological index, namely MCFI, to grade the fat-wrapping around the gut based on the extent of bowel circumference encompassed by the vessels in fat. Two radiologists [X.L. and B.L., with 8–10 years of experience in abdominal imaging] unaware of pathological data measured MCFI on the designated segments, and the results from one of them were randomly selected for analyses. The results from the other radiologist are shown in the Supplementary Materials. Volume data acquired in the arterial or venous phase of enhancement CTE scanning were post-processed on the Vitrea workstation [Canon Medical System]. To determine the extent to which mesenteric vessels overlapped the bowel circumference, a view of the affected intestine was obtained by orientating the slice/section perpendicular to the longitudinal axis of the intestine. This was achieved using multi-planar reconstruction and maximum-intensity projection. Once completed, volume rendering reconstruction was applied to enable vessel visualization as these overlapped the intestine [Supplementary Fig. 1]. The circumference of the intestine was subdivided into eighty equal zones and a score of 1 was applied for each zone overlapped by mesenteric vessels [Figure 2].
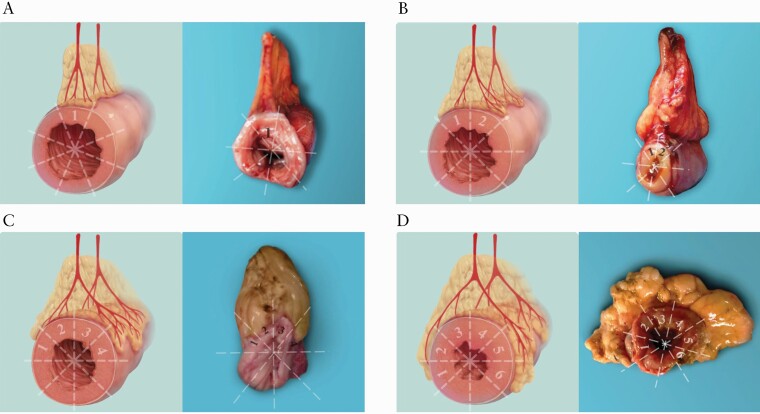
Figure 2.
Schematic diagrams and the corresponding resected specimens demonstrating fat wrapping around the gut graded by MCFI which was modified from a prior study.2 The bowel circumference on its axial plane is divided into eight equal areas with one score for each area. The MCFI, which reflects the degree of mesenteric fat wrapping around the gut, is scored from 1 to 8 according to the areas of bowel surface covered by the corresponding mesenteric vessels. In the case with a MCFI of 1 [A], the MAT is normal or shows minimal fat wrapping. With an MCFI of 2 [B], the fat wrapping commences at the bowel margin of the mesentery but is limited. With an MCFI of 4 [C], the fat wrapping increases and covers nearly 50% of the intestinal circumference. With an MCFI of 6 [D], the MAT thickening is more pronounced, and the fat wrapping extends to 6/8 of the bowel circumference. MCFI, mesenteric creeping fat index; MAT, mesenteric adipose tissue
2.3.2. Visceral to subcutaneous fat area ratio
Using a fat assessment software on the Vitrea workstation, visceral and subcutaneous fat areas of CD patients were automatically measured on the basis of the pixel count on CT images8 at the L3 and L4 vertebral levels, respectively [Supplementary Fig. 2]. The visceral to subcutaneous fat area ratio was automatically calculated by the software, which was operated by a radiologist [L.H., with 6 years of experience in abdominal imaging] unaware of the pathological data.
2.3.3. Fibrofatty proliferation score
In previous studies,7,13 the volume of mesenteric fat adjacent to a bowel segment and displacement of the intestine have been combined as the ‘fibrofatty proliferation score’ to semi-quantify mesenteric changes. Mesenteric thickening was semi-quantified radiographically using the ‘fibrofatty proliferation score’ by a radiologist [Z.F., with 5 years of experience in abdominal imaging and unaware of pathological data] according to this system: 0 [absence of changes], 1 [mild changes] and 2 [moderate or severe changes] [Supplementary Fig. 3].
2.3.4. Intestinal stricture index
Stricture formation was assessed by a radiologist [S.F., with 20 years of experience in abdominal imaging] using a radiological stricture index: the ratio of the maximal upstream luminal diameter is divided by the minimum luminal diameter apparent within the stricturing region.14
2.4. Gross anatomical and histopathological reference
2.4.1. Macroscopical evaluation: CF grading in surgical specimens
In the prospective cohort, macroscopic CF was assessed by a radiologist [C.S., with 20 years of experience in abdominal CT] and a surgeon [Z.C., with 10 years of experience in intestinal resection for CD] by consensus. Similar to the evaluation of MCFI, the degree of CF wrapping around the bowel circumference was described using ‘macroscopic fat wrapping score’ modified from a previous study2 and was scored from 1 to 8 according to the extent of bowel surface overlapped by MAT [Figure 2]. This score in the retrospective cohort was not performed due to the unavailable images of surgical specimens.
2.4.2. Microscopic evaluation: Inflammation, fibrosis, and smooth muscle hypertrophy/hyperplasia
A full-thickness, well-orientated and transverse sample of the surgical specimen was obtained from the mesenteric border of the gut which was in the mid-point of the stenotic segment based on the degree of narrowing by inspection performed by the radiologist [C.S.] and the surgeon [Z.C.], and embedded in paraffin following fixation in formalin. Two consecutive histopathological sections [4 µm thick] were obtained for analysis. One section was stained using haematoxylin and eosin [H&E] to establish the histological inflammation score and the smooth muscle measurement, and the other section was stained with Masson’s trichrome to generate the histological fibrosis score.
