Abstract
Background
Cutibacterium species are common pathogens in periprosthetic joint infections (PJI). These infections are often treated with β-lactams or clindamycin as monotherapy, or in combination with rifampin. Clinical evidence supporting the value of adding rifampin for treatment of Cutibacterium PJI is lacking.
Methods
In this multicenter retrospective study, we evaluated patients with Cutibacterium PJI and a minimal follow-up of 12 months. The primary endpoint was clinical success, defined by the absence of infection relapse or new infection. We used Fisher’s exact tests and Cox proportional hazards models to analyze the effect of rifampin and other factors on clinical success after PJI.
Results
We included 187 patients (72.2% male, median age 67 years) with a median follow-up of 36 months. The surgical intervention was a 2-stage exchange in 95 (50.8%), 1-stage exchange in 51 (27.3%), debridement and implant retention (DAIR) in 34 (18.2%), and explantation without reimplantation in 7 (3.7%) patients. Rifampin was included in the antibiotic regimen in 81 (43.3%) cases. Infection relapse occurred in 28 (15.0%), and new infection in 13 (7.0%) cases. In the time-to-event analysis, DAIR (adjusted hazard ratio [HR] = 2.15, P = .03) and antibiotic treatment over 6 weeks (adjusted HR = 0.29, P = .0002) significantly influenced treatment failure. We observed a tentative evidence for a beneficial effect of adding rifampin to the antibiotic treatment—though not statistically significant for treatment failure (adjusted HR = 0.5, P = .07) and not for relapses (adjusted HR = 0.5, P = .10).
Conclusions
We conclude that a rifampin combination is not markedly superior in Cutibacterium PJI, but a dedicated prospective multicenter study is needed.
Keywords: Cutibacterium species, Propionibacterium species, periprosthetic joint infections, rifampin, antibiotic treatment
In this retrospective study, we observed no significant benefit of using rifampin to avoid relapses or new infections but a benefit when the prosthesis was removed or exchanged and an antibiotic treatment was given for at least 6 weeks.
Cutibacterium species (mainly Cutibacterium acnes and Cutibacterium avidum), are, after staphylococci and streptococci, among the most frequently isolated pathogens causing periprosthetic joint infections (PJIs) [1]. C. acnes predominantly infects shoulder and hip implants [2], whereas C. avidum is associated with hip arthroplasty infection [3–6]. In general, PJIs are difficult to cure because bacteria grow as biofilms on implants. In biofilms, the sessile bacteria are embedded in a matrix of extracellular polymeric substances, which are at least partially produced by the bacteria themselves; bacteria in biofilms are protected against the immune system [7, 8]. Sessile bacteria have a low metabolism and consequently replicate at a slow rate [7].
Rifampin has a low minimal bactericidal concentration against sessile Staphylococcus aureus and coagulase-negative staphylococci [9]. Accordingly, rifampin has been shown to cure experimental implant-associated staphylococcal infections in animal models and combination with rifampin has been found to be more efficacious than standard therapy in observational studies as well as in a controlled trial of patients with orthopedic device-associated infection managed with debridement and retention of prosthesis (DAIR) [10–14]. In analogy to treatment concepts for staphylococcal infections, antibiotic regimens including rifampin are used to treat Cutibacterium PJIs in some orthopedic centers because of low minimal bactericidal concentration. In small case series, rifampin was combined with clindamycin [15, 16] or amoxicillin [17]. Furustrand et al showed in a guinea pig model that antibiotic regimens containing rifampin in Cutibacterium infections yielded favorable results when an implant is present [18]. Rifampin cured 63% of the infected cages in combination with daptomycin, 46% with vancomycin, and 25% with levofloxacin, whereas monotherapy with daptomycin, vancomycin, or levofloxacin cured only 4%, 17%, and 0% of infections, respectively. Thus, combinations with rifampin were superior to single regimens without rifampin in this study, though β-lactams were not administered to animals. However, no large study evaluating rifampin in humans is available. Because of the lack of large clinical studies, it is unclear if addition of rifampin is indeed necessary for cure of Cutibacterium PJI.
In a large cohort of patients with Cutibacterium PJI, we tested the hypothesis that adding rifampin to an antibiotic regimen for cure of infection is not superior to antibiotic regimen without rifampin. Moreover, we hypothesized that the choice of surgical treatment concept is a major element determining successful outcome of these infections.
METHODS
Study Setting and Population
This is a multicenter retrospective study including patients from 9 countries (18 centers) with a PJI diagnosis between 2005 and 2018. We evaluated patients with Cutibacterium PJI, defined by growth of C. acnes, C. avidum, or Cutibacterium granulosum from at least 2 different diagnostic samples including tissue biopsies, sonication fluid, or synovial fluid. Samples for microbiology were cultivated for 14 days in 13/18 (72.2%) and 6 to 10 days in 5/18 (27.8%) of the study centers. We recorded information about clinical presentation, antibiotic and surgical treatment, and infection outcome. The case report form relies on the PJI database app developed by the study group ESGIAI supported by ESCHMID (https://apps.apple.com/us/app/pji-database/id1331588615). We only included patients who underwent surgery for curative management of Cutibacterium PJI (ie, 1-stage or 2-stage exchange of the prosthesis [with or without spacer implantation]), DAIR, or explantation without new prosthesis. Patients were followed until infection relapse, new infection, or death with a minimum follow-up of 12 months after the surgical intervention for Cutibacterium PJI. We did not include cases with only 1 positive Cutibacterium sample but treated as infection, polymicrobial infection, an antibiotic treatment longer than 6 months or labelled as lifelong suppressive treatment, no surgical treatment at all, or insufficient or a short follow-up of less than 1 year.
Definitions
We distinguished between early acute infections with time to septic surgery less than 4 weeks after last surgery and chronic infections with time to septic surgery longer than 4 weeks. The primary endpoint of our study was treatment failure, defined as either infection relapse, new infection, or death from PJI. Infection relapse was defined as proven when persisting signs or symptoms of infection (pain, swelling, redness, wound secretion, or elevated serum inflammatory parameters) were present and 2 new diagnostic samples microbiologically identified the same Cutibacterium species. We defined it as possible when not microbiologically proven but suggested by persisting symptoms or signs of infection. A new infection was defined as a microbiologically proven infection in case of a new pathogen detected in ≥2 diagnostic samples during the follow-up period. The follow-up time started at the date of the initial surgery for Cutibacterium PJI, specifically, the date of explantation in case of a 2-stage exchange of the prosthesis.
Antibiotic Treatment
Patients were grouped into a rifampin group in case rifampin was used after the surgery for Cutibacterium PJI for at least 1 week, with a sensitivity analysis using the thresholds of at least 4 weeks and at least 6 weeks. Antibiotic treatment duration was calculated as the total duration for all drugs (including rifampin) combined, as well as for intravenous (IV), oral (PO), and rifampin use.
Statistical Analyses
Patient characteristics between the group of patients who received rifampin and those who did not receive rifampin were analyzed using t tests (continuous variables) and chi-squared tests (categorical variables). The effect of adding rifampin to the antibiotic regimen on clinical success was tested in 2 ways, cross-sectionally as well as longitudinally (time to treatment failure): first, a cross-sectional analysis (chi-squared test) was used to analyze the effect of rifampin on the outcome (failure, relapse, new infection) stratified by surgical strategy. Second, the time to treatment failure and time to relapse was assessed using Cox proportional hazards models, with explanatory variables including rifampin as well as the most important demographic and clinical parameters. Proportional hazards assumptions were analyzed using Schoenfeld residuals available in the R package survival [19]. Models were adjusted for total duration of antibiotic use as well as the surgical strategy (ie, the 2 clinically most relevant factors). To assess the effect of the treatment center, we performed a sensitivity analysis using a mixed effect model, where the country was included as a random effect. Statistical analyses were performed using R (version 3.4.4). Moreover, to assess the effect of the most commonly used treatment regimen in literature (clindamycin/rifampin), we performed a sensitivity analysis in which we looked at hazard ratios of other rifampin combinations than clindamycin, clindamycin alone, and other monotreatment.
RESULTS
Study Population
We included 187 patients from 9 countries; the median time of follow-up after infection treatment was 36 months. Most patients were male (72.2%) with a median age of 67 years and a median body mass index (BMI) of 28 kg/m2 (Table 1). The median time to PJI after the last surgical procedure was 20 months, with a chronic infection (>1 month) in 177 (94.7%) patients and early postoperative infection (<1 month) in 10 (5.3%). All but one had a treatment failure in the late postoperative phase. The one patient with an early postoperative treatment failure had a new infection (Supplementary Table 1). The most common joint prostheses were hip (51.9%), shoulder (37.3%), and knee (9.1%). In most cases, the isolated pathogen in 2 or more diagnostic samples was C. acnes (84.5%) (Table 2).
Table 1.
Baseline Characteristics of 187 Cases Treated for a Cutibacterium PJI With (n = 81, 43.3%) and Without (n = 106, 56.7%) a Rifampin Combination
| All Patients | With Rifampin | Without Rifampin | Comparison (P Value) | ||
|---|---|---|---|---|---|
| Total | 187 | 81 | 106 | ||
| General patient information | |||||
| Follow-up time | Months (median, IQR) | 36 [23, 60] | 43 [25, 70] | 33 [21, 47] | .0344 |
| Sex | Male | 135/187 (72.2%) | 60/81 (74.1%) | 75/106 (70.8%) | .7359 |
| Female | 52/187 (27.8%) | 21/81 (25.9%) | 31/106 (29.2%) | ||
| Age | Median, IQR | 67 [58, 74] | 65 [57, 72] | 68 [59, 76] | .1959 |
| Body mass index | Median, IQR | 28 [26, 32] | 28 [25, 30] | 29 [27, 32] | .2525 |
| Country | Country 1 | 105/187 (56.1%) | 62/81 (76.5%) | 43/106 (40.6%) | .0002 |
| Country 2 | 28/187 (15.0%) | 10/81 (12.3%) | 18/106 (17.0%) | ||
| Country 3 | 19/187 (10.2%) | 4/81 (4.9%) | 15/106 (14.2%) | ||
| Country 4 | 13/187 (7.0%) | 0/81(0.0%) | 13/106 (12.3%) | ||
| Country 5 | 7/187 (3.7%) | 1/81 (1.2%) | 6/106 (5.7%) | ||
| Country 6 | 7/187 (3.7%) | 2/81 (2.5%) | 5/106 (4.7%) | ||
| Country 7 | 6/187 (3.2%) | 2/81 (2.5%) | 4/106 (3.8%) | ||
| Country 8 | 1/187 (0.5%) | 0/81 (0.0%) | 1/106 (0.9%) | ||
| Country 9 | 1/187 (0.5%) | 0/81 (0.0%) | 1/106 (0.9%) | ||
| Prosthesis | |||||
| Joint prosthesis | Hip | 97/187 (51.9%) | 40/81 (49.4%) | 57/106 (53.8%) | .3501 |
| Shoulder | 70/187 (37.4%) | 34/81 (42.0%) | 36/106 (34.0%) | ||
| Knee | 17/187 (9.1%) | 7/81 (8.6%) | 10/106 (9.4%) | ||
| Other (foot, elbow) | 3/187 (1.6%) | 0/81 (0.0%) | 3/106 (2.8%) |
Body mass index was defined as the weight (in kg) divided by the height (in m) squared.