2.4.2.1. Inflammation and fibrosis
A pathologist [Q. C.] with 10 years of experience in bowel pathology without CTE information graded the bowel inflammation on H&E section and fibrosis on Masson’s trichrome section from 0 to 4 using a semi-quantitative scoring system [Supplementary Table 1].15 Histological scores were categorized as mild [score 0–2] or moderate–severe [score 3–4] groups. The fibrosis-predominant strictures [moderate–severe fibrosis but mild inflammation within the bowel strictures] and inflammation-predominant strictures [moderate–severe inflammation but mild fibrosis] were categorized according to the histological results.16
2.4.2.2. Smooth muscle hypertrophy/hyperplasia
The maximum thickness of both muscularis mucosae and muscularis propria were determined [in mm] using CaseViewer software [version 2.3, 3DHISTECH] at the level of the intestine at which stricturing was most severe on macroscopic evaluation as mentioned before. This was completed by the pathologist [Q.C.] using an established technique.17
2.5. Statistical analysis
Statistical analysis was performed via two-sided comparisons, with significance defined as p < 0.05 using SPSS version 20.0 software [IBM]. Normally distributed data were expressed as mean ± standard deviation, and non-normally distributed data were presented as median (interquartile range [IQR]). Differences in macroscopic fat wrapping score and MCFI between moderate–severely and mildly fibrotic strictures and between different histological types of bowel strictures were tested using a Mann–Whitney U test. A bivariate correlation analysis was performed using Spearman’s rank correlation. A correlation [r] < 0.01 was considered as none, 0.01–0.24 as minimal, 0.25–0.49 as fair, 0.50–0.74 as moderate to good, and 0.75–1.00 as very good to excellent. Area under the receiver operating characteristics [ROC] curve [AUC] values were calculated to determine the accuracy of MCFI for differentiating different severity of bowel fibrosis. An AUC of 0.50–0.69 was considered as mild, 0.70–0.89 as moderate and 0.90–1.00 as good. The optimal threshold was determined using ROC curve analysis following Youden’s index. The inter-observer agreement for MCFI was tested using the kappa statistic. The heat map of the correlation matrix among radiological parameters and histological findings was generated using open-source Python version 3.7.7 software.
3. Results
3.1. Clinical data
Ninety-one patients in the retrospective cohort and 30 patients in the prospective cohort were included in the final analysis. Only one readily identifiable stricture on macroscopic inspection was included for each patient. A fair correlation between C-reactive protein and MCFI was found in the retrospective cohort [r = 0.274, p = 0.009] whereas no significant correlation was shown in the prospective cohort [r = 0.258, p = 0.168]. No significant correlation between disease duration and MCFI was shown in both the retrospective [r = 0.024, p = 0.818] and the prospective [r = 0.148, p = 0.436] cohorts. Demographic and clinical data are summarized in Table 1. Radiological and histopathological characteristics of intestinal strictures are presented in Table 2.
Table 1.
Demographic and clinical characteristics in patients with Crohn’s disease
| Retrospective cohort [n = 91] | Prospective cohort [n = 30] | |
|---|---|---|
| Gender, n [female/male] | 24/67 | 10/20 |
| Age, years [mean ± SD] | 32.11 ± 10.28 | 31.70 ± 11.93 |
| Disease duration, months (median [IQR]) | 36 [12–70] | 48 [12–78] |
| Interval between CT and surgery, days (median [IQR]) | 14 [10–29] | 23 [12–48] |
| Smoking, n [%] | 22 [24.18%] | 2 [6.67%] |
| BMI (median [IQR]) | 16.70 [15.20–17.99] | 17.85 [16–19.13] |
| Biologics use preoperatively, n [%] | 14 [15.38%] | 6 [20%] |
| Perianal surgery history, n [%] | 16 [17.58%] | 1 [3.33%] |
| Type of surgery, n [%] | ||
| Ileocolic resection | 49 [53.85%] | 20 [66.67%] |
| Partial small bowel resection | 20 [21.98%] | 3 [10%] |
| Partial colon resection | 7 [7.69%] | 3 [10%] |
| Ileocolic + partial small bowel resection | 8 [8.79%] | 4 [13.33%] |
| Ileocolic + partial colon resection | 6 [6.59%] | 0 |
| Partial small bowel + partial colon resection | 1 [1.10%] | 0 |
| Location of specimen, n [%] | ||
| Jejunum | 4 [4.40%] | 0 |
| Proximal ileum | 10 [10.99%] | 4 [13.33%] |
| Terminal ileum | 38 [41.76%] | 15 [50%] |
| Ileocolon | 12 [13.19%] | 2 [6.67%] |
| Colon | 27 [29.67%] | 9 [30%] |
| CDAI, n [%] | ||
| < 150 | 10 [10.99%] | 9 [30%] |
| 150–220 | 17 [18.68%] | 4 [13.33%] |
| 220–450 | 57 [62.64%] | 16 [53.33%] |
| > 450 | 7 [7.69%] | 1 [3.33%] |
| CRP, mg/L (median [IQR]) | 26.44 [10.34–69.50] | 26.16 [11.06–46.70] |
| ESR, mm/h (median [IQR]) | 32 [18–54] | 44.50 [18–51] |
BMI, body mass index; CDAI, Crohn’s disease activity index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range; SD, standard deviation.
Table 2.
Radiological and histopathologic characteristics of CD stricture
| Retrospective cohort [n = 91] | Prospective cohort [n = 30] | |
|---|---|---|
| Bowel strictures on CT, mm [median [IQR]] | ||
| Luminal diameters of stenosed segments | 4.40 [3.58–5.94] | 5.81 [4.55–14.00] |
| Luminal diameters of upstream segments | 34.50 [25.70–43.10] | 26.60 [15.68–40.35] |
| Intestinal stricture index | 7.07 [4.88–12.15] | 4.18 [1.34–8.67] |
| Fibrosis score, [median [IQR]] | 3 [2–3] | 2 [1–3] |
| 0 | 0 | 0 |
| 1 | 1 [1.10%] | 8 [26.67%] |
| 2 | 23 [25.27%] | 9 [30%] |
| 3 | 49 [53.85%] | 9 [30%] |
| 4 | 18 [19.78%] | 4 [13.33%] |
| Inflammation score (median [IQR]) | 3 [3–4] | 2 [1–4] |
| 0 | 0 | 0 |
| 1 | 0 | 10 [33.34%] |
| 2 | 16 [17.58%] | 9 [30%] |
| 3 | 40 [43.96%] | 3 [10%] |
| 4 | 35 [38.46%] | 8 [26.67%] |
| Maximum thickness of bowel smooth muscle, mm [median [IQR]] | ||
| Muscularis mucosae | 0.93 [0.54–1.44] | 0.80 [0.09–1.45] |
| Muscularis propria | 4.86 [3.31–6.18] | 3.79 [1.99–4.56] |
| Circular muscularis propria | 3.31 [2.01–4.42] | 2.39 [1.30–3.33] |
| Longitudinal muscularis propria | 1.29 [0.88–2.00] | 0.92 [0.68–1.64] |
CD, Crohn’s disease; IQR, interquartile range.