Table 2.
Infection Characteristics of 187 Patients With a Cutibacterium PJI Treated With Rifampina (n = 81) and Without (n = 106)
| Total | With Rifampin | Without Rifampin | Comparison (P Value) | ||
|---|---|---|---|---|---|
| Total | 187 | 81 | 106 | ||
| Cutibacterium species | Cutibacterium acnes | 158/187 (84.5%) | 66/81 (81.5%) | 91 106 (85.8%) | .6189 |
| Cutibacterium avidum | 20/187 (10.7%) | 10/81 (12.3%) | 10/106 (9.4%) | ||
| Cutibacterium granulosum | 9/187 (4.8%) | 4/81 (4.9%) | 5/106 (4.7%) | ||
| Clinical presentation | |||||
| Sinus tract | n, % | 19/187 (10.2%) | 4/81 (4.9%) | 15/106 (14.2%) | .0685 |
| Pain | n, % | 164/187 (87.7%) | 67/81 (82.7%) | 97/106 (91.5%) | .1119 |
| Pathogenesis outcome | |||||
| Time to PJI | Months (median, IQR) | 20 [6, 41] | 20 [4, 42] | 18 [8, 39] | .1304 |
| Acute early (≤4 wk after last surgery) | n, % | 10/187 (5.3%) | 7/81 (8.6%) | 3/106 (2.8%) | |
| Chronic late (>4 wk after last surgery) | n, % | 177/187 (94.7%) | 74/81 (91.4%) | 103/106 (97.2%) | |
| Antibiotic treatment | |||||
| Overall antibiotic treatment durationb | Weeks (median, IQR) | 12 [7, 13] | 12 [11, 14] | 9 [6, 12] | .0013 |
| Overall duration >6 wk | n, % | 141/187 (75.4%) | 69/81 (85.2%) | 72/1066 (67.9%) | |
| IV antibiotics duration | days (median, IQR) | 14 [10, 24.5] | 14 [9, 18] | 16 [10.2, 28] | .0087 |
| IV antibiotics | n, % | 174/187 (93.0%) | 73/81 (90.1%) | 101/106 (95.3%) | .2781 |
| IV duration >14 d | n, % | 89/187 (47.6%) | 30/81 (37.0%) | 59/106 (55.7%) | |
| PO antibiotics duration | Weeks (median, IQR) | 9 [4, 11] | 10 [7, 12] | 7 [4, 10] | <.0001 |
| Rifampin duration | Weeks (median, IQR) | 10 [6, 12] | |||
| Rifampin duration >6 wk | n, % | 58/81 (71.6%) | |||
| Treatment: surgical concept | DAIR | 34/187 (18.2%) | 15/81 (18.5%) | 19/106 (17.9%) | .0368 |
| 1-stage exchange of prosthesis | 51/187 (27.3%) | 3181 (38.3%) | 20/106 (18.9%) | ||
| 2-stage exchange of prosthesis with spacer | 63/187 (33.7%) | 20/81 (24.7%) | 43/106 (40.3%) | ||
| 2-stage exchange of prosthesis without spacer | 32/187 (17.1%) | 12/81 (14.8%) | 20/106 (18.9%) | ||
| Explantation without new prosthesis | 7/187 (3.7%) | 3/81 (3.7%) | 4/106 (3.8%) | ||
| Outcome | |||||
| Overall failurec | (n, %) | 38/187 (20.3%) | 10/81 (12.3%) | 28/106 (26.5%) | .0288 |
| Relapse | Proven and possible (n, %) | 28/187 (15.0%) | 8/81 (9.9%) | 20/106 (18.9%) | .1334 |
| Proven (n, %) | 16/28 (57.1%) | 5/8 (62.5%) | 11/20 (55.0%) | ||
| Possible (n, %) | 12/28 (42.9%) | 3/8 (37.5%) | 9/20 (45.0%) | ||
| Relapse: time of occurrence | At implantation (n, %) | 11/28 (39.3%) | 3/8 (37.5%) | 8/20 (40.0%) | |
| During AB treatment (n, %) | 8/28 (28.6%) | 2/8 (25.0%) | 6/20 (30.0%) | ||
| After AB treatment stop (n, %) | 9/28 (32.1%) | 4/8 (50.0%) | 5/20 (25.0%) | ||
| New infection | n, % | 13/187 (7.0%) | 2/81 (2.5%) | 11/106 (10.4%) | .0692 |
| New infection: time of occurrence | At implantation (n, %) | 2/13 (15.4%) | 0/2 (0.0%) | 2/11 (18.2%) | |
| During AB treatment (n, %) | 2/13 (15.4%) | 0/2 (0.0%) | 2/11 (18.2%) | ||
| After AB treatment stop (n, %) | 9/13 (69.2%) | 1/2 (50.0%) | 8/11 (72.7%) | ||
| Death | Overall (n, %) | 13/187 (7.0%) | 4/81 (4.9%) | 9/106 (8.5%) | .5116 |
| From PJI (n, %) | 0/187 (0.0%) | 0/81 (0.0%) | 0/106 (0.0%) |
Abbreviations: AB, antibiotic; DAIR, debridement and retention of prosthesis; IQR, interquartile ratio; IV, intravenous; PO, oral.