3.2. Relationship between CF-related parameters and CD fibrostenosis
3.2.1. Retrospective cohort
3.2.1.1 MCFI is associated with CD fibrostenosis
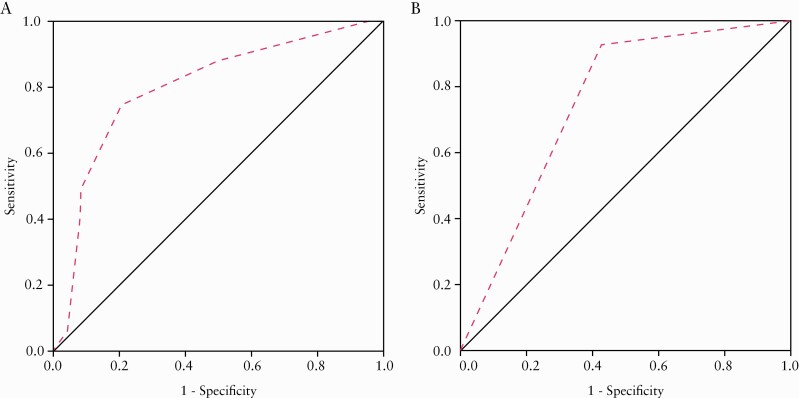
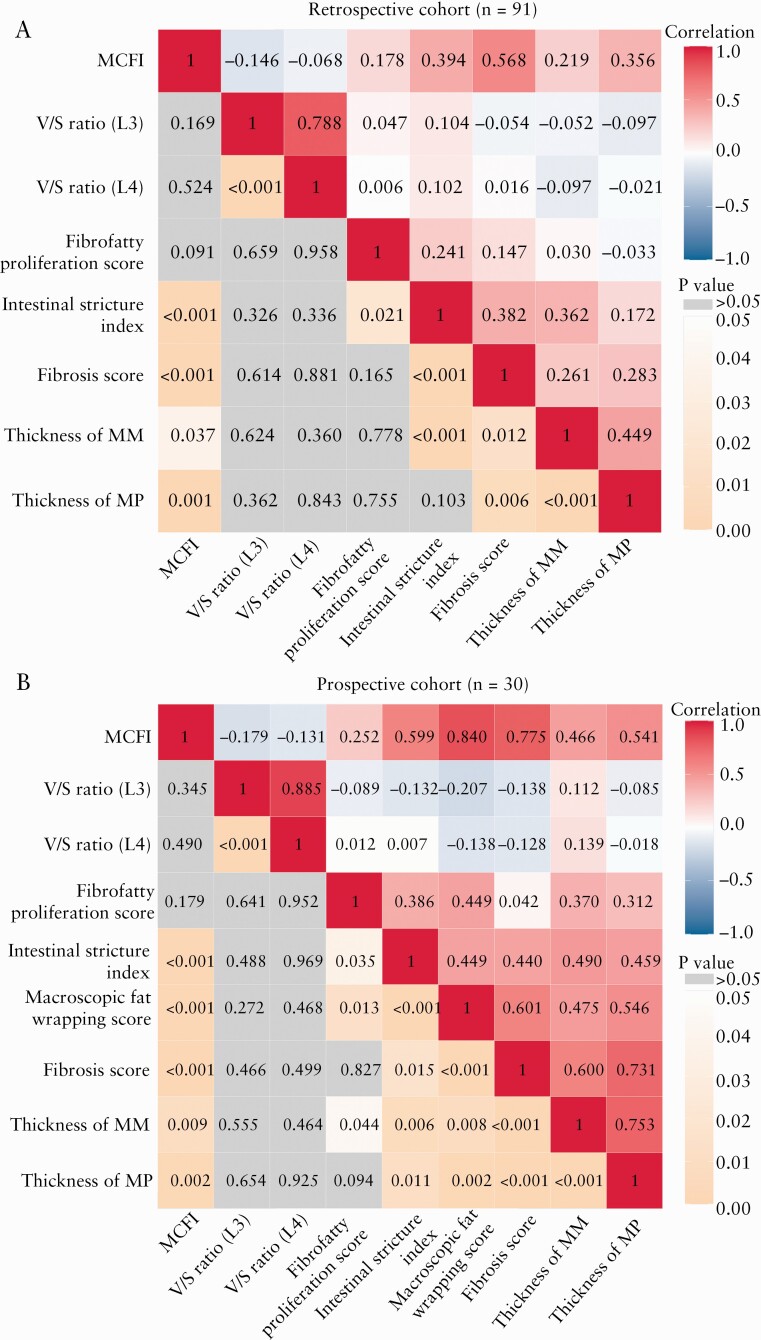
In the retrospective cohort, a good correlation [r = 0.568, p = 0.000] was observed between MCFI and histological fibrosis scores. The MCFI of moderate–severely fibrotic strictures (median [IQR], 4 [3–6]) was higher than that of mildly fibrotic strictures (median [IQR], 2.5 [2–3]) [Z = −4.426, p = 0.000]. The MCFI had a moderate accuracy (AUC = 0.799; 95% confidence interval [CI], 0.690–0.907; p = 0.000; Figure 3A) in differentiating moderate–severely from mildly fibrotic strictures. Using an MCFI of > 3 as a threshold value, sensitivity and specificity were 88.10% and 50%, respectively. A minimal to fair correlation was observed between MCFI and both the thickness of muscularis mucosae [r = 0.219, p = 0.037] and muscularis propria [r = 0.356, p = 0.001]. A fair correlation was also observed between MCFI and intestinal stricture index [r = 0.394, p = 0.000] while no significant correlation was found between MCFI and luminal diameter of the upstream segment [r = 0.040, p = 0.705]. Inter-observer agreement levels of MCFI between both radiologists was acceptable in the retrospective cohort [κ= 0.822, p = 0.000].
Figure 3.
[A] On ROC analysis, MCFI had a moderate accuracy [AUC = 0.799; 95% CI, 0.690–0.907; p = 0.000] for distinguishing moderate–severely fibrotic from mildly fibrotic strictures in the retrospective cohort. [B] Using an MCFI > 3 derived from the retrospective cohort as the cutoff level for distinguishing moderate–severely fibrotic strictures from mildly fibrotic strictures in the prospective cohort, the AUC of MCFI was 0.756 [95% CI, 0.579–0.932; p = 0.018]. ROC, receiver operating characteristics; MCFI, mesenteric creeping fat index; AUC, area under the ROC curve; CI, confidence interval
3.2.1.2 No or weak correlations between fibrofatty proliferation score, visceral to subcutaneous fat area ratio and CD fibrostenosis
In the retrospective cohort, no significant correlation was apparent between fibrofatty proliferation score and histological findings [all p > 0.05]. A minimal correlation was shown between fibrofatty proliferation score and intestinal stricture index [r = 0.241, p = 0.021]. However, no correlation was apparent between visceral to subcutaneous fat area ratio with histological findings and with intestinal stricture index [all p > 0.05].
3.2.2. Prospective cohort
3.2.2.1. MCFI is validated to associate with CD fibrostenosis
In the prospective validation cohort, a very good correlation was observed between MCFI and histological fibrosis scores [r = 0.775, p = 0.000]. The MCFI accurately differentiated moderate–severely (median [IQR], 4 [4–5]; Figure 4A, B) from mildly (median [IQR], 3 [2–4]; Figure 4C, D) fibrotic strictures [Z = −3.069, p = 0.002]. Using the MCFI threshold of > 3, AUC, sensitivity and specificity were 0.756 [95% CI, 0.579–0.932; p = 0.018; Figure 3B], 92.31% [12/13] and 58.82% [10/17], respectively. Fair to moderate correlations were apparent between MCFI and thickness of muscularis mucosae [r = 0.466, p = 0.009] and between MCFI and thickness of the muscularis propria [r = 0.541, p = 0.002]. A fair to moderate correlation was apparent between MCFI and intestinal stricture index [r = 0.599, p = 0.000] and between MCFI and luminal diameter of the upstream segment [r = 0.369, p = 0.045]. Inter-observer agreement levels of MCFI between both radiologists were acceptable in the prospective cohort [κ = 0.805, p = 0.000].
Figure 4.
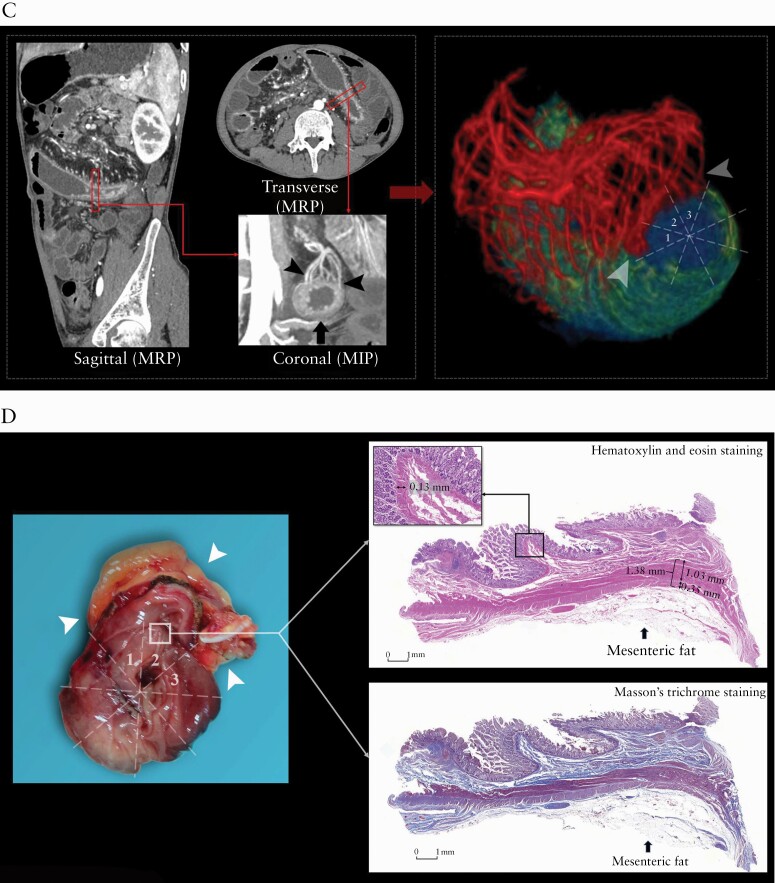
Images from a 20-year-old male patient with CD [A, B]. [A] Coronal, sagittal and transverse post-contrast enhanced CT images showing marked bowel wall thickening and luminal narrowing in the descending colon [arrow]; the MCFI of the designated segment [arrow] reconstructed from the adjacent mesenteric vessels [arrowheads] using MPR and MIP is scored as 6. On VR reconstruction, the three-dimensional anatomical relationship between the designated gut [green area] and the mesenteric vessels [red areas] is shown and the MCFI with a score of 6 is verified. [B] Macroscopic specimen showing the creeping fat [arrowhead] wrapping around the resected bowel segment with a macroscopic fat wrapping score of 6. H&E and Masson’s trichrome staining depict severe inflammation [score = 4] and severe transmural fibrosis [blue area; score = 4] in the area corresponding to creeping fat [arrow]; the maximum thickness of muscularis mucosae and muscularis propria are 1.58 and 4.74 mm, respectively. ×20 magnification. Images from a 30-year-old male patient with CD [C, D]. [C] Coronal, sagittal and transverse post-contrast enhanced CT images showing bowel wall thickening and luminal narrowing in the jejunum [arrow]; the MCFI of the designated segment [arrow] reconstructed from the adjacent mesenteric vessels [arrowheads] using MPR and MIP is scored as 3. On VR reconstruction, the three-dimensional anatomical relationship between the designated gut [green area] and the mesenteric vessels [red areas; arrowheads] is shown and the MCFI with a score of 3 is verified. [D] Macroscopic specimen showing the creeping fat [arrowhead] wrapping around the resected bowel segment with a macroscopic fat wrapping score of 3. H&E and Masson’s trichrome staining depict mild inflammation [score = 1] and mild fibrosis [blue area; score = 2]; the maximum thickness of muscularis mucosae and muscularis propria are 0.13 and 1.38 mm, respectively. ×20 magnification. CD, Crohn’s disease; MCFI, mesenteric fat creeping index; MPR, multiplanar reconstruction; MIP, maximum intensity projection; VR, volume rendering; H&E, hematoxylin and eosin
Correlations between MCFI and histological findings/intestinal stricture index in small bowels and colons [Supplementary Table 2] and in bowel segments with and without penetrating diseases [Supplementary Table 3] are included in the Supplementary Materials. Of note, similar strengths of correlations between MCFI and thickness of muscularis mucosae/muscularis propria were found in small bowels [r = 0.239–0.508] compared with colons [r = 0.207–0.554] in both cohorts.