aRifampin doses were prescribed as 450 mg twice daily in 44.4%, 600 mg once daily in 27.8%. In 33.3%, doses were not recorded.
bIn patients treated with a 2-stage exchange of prosthesis with a long interval of at least 6 weeks of antibiotic treatment followed by an antibiotic-free window of 2 weeks, antibiotic treatment duration was counted until the start of the antibiotic window. In those patients with a 2-stage exchange and a short first interval of antibiotic treatment, IV and PO antibiotic treatment duration was combined after the explantation and the implantation date of the prosthesis.
cSeveral patients had an infection relapse as well as a new infection.
Two-stage exchange of the prosthesis was performed in 95 (50.8%), 1-stage exchange of the prosthesis in 51 (27.3%), and DAIR in 34 (18.2%) patients (see Supplementary Table 1 for the surgical strategy in acute versus chronic cases). The median overall antibiotic duration was 12 weeks; median = 14 days for IV antibiotics and median = 9, weeks for PO antibiotic treatment. Most patients (174, 93.0%) were prescribed IV antibiotics. Rifampin was prescribed in 81 (43.3%) cases; the median duration of rifampin use was 10 weeks (Table 2 and Supplementary Figure 1).
Rifampin
There were no significant differences regarding gender, age, BMI, and the involved joint prosthesis between patients who received rifampin and those who did not. There was, however, a difference regarding the surgical strategy: among patients who received rifampin, 31/81 (38.3%) had a 1-stage exchange and 32/81 (39.5%) a 2-stage exchange of prothesis; whereas among patients who did not receive rifampin 20/106 (18.9%) had a 1-stage exchange and 63/106 (59.4%) had a 2-stage exchange (P = .37).
We also saw differences in the countries in which the patients were treated, ranging between no patients and more than one-half of patients receiving rifampin (Supplementary Table 2). Moreover, there was no clear time trend in prescribing rifampin during the study time frame (Supplementary Figure 2). Overall, follow-up time and the overall antibiotic duration was longer in patients who received rifampin compared to those who did not, for all antibiotics combined or IV or PO antibiotics separately (Table 2). The combination treatment with rifampin is documented in Supplementary Table 3.
Outcome
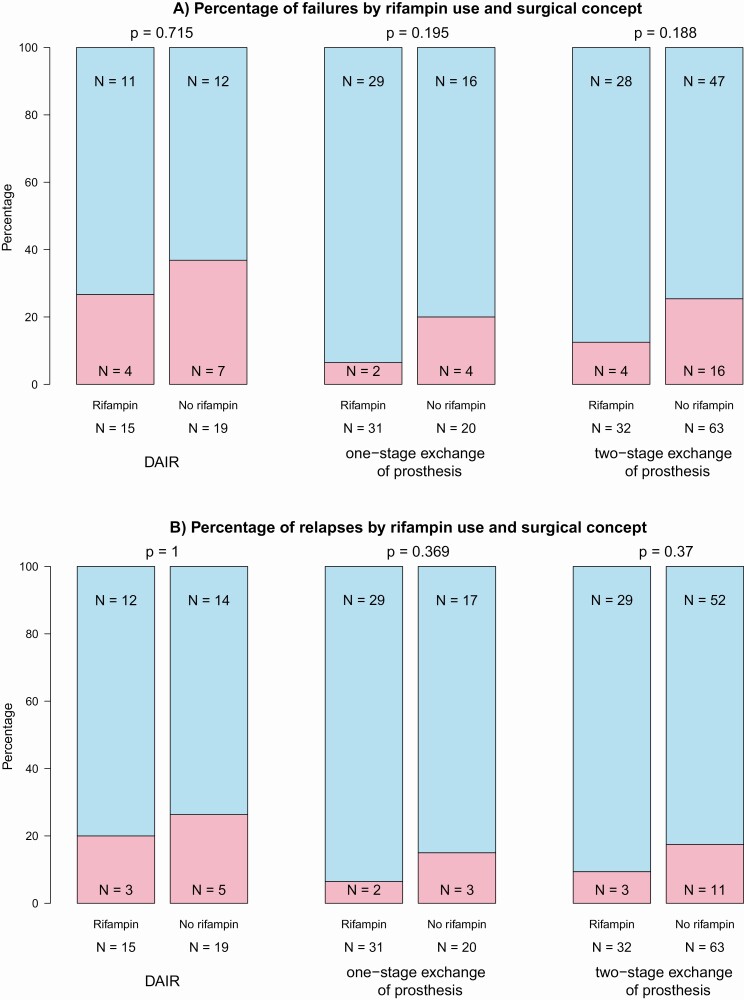
Overall, treatment failure (relapse and new infection) manifested in 38 (20.3%) cases. Infection relapses occurred in 28 (15.0%) cases (proven relapse: 16; possible relapse: 12), and new infection in 13 (7.0%). During follow-up, 13 (7.0%) patients died, but PJI did not result in death (Table 2). Among the patients treated with rifampin, treatment failure was observed in 10 (12.3%) cases compared with 28 (26.4%) cases among patients not treated with rifampin (P = .029). This difference, however, was not significant for relapse and new infection separately, which was observed in 8 (9.9%) and 2 (2.5%) cases of patients who received rifampin, respectively, compared with 20 (18.9%) and 11 (10.4%) cases in patients who did not receive rifampin (P = .13 for relapse and P = .069 for new infection). Stratified by surgical strategy, the frequency of treatment failures was highest in patients for whom DAIR was performed (11/34, 32.4%) compared with 1-stage (6/51, 11.7%) or 2-stage exchange (20/95, 21.1%) of prosthesis. In each group (DAIR, 1-stage exchange, 2-stage exchange), fewer treatment failures in patients who received rifampin were observed. This difference was not significant looking cross-sectionally at overall treatment failures of relapses only (Figure 1). In a sensitivity analysis, we restricted to the first 3 years after surgical intervention with no significant difference either (see Supplementary Figure 3).