3.2.2.2. No or weak correlations between fibrofatty proliferation score, visceral to subcutaneous fat area ratio and CD fibrostenosis
In the prospective cohort, a fair correlation was observed between fibrofatty proliferation score and thickness of muscularis mucosae [r = 0.370, p = 0.044]. No correlation was apparent between fibrofatty proliferation score and other histological findings [all p > 0.05]. A fair correlation was shown between fibrofatty proliferation score and intestinal stricture index [r = 0.386, p = 0.035]. However, no correlation was apparent between visceral to subcutaneous fat area ratio with histological findings and with intestinal stricture index [all p > 0.05]. The correlations among CF-related parameters, histological findings and intestinal stricture index are shown in Figure 5.
Figure 5.
Correlation coefficient matrix of creeping fat-related parameters, histological findings and intestinal stricture index in the retrospective cohort [A] and the prospective cohort [B]. The maximum positive correlation is given at a correlation coefficient of 1 [red] and the maximum negative correlation at −1 [blue] in the top right portion of the matrix graph; the dark areas indicate a strong correlation, and the lighter coloured regions indicate a relatively weak correlation. The p values of the corresponding correlation analyses [orange and gray] are shown in the bottom left portion of the matrix graph; the dark orange areas indicate a p value close to 0, the lighter orange regions indicate a p value close to 0.05, and the gray areas a p value > 0.05. MCFI, mesenteric creeping fat index; V/S ratio [L3], visceral to subcutaneous fat area ratio [L3 level]; V/S ratio [L4], visceral to subcutaneous fat area ratio [L4 level]; MM, muscularis mucosae; MP, muscularis propria
3.2.2.3. Macroscopic CF is associated with CD fibrostenosis
In the prospective cohort, the mean macroscopic fat wrapping score was 4.33 ± 1.54 with a range of 1–8. There was a good correlation between macroscopic fat wrapping and histological fibrosis scores [r = 0.601, p = 0.000]. The macroscopic fat wrapping scores of moderate–severely fibrotic strictures (median [IQR], 5 [4–5.5]) were significantly higher than those of mildly fibrotic strictures (median [IQR], 4 [2.5–5.5]) [Z = −2.202, p = 0.028]. A fair to moderate correlation was also observed between macroscopic fat wrapping scores and thickness of muscularis mucosae [r = 0.475, p = 0.008], and between macroscopic fat wrapping scores and thickness of the muscularis propria [r = 0.546, p = 0.002]. The macroscopic fat wrapping score also directly correlated with intestinal stricture indices [r = 0.642, p = 0.000].
3.3. MCFI may reveal the severity of macroscopic fat wrapping
In the prospective cohort, the median MCFI was 4 [IQR, 2–4] with a range of 1–6 [Table 3]. Examination of surgical specimens identified a topographical coupling of mesenteric vessels along the surface of the intestine and CF extent [Figure 6]. An excellent correlation was found between MCFI and macroscopic fat wrapping scores based on examination of surgical specimens [r = 0.840, p = 0.000].
Table 3.
MCFI, visceral to subcutaneous fat area ratio and fibrofatty proliferation score in patients with Crohn’s disease
| Retrospective cohort [n = 91] | Prospective cohort [n = 30] | |
|---|---|---|
| MCFI (median [IQR]) | 4 [3–6] | 4 [2–4] |
| 1 | 1 [1.10%] | 2 [6.67%] |
| 2 | 19 [20.88%] | 7 [23.33%] |
| 3 | 16 [17.58%] | 2 [6.67%] |
| 4 | 20 [21.98%] | 16 [53.33%] |
| 5 | 5 [5.49%] | 0 |
| 6 | 25 [27.47%] | 3 [10%] |
| 7 | 5 [5.49%] | 0 |
| 8 | 0 | 0 |
| Fibrofatty proliferation score (median [IQR]) | 0 [0–1] | 0 [0–0] |
| 0 | 62 [68.13%] | 24 [80%] |
| 1 | 22 [24.18%] | 5 [16.67%] |
| 2 | 7 [7.69%] | 1 [3.33%] |
| Visceral to subcutaneous fat area ratio [L3] (median [IQR]) | 1.78 [0.65–3.60] | 1.01 [0.55–2.06] |
| Visceral to subcutaneous fat area ratio [L4] (median [IQR]) | 1.17 [0.66–2.61] | 0.91 [0.48–1.24] |
MCFI, mesenteric creeping fat index; IQR, interquartile range.