Figure 1.
Outcome of Cutibacterium PJIs stratified by surgical strategy (DAIR, 1-stage exchange, 2-stage exchange), either looking at failures in general (A) or at relapses only (B). Abbreviations: DAIR, debridement and retention of prosthesis; PJI, periprosthetic joint infection.
Dynamic of Treatment Failure Overall and Relapses
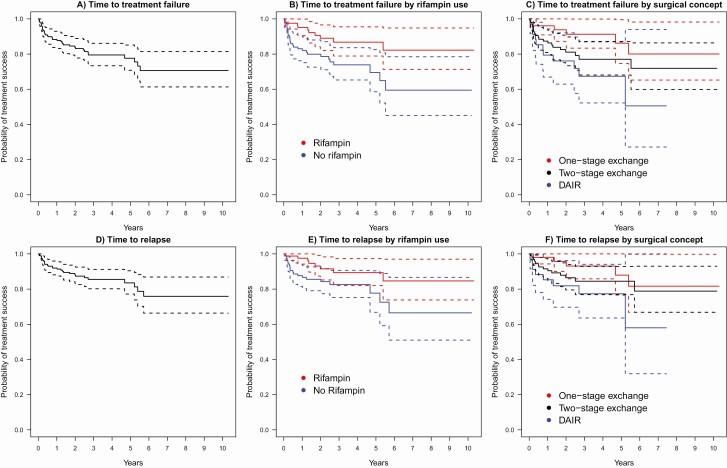
The median time to treatment failure was 19.3 months (interquartile range [IQR] = 7.0-58.1) (Figure 2A). We observed increased hazards of treatment failure in patients not treated with rifampin compared with patients treated with rifampin (unadjusted hazard ratio (HR) = 2.50; 95% confidence interval [CI], 1.21-5.16; P = .013; Figure 2B) as well as increased hazards of treatment failure in patients who underwent DAIR compared with 1-stage exchange (unadjusted HR = 3.4; 95% CI, 1.26-9.27; P = .016) or 2-stage exchange (unadjusted HR = 1.6; 95% CI, 0.78-3.42; P = .19) of prostheses (Figure 2C).
Figure 2.
A, Kaplan-Meier curve of all patients (n = 187), with 38 having treatment failure (ie, infection relapse or new infection); B, Kaplan-Meier curve of patients using rifampin (n = 81, 10 failures) and not using rifampin (n = 106, 28 failures); C, Kaplan-Meier curve of patients stratified by surgical strategy: 1-stage exchange (n = 51, 6 failures), 2-stage exchange (n = 95, 20 failures), DAIR (n = 34, 11 failures); D, Kaplan-Meier curve of all patients (n = 187), with 28 having an infection relapse; E, Kaplan-Meier curve of patients prescribed rifampin (n = 81, 8 relapses) or not (n = 106, 20 relapses); F, Kaplan-Meier curve of patients stratified by surgical strategy: 1-stage exchange (n = 51, 5 relapses), 2-stage exchange (n = 95, 14 relapses), DAIR (n = 34, 8 relapses). Abbreviation: DAIR, debridement and retention of prosthesis.
The median time to infection relapse was 23.3 months (IQR = 8.6-60.5) (Figure 2D). Again, increased hazards of infection relapse were observed in patients who did not receive rifampin (unadjusted HR = 2.28; 95% CI, 1.00-5.18; P = .05) (Figure 2E) and patients who underwent DAIR as compared with 1-stage exchange (HR = 3.08; 95% CI, 1.0-9.52; P = .05) or 2-stage exchange (HR = 1.76; 95% CI, 0.74, 4.23; P = .20) of prostheses (Figure 2F). The median time to new infection was 11.4 months (IQR = 6.8-39.1).
Effect of Rifampin and Other Factors
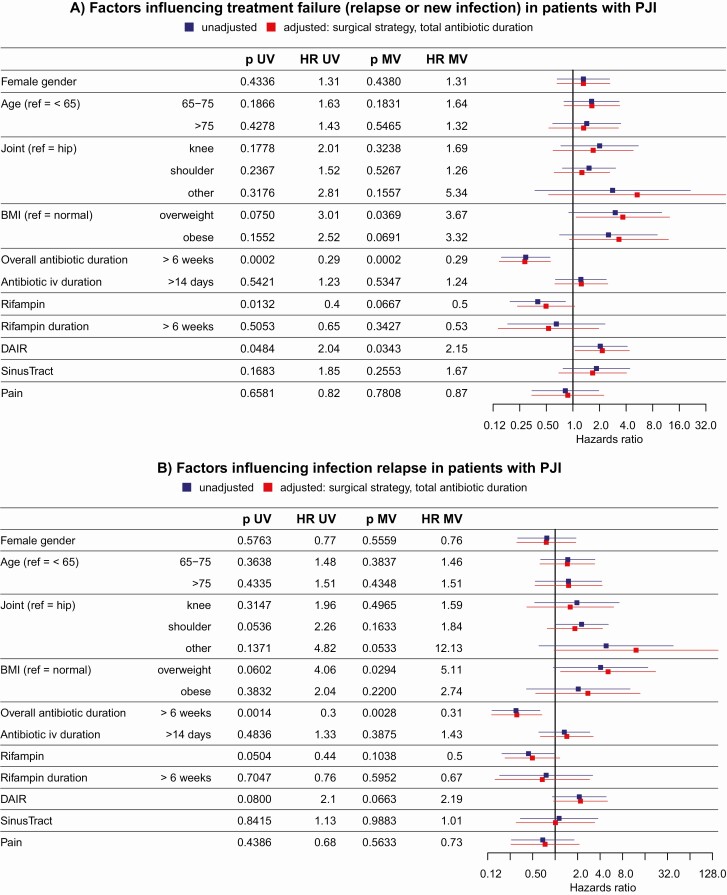
The effect of adding rifampin to the antibiotic combination therapy was not significant after adjusting for surgical strategy and overall duration of antibiotic treatment (adjusted HR = 0.50; 95% CI, 0.23-1.05; P = .07) (Figure 3A). However, using DAIR instead of a surgical strategy involving the change of prosthesis was significantly associated with higher hazards of treatment failure, even after adjusting for antibiotic duration (HR = 2.15; 95% CI, 1.06-4.37; P = .03). Moreover, an overall antibiotic duration of more than 6 weeks was associated with a reduced hazard for treatment failure even after adjusting for surgical strategy (adjusted HR = 0.29; 95% CI, 0.15-0.56; P = .0002). Similar results were obtained for relapse only (Figure 3B). In a sensitivity analysis, we only grouped patients into the rifampin stratum in case the intake lasted at least 4 or 6 weeks, respectively, and obtained similar results (Supplementary Table 4). Most patients included in this study came from 1 country (n = 105) (country 1, Table 1) but the rate of rifampin strongly differed between countries. Therefore, we used a mixed effects model to include the effect of different countries, again leading to similar results (Supplementary Figure S4).
Figure 3.
Factors influencing failure overall (A) and relapse (B). Abbreviations: BMI, body mass index (overweight: BMI > 25, obese: BMI > 30); DAIR, debridement and implant retention; HR, hazards ratio; IV, intravenous; MV, multivariable; PJI, periprosthetic joint infections; UV, univariable.
Because of the heterogeneity of our study population with different antibiotic regimens (Supplementary Table 3), we did not stratify treatment outcome for all different antibiotic regimens. However, in a sensitivity analysis, success rate was highest for the combination with rifampin and clindamycin, although the difference was not statistically significant (Supplementary Table 3 and Supplementary Figure 5).
DISCUSSION
In this multicenter study, we included 187 patients with Cutibacterium PJI and evaluated the added value of rifampin as part of antibiotic regimens following septic surgery. We observed an overall successful treatment outcome in 79.7% cases, with relapses in 15% and new infection in 7% cases. We observed a tentative evidence for a beneficial effect of adding rifampin to the antibiotic treatment—though not statistically significant, the hazards for developing treatment failure were halved in the group of patients treated with rifampin. A statistically significant effect halving the hazards of developing treatment failure was observed for choosing the exchange of the prosthesis instead of DAIR to successfully treating Cutibacterium PJI and an antibiotic treatment of at least 6 weeks.
In this largest case series up to now on Cutibacterium PJI, we show that clinical success is mainly dominated by performing a surgical approach with removal or exchange of the prosthesis instead of a DAIR procedure. As described in the treatment concept by Zimmerli et al [20] and treatment outcome studies [21–24], the proper selection of patients for DAIR achieves high clinical success. Barberan et al showed in 60 staphylococcal PJIs [25] that the treatment success rate with a DAIR regimen decreased from 83.4% when symptoms lasted shorter than month to 65.2% when between 2 and 6 months and to 30.8% when more than 6 months of symptoms. We counted a chronic infection in 94.7%, with a median time to infection of 11.4 months, in which an exchange of the prosthesis should be performed because of mature biofilm. However, a DAIR without removal of the implant approach was chosen in a higher proportion of the patients with 18.2% even though some of these patients had a chronic infection. Looking at patients with an exchange of the prostheses, we observed fewer treatment failures when performing 1-stage exchange as compared with 2-stage exchange (Figure 1). Despite the reduced risk for the patients by having only 1 operation instead of 2 operations, 1-stage exchange is so far rarely the concept of choice. However, several studies highlighted the good clinical outcome of 1-stage exchange [26–29].