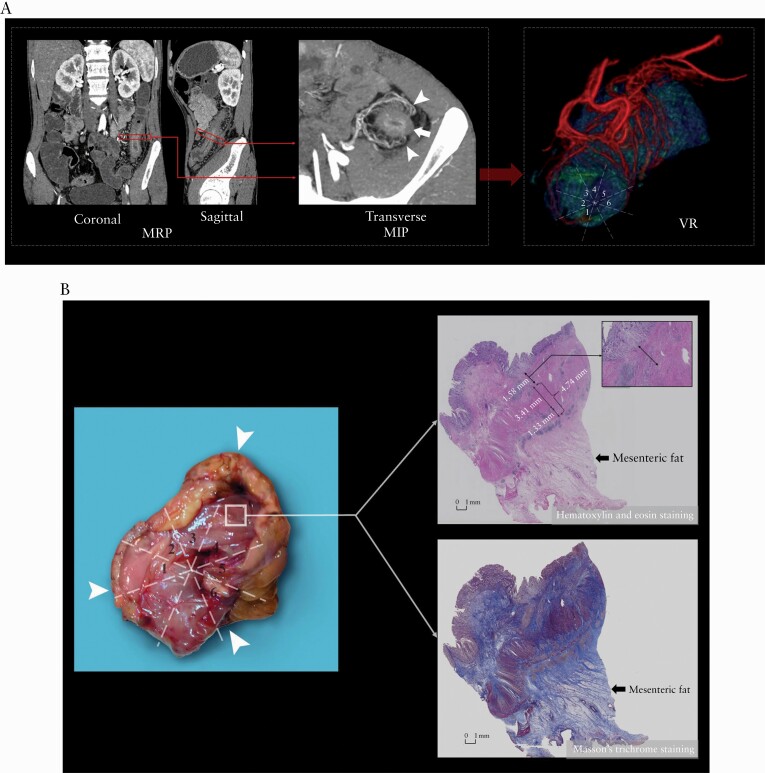
Figure 6.
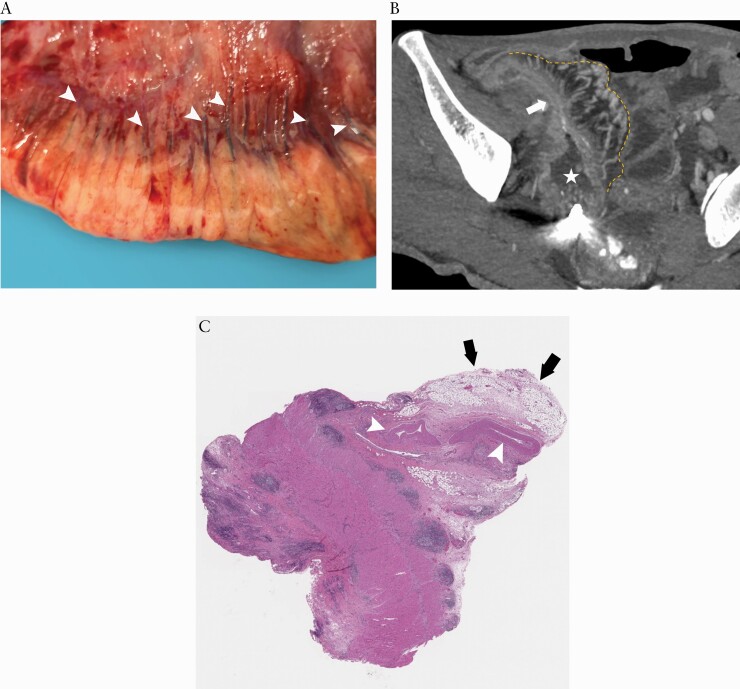
Surgical specimen [A] identified a topographical coupling of small mesenteric vessels [arrowheads] along the surface of the intestine and extending creeping fat. This anatomical manifestation is the basis for creating the mesenteric creeping fat index. On the corresponding coronal CTE using MIP reconstruction with a thickness of 4.90 mm [B], the resected small bowel [arrow] with prestenotic dilatation [asterisk] has prominent perienteric vasculature [i.e. comb sign] in the creeping fat [yellow dotted line], which is consistent with the surgical specimen. H&E staining section [C] shows enlarged mesenteric vessels in the MAT. CTE, CT enterography; MIP, maximum intensity projection; H&E, haematoxylin and eosin; MAT, mesenteric adipose tissue
3.4. Difference in MCFI between fibrosis-predominant strictures and inflammation-predominant strictures
A fair correlation between MCFI and histological inflammation scores was shown in the retrospective [r = 0.311, p = 0.003] and the prospective [r = 0.497, p = 0.005] cohorts. Of 91 bowel specimens in the retrospective cohort, 12 were from fibrosis-predominant strictures and 20 were from inflammation-predominant strictures. The MCFI of fibrosis-predominant strictures (median [IQR], 4 [3, 5.5]) was significantly higher than that of inflammation-predominant strictures (median [IQR], 3 [2, 3.75]) [Z = −2.370, p = 0.018]; this analysis in the prospective cohort was not performed due to the limited sample size of fibrosis-predominant [n = 3] and inflammation-predominant [n = 1] strictures.
4. Discussion
This study identified an association between the severity of CF, and the severity of intestinal fibrostenosis. To non-invasively quantify the degree of CF, a novel index [MCFI] was developed using CTE. We noted a strong correlation between MCFI and macroscopic fat wrapping, as well as with the histological degree of fibrostenosis. Moreover, the MCFI outperformed the visceral to subcutaneous fat area ratio, and also the fibrofatty proliferation score, with regard to characterization of CD fibrostenosis.