We observed an overall treatment success rate of 80% and 85% when only looking at relapses, which was not significantly different in patients treated with rifampin versus those without (89.9% vs 81.5%). This is in line with the study by Jacobs et al [15] analyzing 60 patients with Cutibacterium PJI and observing an overall success rate of 86% after 2 years follow-up and no significant difference between clindamycin/rifampin versus clindamycin alone. However, caution is needed when prescribing rifampin in combination antibiotic therapy. Besides several known side effects of rifampin (eg, nausea, hepatitis) and drug interactions, emergence of resistance to rifampin is a complication when used in staphylococcal infections [30]. There are a few reports also describing rifampin resistance in Cutibacterium [31–34]. Because Cutibacterium isolates from relapse cases were not stored as a routine, we could not determine whether emergence of resistance is a relevant problem in our cohort.
Besides the chosen surgical strategy, the length of antibiotic treatment was an important factor for clinical success in our study. We found that antibiotic regimens of more than 6 weeks were superior to regimens less than 6 weeks, which we interpreted as a need for treating biofilm infections. Infectious Diseases Society of America guidelines also recommend an antibiotic treatment of at least 6 weeks [35]. Compared with the success rate of 86% in the paper of Jacobs et al [15] with 60 Cutibacterium PJI, our lower success rate of 79% overall could be due to treating for less than 6 weeks in 7.5% (14 of 187) of the cases. We did not detect any difference between IV antibiotic duration of more or less than 14 days. An IV treatment of 2 to 4 weeks to treat PJIs was suggested in the review article by Zimmerli et al in 2004 [20], with the rationale of a better bone penetration with IV antibiotics [36]. In line with our results, a benefit of IV treatment longer than 7 days was not shown in the recently published oral versus intravenous antibiotics trial [37, 38].
This study has several strengths and limitations. A strength is that we were able to include a large number of cases from different countries, with in-depth patient and clinical information, with a curative treatment regimen, and a long follow-up time. Gathering data from different centers increases the risk of different ways of data management; hence, a center bias in our results cannot be excluded. We included a sensitivity analysis in which we performed a mixed effects approach including the center as random effect. Moreover, because of the retrospective nature of this project—as compared with a prospective clinical trial—optimal data quality cannot be guaranteed, included missing information. However, huge efforts were taken to clean the data and retrospectively get information about missing and inconsistent data, leading to good quality in the main outcome variables. One limitation concerns the definition of infection relapse. First, we included not only microbiologically proven relapses but also probable relapses. Second, we included relapses happening more than 2 years after septic surgery. It could be the case that these relapses are actually new infections with another Cutibacterium isolate, which is not distinguishable without characterizing the isolates. To overcome this problem, we concentrated on analyzing clinical success (ie, combining relapses and new infections) and analyzed relapses separately.
We conclude that a rifampin combination is not markedly superior, although considering the mixed data both in the literature and this study’s results, it is still inconclusive as to whether rifampin should be recommended. Hence, this emphasizes the need for a dedicated prospective multicenter study. However, our study results suggest to insist on changing the prosthesis and treating with antibiotics for at least 6 weeks in Cutibacterium PJI.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Richard Alexander Kuehl for patient data, Roberto Speck for scientific support, and the ESCMID Study Group for Implant-Associated Infections (ESGIAI) for their support.
Potential conflicts of interest. R. D. K. reports grants from Swiss National Science Foundation, outside the submitted work. B. J. reports grants and personal fees from Medacta International, grants from Depuy J&J, and other support from Bonebridge, outside the submitted work. R. T. reports personal fees from Medacta, personal fees from Zimmer Biomet, outside the submitted work, and is the EBJIS president. R. P. reports grants from Merck, ContraFect, TenNor Therapeutics Limited, and Shionogi. R. P. is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, and Qvella; monies are paid to Mayo Clinic. R. P. is also a consultant to Netflix. In addition, R. P. has a patent on Bordetella pertussis/parapertussis polymerase chain reaction issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. R. P. receives an editor’s stipend from the Infectious Disease Society of America, and honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. J. L. T. reports grants from Correvio, outside the submitted work. J. E. reports grants from BIOFIRE and congress costs from Pfizer, outside the submitted work. T. S. K. reports personal fees from InfectoPharm, grants from B. Braun Medical, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27:302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossard DA, Ledergerber B, Zingg PO, et al. Optimal length of cultivation time for isolation of Propionibacterium acnes in suspected bone and joint infections is more than 7 days. J Clin Microbiol 2016; 54:3043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achermann Y, Liu J, Zbinden R, et al. Propionibacterium avidum: a virulent pathogen causing hip periprosthetic joint infection. Clin Infect Dis 2018; 66:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeller VA, Letembet VA, Meyssonnier VA, Heym B, Ziza JM, Marmor SD. Cutibacterium (formerly Propionibacterium) avidum: a rare but avid agent of prosthetic hip infection. J Arthroplasty 2018; 33:2246–50. [DOI] [PubMed] [Google Scholar]
- 5.Marmor S, Zeller V, Letembet-Ippet V-A, et al. Propionibacterium avidum, un agent rare d’infection de prothèse articulaire de hanche. Rev Chir Orthopédique Traumatol 2017; 103:S88–9. [Google Scholar]
- 6.Renz N, Mudrovcic S, Perka C, Trampuz A. Orthopedic implant-associated infections caused by Cutibacterium spp.—a remaining diagnostic challenge. PLoS One 2018; 13:e0202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton JW, Cheng KJ, Geesey GG, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol 1987; 41:435–64. [DOI] [PubMed] [Google Scholar]
- 8.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284:1318–22. [DOI] [PubMed] [Google Scholar]
- 9.Baldoni D, Haschke M, Rajacic Z, Zimmerli W, Trampuz A. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob Agents Chemother 2009; 53:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleh-Mghir A, Muller-Serieys C, Dinh A, Massias L, Crémieux AC. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:4589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 2011; 53:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis 1992; 14:1251–3. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt M, Stein A, Argenson JN, Roiron R, Groulier P, Raoult D. Oral treatment of Staphylococcus spp. infected orthopaedic implants with fusidic acid or ofloxacin in combination with rifampicin. J Antimicrob Chemother 1997; 39:235–40. [DOI] [PubMed] [Google Scholar]
- 14.Garrigós C, Murillo O, Euba G, et al. Efficacy of tigecycline alone and with rifampin in foreign-body infection by methicillin-resistant Staphylococcus aureus. J Infect 2011; 63:229–35. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs AM, Van Hooff ML, Meis JF, Vos F, Goosen JH. Treatment of prosthetic joint infections due to Propionibacterium. Similar results in 60 patients treated with and without rifampicin. Acta Orthop 2016; 87:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect 2007; 55:119–24. [DOI] [PubMed] [Google Scholar]
- 17.Levy PY, Fenollar F, Stein A, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis 2008; 46:1884–6. [DOI] [PubMed] [Google Scholar]
- 18.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 2012; 56:1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81:515–26. [Google Scholar]
- 20.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004; 351:1645–54. [DOI] [PubMed] [Google Scholar]
- 21.Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 2004; 32:222–8. [DOI] [PubMed] [Google Scholar]
- 22.Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect 2006; 12:433–9. [DOI] [PubMed] [Google Scholar]
- 23.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996; 78:512–23. [DOI] [PubMed] [Google Scholar]
- 24.Betsch BY, Eggli S, Siebenrock KA, Täuber MG, Mühlemann K. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin Infect Dis 2008; 46:1221–6. [DOI] [PubMed] [Google Scholar]
- 25.Barberán J, Aguilar L, Carroquino G, et al. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med 2006; 119:993.e7–10. [DOI] [PubMed] [Google Scholar]
- 26.Nagra NS, Hamilton TW, Ganatra S, Murray DW, Pandit H. One-stage versus two-stage exchange arthroplasty for infected total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2016; 24:3106–14. [DOI] [PubMed] [Google Scholar]
- 27.Kunutsor SK, Beswick AD, Whitehouse MR, Blom AW. One- and two-stage surgical revision of infected elbow prostheses following total joint replacement: a systematic review. BMC Musculoskelet Disord 2019; 20:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sevelda F, Fink B. One-stage exchange of septic shoulder arthroplasty following a standardized treatment algorithm. J Shoulder Elbow Surg 2018; 27:2175–82. [DOI] [PubMed] [Google Scholar]
- 29.Ilchmann T, Zimmerli W, Ochsner PE, et al. One-stage revision of infected hip arthroplasty: outcome of 39 consecutive hips. Int Orthop 2016; 40:913–8. [DOI] [PubMed] [Google Scholar]
- 30.Achermann Y, Eigenmann K, Ledergerber B, et al. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): a matched case-control study. Infection 2013; 41:431–7. [DOI] [PubMed] [Google Scholar]
- 31.Corvec S, Guillouzouic A, Aubin GG, et al. Rifampin-resistant Cutibacterium (formerly Propionibacterium) namnetense superinfection after staphylococcus aureus bone infection treatment. J Bone Jt Infect 2018; 3:255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furustrand Tafin U, Aubin GG, Eich G, Trampuz A, Corvec S. Occurrence and new mutations involved in rifampicin-resistant Propionibacterium acnes strains isolated from biofilm or device-related infections. Anaerobe 2015; 34:116–9. [DOI] [PubMed] [Google Scholar]
- 33.Furustrand Tafin U, Trampuz A, Corvec S. In vitro emergence of rifampicin resistance in Propionibacterium acnes and molecular characterization of mutations in the rpoB gene. J Antimicrob Chemother 2013; 68:523–8. [DOI] [PubMed] [Google Scholar]
- 34.Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Med Mal Infect 2014; 44:241–50. [DOI] [PubMed] [Google Scholar]
- 35.Osmon DR, Berbari EF, Berendt AR, et al. ; Infectious Diseases Society of America . Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 36.Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMeekin N, Geue C, Briggs A, et al. ; OVIVA collaborators . Cost-effectiveness of oral versus intravenous antibiotics (OVIVA) in patients with bone and joint infection: evidence from a non-inferiority trial. Wellcome Open Res 2019; 4:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarborough M, Li HK, Rombach I, et al. Oral versus intravenous antibiotics for bone and joint infections: the OVIVA non-inferiority RCT. Health Technol Assess 2019; 23:1–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.