CF plays an active role in the pathogenesis of CD.8,18–20 A recent cellular and molecular study21 showed that gut bacteria in CD translocate to MAT and then promote the formation of CF; conversely, CF may be a defence mechanism partly mediated by M2-like macrophages and specific bacteria to limit gut bacterial spread. Given this association, it is important to characterize the role of CF in CD fibrostenosis. We recently demonstrated that adipocytes from CF secrete increased levels of free fatty acids, which in turn create a pronounced effect on intestinal smooth muscle cell proliferation.22 However, clinical studies of CF in CD fibrostenosis remain limited in number, and research on the relationship between CF and intestinal smooth muscle hypertrophy/hyperplasia is relatively sparse. In the present study, the extent of macroscopic CF wrapping around the gut increased in tandem with the severity of fibrosis, as well as with the thickness of muscularis mucosae and muscularis propria, and inversely with luminal diameter. This supports the findings of other groups.1,2,4
The above findings also support efforts to develop a means of non-invasively assessing the extent of fat wrapping around the gut. To address this issue, the extent to which peri-enteric mesenteric vessels enwrap the intestine was determined radiologically and then used to generate an index, the MCFI. This is similar to the ‘mesenteric disease activity index’ reported by Coffey et al..2 According to the latter index, mild mesenteric disease corresponded to minimal fat-wrapping, moderate mesenteric disease corresponded to < 25% fat-wrapping and severe disease corresponded to > 25% fat-wrapping.2 The MCFI exploits the established reported relationship between mesenteric vessels and CF.4,9,23,24 Additional studies found that expansion of adipose tissue deposits occurs in tandem with angiogenesis, and this may be related to signals derived from proliferating adipocytes.9,25,26 In CD, angiogenesis in MAT correlates with increases in visceral fat.23 These were directly confirmed by our results and other studies4,24 in which the number of blood vessels increased in MAT and these vessels showed a similar wrapping magnitude around the gut as with CF.
According to the results of the present study, MCFI may be an accurate means of non-invasively determining the degree to which the mesentery wraps around the intestine as CF. This suggestion was further supported by the correlation observed between MCFI and severity of fibrostenosis. Some studies indicate that the MAT must cover at least 50% of the bowel circumference in order to diagnose the presence of CF.27,28 It is likely that this criterion is too stringent as lower degrees of CF were more frequently identified.3 Consistent with the data from Coffey et al.,2 MCFI of > 3 [i.e. 4–8] often indicated the coexistence of moderate–severe bowel fibrostenosis. The MCFI threshold was further tested in a prospective cohort and it was associated with moderate accuracy and high sensitivity.
Of note, a stronger correlation was observed between the MCFI and fibrostenosis than was apparent between either visceral to subcutaneous fat area ratio and fibrofatty proliferation score and fibrostenosis. Although this ratio may be useful in reflecting the severity of CD stricturing,8,29 our study did not identify a correlation between the ratio and fibrostenosis. It is feasible that this relates to variations in the measurement of levels of visceral adiposity as there is no consensus as to how this is defined. As a result, retroperitoneal fat is sometimes grouped under the term visceral fat, when retroperitoneal fat is somatic in nature and position. The result is that impressions of levels of visceral adiposity are interpreted as being greater than actual levels. It is possible that the improved accuracy of the MCFI relates to the fact that it focuses on fat at the intestine, and not other areas.
This is one of the few studies available assessing the relationship between the severity of CF and smooth muscle hyperplasia/hypertrophy which have been recently reported to be the major contributors to intestinal stricture formation in CD,5,6 but there is a deficit in studies addressing this issue. As our study identified a relationship between the severity of CF on cross-sectional imaging and the smooth muscle hyperplasia/hypertrophy, it adds knowledge to this area. Another interesting point is whether the relationship between MCFI with smooth muscle hyperplasia/hypertrophy in the small bowel is different from that in the colon. Many genetic studies30–32 have suggested that the small bowel and colonic CD represent two distinct disease entities. Similarly, CF that is adjacent to the small bowel might also be different from that adjacent to the colon, according to the study of Kredel et al.33 in which small bowel CF showed distinct adipocyte hyperplasia combined with dense T-cell infiltration and fibrosis whereas colonic fat lacked these findings in CD. However, in our study, similar correlations between MCFI and thickness of smooth muscle were found between small bowel strictures and colonic strictures, indicating that CF may produce an equal effect on intestinal smooth muscle hyperplasia/hypertrophy in these two locations. Moreover, our results also suggested that CF, bowel fibrosis and bowel inflammation are closely related, although it appears that the relationship between CF and bowel fibrosis was stronger.
However, this study also had certain limitations. First, a control ‘normal’ group was not included as this would have meant excision of normal intestine, for correlation with radiological findings. It is feasible that mesenteric adipose tissue from apparently normal regions of the mesentery may also play a role in the very early stages of CF.34 Second, sample sizes in both the retrospective and the prospective cohorts were limited. Whilst this is generally a feature of studies in CD, the collation of results of multiple centres could increase sample size enough to strengthen the conclusions. Third, we used CTE rather than magnetic resonance enterography to develop the MCFI because modern low-radiation CT scanners are safe for CD patients and have a higher spatial resolution that may be more suitable for this preliminary study. MCFI reconstructed by magnetic resonance enterography is worthy of further study. Lastly, the body mass index of the patients with CD enrolled in this study was generally low. Although CF is a distinctive body-weight-independent accumulation of MAT wrapped around the bowel segments affected by CD,35 the relationship between MCFI and intestinal fibrotic stricture needs to be confirmed in CD patients with higher body mass index in a future study.
In conclusion, MCFI is a non-invasive radiological index that accurately quantifies the degree of CF. It outperforms other established indices such as the visceral to subcutaneous fat area ratio and fibrofatty proliferation score in assessing the severity of fibrostenosis. Give these findings, the MCFI should be further investigated as a non-invasive means of determining the severity of CF and associated mural changes in CD.
Supplementary Material
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers: 82070680, 81970483, 82072002, 81870451, 81600508, 81770654, 81971684, 81771908].
Conflict of Interest
All authors declare no competing interests.
Author Contributors
R.M. and S.T.F. managed the project. X.H.L. and S.T.F. performed the study design, data analysis and data interpretation. Data collection was done by Q.H.C., L.H., Z.N.F., B.L.L., Y.Q., Z.H.C. and C.H.S.. X.H.L wrote the manuscript. R.M., J.C.C., M.E.B., D.B., M.I., F.R., S.G., Z.P.L., S.T.F., Y.L. and M.H.C. edited the manuscript. All authors reviewed and commented on the manuscript and approved the final version.
Data Availability Statements
Data are available on request.
References
- 1.Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn’s disease: evidence for a possible causative link. J Pathol 2000;190:196–202. [DOI] [PubMed] [Google Scholar]
- 2.Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis 2018;12:1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey JC, O’Leary DP, Kiernan MG, Faul P. The mesentery in Crohn’s disease: friend or foe? Curr Opin Gastroenterol 2016;32:267–73. [DOI] [PubMed] [Google Scholar]
- 4.Zuo L, Li Y, Zhu W, et al. Mesenteric adipocyte dysfunction in Crohn’s disease is associated with hypoxia. Inflamm Bowel Dis 2016;22:114–26. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017;11:92–104. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Ko HM, Torres J, et al. Luminally polarized mural and vascular remodeling in ileal strictures of Crohn’s disease. Hum Pathol 2018;79:42–9. [DOI] [PubMed] [Google Scholar]
- 7.Rimola J, Alfaro I, Fernández-Clotet A, et al. Persistent damage on magnetic resonance enterography in patients with Crohn’s disease in endoscopic remission. Aliment Pharmacol Ther 2018;48:1232–41. [DOI] [PubMed] [Google Scholar]
- 8.Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol 2011;9:684–687.e1. [DOI] [PubMed] [Google Scholar]
- 9.Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 2014;1842:463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther 2018;48:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XH, Mao R, Huang SY, et al. Characterization of degree of intestinal fibrosis in patients with crohn disease by using magnetization transfer MR imaging. Radiology 2018;287:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner M, Ko HM, Chatterji M, et al. Magnetic resonance imaging predicts histopathological composition of ileal Crohn’s disease. J Crohns Colitis 2018;12:718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai T, Katsuno T, Saito K, et al. Mesenteric findings of CT enterography are well correlated with the endoscopic severity of Crohn’s disease. Eur J Radiol 2017;89:242–8. [DOI] [PubMed] [Google Scholar]
- 14.Orscheln ES, Dillman JR, Towbin AJ, Denson LA, Trout AT. Penetrating Crohn disease: does it occur in the absence of stricturing disease? Abdom Radiol (NY) 2018;43:1583–9. [DOI] [PubMed] [Google Scholar]
- 15.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis 2012;18:849–56. [DOI] [PubMed] [Google Scholar]
- 16.Du JF, Lu BL, Huang SY, et al. A novel identification system combining diffusion kurtosis imaging with conventional magnetic resonance imaging to assess intestinal strictures in patients with Crohn’s disease. Abdom Radiol (NY) 2020. doi: 10.1007/s00261-020-02765-3. [DOI] [PubMed] [Google Scholar]
- 17.Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 2019;156:1082–1097.e11. [DOI] [PubMed] [Google Scholar]
- 18.Mao R, Kurada S, Gordon IO, et al. The mesenteric fat and intestinal muscle interface: creeping fat influencing stricture formation in Crohn’s disease. Inflamm Bowel Dis 2019;25:421–6. [DOI] [PubMed] [Google Scholar]
- 19.Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment Pharmacol Ther 2017;45:1255–64. [DOI] [PubMed] [Google Scholar]
- 20.Büning C, von Kraft C, Hermsdorf M, et al. Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis 2015;21:2590–7. [DOI] [PubMed] [Google Scholar]
- 21.Ha CWY, Martin A, Sepich-Poore GD, et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 2020;183:666–683.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao R, Doyon G, Kurada S, et al. Creeping-fat derived free fatty acids induce hyperplasia of intestinal muscularis propria muscle cells – a novel link between fat and intestinal stricture formation in Crohn’s disease. Gastroenterology 2018;154:131. [Google Scholar]
- 23.Li Y, Zhu W, Zuo L, Shen B. The role of the mesentery in Crohn’s disease: the contributions of nerves, vessels, lymphatics, and fat to the pathogenesis and disease course. Inflamm Bowel Dis 2016;22:1483–95. [DOI] [PubMed] [Google Scholar]
- 24.Brahme F, Lindström C. A comparative radiographic and pathological study of intestinal vaso-architecture in Crohn’s disease and in ulcerative colitis. Gut 1970;11:928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 2017;127:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemoine AY, Ledoux S, Larger E. Adipose tissue angiogenesis in obesity. Thromb Haemost 2013;110:661–8. [DOI] [PubMed] [Google Scholar]
- 27.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut 2007;56:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink C, Karagiannides I, Bakirtzi K, Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm Bowel Dis 2012;18:1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant RV, Schultz CG, Ooi S, et al. Visceral adipose tissue is associated with stricturing Crohn’s disease behavior, fecal calprotectin, and quality of life. Inflamm Bowel Dis 2019;25:592–600. [DOI] [PubMed] [Google Scholar]
- 30.Newman B, Silverberg MS, Gu X, et al. CARD15 and HLA DRB1 alleles influence susceptibility and disease localization in Crohn’s disease. Am J Gastroenterol 2004;99:306–15. [DOI] [PubMed] [Google Scholar]
- 31.Cuthbert AP, Fisher SA, Mirza MM, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 2002;122:867–74. [DOI] [PubMed] [Google Scholar]
- 32.Dulai PS, Singh S, Vande Casteele N, et al. Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol 2019;17:2634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kredel LI, Jödicke LJ, Scheffold A, et al. T-cell composition in ileal and colonic creeping fat – separating ileal from colonic Crohn’s disease. J Crohns Colitis 2019;13:79–91. [DOI] [PubMed] [Google Scholar]
- 34.Zulian A, Cancello R, Micheletto G, et al. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut 2012;61:86–94. [DOI] [PubMed] [Google Scholar]
- 35.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation – visceral obesity and creeping fat. Front Immunol 2014;5:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.